Abstract
A highly sensitive and selective liquid chromatography-tandem mass spectrometry method for the determination of tetrahydrocannabinol (THC), cannabidiol, and rimonabant in rat plasma was developed. Analytes and the internal standard were extracted from plasma using a combination of protein precipitation followed by liquid-liquid extraction. Chromatographic separation was done using Waters Symmetry C18, 4.6 × 150 mm, 5 um column using 10 mm ammonium formate buffer and methanol. The total run time was 6 min, and separation was achieved using isocratic elution at a flow rate of 1 mL/min using a 10:90 (aqueous: organic) ratio. The ionization of the analytes was optimized using electrospray ionization in positive mode, and multiple reaction mode was used for this analysis. This method showed linearity from 0.1 to 100 ng/ml for all the analytes and was validated according to FDA Bioanalytical Method Validation Guidance in terms of accuracy, precession, linearity, stability, matrix effect, recovery, and stability. This method was successfully applied to characterize the pharmacokinetics of THC in rats after continuous passive smoke exposure for 50 min when rimonabant was co-administered with cannabis smoke. Maximum concentration (Cmax) for THC was observed immediately after rats were removed from the exposure chamber (10 min post completion) which declined with a terminal half-life of 3.7 h and clearance was calculated to be 1.1 (L/h). Rimonabant (i.p) at a dose of 3 mg/kg was rapidly absorbed and maximum concentration (Cmax) was seen at 11 min which declined with a terminal half-life of 5.4 h and clearance was calculated to be 2.0 (L/h). Exposure AUCinf (h* μ/L) for THC and rimonabant were 13.9 and 457.6 respectively. As this method was highly sensitive and required only 50 L of plasma, it is applicable in rodent models that assess the exposure-response relationships of these drugs.
Keywords: Marijuana, Δ9-Tetrahydrocannabinol (THC), SR141716A (rimonabant), Cannabidiol (CBD), UFLC-MS/MS
1. Introduction
Cannabis refers to the dried flowers and small leaves of the female hemp (Cannabis sativa) plant and is among the oldest crops in the world. The plant is known to contain more than 400 compounds, at least 60 of which are cannabinoids, and of which delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most commonly researched [1]. THC is one of the most commonly used illicit drugs across the globe and smoking is the most prominent method of administration [2]. Though it is considered a Schedule I substance in the United States, THC (Marinol®) is prescribed to stimulate appetite to treat AIDS-related anorexia. In Europe, the drug Sativex®, which contains a combination of 9-tetrahydrocannabinol (THC) and cannabidiol (CBD), is available as a therapy for multiple sclerosis [3,4]. Both THC and CBD bind to the cannabinoid-1 receptor (CB1R), located primarily in the central nervous system, to elicit many of their pharmacological actions [5].
SR141716 A (rimonabant) is highly selective for CB1Rs and has both antagonist and inverse agonistic properties. It was developed by Sanofi Aventis to treat obesity, but was later withdrawn from the market for safety reasons [6,7]. Rimonabant has also been shown to antagonize symptoms in the “cannabinoid tetrad” (analgesia, hypomotility, hypothermia, and catalepsy) in mice [8] and is one of the compounds most commonly used to study and compare effects of THC and synthetic cannabinoids in various animal studies [8–11]. Rimonabant is typically administrated intraperitoneally (i.p) to counteract the effects of THC and synthetic CB1 agonists, and thus understanding the pharmacokinetics of rimonabant would allow better understanding of the doses at which the CB1 agonist effects are antagonized.
Various bioanalytical methods have been reported to quantify cannabinoids in both rat and human plasma; however, most methods developed for rats have 5–10 ng/mL as their LLOQ [12,13]. THC is known to have a long half-life due to its lipophilic nature, and hence its redistribution from fat tissue becomes a rate-limiting step that contributes to this prolonged half-life. Development of a method with a lower LLOQ will enable better characterization of THC distribution and elimination. Other methods developed in rats were developed in blood, and require more than 100 L of the matrix, which would be challenging when performing a pharmacokinetic study [12,14–16]. Decreasing the volume typically causes low sensitivity for effective quantification of a compound. Hence, the main aim of this study was to develop a sensitive and specific analytical method using LC–MS/MS with a short run time to determine the plasma concentrations of THC and CBD, and the CB1 antagonist rimonabant, simultaneously in rat plasma. One of the primary motivations for the development and validation of this new method, despite the availability of previously published methods, is to apply it to develop an animal model for passive cannabis smoke inhalation, in which low concentrations of cannabinoids are expected due to low and variable bioavailability. Most previously published studies were able to quantify cannabinoids following i.p. administration, for which plasma concentrations are higher. In this study we aimed to characterize the pharmacokinetics of these compounds after rats were exposed to smoke generated by burning cannabis cigarettes containing 5.3% THC (< 0.001% CBD) and freshly prepared rimonabant injected (i.p) immediately upon removal from the smoke exposure chamber (Fig. 1).
Fig. 1.
Chemical structures of (a)Δ9-tetrahydrocannabinol (b) cannabidiol and (c) rimonabant.
We report a highly sensitive liquid chromatography tandem mass spectrometery (LC–MS/MS) method to simultaneously quantify THC, CBD, and rimonabant, which requires a low volume of rat plasma (50L) and has a LLOQ of 100pg mL−1 for all three compounds. This method was then applied to characterize the pharmacokinetics of THC and rimonabant up to 10 h following passive smoke inhalation and co-administration of rimonabant (Fig. 2).
Fig. 2.
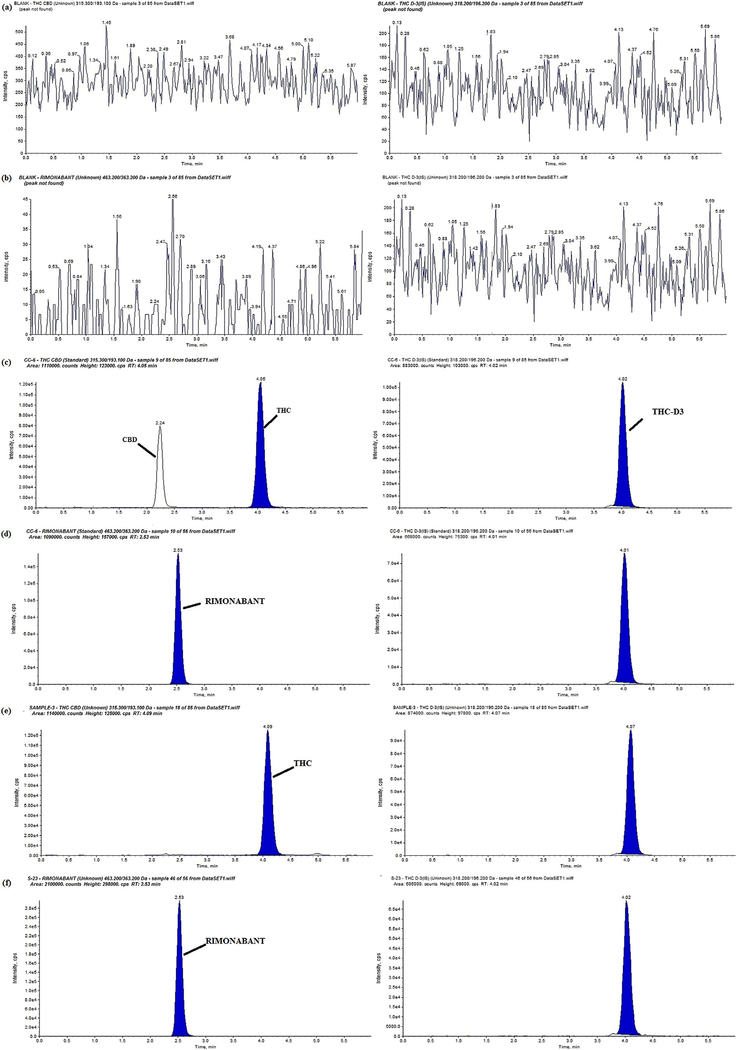
Representative MRM ion-chromatograms of (a) blank rat plasma (THC, CBD and IS), (b) blank rat plasma (Rimonabant & IS), (c) Calibration curve sample chromatogram THC (4.05 min) & CBD, (2.24 min) min), (d) Calibration curve sample chromatogram Rimonabant (2.63 min), (e) chromatogram of PK sample of THC, CBD and IS and (f) chromatogram of PK sample of Rimonabant & IS.
2. Materials and methods
2.1. Chemicals and reagents
Tetrahydrocannabinol, cannabidiol, and deuterium-labeled delta-9-THC (THC-D3) for mass spectroscopy (∼99% pure) were purchased from Ceriliant (Texas, USA). Double distilled water was obtained in-house (Department of Pharmaceutics, University of Florida). All other reagents and chemicals such as methanol, ace-tonitrile, ammonium formate, and formic acid were purchased from Fisher Scientific (PA, USA)
2.2. Instrumentation and chromatographic conditions
The Shimadzu UFLC-Nexera X2 system (Kyoto, Japan) consisted of an LC-30CE pump, SIL-30AC autosampler, CTO-30 A column oven and DGU-20ASR degasser. The ionization and detection of the analytes were carried out on the ABSCIEX API 5500 Q-Trap mass spectrometer (AB Sciex, Framingham, MA, USA). All data analysis was performed with Analyst 1.6 software (AB Sciex, Framingham, MA, USA). The mobile phase A was 10 mM ammonium formate buffer with 0.1% formic acid, and mobile phase B consisted of methanol in the ratio 10:90 (A:B). Separations were achieved using isocratic elution at a flow rate of 1 mL/min. The sample injection volume was 20 L and the total analysis run time of a single injection was six minutes.
Bioanalysis of the analytes was performed in the positive electrospray ionization (ESI) mode. The mass spectrometric detection of the analytes and the internal standard (IS) were performed in the multiple reaction monitoring modes (MRM). Tuning by infusing the analytes (10 μ L/min) and IS was performed to determine various compound-dependent and source-dependent parameters
2.3. Stock, standard and quality control samples
Individual stock solutions (1 mg/ml) of THC, CBD, and THCD3 were prepared in methanol, and rimonabant was prepared in DMSO. A mixed stock containing THC, CBD and rimonabant then was prepared in methanol at a concentration of 10 μ g/mL. From that mixed stock, working standards were freshly prepared and spiked (5 l) into blank plasma (45 μL) to obtain concentrations of 0.1–100 ng/mL. Five different quality control (QC) samples viz, lower limit of quantification (LLOQ), low quality control (LQC), middle quality control 1 (MQC1), middle quality control 2 (MQC2) and high quality control (HQC) were prepared daily in 5 replicates. All stocks were stored in the freezer at −80°C and found to be stable during the entire validation. The working stock solution of THC-D3 was prepared at a concentration of 1 μg/ml in methanol.
2.4. Extraction procedure
The extraction of cannabinoids and rimonabant from rat plasma was done using a two-step process involving protein precipitation (PPT) followed by liquid-liquid extraction (LLE). THC-D3 was diluted in acetonitrile to obtain a concentration of 100 ng/mL, and 100 μL of the solution was added in order to precipitate the proteins. The samples were vortexed for 10 s, and 1000 L of hexane was added to each sample. After vortexing for another 30 s, the samples were centrifuged at 10,000 rpm for 10 min. To finish the extraction, 900 μ L of the supernatant was transferred into a new vial and dried using a nitrogen evaporator for 10 min. Dried samples were then reconstituted with 100 L of methanol, and 20 L was injected into the chromatographic system.
2.5. Assay validation procedure
The LC–MS/MS method was validated as per USFDA Bioanalytical Method Validation guidelines, covering validation parameters including linearity, specificity, selectivity, accuracy and precision, matrix effects, stability, and recovery.
2.6. Calibration curve and linearity
The calibration curve consisted of a blank sample (blank plasma without analyte or IS), a zero sample (plasma + IS) and 8 non-zero samples (0.1–100 ng/ml).The calibrators used in the curve were 0.10, 0.16, 0.509, 1.37, 4.55, 18.2, 52.0 and 100 ng/mL. The calibration curve was plotted by using the peak area ratio of the analyses and the IS against the standard nominal concentration of calibration standards in the matrix.
2.7. Selectivity and sensitivity
Selectivity was investigated by analyzing processed blank plasma collected from six different batches with samples spiked with analytes at LLOQ. Specificity was established as the absence of interfering peaks at the retention time of the analytes and IS. Sensitivity was established as the lowest analyte concentration (LLOQ) that was within the acceptable accuracy and precision limits.
2.8. Intra and inter-day precision and accuracy
Intra- and inter-day accuracy and precision were determined by analyzing 5 replicates of QCs (LLOQ, LOQ, M1QC, M2QC and HQC) in rat plasma. The results were considered to be acceptable if the coefficient of variation (%CV) was within 15% for LQC, M1QC, and HQC, except for the LLOQC, for which the criterion for acceptability was 20% CV deviation from the nominal concentration value.
2.9. Recovery
Recovery was conducted at 5 QC levels (LLOQ, LQC, M1QC, M2QC and HQC). The peak areas of the extracted samples were compared with non-extracted acetonitrile samples to obtain recovery.
2.10. Matrix effect
The matrix effect on ion suppression or enhancement was addressed by comparing peak areas of the QCs (LLOQ, LQC, M1QC, M2QC and HQC) spiked in processed blank samples to QCs spiked in neat solutions performed in six replicates.
2.11. Stability
The stability of all of the analytes was assessed at LQC and HQC levels. Three cycles of freeze-thaw, bench top, short term and long term conditions were performed. Samples were considered stable if the accuracy and precision were within the acceptable limits. Six replicates each of LQC and HQC were used in the assay.
2.12. In-vivo pharmacokinetic study
The validated bioanalytical method described above was applied to determine concentrations of THC and rimonabant obtained from in vivo studies. Cannabis cigarettes containing 5.3% THC were obtained from the NIDA Drug Supply Program, and the protocol for this study (protocol number 201,607,852) was approved by the Institutional Animal Care and Use Committee at the University of Florida. Male Wistar rats (n = 4) weighing 275–300 g and having already undergone surgery to implant jugular vein catheters were ordered from Envigo. Rats were housed in the vivarium in the McKnight Brain Institute at University of Florida, and kept in a temperature controlled, 12 h light–dark cycle environment with free access to water and food. After 5 days of acclimation, rats were exposed to smoke from burning 5 sequentially-smoked cannabis cigarettes, each weighing ∼0.9 g and containing 5.3% THC and < 0.001% CBD. Smoke exposure was conducted in a Teague Enterprises TE-10 Smoking Machine (Davis, CA, USA) as described previously [17]. The total duration of smoke exposure was 50 min, as each cigarette took ∼10 min to burn completly. Ten puffs (2 s perpuff, 1 min inter-puff interval) were obtained from each cigarette. Mainstream smoke from each puff was directed into the exposure chamber, in which rats were individually housed in standard rat home cages. Immediately following the final cigarette, rats were removed from the exposure chamber and given i.p. injections of freshly prepared rimonabant (3 mg/kg). Rimonabant was dissolved in a mixture of DMSO and Tween 80, and the volume was made up with 0.9% saline to achive the desired concentration (20:5:75). Blood (0.2 mL) was drawn from the jugular vein catheter at 10, 20, 40, 60,120, 240, 360, 480 and 600 min following smoke exposure. Plasma was separated by centrifugation (3000 g, 10 min, at 4°C) and stored at −80 °C until analysis.
3. Results and discussion
3.1. LC–MS/MS method development
Various LC conditions were optimized during the method development to obtain peaks with the best sensitivity and symmetry. Mobile phases used to achieve chromatographic separation consisted of methanol and 10 mM ammonium formate buffer containing 0.1% formic acid. The robustness of the method was evaluated against various columns from 50 m to 150 m in length. The best separation with good sensitivity and peak shapes was achieved with a Waters Symmetry C18 column (150 mm × 4.6 mm i.d. 5 m). ESI positive mode was finalized for ionization after comparison between ESI and atmospheric pressure chemical ionization source (APCI). Greater sensitivity was observed with ESI relative to APCI. The most abundant parent/daughter ions and compound-dependent and source-dependent parameters are shown in Table 1. A stable isotope-labelled analyte or structural analogue is desirableas the IS in mass spectrometry; hence THC-D3 was chosen as the IS.
Table 1.
Optimized source and compound dependent parameters for THC, CBD, Rimonabant, and THC-d3.
| Source Parameters | Value |
|---|---|
| Curtain Gas (psi) | 10 |
| Collision Gas (psi) | Medium |
| IonSpray Voltage (eV) | 5500 |
| Source Temperature (°C) | 500 |
| Ion Source Gas 1 (psi) | 45 |
| Ion Source Gas 2 (psi) | 35 |
| Compound Parameters | THC | CBD | Rimonabant | THC-D3 |
|---|---|---|---|---|
| Q1 Mass (Da) | 315.3 | 167.2 | 463.2 | 318.2 |
| Q3 Mass (Da) | 193.1 | 177.4 | 363.2 | 196.2 |
| DP (ev) | 74 | 74 | 90 | 74 |
| CE (ev) | 33 | 33 | 62 | 33 |
| EP (ev) | 13 | 13 | 13 | 13 |
| CXP (ev) | 9 | 9 | 9 | 9 |
| Dwell Time (msec) | 200 | 200 | 200 | 200 |
DP- Declustering potential.
CE- Collision energy.
EP-Entrance potential.
CXP- Collision exit potential.
3.2. Method Validation
3.2.1. Selectivity
The method showed good selectivity as no endogenous peaks interfered with the three analyte peaks. The retention times of CBD, THC and rimonabant were 2.6, 4.8 and 2.6 min respectively. The peaks of the IS and analytes showed little variability, with the relative standard deviation (R.S.D) within the acceptable ± 15%.
3.2.2. Calibration curve and linearity
A calibration curve for rat plasma ranging from 0.1 to 100 ng/mL was constructed using 8 calibrators for each analytes. The average intra-day and inter-day regression coefficient was found to be >0.99. With this method the LLOQ with %R.S.D < 20 and a signalto-noise ratio (S/N) > 10 was seen at 0.1. Extracted blank sample injected after HQC had a peak area of less than 5% of LLOQ, indicating that there was no carry-over effect.
3.2.3. Accuracy and precision
Accuracy and precision were calculated at 5 different QC levels: LLOQ, LQC, M1QC, M2QC and HQC (n = 5) for 5 days. The intra-day and inter-day variability was less than 15% at all concentrations, indicating that the method was accurate and precise for the given concentration range from 0.1 to 100 ng/mL. These results are shown in Table 2. The CV(%) for intra-day assays for THC, CBD and rimonabant ranged from 3.76 to 7.00, 5.16–8.35 and 5.50–8.10 respectively, and the bias (%) for the intra-day assay for THC, CBD and rimonabant ranged from −7.08–8.00, −1.24–13.60 and −7.3311.53 respectively. The CV(%) for inter-day assays for THC, CBD and rimonabant ranged from 4.40 to 7.84, 6.58–9.68 and 5.81–7.68 respectively, and bias (%) for the inter-day assay for THC, CBD and rimonabant ranged from −0.16–7.11, −2.61–10.33 and −3.87–9.28 respectively.
Table 2.
Accuracy and precision for within batch and between-batch QC samples.
| Level | Nominal Conc. (ng/mL) | Intra-day |
Intra-day |
||
|---|---|---|---|---|---|
| CV(%) | % Bias | CV(%) | % Bias | ||
| THC | |||||
| LLOQ | 0.1 | 3.76 | 8.00 | 4.40 | 7.11 |
| LQC | 0.3 | 4.48 | −7.08 | 7.84 | 3.53 |
| M1QC | 5 | 4.34 | 4.52 | 6.57 | −0.16 |
| M2QC | 40 | 6.17 | 6.93 | 6.45 | 3.93 |
| HQC | 80 | 7.00 | 3.27 | 6.37 | 2.93 |
| CBD | |||||
| LLOQ | 0.1 | 5.16 | 13.60 | 6.58 | 10.33 |
| LQC | 0.3 | 6.54 | 2.80 | 7.10 | 7.24 |
| M1QC | 5 | 5.88 | −1.24 | 8.29 | −2.61 |
| M2QC | 40 | 8.35 | 5.30 | 10.24 | 1.74 |
| HQC | 80 | 7.73 | 10.55 | 9.68 | 0.62 |
| Rimonabant | |||||
| LLOQ | 0.1 | 8.10 | 11.53 | 6.26 | 9.28 |
| LQC | 0.3 | 7.48 | −7.33 | 7.68 | −3.87 |
| M1QC | 5 | 5.86 | −4.01 | 7.61 | −1.40 |
| M2QC | 40 | 6.52 | 8.55 | 8.98 | 1.13 |
| HQC | 80 | 5.50 | 10.61 | 5.81 | 8.96 |
3.2.4. Recovery and matrix effect
The mean percentage recovery of the analytes is shown in Table 3. These data suggest that protein precipitation (PPT) followed by liquid-liquid extraction (LLE) provides efficient and reproducible recovery for all three analytes. No significant matrix effect was observed in any of the six tested rat plasma lots at the LLOQC, LQC, M1QC, M2QC and HQC concentrations. The mean recovery (%) for THC, CBD and rimonabant ranged from 74.32 to 79.23, 72.73–77 and 81.90–85.16 respectively.
Table 3.
Mean extraction recovery of analytes in rat plasma.
| Analytes | LLOQ (%) | LQC (%) | M1QC(%) | M2QC (%) | HQC (%) |
|---|---|---|---|---|---|
| THC | 74.32 ± 6.50 | 79.23 ± 5.75 | 77.83 ± 4.26 | 75.84 ± 4.21 | 75.03 ± 3.89 |
| CBD | 77.85 ± 5.55 | 72.73 ± 5.92 | 74.79 ± 5.79 | 72.85 ± 7.77 | 76.02 ± 5.62 |
| Rimonabant | 83.75 ± 6.64 | 82.53 ± 7.24 | 85.16 ± 7.51 | 81.90 ± 5.55 | 82.08 ± 3.57 |
3.2.5. Stability
The results of the stability studies are presented in Table 4. The concentrations for analytes were found to be within the acceptable limits. The mean long term stability (%) for THC, CBD and rimonabant ranged from 97.20 to 108.30, 103.30–106.50 and 96.90–99.40 respectively. The mean short term stability (%) for THC, CBD and rimonabant ranged from 103.20–104.40, 97.50–99.60 and 95.70–104.30 respectively. The mean freeze-thaw stability (%) for THC, CBD and rimonabant ranged from 109.20 to 112.80, 105.21–108.50 and 106.25–109.50 respectively. The auto sampler stability (%) for THC, CBD and rimonabant ranged from 99.97 to 105.30, 96.80–103.60 and 102.30–104.50 respectively.
Table 4.
Stability data of analytes in rat plasma.
| Level | Nominal Concentration (ng/mL) |
Long-term Stability (At −70 ± 10°C, 30 days) | Short-term Stability (At 20°C, 24 hr) | Freeze-Thaw Stability (At, −70 ± 10°C | Autosampler stability (At 20°C, 24 hr) |
|---|---|---|---|---|---|
| % Accuracy | % Accuracy | % Accuracy | % Accuracy | ||
| THC | |||||
| LQC | 0.3 | 108.30 | 103.20 | 112.80 | 105.30 |
| HQC | 80 | 97.20 | 104.40 | 109.20 | 99.97 |
| CBD | |||||
| LQC | 0.3 | 106.50 | 97.50 | 108.50 | 103.60 |
| HQC | 80 | 103.30 | 99.60 | 105.21 | 96.80 |
| Rimonabant | |||||
| LQC | 0.3 | 96.90 | 95.70 | 106.25 | 104.50 |
| HQC | 80 | 99.40 | 104.30 | 109.50 | 102.30 |
3.3. In-vivo pharmacokinetic study
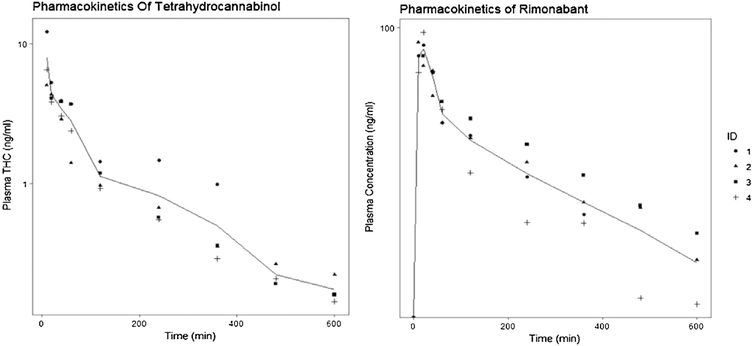
The method was successfully applied to analyze THC and rimonabant from rat plasma after co-administration. The pharmacokinetic profiles of both the compounds are shown in Fig. 3. Upon obtaining the pharmacokinetic profile, a noncompartmental analysis was performed in order to estimate the pharmacokinetic parameters for both the compounds, with the results presented in Table 5. The maximum concentration of THC (Cmax) was observedin the first sample (10 min after rats were removed from the exposure chamber) and the concentration declined with a terminal half-life of 3.7 h; total AUC0-t (h*μg/L) of THC after 50 min of continuous smoke exposure was 12.2 ± 3, AUCinf (h* μg/L) 13.9 ± 3.8. The clearance was calculated to 1.1 (L/h) which is consistent with previously reported clearance values in rats, which range from 1 to 5 (L/h) [12,18]. The maximum concentration (Cmax) of 88.6 (ng/ml) for rimonabant was seen 10–12 min after i.p administration and it declined with a terminal half-life of 5.4 h; total AUC0-t (h* μg/L) of rimonabant after 3 mg/kg was 308.15; AUCinf (h* μg/L) 457.6. The clearance (CL/F) was calculated to 2 (L/h). This LCMS method was successfully able to capture the pharmacokinetics of THC up to 10 h after passive smoke inhalation, when the amount of THC in the body was very low and required a sensitive method of detection. Based on these results we can say that this method can be applied to any rat model in which the effects of THC and rimonabant are being evaluated separately or together to understand the underlying pharmacology of these drugs.
Fig. 3.
Concentration-time profile of THC and Rimonabant.
Table 5.
Pharmacokinetics parameters of THC and rimonabant in Wistar rats.
| Parameter | THC(Inhalation) | Rimonabant(i.p) |
|---|---|---|
| Dose | 0.05 mg/kg | 3 mg/kg |
| T1/2 (h) | 3.7 ± 1.3 | 5.4 ± 0.85 |
| AUC0-t(h*ug/L) | 12.2 ± 3 | 308.15 ± 55 |
| AUCinf(h*ug/L) | 13.9 ± 3.8 | 457.6 ± 86 |
| CL and CL/F**(L/h) | 1.1 ± 0.26 | 2.0 ± 0.3** |
| Vss (L) | 3.9 ± 2.07 | - |
| Tmax (h) | - | 0.2 ± 0.07 |
| Cmax (ng/mL) | - | 88.6 ± 5.64 |
Abbreviations: Cmax, maximum concentration achieved in plasma; Vss, Volume of distribution at steady state; AUC0-t, Area under the curve from 10 min post smoke exposure to 10 h; AUCinf, Area under the curve from 10 min post smoke exposure to infinity; CL, Clearance; t1/2, Terminal half-life; Each value represents mean ± SD**, indicating apparent clearance.
Dose calculations for inhalation studies are usually not straightforward. Some of the sources of variability include particle size, location of the deposited fraction, and mode of exposure. A theoretical estimate can be obtained using various approaches, and previous literature has presented an indirect way of calculating the THC doses for cannabis inhalation studies. Only a fraction of the total amount of THC present is available for inhalation, as some is lost due to pyrolysis and more is lost as sidestream smoke. Previously-published data suggest that roughly 50% of the THC is lost due to pyrolysis and that only 30–45% of the remaining drug is available for inhalation after accounting for loss due to side stream smoke [19]. Based on these assumptions, we calculated the concentration of THC that would be present in the exposure chamber (264 L) for the animals to inhale. After obtaining the concentration of THC inside the exposure chamber we calculated the lung deposited dose using the following equation [20]:
Delivered Dose (mg/kg) = C * T * RMV * DF/BW
RMV = 0.608 * BW(0.852) Where C: Concentration of the drug (mg/L), T: Total time of exposure (min), RMV: Respiratory mean volume, DF: Fraction deposited, BW: Body weight (kg)
The delivered dose of THC to each animal was calculated to be 0.05 mg/kg accounting for 4 cages that were placed inside the exposure chamber, where 10% of the total drug was assumed to be the deposition factor. Typically for rodent 10% is used and 25% is assumed for non-rodent species [20]. Similar calculations have been used to determine the dose of the THC delivered in mice [21].
4. Conclusion
A rapid, sensitive, reproducible, and robust bioanalytical method was developed for detection of THC, CBD, and rimonabant in rat plasma using LC–MS/MS. This method enables the quantification of these analytes at concentrations up to 0.1 ng/mL, which should enable better understanding of the elimination phase of these compounds. Moreover, this method requires only 50 μL for processing, which is useful for repeated sampling regimens in small animals such as rodents. These characteristics make this method cost effective, and render it possible to detect the analytes over extended periods. Finally, this method can be used for detection of analytes in complex matrices such as plasma. To the best of our knowledge, this is the first validated and sensitive method that enables quantification of both CB1 agonists and antagonists in a single run, with demonstrated efficacy in an inhalation pharmacokinetic study.
References
- [1].Huestis MA, Human cannabinoid pharmacokinetics, Chem. Biodivers 4 (2007) 1770–1804, 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Panlilio LV, Goldberg SR, Justinova Z, Cannabinoid abuse and Addiction: clinical and preclinical findings, Clin. Pharmacol. Ther 97 (2015) 616–627, 10.1002/cpt.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA, Plasma cannabinoid pharmacokinetics following controlled oral 9-tetrahydrocannabinol and oromucosal cannabis extract administration, Clin. Chem 57 (2011) 66–75, 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].U. Pharmaceuticals, Marinol® CIII (Dronabinol) Capsules Rx Only, 2006, pp. 3–14.
- [5].Lupica CR, Riegel AC, Hoffman AF, Marijuana and cannabinoid regulation of brain reward circuits, Br. J. Pharmacol 143 (2004) 227–234, 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Egan Amy G., Colman EG, FDA Briefing Document NDA 21–888 Zimulti (Rimonabant) Tablets, 20 Mg Sanofi Aventis Advisory Committee – June 13, 2007, Int. J, 2007. [Google Scholar]
- [7].Moreira FA, Crippa JAS, The psychiatric side-effects of rimonabant, Rev. Bras. Psiquiatr 31 (2009) 145–153, 10.1590/S151644462009000200012. [DOI] [PubMed] [Google Scholar]
- [8].Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH, SR 141716 (rimonabant) precipitates withdrawal in marijuana-dependent mice, Pharmacol. Biochem. Behav 85 (2006) 105–113, 10.1016/jpbb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- [9].Järbe TUC, Gifford RS, Makriyannis A, Antagonism of 9 -THC induced behavioral effects by rimonabant: time-course studies in rats, Circulation 648 (2011) 133–138, 10.1016/j.ejphar.2010.09.006.Antagonism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Niyuhire F, a Varvel S, Martin BR, Lichtman AH, Exposure to marijuana smoke impairs memory retrieval in mice, J. Pharmacol. Exp. Ther 322 (2007) 1067–1075, 10.1124/jpet.107.119594. [DOI] [PubMed] [Google Scholar]
- [11].Katsidoni V, Kastellakis A, Panagis G, Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity, Int. J. Neuropsychopharmacol 16 (2013) 2273–2284, 10.1017/S1461145713000709. [DOI] [PubMed] [Google Scholar]
- [12].Zgair A, Wong JCM, Sabri A, Fischer PM, Barrett DA, Constantinescu CS, Gershkovich P, Development of a simple and sensitive HPLC–UV method for the simultaneous determination of cannabidiol and 9 -tetrahydrocannabinol in rat plasma, J. Pharm. Biomed. Anal. J. Pharm. Biomed 114 (2015) 145–151, 10.1016/j.jpba.2015.05.019. [DOI] [PubMed] [Google Scholar]
- [13].Valiveti S, Stinchcomb AL, Liquid chromatographic-mass spectrometric quantitation of Δ 9-tetrahydrocannabinol and two metabolites in pharmacokinetic study plasma samples, J. Chromatogr. B Anal. Technol. Biomed. Life Sci 803 (2004) 243–248, 10.1016/j.jchromb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- [14].Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE, A vapourized 9-tetrahydrocannabinol ( 9-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents, J. Pharmacol. Toxicol. Methods 70 (2014) 120–127, 10.1016/j.vascn.2014.06.006. [DOI] [PubMed] [Google Scholar]
- [15].Palazzoli F, Citti C, Licata M, Vilella A, Manca L, Zoli M, Vandelli MA, Forni F, Cannazza G, Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), 9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of, J. Pharm.Biomed. Anal 150 (2018) 25–32, 10.1016/j.jpba.2017.11.054. [DOI] [PubMed] [Google Scholar]
- [16].Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, Platt B, Riedel G, Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive beha, Psychopharmacology (Berl.) 219 (2012) 859–873, 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- [17].Bruijnzeel AW, Qi X, Guzhva LV, Wall S, Deng JV, Gold MS, Febo M, Setlow B, Behavioral characterization of the effects of cannabis smoke and anandamide in rats, PLoS One 11 (2016) 1–24, 10.1371/journal.pone.0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Valiveti S, Agu RU, Hammell DC, Paudel KS, Caroline Earles D, Wermeling DP, Stinchcomb AL, Intranasal absorption of Δ9-tetrahydrocannabinol and WIN55, 212–2 mesylate in rats, Eur. J. Pharm. Biopharm 65 (2007) 247–252, 10.1016/j.ejpb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [19].Rosenkrantz H, Braude MC, Acute, subacute and 23-day chronic marihuana inhalation toxicities in the rat, Toxicol. Appl. Pharmacol 441 (1974) 428–441. [DOI] [PubMed] [Google Scholar]
- [20].Alexander DJ, Collins CJ, Coombs DW, Gilkison IS, Hardy CJ, Healey G, Karantabias G, Johnson N, Karlsson A, Kilgour JD, McDonald P, Association of inhalation toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals, Inhal. Toxicol 20 (2008) 1179–1189, 10.1080/08958370802207318. [DOI] [PubMed] [Google Scholar]
- [21].Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR, The pharmacological activity of inhalation exposure to marijuana smoke in mice, Drug Alcohol Depend. 63 (2001) 107–116, 10.1016/S03768716(00)00205-2. [DOI] [PubMed] [Google Scholar]