Abstract
Locomotion is a defining characteristic of animal life and plays a crucial role in most behaviors. Locomotion involves physical activity, which can have far-reaching effects on physiology and neurobiology, both acutely and chronically. In human populations and in laboratory rodents, higher levels of physical activity are generally associated with positive health outcomes, although excessive exercise can have adverse consequences. If and how such relationships occur in wild animals is unknown. Behavioral variation among individuals arises from genetic and environmental factors, their interactions, and also from developmental programming (persistent effects of early-life environment). Although tremendous progress has been made in identifying genetic and environmental influences on individual differences in behavior, early-life effects are not well understood. Early-life effects can in some cases persist across multiple generations following a single exposure and, in principle, may constrain or facilitate the rate of evolution at multiple levels of biological organization. Understanding the mechanisms of such trans-generational effects (e.g., exposure to stress hormones in utero, inherited epigenetic alterations) may prove crucial to explaining unexpected and/or sex-specific responses to selection, as well as limits to adaptation. One area receiving increased attention is early-life effects on adult physical activity. Correlational data from epidemiological studies suggest that early-life nutritional stress can (adversely) affect adult human activity levels and associated physiological traits (e.g., body composition, metabolic health). The few existing studies of laboratory rodents demonstrate that both maternal and early-life exercise can affect adult levels of physical activity and related phenotypes. Going forward, rodents offer many opportunities for experimental studies of (multi-generational) early-life effects, including studies that use maternal exposures and cross-fostering designs.
Keywords: activity-stat, developmental programming, epigenetics, exercise, genotype-by-environment interaction, metabolic imprinting, obesity, wheel running
1.1. Ecological and Evolutionary Importance of Locomotor Behavior
Locomotion is a defining characteristic of animal life and locomotor behavior plays a crucial role in most natural activities, including foraging, searching for mates, escaping from predators, patrolling the home range, dispersal, and migration. Consequently, individual differences in locomotor behavior have the potential to be important determinants of various components of Darwinian fitness, such as survivorship and fecundity (Feder et al. 2010; Careau and Garland, Jr. 2012). However, for animals in the wild, few empirical studies have examined relationships between measures of physical activity (e.g., daily movement distance, home range size) and components of Darwinian fitness (for some examples with lizards, see: Civantos 2000; Clobert et al. 2000; Sinervo et al. 2000).
Although studies of individual variation in physical activity as it relates to fitness components are few and far between, some comparisons of species have identified relationships between locomotor behavior observed in the wild and aspects of locomotor performance as measured under controlled laboratory conditions (e.g., Garland, Jr. 1999; Albuquerque et al. 2015). These relationships imply correlated evolution, likely because they coadapted in response to selection (Huey and Bennett 1987; Bauwens et al. 1995; Angilletta Jr et al. 2006) (but see Artacho et al. 2015). Although most typically it is imagined that past natural selection acted to favor the evolution of appropriate behaviors and physiological abilities, sexual selection may also have played a role (Irschick et al. 2007; Kuijper et al. 2012; Oufiero et al. 2014). In any case, locomotor behavior and activity levels can be viewed as key aspects of an animal’s overall function, behavioral ecology, life history, and evolutionary biology (Dickinson et al. 2000; Nathan 2008; Kuhn et al. 2016; Wallace and Garland, Jr. 2016).
Most voluntary behaviors can be classified as complex traits, and locomotor behavior (physical activity) is no exception (Swallow and Garland, Jr. 2005; Garland, Jr. and Kelly 2006; Garland, Jr. et al. 2011b, 2017). Behaviors result from interactions of the brain, nerves, and muscles, supported by the digestive, circulatory, and thermoregulatory systems. Like all voluntary behaviors, the expression of voluntary activity can be limited by either motivation or ability. An individual with an extremely high motivation to be active will likely be limited by its exercise abilities (presuming that it has ample opportunity to be active), whereas a “super athlete” in terms of physiology will not run a marathon unless duly motivated. This type of interaction is an example of what can be termed phenotypic epistasis (Phillips 2008; Rice 2008).
The complexity of activity levels goes beyond the motivation-ability dichotomy. Total activity (e.g., daily movement distance in the wild: Garland, Jr. 1983, 1999; Goszczynski 1986) will be the product of the amount of time spent moving (duration) and the average speed (intensity), each of which can be limited by motivation or ability. In turn, each of these four lowest-level traits (motivation or ability for both duration and intensity) is affected by genes, environmental factors, gene-by-environment interactions, and developmental programming (a general term describing the entrainment of early-life effects). Moreover, aspects of motivation and ability might share certain components. For example, susceptibility to exercise-induced muscle pain could be affected by properties of muscles as well as integration of pain signals in brain areas that affect motivation and reward. Such shared and interactive effects can also be modified in an acute sense by immediate environmental conditions. For example, a heavy snow fall could pose physical and thermoregulatory challenges that might affect both motivation (e.g., via hunger) and ability (via the physical impediments of moving through deep snow).
The non-additive complexity of interactions among the subordinate traits that determine activity levels means that single-factor manipulations (e.g., Kolb et al. 2010) may be less informative than in simpler, more linear physiological or biomechanical systems. Moreover, selection generally does not act directly on single subordinate traits but rather at higher levels of biological organization, such as behavior and organismal performance (e.g., Garland, Jr. and Kelly 2006; Careau and Garland, Jr. 2012; Storz et al. 2015). Therefore, selection experiments and experimental evolution have become popular approaches to elucidate both the evolution and biological underpinnings of particular complex traits (Garland, Jr. 2003; Swallow and Garland, Jr. 2005; Garland, Jr. and Kelly 2006; Garland, Jr. and Rose 2009; Kawecki et al. 2012; Storz et al. 2015). However, before entertaining the possibility of a phenotypic selection experiment, it is prudent to consider the extent to which a trait is heritable and hence likely to respond to selection.
1.2. Genes, Environment, and (Locomotor) Behavior
Most behaviors that have been studied from the perspective of quantitative genetics show some evidence of narrow-sense heritability within particular study populations, including both humans and rodents (Turkheimer 2000; Visscher et al. 2008; Jensen 2015). Aspects of locomotor behavior measured in various contexts are no exception (DeFries et al. 1978; Roberts et al. 2012; Careau et al. 2013; Kelly and Pomp 2013; de Geus et al. 2014; Gielen et al. 2014).
Most of the existing quantitative genetic studies of locomotor behavior suffer from the typical limitations of quantitative genetics. The total phenotypic variation within a population is statistically partitioned into a minimum of two components (genetic and environmental), which are then treated as veritable black boxes. Depending on the related individuals included in the analysis, rearing conditions, sample size, and the aptness of the fitted statistical model, the genetic and environmental components of variance can be broken down into many more subcomponents, including additive genetic, dominance genetic, epistatic genetic, pre-natal maternal environmental, post-natal maternal environmental, and interactions between the various genetic and/or environmental subcomponents (Falconer and Mackay 1996). Nonetheless, each of these subcomponents is still treated as a black box in that (typically) no attempt is made to identify the specific factors (mechanisms) that act on the phenotype of interest within the categories. For example, if post-natal maternal effects are found to be substantial in a study of rodents, then that study typically does not include measurement of possible maternal factors that might be involved (e.g., milk yield, milk composition, maternal behaviors). This is understandable in that one must first identify the important sources of variation before pursuing detailed studies of those sources, but relatively few studies seem to follow-up on the initial findings.
Moving beyond the statistical descriptors that derive from quantitative genetic analyses, chromosomal regions and specific genes associated with locomotor behavior have been identified in mammals (e.g., Kelly et al. 2012a; Kelly and Pomp 2013; Kostrzewa and Kas 2014). However, much of the individual variation in locomotor behavior is not accounted for by genetics (e.g., Kelly et al. 2012a; Kelly and Pomp 2013; Kostrzewa and Kas 2014), highlighting the importance of environmental effects. Considerable progress has also been made towards understanding the neural and endocrine substrates of variation in activity levels, such as the involvement of dopamine signaling in the brain’s reward pathways in the control of voluntary exercise (e.g., see Rhodes et al. 2005; Knab and Lightfoot 2010; Garland, Jr. et al. 2011b; Keeney et al. 2012; Roberts et al. 2012; Waters et al. 2013; Majdak et al. 2014). However, the possible role of epigenetic processes in the control of locomotor behavior has scarcely been studied (Zhu et al. 2016).
1.3. Genes, Environment, and “Epigenetic” Processes Affect Complex Traits
Many aspects of developmental processes can be influenced by environmental factors (Waterland and Garza 1999). Epigenetic development is one such process that has recently attracted much attention. Modern usage of the term “epigenetics” stems from Conrad Waddington, who used it to bridge Mendelian inheritance and developmental biology (Waddington 1939). Epigenetics was broadly used as an explanation for how cells grew into differentiated cell types in different regions of the body during development, a concept which is still used today (Zhou et al. 2011). Some confusion has ensued because the definition, usage, and conceptualization of the term “epigenetic” has evolved since Waddington’s oft-cited 1942 paper (Waddington 1942). Part of this confusion may stem from the tendency for articles published in biomedically oriented journals not to cite journals in ecology/evolutionary biology/organismal biology (e.g., see Rosvall and Bergstrom 2007; Nesse and Stearns 2008). The inconsistent usage of the term is evident in Table 1. As Burggren and Crews note, “a reading of the epigenetic scientific literature reveals that the use of the word ‘epigenetics’ is currently relatively messy, with ambiguity apparent, especially over the past two decades” (Burggren and Crews 2014). The prefix, epi, of the term “epigenetics” comes from the Greek for “above,” “over,” or “outside.” Thus, epigenetics happens “above” the genetic level. In the 1930s, “epigenetics” was based on the concept of epigenesis, and defined as the manifestation of genotypic variation into phenotypes (Waddington 1953). More complete histories and etymologies of epigenetics can be found elsewhere (Holliday 2006; Deans and Maggert 2015).
Table 1.
Examples of definitions involving epigenetics, ignoring usage prior to Waddington, illustrating how they have changed across the years. All are direct quotations from the original sources (including quotation marks used there), with one exception as indicated. The various definitions also illustrate continuing variation, especially with respect to the breadth of the definition and also whether some degree of inheritance is necessarily implied. Epigenetic effects are one mechanism of early-life effects, the more general topic covered in this paper.
| Author(s) | Year | Definition |
|---|---|---|
| Waddington | 1939 | One might say that the set of organizers and organizing relations to which a certain piece of tissue will be subject during development make up its “epigenetic constitution” or “epigenotype.” |
| Waddington | 1942 | the processes involved in the mechanism by which the genes of the genotype bring about phenotypic effects |
| Holliday | 1987 | concerned with the strategy of genes in unfolding the genetic program in development |
| Atchley and Hall | 1991 | we use the term ‘epigenetic factors’ for those factors that arise external to a developing cell and influence the cell’s DNA extrinsically |
| Riggs et al. | 1996 | The study of mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence |
| Wu and Morris | 2001 | the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence |
| Jaenisch and Bird | 2003 | Many [alterations] in gene expression arise during development and are subsequently retained through mitosis. Stable alterations of this kind are said to be “epigenetic,” because they are heritable in the short term but do not involve mutations of the DNA itself. |
| Holliday | 2006 | the study of nuclear inheritance which is not based on changes in DNA sequence |
| Bird | 2007 | the structural adaptation of chromosomal regions so as to register, signal, or perpetuate altered activity states |
| Jablonka | 2009 | the study of the processes that underlie developmental plasticity and canalization and that bring about persistent developmental effects in both prokaryotes and eukaryotes |
| Jablonka and Raz | 2009 | Epigenetics is the study of the processes that underlie developmental plasticity and canalization and that bring about persistent developmental effects in both prokaryotes and eukaryotes. |
| Jablonka and Raz | 2009 | Epigenetic inheritance is a component of epigenetics. It occurs when phenotypic variations that do not stem from variations in DNA base sequences are transmitted to subsequent generations of cells or organisms. |
| Halfmann and Lindquist | 2010 | encompasses all mechanisms for the inheritance of biological traits that do not involve alterations of the coding sequence of DNA |
| Jablonka | 2013 | The molecular processes that underlie persistent developmental changes are known as epigenetic mechanisms. |
| Tammen et al. | 2013 | Epigenetics is the field of study surrounding stable alterations to the DNA and histone proteins that alter gene expression (Jaenisch and Bird, 2003). |
| Laland et al. | 2014 | Paraphrase: Emerging evolutionary synthesis includes epigenetics among other kinds of extra-genetic inheritance. |
| Waterland | 2014 | Epigenetics is the study of mitotically heritable alterations in gene expression potential that occur without alterations in DNA sequence (Jaenisch and Bird, 2003). |
| Pluess | 2015 | study of changes in organisms caused by modification of gene expression rather than alteration of the DNA |
Holliday (Holliday 1994) and others refined the study of epigenetics to a more molecular level, as in the following definition by Riggs and colleagues: “the study of mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence” (Riggs et al. 1996). But the definition continues to be in flux. Jablonka and Raz (Jablonka and Raz 2009) proposed that although some components of epigenetics are heritable (“transmitted to subsequent generations of cells or organisms”), they need not necessarily be. For example, the mechanical forces involved in muscle contractions in utero are important epigenetic factors in skeletogenesis (Carter et al. 1998), and phenotypic differences in monozygotic twins can be attributed to epigenetic differences accumulated during the lifetime of each twin that would not be inherited by their offspring (Fraga et al. 2005).
Thus, Jablonka and Raz defined epigenetics as “the study of the processes that underlie developmental plasticity and canalization and that bring about persistent developmental effects in both prokaryotes and eukaryotes” (Jablonka and Raz 2009). They next define “epigenetic inheritance” as “… phenotypic variations that do not stem from variations in DNA base sequences [which] are transmitted to subsequent generations of cells or organisms.” Epigenetic inheritance can be transgenerational if the mechanism of heritable epigenetic variation occurs in or is otherwise transmissible to germ line cells. Bonduriansky (Bonduriansky 2012) discusses the varied and complex implications that non-genetic inheritance has on the classic Mendelian model of heredity.
Some are proposing to broaden the scope of epigenetics even further, defining it as the study of any mechanisms for the inheritance of biological traits that are not themselves coded into the genome (nucleotide sequence) of the organism (e.g., see Burggren and Crews 2014). This definition encompasses any example of inheritance so long as the biological trait involved is not strictly genetic, regardless of the specific nature of the mechanism involved (DNA methylation, histone modification, etc.).
Jablonka and Raz (Jablonka and Raz 2009) also proposed “broad” and “narrow” sense descriptions of epigenetics to define different modes of inheritance. In the narrow sense, there must be transmission of biological variation not due to variation in DNA nucleotide sequences from mother to daughter cell. The narrow aspect of epigenetic inheritance focuses on sexual and asexual reproduction. The cell, rather than multicellular individuals, is the unit of transmission. When defining epigenetics, many researchers may restrict its usage to the system they are studying. Holliday, for example, proposed that epigenetics is “the study of the mechanisms of temporal and spatial control of gene activity during the development of complex organisms” (Holliday 1990). Indeed, it is not uncommon to find researchers insisting upon a direct mechanism of gene regulation for a biological trait to qualify something as “epigenetic.” Prions are an apparent exception to these strict considerations (Halfmann and Lindquist 2010). Here, we use the term “epigenetic” to refer to mitotically heritable alterations in gene expression potential that are not caused by changes in DNA sequence (Waterland and Michels 2007).
The expression of behavior is reliant, to a certain extent, on the expressional outcomes of the genome. Some behaviors are innate or instinctual, whereas others arise only in individuals who were exposed to certain experiences. To illustrate the latter point, consider the work of Meaney, Szyf, and their colleagues, which experimentally demonstrates the transmission of epigenetic factors that result in an anxiety phenotype in laboratory rats. Rats who were neglected early in life as pups by their mother grew into adults with higher corticosterone levels. Neglected rats also showed higher methylation of the promoter region of the glucocorticoid receptor (GR) gene in the hippocampus, which blocks promoter factors from binding and represses the gene, thus down-regulating GR expression. The result was diminished resilience to stress, which manifested as higher levels of anxiety in the animal. Adult rats who experienced higher levels of anxiety did not lick or groom their own pups as often as did rats with lower levels of anxiety and higher levels of GR expression (Caldji et al. 2001; Meaney and Szyf 2005). To be clear, there does not appear to be any biological transmission of chemical epigenetic factors from mother to offspring. Rather, the behavior of the parent had epigenetically altered expression of a gene in the offspring, which made the offspring phenotypically resemble the parent. Such phenomena have been termed ‘recapitulation,’ which is distinct from transgenerational epigenetic inheritance (Waterland 2014).
1.4. Causes and Consequences of Early-life Effects
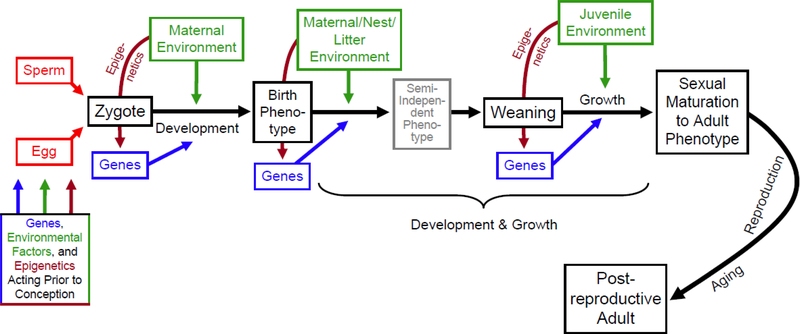
“Early-life” effects potentially can occur at any point prior to sexual maturity, while the brain and body continue to develop, including alterations of sperm or eggs that occur prior to conception, during gestation or egg development, around birth, and while experiencing maternal or paternal provisioning and care (e.g., via lactation) (Figure 1). This definition indicates that “early-life” ends at sexual maturity, which seems an appropriate developmental milestone.
Figure 1.
Illustration of genetic, environmental, and epigenetic factors acting at various stages of an organism’s life history. (For simplicity, genotype-by-environment interactions are not depicted.) Epigenetic mechanisms are one of several developmental processes that are influenced by environment, especially during so-called critical periods (Waterland and Garza 1999). Mechanisms of epigenetic effects, which alter gene expression without changing DNA sequences, include DNA methylation and histone modification. “Ontogeny” can be used to describe the entire sequence from fertilization (conception) through development, growth, sexual maturation, aging, and senescence. Additional genes (not shown) may act not only at specific ages or stages but generally across most or all of ontogeny. Similarly, some environmental factors (e.g., pH of a lake in which fish live) may last for the entire lifecycle (subject to seasonal cycles). For many animals, immediate maternal environmental effects stop at weaning (e.g., for mice in a lab setting), but this is not necessarily the case for humans of for other animals in the wild, especially for species in which offspring tend to inherit their parents’ home ranges or territories.
Genetic and environmental factors (including those affecting epigenetic processes) may act in an age- or stage-specific manner, or their effects may extend over fairly long portions of an organism’s total life cycle. Long-lasting environmental and epigenetic effects are particularly likely if they occur during critical periods of development. This concept has led to growing recognition of the importance of “developmental programming,” “fetal programming,” “biological embedding,” and the Developmental Origins of Health and Disease (DOHAD) hypothesis (Waterland and Garza 1999; Ross and Desai 2005; Breier et al. 2006; Taylor and Poston 2007; Waterland and Michels 2007; Hanson and Gluckman 2008; McGowan et al. 2008; Samuelsson et al. 2008; Langley-Evans 2009; Metges 2009; Alfaradhi and Ozanne 2011; Sullivan et al. 2011; Hertzman 2012; Bahls et al. 2014; Grissom et al. 2014; Ong and Muhlhausler 2014; Öst et al. 2014; Barboza Solís et al. 2015).
Considered broadly, early-life effects might occur through variation in any environmental factor, whether abiotic or biotic. For example, most ectothermic vertebrates experience variation in temperature as developing embryos while inside the mother, incubating in a nest, or shortly after birth or hatching -- and this variation has the potential to alter developmental processes in ways that could have lasting effects on various phenotypic traits (e.g., Mitchell et al. 2013). As another example, birds, during the egg stage, are potentially exposed to a relatively wide range of variation in temperature, which could have long-lasting effects. Indeed, birds have become convenient model systems for studies of early-life effects because the egg stage facilitates separating prenatal versus postnatal effects (Crino and Breuner 2015; Ben-Ezra and Burness 2017; Lynn and Kern 2017).
In mammals, lasting effects on adult traits can be caused by variation in amount or quality of maternal care, transmission of hormones and nutrients to offspring in utero or during lactation, general levels of energy availability, the social environment, and occurrence of various physical or psychological stressors (Alfaradhi and Ozanne 2011; Connor et al. 2012). Some of the effects may be mediated by such intermediate phenotypes as alterations in fetal growth rates, whereas others may be more direct. The nature of a given early-life effect can differ between pre- and postnatal periods (Simar et al. 2012). Certain early-life effects can amplify across successive generations, i.e., effects accumulate transgenerationally (Waterland et al. 2008). Moreover, they can interact with other sources of environmental and genetic variation, including sex (Whitaker et al. 2012), in ways that facilitate or constrain the rate of evolution at multiple levels of biological organization (Badyaev 2008; Paranjpe et al. 2013). Hence, understanding early-life effects may prove crucial to explaining sex-specific responses to selection (Garland, Jr. et al. 2011a; Keeney et al. 2012) and limits to behavioral evolution (Careau et al. 2013, 2015).
Of various potential biological mechanisms mediating the persistent effects of early-life exposures (Waterland and Garza 1999), induced alterations in epigenetic regulation likely play an important role in developmental programming of physical activity (Waterland 2014; Zhu et al. 2016). In particular, the epigenetic mechanism of DNA methylation is a prime candidate (Alvarado et al. 2014); developmental establishment of DNA methylation is affected by environment (Jirtle and Skinner 2007) and, once established, is maintained with high fidelity (Cedar and Bergman 2009), enabling the life-scale stability that is the hallmark of developmental programming.
Alterations in epigenetic regulation need to be understood at the level of the intermediate phenotypes (endophenotypes) or subordinate traits that lead ultimately to dysregulation of organismal function and behavior. Additionally, despite the overwhelming current focus of the developmental origins field on epigenetics as an underlying mechanism, it is essential to recognize that epigenetics is just one of multiple developmental mechanisms that are likely coordinately impacted by environment (Waterland and Garza 1999; Waterland 2014). In particular, one mechanism that previously received much more attention is induced alterations in morphological development (Waterland and Garza 1999). During various critical ontogenetic periods, both epigenetic and morphological development are underway, and are intimately intertwined. For example, in the mouse, during postnatal development of arcuate nucleus of the hypothalamus, cell-type specific maturation of DNA methylation (Li et al. 2014) occurs at the same time that neuronal projections are forming from the arcuate nucleus to other regions of the hypothalamus (Bouret et al. 2004). Hence, although it is useful to list potential mechanisms of developmental programming, studying these interacting processes in an integrative fashion will be essential to gain meaningful insights into how environment affects developmental outcomes, including physical activity behavior (Waterland 2014).
1.5. Early-life Effects on Adult Physical Activity in Humans and Rodents
Human studies suggest that early-life experiences from conception to sexual maturity can alter adult levels of voluntary exercise (VE) and/or spontaneous physical activity (SPA). For example, in a prospective birth-cohort study using accelerometers, parents’ physical activity during pregnancy and early in the child’s life was positively associated with the child’s physical activity at 11–12 years (Mattocks et al. 2008). Adults exposed to the Dutch Famine during gestation have increased adiposity and more atherogenic lipid profiles that may be related to decreased physical activity (Lussana et al. 2008; Stein et al. 2009). The mechanisms underlying these sorts of effects in humans are poorly understood (Dishman et al. 1985; Sallis and Hovell 1990; Andersen et al. 2009; Koeneman et al. 2011), in part because existing human studies are mostly correlational, cross-sectional (Trost et al. 2002), or of questionable methodological quality (e.g., use of questionnaires to gauge physical activity: Hallal et al. 2006; Stein et al. 2009; Koeneman et al. 2011).
Numerous rodent studies establish that early-life nutritional and other environmental exposures can affect adult body composition, appetite, dietary preferences, reward signaling, etc. (e.g., Frazier et al. 2008; Teegarden et al. 2009; Vucetic et al. 2010, 2012; Ozanne and Siddle 2011). All of these traits are potentially related to levels of physical activity (Garland, Jr. et al. 2011b). Far fewer rodent studies address early-life effects on adult VE or SPA (Breier et al. 2006; Dai et al. 2012; Sun et al. 2013) (see also Donovan et al. 2013 on sheep).
In rats, for example, supplementing the maternal diet with sunflower oil caused elevated offspring SPA (Brenneman and Rutledge 1982), whereas maternal undernourishment reduced it (Vickers et al. 2003). Adult offspring of diet-induced obese mice were hyperactive in home cages (Samuelsson et al. 2008), but rats were not (Samuelsson et al. 2010). Mouse maternal diets with partial substitution of protein by fat and carbohydrate can cause cage hyperactivity in adult offspring (Roghair et al. 2009). Mice that experienced undernutrition in utero showed reduced wheel running at night but increased running during the day (Sutton et al. 2010). Maternal protein undernutrition in mice can interact with post-weaning diet and have sex-specific effects on SPA (Whitaker et al. 2012). In mice, overnutrition during the suckling period causes blunted SPA in adult female but not male offspring (Li et al. 2013). Fetal growth restriction followed by postnatal catch-up growth induces to a persistent decrease in SPA, also only in female offspring (Baker et al. 2015). Maternal wheel exercise before and during gestation can reduce the negative effects of a maternal high-fat diet on offspring metabolic health (Stanford et al. 2015). Surprisingly, standing exercise to obtain food/water during pregnancy caused male (but not female) offspring to have increased % body fat as adults (Rosa et al. 2013).
Two recent studies from our laboratories clearly demonstrate early-life effects on adult physical activity in mice. Acosta et al. (Acosta et al. 2015) housed male mice from selectively bred High Runner lines and from their non-selected control lines in standard cages or in the same cages with attached wheels for 3 weeks, beginning just after weaning. All mice then experienced two months without wheel access. Early-life wheel access increased adult voluntary exercise, but not cage activity, in both High Runner and control lines of mice. The effect on adult plasma leptin concentrations depended on genetic background, causing a decrease in High Runner mice but an increase in mice from control lines (after adjusting for variation in fat pad mass). In a study of inbred C57BL/6J mice, females were housed with locked or unlocked wheels before and during pregnancy. Females with unlocked wheels ran throughout pregnancy, though daily distance and velocity declined as pregnancy progressed. As adults, offspring of the mothers who exercised during pregnancy were more physically active both on wheels and in their cages, with some variation between the sexes (Eclarinal et al. 2016).
Overall, existing rodent studies provide clear evidence that factors experienced by mothers prior to conception, by individuals in utero or during the suckling period, and even during later phases of early life can have important effects on adult physical activity or such associated traits as body composition and aerobic capacity. The biological underpinnings of these effects are not yet understood. Nevertheless, they may have important implications for adult life, including various aspects of behavior, energetics, and health.
1.6. Health Impacts of Locomotor Behavior
As outlined in the previous section, a variety of studies now suggest or demonstrate that adult locomotor behavior can be influenced by early-life effects (e.g., Levin 2008; Gardner and Rhodes 2009; Meek et al. 2010; Donovan et al. 2013; Eclarinal et al. 2016). Locomotor behavior involves physical activity, whose primary components are generally termed voluntary exercise (VE) and spontaneous physical activity (SPA) in studies of humans and laboratory rodents (Garland, Jr. et al. 2011b). The biological determinants of VE and SPA differ, and in sex-specific ways (Dishman et al. 2006; Garland, Jr. et al. 2011b). Considerable research implicates both VE and SPA as key contributors to energy expenditure in humans, depending on the population, sex, and age considered. In general, higher levels of physical activity are associated with a wide range of positive health outcomes in humans (Haskell et al. 2007). In humans, deficiencies specifically in VE and/or SPA have been associated with numerous socially and economically important diseases and disorders, including obesity, metabolic syndrome, cardiovascular disease, Type 2 diabetes, and certain cancers (Booth and Lees 2006a; Booth et al. 2007). Similar studies have yet to be done for wild populations of other vertebrates. Nonetheless, it is reasonable to propose the working hypothesis that physical activity will be positively related to aspects of animal health in nature (see also Halsey 2016).
Excessive exercise can have adverse effects in both humans and other vertebrates, including loss of body mass, bone and joint degeneration, chronic localized pain, hormonal dysfunction, sleep disturbances, changes in emotions, an inability to sustain intense exercise, and an increase in the amount of time required for recovery from exercise (Garland, Jr. et al. 1987; Bruin et al. 1994; Meeusen et al. 2007; Scheurink et al. 2010; Matzkin et al. 2015). Whether overtraining, activity-induced anorexia, amenorrhea or other negative consequences of excessive physical activity occur in non-human vertebrates living in the wild has, to our knowledge, not been studied.
1.7. Ecological Relevance, Anthropogenic Disturbances, and Conservation Biology of Early-life Effects on Locomotor Behavior
Early-life effects fall under the general heading of (developmental) phenotypic plasticity (Garland, Jr. and Kelly 2006; Kelly et al. 2012b; Martin et al. 2015). A tradition in functional biology has been to assume that most if not all cases of phenotypic plasticity (e.g., during acclimation to changed temperature conditions) are adaptive in the sense of increasing organismal performance in the altered conditions and hence also positively affecting Darwinian fitness. However, this hypothesis (often found as an unstated assumption) deserves critical testing, and many such tests have failed to support it (Wilson and Franklin 2002; Angilletta Jr et al. 2006; Kelly et al. 2012b; Bateson et al. 2014). This should be born in mind as we consider the ecological relevance of early-life effects on adult locomotor behavior. In particular, one could argue that most early-life perturbations would be likely to have adverse effects because organisms have been fine-tuned through many generations of natural selection in a relatively stable environment. Early-life perturbations that stem from “unusual” or unfamiliar environmental conditions (e.g., smoking cigarettes: Pembrey et al. 2006; Barboza Solís et al. 2015) are not necessarily “anticipated” and so might well have adverse consequences. In general, non-adaptive plasticity is expected to occur with fairly high frequency any time an organism experiences an environmental change that is novel in the context of its evolutionary history (Garland, Jr. and Kelly 2006; Kelly et al. 2012b; and references therein).
Early-life modifications of locomotor behavior and of many other traits could have major adaptive -- or maladaptive -- significance in natural populations (Meyers and Bull 2002; Ghalambor et al. 2007; Badyaev 2008; Duckworth 2009; Kuijper et al. 2014; Dantzer 2015). For example, mothers experiencing poor nutritional conditions might be at a selective advantage if they could somehow increase the locomotor tendencies of their offspring, such that they might be more likely to disperse from unfavorable habitats (Le Galliard et al. 2004). Although these sorts of scenarios have been widely discussed with respect to manipulation of the sex ratio of offspring (Sheldon and West 2004), relatively little attention has been given to the possibility that offspring locomotor behavior (in terms of ability and/or propensity) might be similarly modified. A few existing studies of lizards (de Fraipont et al. 2000; Meylan et al. 2012; Bestion et al. 2014) and of small mammals (Nunes et al. 1998) in the wild demonstrate that it can, and some of these studies also implicate hormonal mechanisms. These previous studies of wild animals, however, have not resolved effects on different components of physical activity, such as voluntary exercise vs. spontaneous physical activity (Garland, Jr. et al. 2011b).
At the level of the population, early-life effects might either decrease (canalize) or increase the range of individual variation, and they might increase or decrease the magnitude of trait correlations. In natural populations experiencing poor nutrition or other types of environmental deterioration that causes physical or psychological stress, the ecological consequences of any resultant effects on activity levels would seem difficult to predict from current knowledge. For example, habitat fragmentation often leads to increased early-life (or parental) interactions with humans, their technology, noise pollution, feral cats, and so forth. Many of these interactions would be stressful in the generally accepted sense of that term, but we have little evidence to suggest whether this might cause individuals to move more or less as adults. If they moved less, then it might facilitate their staying within the safe confines of a small habitat fragment. If they moved more, then it might facilitate their dispersal to a larger piece of suitable habitat, which would have implications for potential range shifts under climate change (see also Feder et al. 2010; Valladares et al. 2014). In general, the ecological consequences and conservation relevance of early-life effects with respect to habitat loss and climate change are poorly understood. For example, two recent reviews of conservation physiology and behavior contain little or no mention of plasticity, early-life effects, epigenetics, locomotor behavior or physical activity (Cooke et al. 2013, 2014).
In addition to ecological consequences of early-life effects on the overall amount of physical activity (e.g., total daily movement distance: Garland, Jr. 1983; Goszczynski 1986; Chappell et al. 2013), the frequency, duration, intensity, and timing of activity could be crucial. For example, if a normally nocturnal animal were to become active during the day it might lead to dire consequences for predation risk. Similarly, if the seasonal timing of the zugunruhe were altered, then entire migrations might fail.
1.8. Experimental Designs to Explore Early-life Effects on Adult Physical Activity
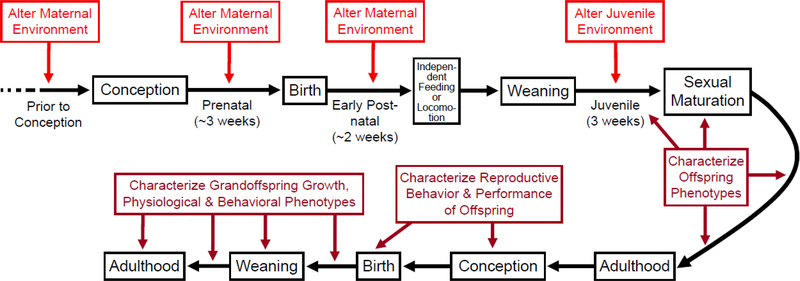
We conclude with some thoughts regarding the use of laboratory strains of rodents for investigating early-life effects. Laboratory rodents (especially mice and rats) are the most widely used animal models for studies of exercise effects on various aspects of health, physiology, and neurobiology -- and many of the general principles of those studies (Kregel et al. 2006) are directly applicable to early-life studies. Figure 2 depicts various stages at which maternal and/or offspring environment can be experimentally manipulated to elucidate early-life effects on adult phenotypes, as well as possible trans-generational effects.
Figure 2.
Potential experimental design to elucidate early-life effects on adult phenotypes, as well as possible trans-generational effects. Life-history and developmental stages are based on placental mammals, and specific timing of events is based on house mice. The maternal environment and/or the juvenile environment can be modified in many ways, including through changes in diet or the providing of access to exercise wheels (Acosta et al. 2015). During the post-natal period from birth to weaning, such factors as litter size or ambient temperature could be manipulated, thus providing other avenues of environmental manipulation that do not derive solely and directly from the mother. Cross-fostering designs also have much to offer, as discussed in the text.
Although not depicted in Figure 2, cross-fostering designs (e.g., Dohm et al. 2001; Plyusnina et al. 2009; Sadowska et al. 2013; Baker et al. 2015; Zhu et al. 2016) are a powerful way to explore early-life maternal effects (and, in some species, even paternal effects). For example, it is common for investigators to provide a nutritional or other exposure to female rodents before and during pregnancy and lactation, then examine effects on the offspring. In such designs, cross-fostering offspring at birth (i.e., “swapping” pups between exposed and unexposed dams) enables one to distinguish whether exposure during fetal or early postnatal (suckling period) development is essential for effect persistence. Another important design consideration is to study inbred populations of animals, so as to take genetic variation (and potential genetic selection at the gametic level [Waterland 2014]) out of the picture. This is essential to understanding more pure epigenetic (or, at least, not genetically mediated) effects. On the other hand, in a natural outbred population there will always be genetic variation. So, performing such experiments (Figure 2) in both isogenic and outbred populations, to see which gives stronger transgenerational effects, would give insights into how much of these effects are mediated by genetic selection vs. transgenerational effects of developmental programming.
The theoretical construct of ‘metabolic imprinting’ (Waterland and Garza 1999) should be useful to guide studies into the fundamental biological mechanisms of early-life effects on physical activity. In particular, metabolic imprinting postulates that the persistence of early-life effects is mediated by primary imprints, which can be characterized at the morphological, cellular, and molecular level. By definition, the primary imprint must be present directly after the early life exposure, and persist to adulthood. In this regard, it is noteworthy that most studies of early-life influences on physical activity test offspring at only one age, usually either directly after the exposure or in adulthood. Testing physical activity behavior at multiple ages provides an essential test of effect persistence, and in some cases such will indicate latent effects in which altered physical activity behavior is not apparent until sexual maturation (Li et al. 2013). Such findings may provide insights into a potential role of sex hormones on central regulation of physical activity. Indeed, early-life effects should be explored in both sexes. Recent work in mice suggests that females may be particular vulnerable to developmental programming of SPA (Li et al. 2013); (Baker et al. 2015). Rather than an annoyance, sex-specific effects should be viewed as an opportunity to test mechanistic hypotheses.
Various approaches can be envisioned to test the hypothesis that epigenetic mechanisms play a key role in developmental programming of physical activity. For example, dietary methyl donor supplementation of female mice before and during pregnancy both promotes DNA methylation in their offspring (Waterland and Jirtle 2003), and prevents transgenerational amplification of obesity (Waterland et al. 2008) that is associated with blunted physical activity (Baker et al. 2015). It will be important to determine if methyl donor supplementation both induces epigenetic changes in the central nervous system and normalizes lifelong physical activity in this model. Transgenic mice with cell-type specific perturbation of the DNA methylation machinery may also be useful. For example, cre-lox technology can be used to knock out the expression of the de novo methyltransferase Dnmt3a in specific types of neurons to test the overall principle that epigenetic mechanisms are important in regulation of physical activity behavior.
Most of the health- and obesity-related literature with rodent models uses, as a “control” group, animals housed without any opportunity for voluntary exercise. However, almost all laboratory (e.g., see Figure 3 in Garland, Jr. et al. 2017) and wild (Dewsbury 1980; Meijer and Robbers 2014) rodents will exhibit a substantial amount of wheel running, if given the opportunity. Thus, rodents housed without wheels or other facilities for physical exercise should be considered as “activity-restricted,” though this is rarely acknowledged. Therefore, a strong case can be made that physically active subjects should be the “control” group, rather than rodents housed without access to wheels, climbing towers (Lionikas and Blizard 2008) or other opportunities to exhibit voluntary exercise (Booth and Lees 2006b; Garland, Jr. et al. 2011b). Similarly, the majority of such studies focus on the potential adverse effects of, for example, high-fat or low-protein diet, with fewer emphasizing the possible positive effects of certain types of diets. We would urge future studies to cast a wider net, potentially including housing conditions without and with opportunities for voluntary exercise, application of forced exercise, diets with expected positive and negative effects, both sexes, and multiple genetic backgrounds (Svenson et al. 2007; Mathes et al. 2011; Meek et al. 2014). This is a tall order, but important if we are to move further towards a comprehensive understanding of early-life effects on adult phenotypes. From an ecological perspective, future studies could attempt to place such experiments into a more naturalistic context by, for example, experimental releases of animals treated differently during early-life stages, then tracking individuals to determine effects on components of Darwinian fitness, such as survival and reproductive success.
Acknowledgments
We thank Layla Hiramatsu for helpful discussions of several of the ideas presented here. Supported by NIH/NICHD R21HD084856 to TG. RAW is supported by USDA/ARS CRIS 6250–51000-055. MDC is supported by an NSF Graduate Research Fellowship.
Literature Cited
- Acosta W, Meek TH, Schutz H, Dlugosz EM, Vu KT, and Garland T Jr. 2015. Effects of early-onset voluntary exercise on adult physical activity and associated phenotypes in mice. Physiology & Behavior 149:279–286. [DOI] [PubMed] [Google Scholar]
- Albuquerque R.L. de, Sanchez G, and Garland T. 2015. Relationship between maximal oxygen consumption and home range area in mammals. Physiological and Biochemical Zoology 88:660–67. [DOI] [PubMed] [Google Scholar]
- Alfaradhi MZ and Ozanne SE. 2011. Developmental programming in response to maternal overnutrition. Frontiers in Genetics 2:Article 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado S, Fernald RD, Storey KB, and Szyf M. 2014. The dynamic nature of DNA methylation: a role in response to social and seasonal variation. Integrative and Comparative Biology 54:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen LG, Ängquist L, Gamborg M, Byberg L, Bengtsson C, Canoy D, Eriksson JG, et al. 2009. Birth weight in relation to leisure time physical activity in adolescence and adulthood: meta-analysis of results from 13 nordic cohorts. PLoS ONE 4:e8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta MJ Jr, Bennett AF, Guderley H, Navas CA, Seebacher F, and Wilson RS. 2006. Coadaptation: a unifying principle in evolutionary thermal biology. Physiological and Biochemical Zoology 79:282–294. [DOI] [PubMed] [Google Scholar]
- Artacho P, Saravia J, Ferrandière BD, Perret S, and Le Galliard J-F. 2015. Quantification of correlational selection on thermal physiology, thermoregulatory behavior, and energy metabolism in lizards. Ecology and Evolution 5:3600–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley WF and Hall BK. 1991. A model for development and evolution of complex morphological structures. Biological Reviews 66:101–157. [DOI] [PubMed] [Google Scholar]
- Badyaev AV 2008. Maternal effects as generators of evolutionary change: a reassessment. Annals of the New York Academy of Sciences 1133:151–161. [DOI] [PubMed] [Google Scholar]
- Bahls M, Sheldon RD, Taheripour P, Clifford KA, Foust KB, Breslin ED, Marchant-Forde JN, et al. 2014. Mother’s exercise during pregnancy programmes vasomotor function in adult offspring. Experimental Physiology 99:205–219. [DOI] [PubMed] [Google Scholar]
- Baker MS, Li G, Kohorst JJ, and Waterland RA. 2015. Fetal growth restriction promotes physical inactivity and obesity in female mice. International Journal of Obesity 39:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza Solís C., Kelly-Irving M, Fantin R, Darnaudéry M, Torrisani J, Lang T, and Delpierre C. 2015. Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proceedings of the National Academy of Sciences 112:E738–E746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Gluckman P, and Hanson M. 2014. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. The Journal of Physiology 592:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwens D, Garland T Jr, Castilla AM, and Van Damme R. 1995. Evolution of sprint speed in lacertid lizards: morphological, physiological and behavioral covariation. Evolution 848–863. [DOI] [PubMed] [Google Scholar]
- Ben-Ezra N and Burness G. 2017. Constant and cycling incubation temperatures have long-term effects on the morphology and metabolic rate of Japanese quail. Physiological and Biochemical Zoology 90:in press. [DOI] [PubMed] [Google Scholar]
- Bestion E, Teyssier A, Aubret F, Clobert J, and Cote J. 2014. Maternal exposure to predator scents: offspring phenotypic adjustment and dispersal. Proceedings of the Royal Society of London B: Biological Sciences 281:20140701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A 2007. Perceptions of epigenetics. Nature 447:396–398. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R 2012. Nongenetic inheritance for behavioral ecologists. Behavioral Ecology 24:326–327. [Google Scholar]
- Booth FW, Laye MJ, Lees SJ, Rector RS, and Thyfault JP. 2007. Reduced physical activity and risk of chronic disease: the biology behind the consequences. European Journal of Applied Physiology 102:381–390. [DOI] [PubMed] [Google Scholar]
- Booth FW and Lees SJ. 2006a. Fundamental questions about genes, inactivity, and chronic diseases. Physiological Genomics 28:146–157. [DOI] [PubMed] [Google Scholar]
- Booth FW and Lees SJ. 2006b. Physically active subjects should be the control group. Medicine & Science in Sports & Exercise 38:405–406. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, and Simerly RB. 2004. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110. [DOI] [PubMed] [Google Scholar]
- Breier BH, Krechowec SO, and Vickers MH. 2006. Programming of obesity—Experimental evidence Pp. 145–156 in Wintour EM and Owens JA eds. Early Life Origins of Health and Disease. Springer. [Google Scholar]
- Brenneman DE and Rutledge CO. 1982. Effect of dietary lipid on locomotor activity and response to psychomotor stimulants. Psychopharmacology 76:260–264. [DOI] [PubMed] [Google Scholar]
- Bruin G, Kuipers H, Keizer HA, and Vander Vusse GJ. 1994. Adaptation and overtraining in horses subjected to increasing training loads. Journal of Applied Physiology 76:1908–1913. [DOI] [PubMed] [Google Scholar]
- Burggren WW and Crews D. 2014. Epigenetics in comparative biology: why we should pay attention. Integrative and Comparative Biology 54:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Liu D, Sharma S, Diorio J, Francis D, Meaney MJ, and Plotsky PM. 2001. Development of individual differences in behavioral and endocrine responses to stress: role of the postnatal environment Pp. 271–292 in Handbook of Physiology, The Endocrine System, Coping with the Environment: Neural and Endocrine Mechanisms. [Google Scholar]
- Careau V and Garland T Jr. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiological and Biochemical Zoology 85:543–571. [DOI] [PubMed] [Google Scholar]
- Careau V, Wolak ME, Carter PA, and Garland T Jr. 2013. Limits to behavioral evolution: the quantitative genetics of a complex trait under directional selection. Evolution 67:3102–3119. [DOI] [PubMed] [Google Scholar]
- Careau V, Wolak ME, Carter PA, and Garland T Jr. 2015. Evolution of the additive genetic variance–covariance matrix under continuous directional selection on a complex behavioural phenotype. Proceedings of the Royal Society B: Biological Sciences 282:20151119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DR, Mikić B, and Padian K. 1998. Epigenetic mechanical factors in the evolution of long bone epiphyses. Zoological Journal of the Linnean Society 123:163–178. [Google Scholar]
- Cedar H and Bergman Y. 2009. Linking DNA methylation and histone modification: patterns and paradigms. Nature Reviews Genetics 10:295–304. [DOI] [PubMed] [Google Scholar]
- Chappell MA, Szafranska PA, Zub K, and Konarzewski M. 2013. The energy cost of voluntary running in the weasel Mustela nivalis. Journal of Experimental Biology 216:578–586. [DOI] [PubMed] [Google Scholar]
- Civantos E 2000. Home-range ecology, aggressive behaviour, and survival in juvenile lizards, Psammodromus algirus. Canadian Journal of Zoology 78:1681–1685. [Google Scholar]
- Clobert J, Oppliger A, Sorci G, Ernande B, Swallow JG, and Garland T. 2000. Trade-offs in phenotypic traits: endurance at birth, growth, survival, predation and susceptibility to parasitism in a lizard, Lacerta vivipara. Functional Ecology 14:675–684. [Google Scholar]
- Connor KL, Vickers MH, Beltrand J, Meaney MJ, and Sloboda DM. 2012. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. The Journal of Physiology 5909:2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Blumstein DT, Buchholz R, Caro T, Fernández-Juricic E, Franklin CE, Metcalfe J, et al. 2014. Physiology, behavior, and conservation. Physiological and Biochemical Zoology 87:1–14. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, and Chown SL. 2013. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conservation Physiology 1:cot001–cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino OL and Breuner CW. 2015. Developmental stress: evidence for positive phenotypic and fitness effects in birds. Journal of Ornithology. [Google Scholar]
- Dai Y, Thamotharan S, Garg M, Shin B-C, and Devaskar SU. 2012. Superimposition of postnatal calorie restriction protects the aging male intrauterine growth-restricted offspring from metabolic maladaptations. Endocrinology 153:4216–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer B 2015. Mothers shape ecological communities: Individual hormonal responses affect the distributions and abundances of bird species. Science 347:822–823. [DOI] [PubMed] [Google Scholar]
- de Fraipont M, Clobert J, John-Alder H, and Meylan S. 2000. Increased pre-natal maternal corticosterone promotes philopatry of offspring in common lizards Lacerta vivipara. Journal of Animal Ecology 69:404–413. [Google Scholar]
- de Geus EJC, Bartels M, Kaprio J, Lightfoot JT, and Thomis M. 2014. Genetics of regular exercise and sedentary behaviors. Twin Research and Human Genetics 17:262–271. [DOI] [PubMed] [Google Scholar]
- Deans C and Maggert KA. 2015. What do you mean, “epigenetic”? Genetics 199:887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFries JC, Gervais MC, and Thomas EA. 1978. Response to 30 generations of selection for open-field activity in laboratory mice. Behavior Genetics 8:3–13. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA 1980. Wheel-running behavior in 12 species of muroid rodents. Behavioural Processes 5:271–280. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, and Lehman S. 2000. How animals move: an integrative view. Science 288:100–106. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud H-R, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, et al. 2006. Neurobiology of exercise. Obesity 14:345–356. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Sallis JF, and Orenstein DR. 1985. The determinants of physical activity and exercise. Public health reports 100:158–171. [PMC free article] [PubMed] [Google Scholar]
- Dohm MR, Hayes JP, and Garland T Jr. 2001. The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan EL, Hernandez CE, Matthews LR, Oliver MH, Jaquiery AL, Bloomfield FH, and Harding JE. 2013. Periconceptional undernutrition in sheep leads to decreased locomotor activity in a natural environment. Journal of Developmental Origins of Health and Disease 4:296–299. [DOI] [PubMed] [Google Scholar]
- Duckworth RA 2009. Maternal effects and range expansion: a key factor in a dynamic process? Philosophical Transactions of the Royal Society B: Biological Sciences 364:1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eclarinal JD, Zhu S, Baker MS, Fiorotto ML, and Waterland RA. 2016. Maternal exercise during pregnancy promotes physical activity in adult offspring. FASEB J 30:2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS and Mackay TFC. 1996. Introduction to quantitative genetics 4th edition. Pearson, Harlow, England. [Google Scholar]
- Feder ME, Garland T Jr., Marden JH, and Zera AJ. 2010. Locomotion in response to shifting climate zones: not so fast. Annual Review of Physiology 72:167–190. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, et al. 2005. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America 102:10604–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CRM, Mason P, Zhuang X, and Beeler JA. 2008. Sucrose exposure in early life alters adult motivation and weight gain. (Hendricks M, ed.)PLoS ONE 3:e3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS and Rhodes P. 2009. Developmental origins of obesity: programming of food intake or physical activity? (Koletzko B, ed.)Advances in Experimental Medicine and Biology 646:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T Jr. 1983. Scaling the ecological cost of transport to body mass in terrestrial mammals. American Naturalist 121:571–587. [Google Scholar]
- Garland T Jr. 1999. Laboratory endurance capacity predicts variation in field locomotor behaviour among lizard species. Animal Behaviour 57:77–83. [DOI] [PubMed] [Google Scholar]
- Garland T Jr. 2003. Selection experiments: an under-utilized tool in biomechanics and organismal biology Pp. 23–56 in Bels VL, Gasc J-P, and Casinos A eds. Vertebrate biomechanics and evolution. BIOS Scientific Publishers Ltd., Oxford, U.K. [Google Scholar]
- Garland T Jr., Else PL, Hulbert AJ, and Tap P. 1987. Effects of endurance training and captivity on activity metabolism of lizards. American Journal of Physiology (Regulatory, Integrative and Comparative Physiology) 252(21):R450–R456. [DOI] [PubMed] [Google Scholar]
- Garland T Jr. and Kelly SA. 2006. Phenotypic plasticity and experimental evolution. Journal of Experimental Biology 209:2344–2361. [DOI] [PubMed] [Google Scholar]
- Garland T Jr., Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, et al. 2011a. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proceedings of the Royal Society B: Biological Sciences 278:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T Jr. and Rose MR, eds. 2009. Experimental evolution: concepts, methods, and applications of selection experiments. University of California Press, Berkeley. [Google Scholar]
- Garland T Jr., Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, et al. 2011b. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. Journal of Experimental Biology 214:206–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T Jr., Zhao M, and Saltzman W. 2017. Hormones and the evolution of complex traits: insights from artificial selection on behavior. Integrative and Comparative Biology 57:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, McKAY JK, Carroll SP, and Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21:394–407. [Google Scholar]
- Gielen M, Westerterp-Plantenga MS, Bouwman FG, Joosen AMCP, Vlietinck R, Derom C, Zeegers MP, et al. 2014. Heritability and genetic etiology of habitual physical activity: a twin study with objective measures. Genes & Nutrition 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goszczynski J 1986. Locomotor activity of terrestrial predators and its consequences. Acta Theriologica 31:79–95. [Google Scholar]
- Grissom NM, Lyde R, Christ L, Sasson IE, Carlin J, Vitins AP, Simmons RA, et al. 2014. Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology 39:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R and Lindquist S. 2010. Epigenetics in the extreme: prions and the inheritance of environmentally acquired traits. Science 330:629–632. [DOI] [PubMed] [Google Scholar]
- Hallal PC, Wells JCK, Reichert FF, Anselmi L, and Victoria CG. 2006. Early determinants of physical activity in adolescence: prospective birth cohort study. BMJ 332:1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey LG 2016. Do animals exercise to keep fit? (Montgomery I, ed.)Journal of Animal Ecology n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Hanson MA and Gluckman PD. 2008. Developmental origins of health and disease: new insights. Basic & Clinical Pharmacology & Toxicology 102:90–93. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, et al. 2007. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine & Science in Sports & Exercise 39:1423–1434. [DOI] [PubMed] [Google Scholar]
- Hertzman C 2012. Putting the concept of biological embedding in historical perspective. Proceedings of the National Academy of Sciences 109:17160–17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R 1987. The inheritance of epigenetic defects. Science 238:163–170. [DOI] [PubMed] [Google Scholar]
- Holliday R 1990. DNA methylation and epigenetic inheritance. Philosophical Transactions of the Royal Society of London B 326:329–338. [DOI] [PubMed] [Google Scholar]
- Holliday R 1994. Epigenetics: an overview. Developmental Genetics 15:453–457. [DOI] [PubMed] [Google Scholar]
- Holliday R 2006. Epigenetics: a historical overview. Epigenetics 1:76–80. [DOI] [PubMed] [Google Scholar]
- Huey RB and Bennett AF. 1987. Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41:1098–1115. [DOI] [PubMed] [Google Scholar]
- Irschick DJ, Herrel A, Vanhooydonck B, and Damme RV. 2007. A functional approach to sexual selection. Functional Ecology 21:621–626. [Google Scholar]
- Jablonka E 2013. Epigenetic inheritance and plasticity: The responsive germline. Progress in Biophysics and Molecular Biology 111:99–107. [DOI] [PubMed] [Google Scholar]
- Jablonka E and Raz G. 2009. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. The Quarterly Review of Biology 84:131–176. [DOI] [PubMed] [Google Scholar]
- Jaenisch R and Bird A. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics Supplement 33:245–254. [DOI] [PubMed] [Google Scholar]
- Jensen P 2015. Adding “epi-” to behaviour genetics: implications for animal domestication. Journal of Experimental Biology 218:32–40. [DOI] [PubMed] [Google Scholar]
- Jirtle RL and Skinner MK. 2007. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics 8:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, and Whitlock MC. 2012. Experimental evolution. Trends Ecol Evol (Amst) 27:547–560. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Meek TH, Middleton KM, Holness LF, and Garland T Jr. 2012. Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior. Pharmacology Biochemistry and Behavior 101:528–537. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Nehrenberg DL, Hua K, Garland T Jr., and Pomp D. 2012a. Functional genomic architecture of predisposition to voluntary exercise in mice: expression QTL in the brain. Genetics 191:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SA, Panhuis TM, and Stoehr AM. 2012b. Phenotypic plasticity: molecular mechanisms and adaptive significance. Comprehensive Physiology 2:1417–1439. [DOI] [PubMed] [Google Scholar]
- Kelly SA and Pomp D. 2013. Genetic determinants of voluntary exercise. Trends in Genetics 29:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM and Lightfoot JT. 2010. Does the difference between physically active and couch potato lie in the dopamine system? International Journal of Biological Sciences 6:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeneman MA, Verheijden MW, Chinapaw MJ, and Hopman-Rock M. 2011. Determinants of physical activity and exercise in healthy older adults: a systematic review. Int J Behav Nutr Phys Act 8:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb EM, Kelly SA, Middleton KM, Sermsakdi LS, Chappell MA, and Garland T Jr. 2010. Erythropoietin elevates VO2,max but not voluntary wheel running in mice. Journal of Experimental Biology 213:510–519. [DOI] [PubMed] [Google Scholar]
- Kostrzewa E and Kas MJ. 2014. The use of mouse models to unravel genetic architecture of physical activity: a review. Genes, Brain and Behavior 13:87–103. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Allen DL, Booth FW, Fleshner MR, Henriksen EJ, Musch TI, Leary DSO, et al. 2006. Resource book for the design of animal exercise protocols. American Physiological Society, Bethesda, Maryland. [Google Scholar]
- Kuhn SL, Raichlen DA, and Clark AE. 2016. What moves us? How mobility and movement are at the center of human evolution. Evolutionary Anthropology: Issues, News, and Reviews 25:86–97. [DOI] [PubMed] [Google Scholar]
- Kuijper B, Johnstone RA, and Townley S. 2014. The evolution of multivariate maternal effects. PLoS Computational Biology 10:e1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper B, Pen I, and Weissing FJ. 2012. A guide to sexual selection theory. Annual Review of Ecology, Evolution, and Systematics 43:287–311. [Google Scholar]
- Laland K, Uller T, Feldman M, Sterelny K, Muller GB, Moczek A, Jablonka E, et al. 2014. Does evolutionary theory need a rethink? Nature 514:161–164. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC 2009. Nutritional programming of disease: unravelling the mechanism. Journal of Anatomy 215:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Galliard J-FL, Clobert J, and Ferriere R. 2004. Physical performance and darwinian fitness in lizards. Nature 432:502–505. [DOI] [PubMed] [Google Scholar]
- Levin BE 2008. Epigenetic influences on food intake and physical activity level: review of animal studies. Obesity 16:S51–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Kohorst JJ, Zhang W, Laritsky E, Kunde-Ramamoorthy G, Baker MS, Fiorotto ML, et al. 2013. Early postnatal nutrition determines adult physical activity and energy expenditure in female mice. Diabetes 62:2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang W, Baker MS, Laritsky E, Mattan-Hung N, Yu D, Kunde-Ramamoorthy G, et al. 2014. Major epigenetic development distinguishing neuronal and non-neuronal cells occurs postnatally in the murine hypothalamus. Human Molecular Genetics 23:1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionikas A and Blizard DA. 2008. Diverse effects of stanozolol in C57BL/6J and A/J mouse strains. European Journal of Applied Physiology 103:333–341. [DOI] [PubMed] [Google Scholar]
- Lussana F, Painter RC, Ocke MC, Buller HR, Bossuyt PM, and Roseboom TJ. 2008. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. American Journal of Clinical Nutrition 88:1648–1652. [DOI] [PubMed] [Google Scholar]
- Lynn SE and Kern MD. 2017. Ecologically relevant cooling early in life alters pre-fledging adrenocortical response in free-living songbirds. Physiological and Biochemical Zoology 90:in press. [DOI] [PubMed] [Google Scholar]
- Majdak P, Bucko PJ, Holloway AL, Bhattacharya TK, DeYoung EK, Kilby CN, Zombeck JA, et al. 2014. Behavioral and pharmacological evaluation of a selectively bred mouse model of home cage hyperactivity. Behavior Genetics 44:516–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Ghalambor CK, and Woods HA, eds. 2015. Integrative organismal biology. Wiley Blackwell, Hoboken, New Jersey. [Google Scholar]
- Mathes WF, Aylor DL, Miller DR, Churchill GA, Chesler EJ, de Villena FP-M, Threadgill DW, et al. 2011. Architecture of energy balance traits in emerging lines of the Collaborative Cross. AJP: Endocrinology and Metabolism 300:E1124–E1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattocks C, Ness A, Deere K, Tilling K, Leary S, Blair SN, and Riddoch C. 2008. Early life determinants of physical activity in 11 to 12 year olds: cohort study. BMJ 336:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin E, Curry EJ, and Whitlock K. 2015. Female athlete triad: past, present, and future. J Am Acad Orthop Surg 23:424–432. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Meaney MJ, and Szyf M. 2008. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Research 1237:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ and Szyf M. 2005. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience 7:103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek TH, Eisenmann JC, and Garland T Jr. 2010. Western diet increases wheel running in mice selectively bred for high voluntary wheel running. International Journal of Obesity 34:960–69. [DOI] [PubMed] [Google Scholar]
- Meek TH, Eisenmann JC, Keeney BK, Hannon RM, Dlugosz EM, and Garland T Jr. 2014. Effects of early-life exposure to Western diet and wheel access on metabolic syndrome profiles in mice bred for high voluntary exercise. Genes, Brain and Behavior 13:322–332. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Watson P, Hasegawa H, Roelands B, and Piacentini MF. 2007. Brain neurotransmitters in fatigue and overtraining. Applied Physiology, Nutrition, and Metabolism 32:857–864. [DOI] [PubMed] [Google Scholar]
- Meijer JH and Robbers Y. 2014. Wheel running in the wild. Proceedings of the Royal Society B: Biological Sciences 281:20140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metges CC 2009. Early nutrition and later obesity: animal models provide insights into mechanisms Pp. 105–112 in Koletzko B, Decsi T, Molnar D, and Hunty A eds. Early Nutrition Programming and Health Outcomes in Later Life. Springer. [DOI] [PubMed] [Google Scholar]
- Meyers LA and Bull JJ. 2002. Fighting change with change: adaptive variation in an uncertain world. Trends in Ecology & Evolution 17:551–557. [Google Scholar]
- Meylan S, Miles DB, and Clobert J. 2012. Hormonally mediated maternal effects, individual strategy and global change. Philosophical Transactions of the Royal Society B: Biological Sciences 367:1647–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TS, Warner DA, and Janzen FJ. 2013. Phenotypic and fitness consequences of maternal nest-site choice across multiple early life stages. Ecology 94:336–345. [DOI] [PubMed] [Google Scholar]
- Nathan R 2008. An emerging movement ecology paradigm. Proceedings of the National Academy of Sciences 105:19050–19051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM and Stearns SC. 2008. The great opportunity: Evolutionary applications to medicine and public health. Evolutionary Applications 1:28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Ha C-DT, Garrett PJ, Mueke E-M, Smale L, and Holekamp KE. 1998. Body fat and time of year interact to mediate dispersal behaviour in ground squirrels. Animal Behaviour 55:605–614. [DOI] [PubMed] [Google Scholar]
- Ong ZY and Muhlhausler BS. 2014. Consuming a low-fat diet from weaning to adulthood reverses the programming of food preferences in male, but not in female, offspring of “junk food”-fed rat dams. Acta Physiologica 210:127–141. [DOI] [PubMed] [Google Scholar]
- Öst A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, et al. 2014. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159:1352–1364. [DOI] [PubMed] [Google Scholar]
- Oufiero CE, Meredith RW, Jugo KN, Tran P, Chappell MA, Springer MS, Reznick DN, et al. 2014. The evolution of the sexually selected sword in Xiphophorus does not compromise aerobic locomotor performance. Evolution 68:1806–1823. [DOI] [PubMed] [Google Scholar]
- Ozanne SE and Siddle K. 2011. Developmental origins of insulin resistance and Type 2 diabetes Pp. 60–80 in Byrne Christopher D. and Wild Sarah H. eds. The Metabolic Syndrome, 2nd ed. Blackwell. [Google Scholar]
- Paranjpe DA, Bastiaans E, Patten A, Cooper RD, and Sinervo B. 2013. Evidence of maternal effects on temperature preference in side-blotched lizards: implications for evolutionary response to climate change. Ecology and Evolution 3:1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, and Golding J. 2006. Sex-specific, male-line transgenerational responses in humans. European journal of human genetics 14:159–166. [DOI] [PubMed] [Google Scholar]
- Phillips PC 2008. Epistasis — the essential role of gene interactions in the structure and evolution of genetic systems. Nature Reviews Genetics 9:855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyusnina IZ, Oskina IN, Tibeikina MA, and Popova NK. 2009. Cross-fostering effects on weight, exploratory activity, acoustic startle reflex and corticosterone stress response in Norway gray rats selected for elimination and for enhancement of aggressiveness towards human. Behavior Genetics 39:202–212. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, and Garland T Jr. 2005. Neurobiology of mice selected for high voluntary wheel-running activity. Integrative and Comparative Biology 45:438–455. [DOI] [PubMed] [Google Scholar]
- Rice SH 2008. Theoretical approaches to the evolution of development and genetic architecture. Annals of the New York Academy of Sciences 1133:67–86. [DOI] [PubMed] [Google Scholar]
- Riggs AD, Martienssen RA, and Russo VEA. 1996. Introduction Pp. 1–4 in Russo VEA, Martienssen RA, and Riggs AD eds. Cold Spring Harbor Monograph Archive. [Google Scholar]
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, and Booth FW. 2012. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiology & Behavior 105:661–668. [DOI] [PubMed] [Google Scholar]
- Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Sorenson AR, Scholz TD, et al. 2009. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 296:R651–R662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa BV, Blair HT, Vickers MH, Dittmer KE, Morel PCH, Knight CG, and Firth EC. 2013. Moderate exercise during pregnancy in Wistar rats alters bone and body composition of the adult offspring in a sex-dependent manner. PLoS ONE 8:e82378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MG and Desai M. 2005. Gestational programming: population survival effects of drought and famine during pregnancy. AJP: Regulatory, Integrative and Comparative Physiology 288:R25–R33. [DOI] [PubMed] [Google Scholar]
- Rosvall M and Bergstrom CT. 2007. Maps of information flow reveal community structure in complex networks. Citeseer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska J, Gebczynski AK, and Konarzewski M. 2013. Basal metabolic rate is positively correlated with parental investment in laboratory mice. Proceedings of the Royal Society B: Biological Sciences 280:20122576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF and Hovell MF. 1990. Determinants of exercise behavior. Exercise and Sport Sciences Reviews 18:307–330. [PubMed] [Google Scholar]
- Samuelsson A-M, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, et al. 2008. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51:383–392. [DOI] [PubMed] [Google Scholar]
- Samuelsson A-M, Morris A, Igosheva N, Kirk SL, Pombo JMC, Coen CW, Poston L, et al. 2010. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 55:76–82. [DOI] [PubMed] [Google Scholar]
- Scheurink AJW, Boersma GJ, Nergårdh R, and Södersten P. 2010. Neurobiology of hyperactivity and reward: Agreeable restlessness in Anorexia Nervosa. Physiology & Behavior 100:490–495. [DOI] [PubMed] [Google Scholar]
- Sheldon BC and West SA. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. The American Naturalist 163:40–54. [DOI] [PubMed] [Google Scholar]
- Simar D, Chen H, Lambert K, Mercier J, and Morris MJ. 2012. Interaction between maternal obesity and post-natal over-nutrition on skeletal muscle metabolism. Nutrition, Metabolism and Cardiovascular Diseases 22:269–276. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Miles DB, Frankino WA, Klukowski M, and DeNardo DF. 2000. Testosterone, endurance, and Darwinian fitness: natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Hormones and Behavior 38:222–233. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Lee MY, Getchell KM, So K, Hirshman LJ, and Goodyear LJ. 2015. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AD, Rundle A, Wada N, Goldbohm RA, and Lumey LH. 2009. Associations of gestational exposure to famine with energy balance and macronutrient density of the diet at age 58 years differ according to the reference population used. Journal of Nutrition 139:1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Bridgham JT, Kelly SA, and Garland T Jr. 2015. Genetic approaches in comparative and evolutionary physiology. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 309:R197–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, and Grove KL. 2011. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 93:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Liang N-C, Ewald ER, Purcell RH, Boersma GJ, Yan J, Moran TH, et al. 2013. Early postweaning exercise improves central leptin sensitivity in offspring of rat dams fed high-fat diet during pregnancy and lactation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 305:R1076–R1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Centanni AV, and Butler AA. 2010. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology 151:1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson KL, Von Smith R, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, et al. 2007. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. Journal of Applied Physiology 102:2369–2378. [DOI] [PubMed] [Google Scholar]
- Swallow JG and Garland T Jr. 2005. Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits—an introduction to the symposium. Integrative and Comparative Biology 45:387–390. [DOI] [PubMed] [Google Scholar]
- Tammen SA, Friso S, and Choi S-W. 2013. Epigenetics: The link between nature and nurture. Molecular Aspects of Medicine 34:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PD and Poston L. 2007. Developmental programming of obesity in mammals. Experimental Physiology 92:287–298. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Scott AN, and Bale TL. 2009. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 162:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost SG, Owen N, Bauman AE, Sallis JF, and Brown W. 2002. Correlates of adults’ participation in physical activity: review and update. Medicine & Science in Sports & Exercise 34:1996–2001. [DOI] [PubMed] [Google Scholar]
- Turkheimer E 2000. Three laws of behavior genetics and what they mean. Current Directions in Psychological Science 9:160–164. [Google Scholar]
- Valladares F, Matesanz S, Guilhaumon F, Araújo MB, Balaguer L, Benito-Garzón M, Cornwell W, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. (Thuiller W, ed.)Ecology Letters 17:1351–1364. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, McCarthy D, and Gluckman PD. 2003. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 285:R271–R273. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Hill WG, and Wray NR. 2008. Heritability in the genomics era — concepts and misconceptions. Nature Reviews Genetics 9:255–266. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Carlin JL, Totoki K, and Reyes TM. 2012. Epigenetic dysregulation of the dopamine system in diet-induced obesity. Journal of Neurochemistry 120:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, and Reyes TM. 2010. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151:4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH 1939. An introduction to modern genetics Allen and Unwin, London. [Google Scholar]
- Waddington CH 1942. The epigenotype. Endeavor; 1:18–20. [Google Scholar]
- Waddington CH 1953. Genetic assimilation of an acquired character. Evolution 7:118–126. [Google Scholar]
- Wallace IJ and Garland T Jr. 2016. Mobility as an emergent property of biological organization: insights from experimental evolution. Evolutionary Anthropology 25:98–104. [DOI] [PubMed] [Google Scholar]
- Waterland RA 2014. Epigenetic mechanisms affecting regulation of energy balance: many questions, few answers. Annual Review of Nutrition 34:337–355. [DOI] [PubMed] [Google Scholar]
- Waterland RA and Garza C. 1999. Potential mechanisms of metabolic imprinting that lead to chronic disease. The American Journal of Clinical Nutrition 69:179–197. [DOI] [PubMed] [Google Scholar]
- Waterland RA and Jirtle RL. 2003. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Molecular and Cellular Biology 23:5293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA and Michels KB. 2007. Epigenetic epidemiology of the developmental origins hypothesis. Annual Review of Nutrition 27:363–388. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Travisano M, Tahiliani KG, Rached MT, and Mirza S. 2008. Methyl donor supplementation prevents transgenerational amplification of obesity. International journal of obesity 32:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RP, Pringle RB, Forster GL, Renner KJ, Malisch JL, Garland T Jr., and Swallow JG. 2013. Selection for increased voluntary wheel-running affects behavior and brain monoamines in mice. Brain Research 1508:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker KW, Totoki K, and Reyes TM. 2012. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutrition, Metabolism and Cardiovascular Diseases 22:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS and Franklin CE. 2002. Testing the beneficial acclimation hypothesis. Trends in Ecology & Evolution 17:66–70. [Google Scholar]
- Wu C. -t. and Morris JR. 2001. Genes, genetics, and epigenetics: a correspondence. Science 293:1103–1105. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Kim J, Yuan X, and Braun T. 2011. Epigenetic modifications of stem cells: a paradigm for the control of cardiac progenitor cells. Circulation Research 109:1067–1081. [DOI] [PubMed] [Google Scholar]
- Zhu S, Eclarinal J, Baker MS, Li G, and Waterland RA. 2016. Developmental programming of energy balance regulation: is physical activity more “programmable” than food intake? Proceedings of the Nutrition Society 75:73–77. [DOI] [PubMed] [Google Scholar]