Abstract
The clinical use of deep brain stimulation (DBS) is among the most important advances in the clinical neurosciences in the past two decades. As a surgical tool, DBS can directly measure pathological brain activity and can deliver adjustable stimulation for therapeutic effect in neurological and psychiatric disorders correlated with dysfunctional circuitry. The development of DBS has opened new opportunities to access and interrogate malfunctioning brain circuits and to test the therapeutic potential of regulating the output of these circuits in a broad range of disorders. Despite the success and rapid adoption of DBS, crucial questions remain, including which brain areas should be targeted and in which patients. This Review considers how DBS has facilitated advances in our understanding of how circuit malfunction can lead to brain disorders and outlines the key unmet challenges and future directions in the DBS field. Determining the next steps in DBS science will help to define the future role of this technology in the development of novel therapeutics for the most challenging disorders affecting the human brain.
The use of deep brain stimulation (DBS) to intervene directly in pathological neural circuits has changed the way that brain disorders are treated and understood. DBS is a neurosurgical procedure that involves the implantation of electrodes into specific targets within the brain and the delivery of constant or intermittent electricity from an implanted battery source. Over 160,000 patients worldwide have undergone DBS for a variety of neurological and non-neurological conditions, with numbers increasing each year1. As a clinical tool, DBS offers several advantages over other surgical approaches for neuromodulation. These advantages include the non-lesional nature of DBS, the capacity to titrate stimulation parameters to maximize benefit and reduce adverse effects and the opportunity to directly interface with the circuit pathology that drives overt symptoms. As a scientific tool, DBS can be used to investigate the physiological underpinnings of brain dysfunction, which enables identification and correction of pathological neuronal signatures and helps to drive technological innovation and enhance safety and clinical outcomes2. Furthermore, as a highly focal intervention with anatomic targets typically on the order of millimetres, DBS has contributed to circuit theories of brain dysfunction by demonstrating that localized dysfunction and intervention can have profound influences on brain-wide networks3–5. This duality of DBS as probe and modulator of brain circuitry has led to the investigation of the therapeutic potential of DBS in a broad range of disorders, including those affecting motor, limbic, memory and cognitive functions1. Notwithstanding its advantages, DBS remains an invasive surgical intervention with low but potentially serious attendant risks, including haemorrhage and infection. Although DBS has become standard of care in patients with movement disorders, its use in other disorders is limited to highly refractory patients and conditions, typically in the context of expert multidisciplinary care and clinical research6.
To date, few indications have been approved for DBS, with the vast majority of procedures performed for movement disorders, most commonly Parkinson disease (PD). Indeed, several randomized controlled trials have found that few treatments are as effective as DBS for controlling the troubling motor symptoms of PD7,8. However, despite the success of DBS, PD is paradigmatic of both the promise and challenges of the technique. For example, although DBS is highly effective in properly selected patients with PD, stimulation at the most commonly used targets — the subthalamic nucleus (STN) or globus pallidus internus (GPi) — is ineffective for the treatment of gait and other axial symptoms and does little to improve (or can even exacerbate) speech and affective and cognitive symptoms9,10. Therefore, intervention at a highly focal point is insufficient as a means of addressing dysfunction of multiple circuits. This concept represents an important limitation and challenge for the field. Additional technical and clinical challenges also exist. Technical innovation will focus on the improvement of practicability, including extension of battery life, design of smaller devices and development of more tailored and adaptive stimulation in addition to the integration of wireless technology. Clinically, the main challenge will be to meet the needs of an ageing population worldwide and expand indications for DBS to circuitopathies other than PD, including depression and Alzheimer disease (AD)1. Even within established indications such as PD, key questions remain unanswered. Biomarkers that predict clinical response and aid in patient selection and stimulation parameter settings are still largely lacking. Furthermore, the timing of intervention is controversial, with some strong evidence that early surgery might be more beneficial than late7. Answers to these questions will shape not only which patients are offered surgery but also the direction of the field for years to come.
The scope of DBS is rapidly expanding and parallels our increasing understanding of the nature of brain circuit dysfunction (Table 1). In order to take stock of the field, this Review addresses the status of DBS by highlighting its current challenges and future. We begin by reviewing the putative mechanisms of DBS and its effects on neural tissue and networks, followed by an overview of how preclinical models have informed translational applications. We then provide an overview of the spectrum of clinical applications, from motor to non-motor, including the challenges for both widely used and emerging indications. Finally, we conclude by examining the clinical, technical and ethical challenges that will help to inform future directions of the field.
Table 1. Disorders currently under investigation with deep brain stimulation.
| Disorder | Circuit | Postulated circuit dysfunction | Deep brain stimulation target(s) being studied or that could be considered | Stage of study |
|---|---|---|---|---|
| Parkinson disease, essential tremor or dystonia | Motor |
|
STN, GPi, GPe, VL thalamus, PPN and spinal cord | Standard of care |
| Major depression | Limbic |
|
SCC, NAcc, habenula and medial forebrain bundle | Phase III |
| Obsessive–compulsive disorder | Motor and limbic |
|
NAcc, BNST, ITP, ALIC and STN | Phase II/III |
| Tinnitus | Auditory |
|
Auditory pathways and caudate nucleus | Phase I |
| Tourette syndrome | Motor and limbic |
|
GPi and CM-Pf | Phase I |
| Schizophrenia — positive symptoms | Executive function, cognition and reward |
|
Temporal cortex and NAcc | Preclinical |
| Schizophrenia — negative symptoms | Motivation, reward, cognition and mood |
|
NAcc, VTA and SCC | Preclinical |
| Alzheimer disease | Cognitive and memory circuits |
|
Fornix, entorhinal cortex, hippocampus, cingulate, precuneus, frontal cortex and nucleus basalis | Phase II/III |
| Pain (phantom pain, deafferentation pain, central pain and nociceptive pain) | Sensory systems and interoceptive awareness |
|
Sensory pathways, periventricular and periaqueductal areas, cingulate and insula | Phase I/II |
| Addiction | Reward | NAcc sensitivity to reward | NAcc | Phase I/II |
| Anorexia nervosa | Reward and mood |
|
SCC and NAcc | Phase II |
| Epilepsy | Various | Abnormal excitability and synchrony | CM thalamus, anterior thalamic nucleus, thalamus and seizure focus | Phase II/III |
ALIC, anterior limb of the capsula interna; BNST, bed nucleus of stria terminalis; CM, centromedian; CM-Pf, CM–parafascicular; GPe, globus pallidus externus; GPi, globus pallidus internus; ITP, inferior thalamic peduncle; NAcc, nucleus accumbens; OFC, orbitofrontal cortex; PPN, pedunculopontine nucleus; SCC, subgenual cingulate cortex; STN, subthalamic nucleus; VL, ventral lateral; VS, ventral striatum; VTA, ventral tegmental area. Adapted with permission from REF.1, Elsevier.
Rationale and mechanisms of action
Many hypotheses have been proposed for the mechanisms by which DBS operates (Table 2). Prevailing theories have focused on stimulation-induced disruption of pathological brain circuit activity1,11. The stimulation effects responsible for this disruption occur at the ionic, protein, cellular and network levels to generate improvements in symptoms12 (Fig. 1). Although it is currently unclear which of the wide-ranging effects of DBS are necessary and sufficient to generate therapeutic outcomes, it is clear that high-frequency (~100 Hz) trains of pulses (~0.1 ms) produce network responses that are fundamentally different (for example, inhibitory effects) from low-frequency (~10 Hz) stimulation.
Table 2. Proposed deep brain stimulation mechanisms.
| Concept | Example evidence for | Example evidence against | Refs |
|---|---|---|---|
| Direct inhibition of neural activity | Somatic recordings from neurons close to the stimulating electrode |
|
133,134 |
| Direct excitation of neural activity |
|
Stimulation-induced action potentials intermittently or inconsistently generate postsynaptic responses | 135,136 |
| Information lesion (jamming) |
|
Network interactions remain intact for high-frequency signals | 20,21 |
| Synaptic filtering |
|
Limited understanding of chronic high-frequency driving of synapses | 137,17 |
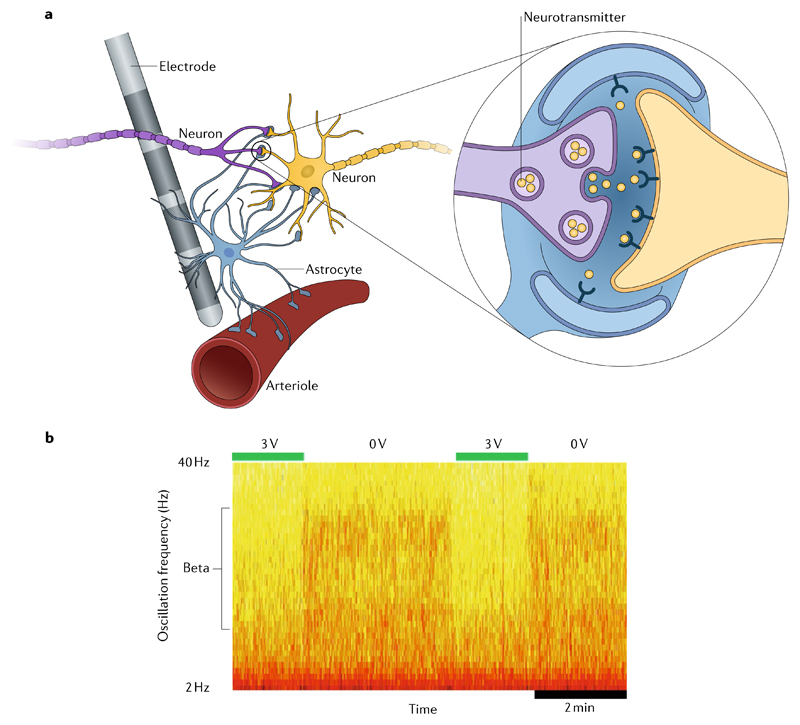
Fig. 1. Deep brain stimulation mechanisms.
a | Neurotransmitters (inset) are released in response to stimulation, leading to calcium waves and subsequent release of gliotransmitters. This release influences synaptic plasticity, leading to arteriole dilation and increased regional blood flow. b | Deep brain stimulation (DBS)-induced changes in local field potentials within the subthalamic nucleus. Activity in the beta band is rapidly reduced with DBS at 3 V and then resumes with stimulation off.
At the ionic level, the purpose of an electrode implanted into the brain and polarized to a negative potential (that is, a cathode) is to redistribute charged particles (such as Na+ and Cl− ions) throughout the extracellular space. This redistribution creates an electric field that can manipulate the voltage sensor of sodium channel proteins imbedded in the membrane of neurons13. At the cellular level, the opening of sodium channels can generate an action potential, which typically initiates in the axon. Stimulation-induced action potentials then propagate in both the orthodromic and antidromic directions to the axon terminals of the neuron. Under the typical conditions of DBS, many axons will be stimulated. The stimulated axons are capable of following stimulation frequencies at ~100 Hz with very high fidelity, but synaptic transmission of these high-frequency signals is a far less robust and much more complicated process than that of axonal transmission14,15. Axon terminals can exhaust their readily releasable pool of neurotransmitters and postsynaptic receptors can depress under such high-frequency activity16,17. Even if these synapses remain functional during DBS, information processing theories dictate that they will become low-pass filters that suppress transmission of low-frequency signals18. This general phenomenon, known as ‘synaptic filtering’, could have a key role in DBS, whereby the neurons and connections that are directly stimulated by DBS hinder the propagation of oscillatory activity patterns within their associated brain networks19.
The basic biophysical effects of DBS provide a context in which to begin to interpret the network activity patterns that are observed in patients. As stimulation frequency remains constant during DBS, the information content of the stimulation signal is effectively zero, which could generate what is known as an ‘information lesion’ in stimulated neurons20. Under this hypothesis, DBS-induced action potentials effectively override any intrinsic activity in the directly stimulated neurons and thereby limit the propagation of oscillatory activity through the network. In addition, the basic concepts of information lesion and synaptic filtering might work in concert to generate robust suppression of low-frequency signals in stimulated brain circuits.
However, not all data support the hypothesis that high-frequency DBS introduces a simple information lesion. Studies in awake and behaving primates have provided some evidence that physiological sensorimotor-related discharge in the pallidum might be maintained at least partially during STN or pallidal DBS21,22. These studies suggest that DBS might act as a filter that permits some sensorimotor-related modulation of the activity of neurons in the stimulated area while selectively blocking transmission of pathological low-frequency oscillations. Likewise, other basal ganglia functions such as motor sequence learning or reward-based decision-making can be preserved during DBS of the STN or globus pallidus23. Nevertheless, the information lesion hypothesis might be reconciled with these observations if physiological coding in the basal ganglia is predominantly supported by mechanisms other than synchronization, which are thereby mostly spared by high-frequency DBS. Indeed, the sparsity of correlations between neurons in the basal ganglia in health supports this model24.
Other network-level factors might also have important roles in the therapeutic mechanisms of DBS for PD. First, the thalamus might act as a low-pass filter by transmitting synchronized inputs from the basal ganglia at frequencies within and below the beta band (12–30 Hz) but not transmitting signals at the high frequencies driven by DBS (>100 Hz)25–28. Second, changes to circuit resonances in PD might maximize the potential for postsynaptic targets to be entrained by low-frequency activity as opposed to the high frequencies driven by DBS29,30. The net result of such factors is that high-frequency DBS might provide an effective local information lesion that blocks the transmission of low-frequency oscillations but, unlike synchronization at low frequency, might have little effect on the function of the wider network27,31. One of the attractions of this schema is that high-frequency DBS then becomes a generic tool that is able to override different forms of pathological low-frequency oscillation, such as those underlying mobile dystonia, tremor and akinesia–rigidity32.
The hypothetical mechanism for DBS outlined above helps to explain only the acute effects of DBS in a subset of movement disorders. It does not explain the longlatency, chronic adaptive changes that occur after DBS in patients with dystonia and can characterize the response to DBS in psychiatric diseases such as depression. One relevant possibility is that low-frequency oscillations are actively reinforced through long-term potentiation, whereas high-frequency stimulation has a lesser effect on plasticity. In this way, replacement of low-frequency patterning with high-frequency stimulation might undo some chronic disease-related phenomena33. Even so, little evidence currently supports an association between psychiatric diseases and pathologically synchronized low-frequency activity within basal ganglia–cortical circuits, which leaves open the possibility that DBS might also work through other mechanisms. One key area of current interest is the effects of DBS on astrocytes, given their role in integrating synaptic information and regulating synaptic plasticity12. The effects of DBS are often delayed and progressive and sometimes take months to achieve maximal benefit in a variety of disorders, including dystonia, depression and epilepsy. Interest is growing in the neuroplastic changes induced by DBS that might be linked to the ability to upregulate the expression of trophic and synaptic proteins with stimulation34.
Insights from animal models
Animal models have played a crucial part in the clinical application of modern DBS in patients with neurological disorders (Table 3). The most evident example is DBS of the STN in PD. The STN was found to have an abnormally increased activity in non-human primates with parkinsonian symptoms caused by treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; an agent toxic to dopaminergic neurons in the STN)35. However, experimental lesions of the STN resulted in clear-cut improvements of rigidity and hypokinesia in the same animal model36,37. Lesions of the STN were so effective at alleviating symptoms that levodopa or apomorphine therapy was not necessary. These findings supported the hypothesis that pathological activity occurs in the STN in PD and that ablation of this area would improve parkinsonian symptoms. In the meantime, DBS-mediated blockage of depolarization, induced by chronic electrical stimulations at high frequencies, was introduced as an alternative for ablation38. The final piece of evidence came again from an animal study. In monkeys rendered parkinsonian by MPTP, high-frequency stimulation of the STN improved motor disability. From these findings, a successful therapy for patients with PD was born39.
Table 3. use of animal models to understand brain circuits.
| Indication | Animal model | Main contribution |
|---|---|---|
| Parkinson disease | MPTP in non-human primate | |
| Epilepsy | Pentylenetetrazol in guinea pigs and rats | |
| Huntington disease | Transgenic rat model |
|
| Compulsivity-related behaviour | Polydipsia rat model |
|
| Depression-like behaviour | CMS rat model |
ANT, anterior nucleus of the thalamus; BDNF, brain-derived neurotrophic factor; BNST, bed nucleus of stria terminalis; CMS, chronic mild stress; GPe, globus pallidus externus; MMT, mammillothalamic tract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; STN, subthalamic nucleus; VMPFC, ventromedial prefrontal cortex.
Another contribution of animal models to clinical application of DBS has been in the field of epilepsy. In a guinea pig model of epilepsy, sectioning of the mammillothalamic tract (MMT) increased the dose threshold for pharmacologically induced seizures40. The MMT is a key component of the circuit of Papez and projects mainly to the anterior nucleus of the thalamus (ANT). In animal models, electrical stimulation of the MMT or the ANT showed anti-epileptic effects41,42. These findings, together with clinical case studies, were the basis for the randomized controlled trial of DBS of the ANT in epilepsy43. This trial helped to provide evidence for the efficacy of DBS in epilepsy. The search for effective targets for DBS in specific types of epilepsy is ongoing44.
In the field of psychiatric disorders, animal models have provided important insights into the mechanisms of action of DBS45. Although early case series showed promising effects of DBS in patients with treatmentrefractory depression, the outcomes of large controlled clinical trials showed limited success46. Data from animal models provided new clues on the potential cause of this discrepancy. DBS has been applied to a number of brain areas in rats exposed to chronic mild stress, and the effects have been evaluated using a battery of behavioural tests encoding motivation, anxiety, anhedonia and behavioural despair47. The regions stimulated have a crucial role in the regulation of negative emotions and are interconnected with a wide range of networks that form a neurocircuitry for affective disorders48,49. The main conclusion of these studies was that different brain regions improve different aspects of mood-related behaviours. High-frequency stimulation of the nucleus accumbens and lateral habenula enhanced motivational aspects of behaviour and reduced anxiety levels, whereas high-frequency stimulation of the ventromedial prefrontal cortex enhanced hedonia and reduced behavioural despair47. These findings suggest that the choice of brain target for DBS should depend on the key symptoms to be treated rather than aiming to resolve a complex and multifaceted disorder such as depression. This approach was demonstrated by one study that looked for potential targets for DBS to treat tics in Tourette syndrome. Stimulation of the anteromedial part of the STN in monkeys that exhibited tic-like behaviour resulted in a reduction of stereotyped movements in these animals50.
Animal studies have also contributed substantially to our understanding of the mechanisms underlying therapeutic and adverse effects of DBS. From an anatomical point of view, we have learned that the effects of high-frequency stimulation go beyond the classic concepts of monosynaptic connectivity. In dopamine-depleted rats, high-frequency stimulation of the STN ameliorated motor disability but induced a remarkable change in mood. This effect was linked to reduced firing of serotoninergic neurons in the midbrain51,52. These brain regions are not connected directly, but high-frequency stimulation nevertheless uncovered a high level of functional connectivity. Furthermore, electrical stimulation approaches have demonstrated that individual STN neurons receive input from motor and limbic areas53. In this way, two distinct behavioural modalities, motion and emotion, can converge, which explains some of the mixed clinical effects of DBS.
Among the major lessons learned from DBS in animal models is the differential effect of the modulation of microcircuits and macrocircuits on key symptoms. This difference also supports current shifts from modelling a disorder towards modelling key symptoms and linking them to specific circuits and neuronal populations or subsets of these circuits and populations. The availability of an increasing number of neuromodulation approaches for animal models — involving electricity, light, sonography and magnetic nanoparticles — is promising and is likely to facilitate new breakthroughs in the field of clinical DBS54.
DBS in movement disorders
Over the past 25 years, DBS has become the standard of care for patients with treatment-refractory motor circuit disorders — most commonly PD, dystonia and essential tremor. DBS is highly effective at controlling motor symptoms but remains very resource intensive. To date, use of DBS has been limited to high-income countries, although use in many developing nations is rising55. Analysis of a US database of hospital discharges between 2002 and 2011 showed that more than 30,000 DBS surgeries were performed during that time56. The numbers of publications on DBS have also risen steeply over the same period, with more than 7,000 manuscripts published between 1991 and 2014 (REF.57). A drop in the number of publications in DBS for PD over the past 5 years might represent progressive scholarly acceptance, whereby the number of investigations that refine or improve a procedure eclipses the total number of reports assessing initial efficacy58.
Parkinson disease
The STN, a key motor relay structure for which dysfunction has been linked to PD symptoms, is the most commonly used target for DBS over the past 10 years59. The GPi is also a common target, and the choice between the STN and GPi is most commonly informed by discussion within a multidisciplinary team and dictated by the patient’s clinical profile and needs. Although randomized studies have shown that STN stimulation might have a greater effect on motor symptoms and dopaminergic medication reduction than GPi stimulation, adverse cognitive and mood effects might be more common after STN stimulation8,60. Numerous studies have also shown that STN DBS provides persistent symptom improvement even 5 or 10 years after surgery, albeit with deterioration of cognition and gait due to the relentless progression of the underlying degenerative disorder61. DBS has been termed ‘the second honeymoon’ in the treatment of PD (with dopaminergic treatment being the first). However, chronic DBS has also created a new phenotype of PD: patients in whom bradykinesia, tremor, rigidity, on–off fluctuations and dyskinesias are improved but who continue to present with progressive gait, speech and cognition problems62. Gait problems, in particular, become important and difficult to manage at late stages of the disease63. DBS of the pedunculopontine nucleus area has been proposed as a measure to improve freezing and postural instability with the goal to reduce related falls, but the selection of appropriate candidates and the difficulty of demonstrating objective benefit have become major obstacles to widespread use of this approach64.
There is a general consensus that a particular type of patient with PD would benefit from DBS — those with advanced disease, motor fluctuations and dyskinesias secondary to chronic levodopa as well as those with refractory and marked tremor. However, findings by the EARLYSTIM study, which suggest that DBS at earlier stages of PD is associated with substantial clinical benefit, have considerably widened the spectrum of patients with PD to whom DBS is offered7. Indeed, trials have now begun to investigate the use of DBS even in patients who do not have motor fluctuations and who can be managed well with medication65. However, given the risk of haemorrhage and infection inherent with DBS, such studies can pose ethical challenges. Ongoing work is now investigating the role of DBS in other challenging cases, including in patients with PD who might be considered too old for surgery — a population that is typically excluded from trials and other surgical interventions66.
Dystonia
The development of DBS for dystonia has lagged about a decade behind its use in PD67. Several randomized sham-controlled trials with blinded delayed-onset stimulation have now demonstrated the efficacy of pallidal DBS for generalized and segmental primary (inherited and idiopathic) dystonia and for cervical dystonia. As a result, DBS has come to play an important part in the treatment of dystonic disorders68,69. For example, pallidal DBS is now considered to be first-line treatment in some childhood generalized dystonias. Age at surgery and duration of dystonia have been identified to be the most important outcome predictors70–72. Genetic background has also been noted to have a major role; for example, the benefit might be superior in patients with DYT1 dystonia compared with those with DYT6 dystonia73. As a result, genetic testing of patients with dystonia who might undergo DBS has been suggested to identify patients who are the most likely to benefit from the procedure74.
The posteroventral lateral GPi has become the most established target for DBS in dystonia75. GPi stimulation provides marked improvement in many manifestations of dystonia with a low frequency of adverse effects. However, bradykinesia and gait problems have been found in patients (especially adults) with segmental dystonia who achieve a good response to DBS; this phenomenon has not yet been fully understood, but it has opened new views on the function of the basal ganglia76,77. Fortunately, these adverse effects usually can be managed by making a compromise between maximal stimulation benefit and the occurrence of these symptoms. Additional targets for DBS that are under investigation for dystonia include the STN and the thalamus. However, despite promising preliminary results of STN DBS, its clinical use thus far has been limited78. Another interesting target is the sensorimotor thalamus, which was regarded as the standard target for dystonia in the era of radiofrequency lesioning79,80. One challenge for future research will be to determine which region in the ventrolateral thalamus would be the ideal target for DBS — the anterior (that is, the Voa according to Hassler), posterior (the Vim) or intermediate regions (the region formerly termed Vop).
The mechanisms by which DBS achieves its clinical effect in dystonia are complex, as demonstrated by the often delayed and progressive improvement exhibited by patients over a period of months. Hypotheses regarding the underlying mechanism include modification of maladaptive plasticity, progressive motor learning, altered inhibition and alterations in pathological oscillatory activity in basal ganglia circuitry81. The long-term benefit of chronic DBS in dystonia is often delayed, requiring weeks or months of stimulation to achieve optimal benefit. Long-term stimulation also seems to produce long-lasting changes in the brain. Interestingly, although dystonia can recur within minutes to hours after stimulation has been turned off in the early postoperative period82, the benefits from stimulation that has been administered for several years can persist for days and weeks after cessation83. DBS might, therefore, act as a true disease-modifying treatment in dystonia, which might justify its use earlier in the course of the disease and not just when conservative treatment either is not available or is poorly effective. Such reasoning has prompted calls for an EARLYSTIM study in dystonia. An unresolved issue is the limited benefit of DBS in acquired forms of dystonia and in patients in whom dystonia is accompanied by other neurological symptoms84. In particular, in this large group of patients, future outcome evaluations need to move beyond the measurement of improvements solely with specific scales that focus on the severity of dystonia. Patient-specific characteristics, such as baseline functional status, need to be considered in addition to changes in disability and quality of life, and the so-called success or failure of therapy in severely disabled patients might need to be redefined85,86.
Tremor
Essential tremor was the first movement disorder indication for which DBS was approved by the FDA in 1997 (REF.38), and, after its efficacy was proved in numerous studies, it has become a routine treatment87. Habituation and the emergence of long-term adverse effects such as dysarthria and gait ataxia in a subset of patients after several years of chronic stimulation remain challenges in DBS treatment of patients with essential tremor88. Whether stimulation of the subthalamic region or direct targeting of fibre tracts in that area would provide better long-term improvement is still unclear. Although DBS is safe and effective, lesional therapies such as radiofrequency ablation, radiosurgery and, increasingly, magnetic-resonance-guided focused ultrasonography are also often considered89. However, DBS remains the procedure of choice for bilateral procedures owing to the increased safety that accompanies the adjustability of the stimulation — something that lesional surgery does not offer. Thalamic DBS has also been used for other types of tremor, including in multiple sclerosis, for which a randomized, blinded trial found substantial clinical benefit90.
Tourette syndrome
DBS for Tourette syndrome was introduced as early as the late 1990s91. Yet, when compared with the development of DBS in other movement disorders, propagation of this treatment modality in Tourette syndrome has been slow. The number of patients with Tourette syndrome who have undergone DBS thus far is estimated to be less than 300 world-wide92. One of the major problems of DBS in this patient group is the complexity of symptoms, which consist of a variety of tics and psychiatric disturbances, such as personality disorders, anxiety, depression, substance abuse and many others. The doubts as to how best to treat Tourette syndrome with DBS are reflected by the uncertainty about the target choice, which includes sub-territories of the basal ganglia and the thalamus that are involved in motor and limbic circuitries92.
Over the years, the efficacy of DBS for Tourette syndrome has been demonstrated in several case series, generally with low patient numbers. According to a meta-analysis, patients with severe symptoms benefited less than those with mild symptoms92. A randomized controlled trial published in 2017 did not detect significant improvement of tics in individuals with Tourette syndrome treated with anteromedial GPi stimulation during the initial blinded phase of the study, but amelioration of tics was confirmed in the open phase of the study93. More studies with randomized controlled designs are needed.
DBS in pain and epilepsy
Pain
Chronic pain was the first indication for chronic DBS, decades before it was considered as a routine treatment for movement disorders94. However, after two large-scale studies in the 1980s and 1990s were stopped for various reasons (including slow patient recruitment), DBS for pain failed to gain widespread popularity and its use was limited to a few specialized centres world-wide95. The evaluation of the results of DBS has been intrinsically more difficult in patients with pain than in patients with movement disorders owing to the subjectivity of the self-assessment of pain. Although nociceptive pain generally can be well controlled with opiate treatment, DBS of targets in the thalamus or in the cingulum is considered for patients with severe refractory neuropathic pain95,96.
Epilepsy
For many decades, the mainstay of surgical treatment of epilepsy has been resective surgery. DBS has been introduced as an option for patients in whom a circumscribed focus amenable for resection cannot be identified. Targets of stimulation include thalamic nuclei such as the ANT or the centromedian–parafascicular complex and the hippocampus itself43,97. Early expectations that DBS would become a central strategy in epilepsy — and possibly replace open resective surgery — were dampened after publication of studies on DBS of the ANT, which demonstrated efficacy but also clearly showed that the majority of patients would not become seizure-free43,98. Closed-loop stimulation, which detects seizure activity with sensing electrodes and delivers electric stimulation to prevent seizure propagation, is a promising technology that needs further exploration99.
DBS for psychiatric indications
Only three DBS indications have received approval by the FDA: PD, dystonia and essential tremor. However, the past two decades have seen rapid advances in our understanding of putative circuits that drive the most common neurological and psychiatric disorders. The success of DBS in modulating dysfunctional motor circuits has spurred the investigation of DBS in other non-motor conditions, predominantly those that affect limbic circuits. Several prospective trials have been conducted to determine whether focal disruption at discrete anatomic targets can affect circuit-wide and network-wide changes in an effort to treat refractory psychiatric symptoms. Although the strategy is promising, several challenges remain. Psychiatric disorders are highly heterogeneous conditions that affect multiple overlapping circuits. These conditions have few (if any) biomarkers to guide treatment or outcomes, and consensus regarding the optimal outcomes to measure is lacking. All of these factors hamper the development of rigorously designed clinical trials. Furthermore, the execution of surgical trials is hampered by substantial challenges surrounding recruitment, in which factors such as heterogeneous referral patterns, a lack of consensus on the definition of treatment resistance and an overall poor awareness and competition for patients across ongoing trials all contribute100. Notwithstanding these challenges, the prospect of a direct interface with pathological brain circuits in a reversible, non-ablative and image-guided fashion continues to spur strong interest in DBS for these emerging indications.
Major depression
Major depression is a common and challenging condition that can substantially affect quality of life, daily functioning and, ultimately, life expectancy101,102. The impact of this disorder on individual patients has not been lost on the generations of researchers who have tried to develop treatments with sustained antidepressant efficacy. Owing to advances in functional imaging, evidence is now emerging that depression is driven by disturbances in key mood-related circuits and that neuromodulation, along with other antidepressant treatments, can contribute to reversals of circuit pathology.
Several brain targets for DBS are currently under investigation for the treatment of depression, including the white matter adjacent to Brodmann area 25 in the subgenual cingulate cortex (SCC)103,104, the anterior limb of the capsula interna (ALIC), the ventral caudate105, the lateral habenula106 and the superolateral branch of the medial forebrain bundle (slMFB)107. To date, none of these targets have convincingly proved to be more effective than the others, and indeed some investigators have suggested that all of these areas represent key nodes in the same affective regulatory circuit. Interestingly, DBS to most targets seems to be associated with sustained efficacy in individual patients, an outcome rarely seen with other therapeutic interventions. Among all of these areas, the SCC has been targeted in the greatest number of patients to date; DBS in this area has been linked to treatment response rates (defined as >50% reduction in the Hamilton Depression Score compared with baseline) of ~60–70%108. However, two industry-sponsored multicentre randomized sham-controlled trials of either SCC or ALIC DBS in depression failed in their primary outcome measure. In the larger of the studies, the SCC BROADEN trial, no difference was found in response rates between the active and sham stimulation arms after 6 months, with the suggestion that total time of active stimulation (time ‘on’) was possibly linked to improved outcomes over time109,110. On a more promising note, approximately one-half of the patients were deemed to have responded to treatment after 18 months to 2 years of open-label stimulation. Both studies were halted after a planned futility analysis of the data from the first patients treated. Such results underscore the challenges of large multicentre trials in a complex, highly heterogeneous disorder such as depression.
Bipolar disorder
Patients with bipolar disorders have extreme and intense emotional states that occur at distinct times, called mood episodes; these disorders occur less frequently than major depression but are as debilitating and are associated with increased risk of suicide. Few patients have been included in DBS studies of major depression, but no evidence indicates that DBS is less effective in bipolar depression than in unipolar depression111. DBS to the SCC, the nucleus accumbens and slMFB seems to be associated with therapeutic effects in bipolar disorders, but randomized, sham-controlled trials have not yet been completed112.
Obsessive–compulsive disorder
Obsessive–compulsive disorder (OCD) is a devastating psychiatric disorder and is marked by severe, egodystonic compulsions and anxiogenic thoughts (that is, obsessions that are associated with time-consuming and subjectively anxiolytic behaviours). Patients often spend hours, at the expense of their relationships, education and careers, engaged in these thoughts and behaviours, which lead in many cases to profound disability and depression. Although psychopharmacological and psychotherapeutic strategies are available that are effective for many patients, up to one-third of patients do not respond to standard, guideline-concordant care and are eligible for neuromodulation.
In 1999, stimulation of the ALIC was proposed as an alternative to irreversible capsulotomy for the treatment of OCD and was among the very first psychiatric indications for DBS113. Early results led to a redefinition of the target as the area just ventral to the ALIC (the ventral capsule and ventral striatum) and/or the nucleus accumbens114–116. In the past few years, several groups have moved the target more posteriorly, aiming at the bed nucleus of the stria terminalis117. As with depression, multiple targets have been proposed for the treatment of OCD with DBS, and most are in the investigational stages at present. After reports of improvements in OCD with STN DBS in patients with comorbid PD and OCD, a French multicentre study explored the effects of DBS in the associative limbic part of the STN118 and found statistically significant reduction of OCD symptoms. Furthermore, patients with OCD treated with DBS in the region of the ventral striatum showed reduced depression, which has led teams in North America and Europe to explore the use of DBS in the treatment of patients with severely refractory depression119,120. In the past few years, stimulation of the slMFB was reported to be associated with sizeable, rapid and sustained efficacy in OCD121. Studies are ongoing in North America, Europe and elsewhere on these applications.
Anorexia nervosa
Anorexia nervosa is a common, pervasive and highly challenging condition with one of the highest mortalities of any psychiatric disorder. Although the physical manifestations of the illness — namely, severe emaciation and malnourishment — are often the most obvious, a growing body of literature has recognized the key role that limbic and emotional circuitry have in triggering and maintaining the illness. The paucity of available treatments in patients with refractory anorexia nervosa and the promising evidence of beneficial effects of DBS in mood-related circuits have led to increased interest in DBS for this condition, whereby the procedure provides a means to directly intervene in illnessdriving circuits and to address high rates of comorbid mood disorder and anxiety. Several open-label, prospective case series have been published investigating the role of DBS in anorexia nervosa. In the largest series to date, 16 patients underwent SCC DBS and were monitored clinically and radiographically for 1 year122. DBS was associated with statistically significant improvements in measures of depression and anxiety and was also linked to sustained changes in cerebral glucose metabolism in key illness-related structures, as measured by fludeoxyglucose–PET. Several months after treatment initiation, patients began to show progressive improvements in weight that were believed to be related to improved control of affective regulation and increased engagement with intensive treatments specific for anorexia nervosa. These results (among others) suggest that the role for DBS in complex conditions such as anorexia nervosa might act as adjuncts to comprehensive and multifaceted treatment plans in highly refractory patients.
DBS in Alzheimer disease
AD is the most common neurodegenerative condition and is marked by progressive declines in memory and cognitive function over decades. Although the past three decades have yielded substantial advances in our understanding of the pathological hallmarks of AD histologically, genetically and radiographically, little therapeutic progress has been made. Current treatment strategies aim to boost acetylcholine availability, reverse known biochemical and metabolic disturbances or clear or prevent amyloid and tau deposition. The ability of DBS to influence activity in key limbic circuits has driven its investigation in AD. Initial studies reported that stimulation in hippocampal outflow pathways led to substantial reversals in hypometabolism and stabilization of cognitive decline in some patients. Several DBS targets for AD have been proposed, including regions immediately anterior to the fornix, entorhinal cortex and the nucleus basalis of Meynert (NBM). Most reports to date have been prospective and have demonstrated that DBS in memory pathways could lead to physiological, network-wide metabolic effects and influence some aspects of memory function. In one study, six patients with AD underwent stimulation of the NBM in a combined 4-week double-blind, 11-month open-label study123. The authors reported that at 12 months, four of six patients responded to treatment. However, a randomized, double-blind, phase II study of fornix DBS in mild AD did not identify a significant difference between active and sham stimulation in the primary cognitive outcome measure at 12 months124. This study did show a statistically significant interaction between patient age and treatment outcomes, whereby patients older than 65 years showed a trend towards improvement in memory and cerebral metabolism at 12 months. Determination of which patients with AD are likely to respond to DBS and which are not remains an area of active investigation. Indeed, the variables that influence outcome are among the inherent challenges of DBS clinical research and can include baseline neuroanatomic substrates, surgical technique and lead placement and choice of target population and outcome measures.
Emerging technology and strategies
The evolution of DBS and its place in the management of patients with refractory brain conditions are intimately related to advances in technology. These advances have shaped not only the device itself and its components (for example, with enhanced tolerability and improvements in battery life and device size) but also the postoperative period, in which safe coupling of DBS to high-resolution imaging can now help to shape our understanding of the clinical effects of stimulation and the effect on brain-wide networks and circuits.
DBS technology
The evolution of hardware and software for spinal cord stimulation in pain management has advanced ahead of that of DBS. Spinal cord stimulation hardware now includes surgical paddles with 32 contacts125, expanded MRI labelling, pulse generators with built-in accelerometers126, the ever-shrinking size of pulse generators, systems with no pulse generator127 and special leads for stimulation of the dorsal root ganglion. The field is also reaping the benefits of new waveforms and software strategies, such as high-frequency, high-density and burst stimulation. DBS, on the other hand, remains a generation behind. We must consider where we, along with industry partners, should focus our efforts to bring DBS technology into the future. A key issue has been that where there is no competition, there is no innovation: for two decades, a lack of competition has persisted in DBS technology, which has suffered from stagnation as a result. Fortunately, competition now exists, which should open the door to new ideas and developments (Box 1).
Box 1. Major initiatives in the deep brain stimulation field.
Advances in control of DBS
Closed-loop DBS. Stimulation can be on demand, such as the triggering of thalamic DBS by arm movement in essential tremor or during seizure activity in epilepsy.
Alternatively, closed-loop DBS can be adaptive, with continuous modulation of DBS by feedback such as the level of beta power in the subthalamic nucleus local field potential in Parkinson disease.
Phase-controlled DBS. Stimulation is delivered at the specific timings (phases) that either increase or attenuate oscillations, as required for therapy. This approach has been piloted in thalamic DBS for tremor.
Model-based control. DBS parameters are selected and modified according to a model of the underlying neural circuitry.
Advances in pattern of DBS
Coordinated reset DBS. This pattern of DBS is intended to disrupt locally synchronized oscillations and change synaptic strengths so that such activity is no longer promoted.
Advances in electrode design
High-resolution electrodes. Thin-film technology and other advances are allowing the development of multi-contact electrodes, which can even be flexible if required. The intention is to provide better control of the stimulation field and high-resolution readouts of neural circuit dysfunction.
Novel IPG design
Miniaturized IPGs. IPGs that are small enough to be embedded in the skull.
Efficient rechargeable batteries. This innovation would enable increased battery life and reduce the risk associated with surgical battery changes.
DBS, deep brain stimulation; IPG, implanted pulse generator.
Similar to any continuous therapy, DBS requires appropriate dosing. Dosing in DBS uses electrical stimulation parameters that control the shape and extent of the electrical field and, within limits, the type of neural elements that are modulated. Although DBS affects a number of electrically responsive neural elements within a given target volume, including cell bodies, dendrites, axons and glial cells, one can simplify (for biophysical reasons) the considerations regarding optimal dosing to the excitation of axons of different conduction velocity and orientation, which are responsible for most of the clinical effects. The principal goal of programming is to maximize the effect of DBS on the fibres that underlie the beneficial effect of the therapy and avoid the recruitment of fibres related to adverse effects (such as corticobulbar fibres that cause dysarthria) at the lowest possible energy costs to improve device longevity.
In current clinical practice, programming is a time-consuming, iterative, trial-and-error process in which certain parameters are set based on experience, stimulation responses are observed and parameters are re-adjusted on the basis of clinical outcome. This process works reasonably well if symptoms can be reliably monitored and respond quickly to parameter changes (for example, tremor or rigidity), enabling a time-limited ‘monopolar review session’, whereby DBS lead contacts are individually tested for efficacy and safety. However, many circuit disorders might not fulfil these criteria, such as dystonia, depression or other conditions involving long-term neuroplastic changes. The resulting risk is an inappropriate dose — often an overdose — of DBS.
In the past few years, we have seen a trend towards a translational approach to programming based on an improved understanding of the biophysical and physiological properties of DBS parameters. This approach has helped to partially overcome the lack of progress in DBS development. DBS devices are now developed with consideration of the specific neurophysiological demands of brain stimulation rather than choices being dictated by electrical engineering and cost considerations across different pacemaker platforms. Dose-finding studies are needed that confirm an appropriate subset of the large DBS parameter space for specific DBS indications. Predictions of suitable parameters can be derived from assumptions about the target volume, target elements and computational models. This method has been successfully used to model the shape and extent of the volume of brain tissue activated by DBS, and tools for this task are now commercially available that enable anatomic visualization of DBS dosing. However, predictive models of the complex and dynamic interactions between temporal pulse parameters and disordered neural communication that underlies circuitopathies are much more difficult to develop.
Patient registries
DBS registries are repositories of clinical and technical information that enable identification and analysis of therapeutic effects and adverse events. An important potential advantage of such registries is aggregation of information on these effects, which enables detection of DBS-mediated improvements in comorbid features of an illness, among other benefits. Furthermore, these registries permit researchers to detect changes in primary outcomes measures, which might influence subsequent study designs128. For example, a case report of DBS in obesity reported substantial improvements on autobiographical memory with stimulation, despite having no effect on obesity. A trial of DBS in AD was then designed that used the same target129. Trial registries with posted, pre-specified outcome measures at the outset of a trial enable researchers to determine which outcomes have been achieved and whether these outcomes need to be modified for subsequent trials.
A central registry for therapeutic DBS trials would enable key stakeholders, including investigators, clinicians and regulators, to access trial-specific information, including study design, outcomes and, crucially, adverse events130. Individualized analyses would then be possible, informed by specific disease treated, the device used, the DBS target employed and the stimulation parameters. Given the heterogeneity of stimulation settings and anatomic targets for some indications in the field, access to a registry would enable queries to be made according to specific criteria. For example, querying the registry for studies that use a specific DBS pulse width or frequency would save other researchers time and could enhance the safety of future studies. The industry would also be able to monitor usage, benefits, risks and adverse events to better inform device design and usability. Potential collaborations between centres embarking on similar, or the same, trials could be more easily facilitated.
Ethical considerations
Implantation of electrodes into deep brain structures to influence their activity raises important ethical questions, especially in new and emerging indications for DBS. This ethical issue is related in part to the fact that DBS, although minimally invasive, is a neurosurgical procedure that is associated with serious surgical risk, including haemorrhage and infection. Furthermore, although DBS is standard of care in PD, it remains highly resource intensive; DBS incurs large capital costs and necessitates a large, expert multidisciplinary team to provide programmes for patients and troubleshoot issues. DBS also commits patients to a lifelong implant, with subsequent battery replacements, which can be problematic in some disorders that affect young adults. Several guidelines have been published that attempt to systematically identify and help to address ethical issues in DBS research and clinical practice6,131,132. Notably, these issues might differ depending on whether established indications, such as PD, or emerging, more experimental indications, such as depression or dementia, are considered. For the former, crucial issues might include resource allocation; fair distribution of and access to novel neurotechnology; and the societal burden, financial or otherwise, of costly and resource-intensive treatments. For emerging indications, the issues might be even more complex, including consent in vulnerable populations, the readiness and rationale for indications for study with DBS, the role of the medical device industry in clinical trials and the use of brain stimulation to enhance healthy, non-pathological function. The next two decades will undoubtedly see rapid advances in our understanding of brain circuitry, and it will be crucial that the ethical issues surrounding those advances are addressed in parallel with the development of rigorously designed, hypothesis-driven clinical trials.
Conclusions
DBS is a powerful tool that can be used to treat brain diseases and investigate their underlying pathophysiology. Rapid advances in the past two decades have led to DBS becoming a standard of care in motor circuit disorders, and several trials have also investigated its efficacy in a number of emerging, non-motor indications. Much of the success of DBS has been driven by preclinical, neurophysiological and computational studies that seek to define its mechanisms and characterize its influence on neural circuitry. Important opportunities and unmet needs in the field include technological innovation focused on improvement of efficiency and tolerability, better integration with imaging and other modalities and capturing the global experience through enhanced study designs and registries. In many ways, the DBS field is still very much evolving, but with an unwavering goal — to treat brain disease as safely and effectively as possible.
Key points.
Deep brain stimulation (DBS) is opening new therapeutic possibilities for neurological and psychiatric disorders.
DBS is enabling neuroscientists to obtain direct measures of cellular activity and to probe the function of neural circuits.
The delivery of DBS at precise locations and the wide range of stimulation parameters available enable unprecedented temporal and spatial control of brain circuits.
The mechanisms of action of DBS at the cell, molecular and systems level are poorly understood and much work remains to be done.
The ethical issues presented by the application of DBS in new patient populations and for new indications require careful consideration.
Acknowledgements
This work was supported by a grant from the World Society for Stereotactic and Functional Neurosurgery. It was coordinated together with the Research Committee of the World Society for Stereotactic and Functional Neurosurgery.
Footnotes
Author contributions
All authors researched data for the article, contributed substantially to discussion of content, contributed to writing of the manuscript and undertook review and/or editing of the manuscript before submission. A.M.L., N.L. and J.K.K. provided overall guidance and oversight of the group writing and review process.
Competing interests
A.M.L. is a consultant to Medtronic, Abbott (formerly St. Jude) and Boston Scientific and is Scientific Director of Functional Neuromodulation. H.B. has received honoraria for speaking from AlphaOmega, Medtronic and Boston Scientific and research support from the Magnet Program of the Israel Ministry of Economics. P.B. has received honoraria for speaking from Medtronic and Boston Scientific. S.C. is a consultant for Boston Scientific and for Medtronic and has received financial support from Medtronic for preclinical research purposes in the field of deep brain stimulation (DBS). K.M. has chaired advisory boards for studies of DBS for obsessive–compulsive disorder sponsored by Medtronic and has received travel and accommodation support to attend meetings from Medtronic and Abbott. C.C.M. is a paid consultant for Boston Scientific Neuromodulation and Kernel as well as a shareholder in the following companies: Surgical Information Sciences, Inc.; Autonomic Technologies, Inc.; Cardionomic, Inc.; Enspire DBS, Inc.; and Neuros Medical, Inc. T.S. has received limited research support for three investigator-initiated studies from Medtronic. M.S. owns stock in General Electric. J.V. receives grants and personal fees from Boston Scientific and is a consultant and paid speaker for Medtronic. J.K.K. is a consultant to Medtronic and Boston Scientific; has received fees for speaking from Abbott; is a past and honorary president of the European Society for Stereotactic and Functional Neurosurgery; and is a past president of the World Society for Stereotactic and Functional Neurosurgery. The other authors have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
With the growing interest in deep brain stimulation (DBS) and its worldwide use, the leadership of the World Society for Stereotactic and Functional Neurosurgery (WSSFN) decided to address the manifold unanswered questions and unmet needs in this rapidly expanding field. To achieve this goal, the WSSFN produced this Review to outline the contemporary discussions, the challenges and the future directions in this area on the basis of a dedicated workshop, which was held 9–11 March 2017. The objective of the workshop was to identify the most pressing current and emerging challenges and unmet needs in the DBS field. Participants from different disciplines were chosen on the basis of their special expertise in neuroscience, neurology, neurosurgery or psychiatry. Specific sections were assigned to two experts, respectively, and the assembled text was then discussed by the whole group during an intensive 2.5-day workshop. Discussion centred around several key topics, including the current clinical status of DBS, the role of preclinical models, emerging science surrounding DBS mechanisms and the role of DBS in motor and non-motor conditions. Additional topics included the ethical challenges surrounding the application of DBS in neurology and psychiatry as well as emerging trends and future directions of the field. The manuscript then underwent several modifications over the next few months until consensus with regard to both relevance and content was reached among the authors.
Hagai Bergman: 0000-0002-2402-6673
Peter Brown: 0000-0002-5201-3044
Thomas E. Schlaepfer: 0000-0003-0612-9692
References
- 1.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn AA, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsman N, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381:1361–1370. doi: 10.1016/S0140-6736(12)62188-6. [DOI] [PubMed] [Google Scholar]
- 4.Laxton AW, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 5.Ballanger B, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum Brain Mapp. 2009;30:3901–3909. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuttin B, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry. 2014;85:1003–1008. doi: 10.1136/jnnp-2013-306580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuepbach WM, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 8.Follett KA, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 9.Eisenstein SA, et al. Acute changes in mood induced by subthalamic deep brain stimulation in Parkinson disease are modulated by psychiatric diagnosis. Brain Stimul. 2014;7:701–708. doi: 10.1016/j.brs.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merola A, et al. Impulse control behaviors and subthalamic deep brain stimulation in Parkinson disease. J Neurol. 2017;264:40–48. doi: 10.1007/s00415-016-8314-x. [DOI] [PubMed] [Google Scholar]
- 11.Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol. 2017;13:548–554. doi: 10.1038/nrneurol.2017.105. [DOI] [PubMed] [Google Scholar]
- 12.McIntyre CC, Anderson RW. Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J Neurochem. 2016;139(Suppl. 1):338–345. doi: 10.1111/jnc.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groome JR. The voltage sensor module in sodium channels. Handb Exp Pharmacol. 2014;221:7–31. doi: 10.1007/978-3-642-41588-3_2. [DOI] [PubMed] [Google Scholar]
- 14.Bucher D, Goaillard JM. Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon. Prog Neurobiol. 2011;94:307–346. doi: 10.1016/j.pneurobio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miocinovic S, et al. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol. 2006;96:1569–1580. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- 16.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum R, et al. Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation. Neurobiol Dis. 2014;62:86–99. doi: 10.1016/j.nbd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindner B, Gangloff D, Longtin A, Lewis JE. Broadband coding with dynamic synapses. J Neurosci. 2009;29:2076–2088. doi: 10.1523/JNEUROSCI.3702-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery EB, Jr, Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol Res. 2000;22:259–266. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- 20.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 21.Agnesi F, Johnson MD, Vitek JL. Deep brain stimulation: how does it work? Handb Clin Neurol. 2013;116:39–54. doi: 10.1016/B978-0-444-53497-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 22.Zimnik AJ, Nora GJ, Desmurget M, Turner RS. Movement-related discharge in the macaque globus pallidus during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2015;35:3978–3989. doi: 10.1523/JNEUROSCI.4899-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wichmann T, DeLong MR. Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics. 2016;13:264–283. doi: 10.1007/s13311-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivlin-Etzion M, Elias S, Heimer G, Bergman H. Computational physiology of the basal ganglia in Parkinson’s disease. Prog Brain Res. 2010;183:259–273. doi: 10.1016/S0079-6123(10)83013-4. [DOI] [PubMed] [Google Scholar]
- 25.Cagnan H, et al. Frequency-selectivity of a thalamocortical relay neuron during Parkinson’s disease and deep brain stimulation: a computational study. Eur J Neurosci. 2009;30:1306–1317. doi: 10.1111/j.1460-9568.2009.06922.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- 27.Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiol Dis. 2012;45:583–590. doi: 10.1016/j.nbd.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- 29.Eusebio A, et al. Resonance in subthalamo-cortical circuits in Parkinson’s disease. Brain. 2009;132:2139–2150. doi: 10.1093/brain/awp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn G, Bujan AF, Fregnac Y, Aertsen A, Kumar A. Communication through resonance in spiking neuronal networks. PLOS Comput Biol. 2014;10:e1003811. doi: 10.1371/journal.pcbi.1003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson CJ, Beverlin B, II, Netoff T. Chaotic desynchronization as the therapeutic mechanism of deep brain stimulation. Front Syst Neurosci. 2011;5:50. doi: 10.3389/fnsys.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guridi J, Alegre M. Oscillatory activity in the basal ganglia and deep brain stimulation. Mov Disord. 2017;32:64–69. doi: 10.1002/mds.26714. [DOI] [PubMed] [Google Scholar]
- 33.Tass PA, Majtanik M. Long-term anti-kindling effects of desynchronizing brain stimulation: a theoretical study. Biol Cybern. 2006;94:58–66. doi: 10.1007/s00422-005-0028-6. [DOI] [PubMed] [Google Scholar]
- 34.Gondard E, et al. Rapid modulation of protein expression in the rat hippocampus following deep brain stimulation of the fornix. Brain Stimul. 2015;8:1058–1064. doi: 10.1016/j.brs.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter MB, Jayaraman A, editors. International Basal Ganglia Society. The Basal Ganglia II: Structure and Function: Current Concepts. Plenum Press; 1987. [Google Scholar]
- 36.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 37.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 38.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 39.Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 40.Mirski MA, Ferrendelli JA. Interruption of the mammillothalamic tract prevents seizures in guinea pigs. Science. 1984;226:72–74. doi: 10.1126/science.6433485. [DOI] [PubMed] [Google Scholar]
- 41.Mirski MA, Fisher RS. Electrical stimulation of the mammillary nuclei increases seizure threshold to pentylenetetrazol in rats. Epilepsia. 1994;35:1309–1316. doi: 10.1111/j.1528-1157.1994.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 42.Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res. 1997;28:89–100. doi: 10.1016/s0920-1211(97)00034-x. [DOI] [PubMed] [Google Scholar]
- 43.Fisher R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 44.Devergnas A, et al. The subcortical hidden side of focal motor seizures: evidence from micro-recordings and local field potentials. Brain. 2012;135:2263–2276. doi: 10.1093/brain/aws134. [DOI] [PubMed] [Google Scholar]
- 45.Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003722. 142rv148. [DOI] [PubMed] [Google Scholar]
- 46.Morishita T, Fayad SM, Higuchi MA, Nestor KA, Foote KD. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11:475–484. doi: 10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim LW, et al. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl Psychiatry. 2015;5:e535. doi: 10.1038/tp.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 49.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 50.Baup N, et al. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J Neurosci. 2008;28:8785–8788. doi: 10.1523/JNEUROSCI.2384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan SK, et al. A combined in vivo neurochemical and electrophysiological analysis of the effect of high-frequency stimulation of the subthalamic nucleus on 5-HT transmission. Exp Neurol. 2012;233:145–153. doi: 10.1016/j.expneurol.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 52.Temel Y, et al. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci USA. 2007;104:17087–17092. doi: 10.1073/pnas.0704144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen ML, et al. Cortico-subthalamic inputs from the motor, limbic, and associative areas in normal and dopamine-depleted rats are not fully segregated. Brain Struct Funct. 2016;222:2473–2485. doi: 10.1007/s00429-016-1351-5. [DOI] [PubMed] [Google Scholar]
- 54.Temel Y, Jahanshahi A. Neuroscience. Treating brain disorders with neuromodulation. Science. 2015;347:1418–1419. doi: 10.1126/science.aaa9610. [DOI] [PubMed] [Google Scholar]
- 55.Jourdain VA, Schechtmann G. Health economics and surgical treatment for Parkinson’s disease in a world perspective: results from an international survey. Stereotact Funct Neurosurg. 2014;92:71–79. doi: 10.1159/000355215. [DOI] [PubMed] [Google Scholar]
- 56.Youngerman BE, Chan AK, Mikell CB, McKhann GM, Sheth SA. A decade of emerging indications: deep brain stimulation in the United States. J Neurosurg. 2016;125:461–471. doi: 10.3171/2015.7.JNS142599. [DOI] [PubMed] [Google Scholar]
- 57.Ineichen C, Christen M. Analyzing 7000 texts on deep brain stimulation: what do they tell us? Front Integr Neurosci. 2015;9:52. doi: 10.3389/fnint.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnurman Z, Kondziolka D. Evaluating innovation. Part 1: the concept of progressive scholarly acceptance. J Neurosurg. 2016;124:207–211. doi: 10.3171/2015.1.JNS142661. [DOI] [PubMed] [Google Scholar]
- 59.Deuschl G, et al. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson’s disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat Disord. 2013;19:56–61. doi: 10.1016/j.parkreldis.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Odekerken VJ, et al. GPi versus STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology. 2016;86:755–761. doi: 10.1212/WNL.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 61.Rizzone MG, et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord. 2014;20:376–381. doi: 10.1016/j.parkreldis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Oroz MC, Moro E, Krack P. Long-term outcomes of surgical therapies for Parkinson’s disease. Mov Disord. 2012;27:1718–1728. doi: 10.1002/mds.25214. [DOI] [PubMed] [Google Scholar]
- 63.Fasano A, Lang AE. Unfreezing of gait in patients with Parkinson’s disease. Lancet Neurol. 2015;14:675–677. doi: 10.1016/S1474-4422(15)00053-8. [DOI] [PubMed] [Google Scholar]
- 64.Hamani C, et al. Pedunculopontine nucleus region deep brain stimulation in Parkinson disease: surgical anatomy and terminology. Stereotact Funct Neurosurg. 2016;94:298–306. doi: 10.1159/000449010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hacker ML, et al. Deep brain stimulation may reduce the relative risk of clinically important worsening in early stage Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:1177–1183. doi: 10.1016/j.parkreldis.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 66.DeLong MR, et al. Effect of advancing age on outcomes of deep brain stimulation for Parkinson disease. JAMA Neurol. 2014;71:1290–1295. doi: 10.1001/jamaneurol.2014.1272. [DOI] [PubMed] [Google Scholar]
- 67.Krauss JK, Pohle T, Weber S, Ozdoba C, Burgunder JM. Bilateral stimulation of globus pallidus internus for treatment of cervical dystonia. Lancet. 1999;354:837–838. doi: 10.1016/S0140-6736(99)80022-1. [DOI] [PubMed] [Google Scholar]
- 68.Volkmann J, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13:875–884. doi: 10.1016/S1474-4422(14)70143-7. [DOI] [PubMed] [Google Scholar]
- 69.Kupsch A, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 70.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81:1383–1389. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- 71.Isaias IU, et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol. 2011;258:1469–1476. doi: 10.1007/s00415-011-5961-9. [DOI] [PubMed] [Google Scholar]
- 72.Lumsden DE, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol. 2013;55:567–574. doi: 10.1111/dmcn.12117. [DOI] [PubMed] [Google Scholar]
- 73.Panov F, et al. Pallidal deep brain stimulation for DYT6 dystonia. J Neurol Neurosurg Psychiatry. 2012;83:182–187. doi: 10.1136/jnnp-2011-300979. [DOI] [PubMed] [Google Scholar]
- 74.Jinnah HA, et al. Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm (Vienna) 2017;124:417–430. doi: 10.1007/s00702-016-1656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moro E, et al. Efficacy of pallidal stimulation in isolated dystonia: a systematic review and meta-analysis. Eur J Neurol. 2017;24:552–560. doi: 10.1111/ene.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blahak C, et al. Micrographia induced by pallidal DBS for segmental dystonia: a subtle sign of hypokinesia? J Neural Transm (Vienna) 2011;118:549–553. doi: 10.1007/s00702-010-0544-y. [DOI] [PubMed] [Google Scholar]
- 77.Schrader C, et al. GPi-DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology. 2011;77:483–488. doi: 10.1212/WNL.0b013e318227b19e. [DOI] [PubMed] [Google Scholar]
- 78.Ostrem JL, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76:870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- 79.Loher TJ, Pohle T, Krauss JK. Functional stereotactic surgery for treatment of cervical dystonia: review of the experience from the lesional era. Stereotact Funct Neurosurg. 2004;82:1–13. doi: 10.1159/000076654. [DOI] [PubMed] [Google Scholar]
- 80.Pauls KA, et al. Deep brain stimulation in the ventrolateral thalamus/subthalamic area in dystonia with head tremor. Mov Disord. 2014;29:953–959. doi: 10.1002/mds.25884. [DOI] [PubMed] [Google Scholar]
- 81.Ruge D, et al. Deep brain stimulation effects in dystonia: time course of electrophysiological changes in early treatment. Mov Disord. 2011;26:1913–1921. doi: 10.1002/mds.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grips E, et al. Patterns of reoccurrence of segmental dystonia after discontinuation of deep brain stimulation. J Neurol Neurosurg Psychiatry. 2007;78:318–320. doi: 10.1136/jnnp.2006.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cif L, et al. The influence of deep brain stimulation intensity and duration on symptoms evolution in an OFF stimulation dystonia study. Brain Stimul. 2013;6:500–505. doi: 10.1016/j.brs.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia. Curr Opin Neurol. 2009;22:406–413. doi: 10.1097/WCO.0b013e32832d9ef3. [DOI] [PubMed] [Google Scholar]
- 85.Gimeno H, Lin JP. The International Classification of Functioning (ICF) to evaluate deep brain stimulation neuromodulation in childhood dystonia-hyperkinesia informs future clinical & research priorities in a multidisciplinary model of care. Eur J Paediatr Neurol. 2017;21:147–167. doi: 10.1016/j.ejpn.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Austin A, Lin JP, Selway R, Ashkan K, Owen T. What parents think and feel about deep brain stimulation in paediatric secondary dystonia including cerebral palsy: a qualitative study of parental decision-making. Eur J Paediatr Neurol. 2017;21:185–192. doi: 10.1016/j.ejpn.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 87.Hariz MI, et al. Multicentre European study of thalamic stimulation for parkinsonian tremor: a 6 year follow-up. J Neurol Neurosurg Psychiatry. 2008;79:694–699. doi: 10.1136/jnnp.2007.118653. [DOI] [PubMed] [Google Scholar]
- 88.Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord. 2008;23:1146–1153. doi: 10.1002/mds.22059. [DOI] [PubMed] [Google Scholar]
- 89.Elias WJ, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375:730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 90.Oliveria SF, et al. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16:691–700. doi: 10.1016/S1474-4422(17)30166-7. [DOI] [PubMed] [Google Scholar]
- 91.Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353:724. doi: 10.1016/s0140-6736(98)05964-9. [DOI] [PubMed] [Google Scholar]
- 92.Baldermann JC, et al. Deep brain stimulation for Tourette-syndrome: a systematic review and meta-analysis. Brain Stimul. 2016;9:296–304. doi: 10.1016/j.brs.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Welter ML, et al. Anterior pallidal deep brain stimulation for Tourette’s syndrome: a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16:610–619. doi: 10.1016/S1474-4422(17)30160-6. [DOI] [PubMed] [Google Scholar]
- 94.Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13:157–165. [PubMed] [Google Scholar]
- 95.Boccard SG, Pereira EA, Moir L, Aziz TZ, Green AL. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery. 2013;72:221–230. doi: 10.1227/NEU.0b013e31827b97d6. discussion 231. [DOI] [PubMed] [Google Scholar]
- 96.Boccard SGJ, et al. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurg. 2017;106:625–637. doi: 10.1016/j.wneu.2017.06.173. [DOI] [PubMed] [Google Scholar]
- 97.Velasco F, et al. Deep brain stimulation for treatment of the epilepsies: the centromedian thalamic target. Acta Neurochir Suppl. 2007;97:337–342. doi: 10.1007/978-3-211-33081-4_38. [DOI] [PubMed] [Google Scholar]
- 98.Salanova V, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jobst BC, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58:1005–1014. doi: 10.1111/epi.13739. [DOI] [PubMed] [Google Scholar]
- 100.Eitan R, et al. One year double blind study of high versus low frequency subcallosal cingulate stimulation for depression. J Psychiatr Res. 2018;96:124–134. doi: 10.1016/j.jpsychires.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 101.Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML. Lifetime suicide risk in major depression: sex and age determinants. J Affect Disord. 1999;55:171–178. doi: 10.1016/s0165-0327(99)00004-x. [DOI] [PubMed] [Google Scholar]
- 102.Whiteford HA, et al. Estimating remission from untreated major depression: a systematic review and meta-analysis. Psychol Med. 2013;43:1569–1585. doi: 10.1017/S0033291712001717. [DOI] [PubMed] [Google Scholar]
- 103.Hamani C, et al. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 104.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Aouizerate B, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- 106.Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 107.Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Madler B. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci. 2012;24:223–236. doi: 10.1176/appi.neuropsych.11080180. [DOI] [PubMed] [Google Scholar]
- 108.Kennedy SH, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 109.Holtzheimer PE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–849. doi: 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- 110.Dougherty DD, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78:240–248. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 111.Bewernick B, Schlaepfer TE. Update on neuromodulation for treatment-resistant depression. F1000Res. 2015;4:1389. doi: 10.12688/f1000research.6633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gippert SM, et al. Deep brain stimulation for bipolar disorder-review and outlook. CNS Spectrums. 2017;22:254–257. doi: 10.1017/S1092852915000577. [DOI] [PubMed] [Google Scholar]
- 113.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 114.Denys D, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 115.Greenberg BD, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sturm V, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 117.Raymaekers S, et al. Long-term electrical stimulation of bed nucleus of stria terminalis for obsessive-compulsive disorder. Mol Psychiatry. 2017;22:931–934. doi: 10.1038/mp.2016.124. [DOI] [PubMed] [Google Scholar]
- 118.Mallet L, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 119.Bewernick BH, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 120.Malone DA, Jr, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coenen VA, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr. 2017;22:282–289. doi: 10.1017/S1092852916000286. [DOI] [PubMed] [Google Scholar]
- 122.Lipsman N, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry. 2017;4:285–294. doi: 10.1016/S2215-0366(17)30076-7. [DOI] [PubMed] [Google Scholar]
- 123.Kuhn J, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol Psychiatry. 2015;20:353–360. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 124.Lozano AM, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J Alzheimers Dis. 2016;54:777–787. doi: 10.3233/JAD-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pilitsis JG, et al. 124 low-back pain relief with a new 32-contact surgical lead and neural targeting algorithm. Neurosurgery. 2016;63(Suppl. 1):151. doi: 10.1093/neuros/nyx519. [DOI] [PubMed] [Google Scholar]
- 126.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014;11:553–563. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deer T, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation. 2016;19:91–100. doi: 10.1111/ner.12381. [DOI] [PubMed] [Google Scholar]
- 128.Schlaepfer TE, Fins JJ. Deep brain stimulation and the neuroethics of responsible publishing: when one is not enough. JAMA. 2010;303:775–776. doi: 10.1001/jama.2010.140. [DOI] [PubMed] [Google Scholar]
- 129.Hamani C, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 130.Synofzik M, Fins JJ, Schlaepfer TE. A neuromodulation experience registry for deep brain stimulation studies in psychiatric research: rationale and recommendations for implementation. Brain Stimul. 2012;5:653–655. doi: 10.1016/j.brs.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 131.Fins JJ, et al. Ethical guidance for the management of conflicts of interest for researchers, engineers and clinicians engaged in the development of therapeutic deep brain stimulation. J Neural Eng. 2011;8 doi: 10.1088/1741-2560/8/3/033001. 033001. [DOI] [PubMed] [Google Scholar]
- 132.Rabins P, et al. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch Gen Psychiatry. 2009;66:931–937. doi: 10.1001/archgenpsychiatry.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55:S13–S16. [PubMed] [Google Scholar]
- 134.Jensen AL, Durand DM. High frequency stimulation can block axonal conduction. Exp Neurol. 2009;220:57–70. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 137.Urbano FJ, Rosato-Siri MD, Uchitel OD. Calcium channels involved in neurotransmitter release at adult, neonatal and P/Q-type deficient neuromuscular junctions. Mol Membr Biol. 2002;19:293–300. doi: 10.1080/0968768021000035087. [DOI] [PubMed] [Google Scholar]
- 138.Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- 139.Temel Y, et al. Motor and cognitive improvement by deep brain stimulation in a transgenic rat model of Huntington’s disease. Neurosci Lett. 2006;406:138–141. doi: 10.1016/j.neulet.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 140.van Kuyck K, Brak K, Das J, Rizopoulos D, Nuttin B. Comparative study of the effects of electrical stimulation in the nucleus accumbens, the mediodorsal thalamic nucleus and the bed nucleus of the stria terminalis in rats with schedule-induced polydipsia. Brain Res. 2008;27:93–99. doi: 10.1016/j.brainres.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 141.Hamani C, et al. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]