Abstract
Purpose:
Corneal endothelial cell regeneration varies by species with nonhuman primates (NHPs) and rabbits displaying low and high proliferative capacities, respectively. Recent studies report that topical application of rho-associated kinase (ROCK) inhibitors accelerates corneal endothelial wound healing in animal models and human patients with endothelial dysfunction. The objectives of this study were to determine the regenerative capacity of canine corneal endothelial cells in vivo and determine their response to a topical ROCK inhibitor, Y27632, following transcorneal freezing.
Methods:
Right eyes of 6 beagles underwent transcorneal freezing; 10 mM of ROCK inhibitor Y27632 or vehicle control was applied topically to both eyes 4 times daily for 56 days. Endothelial cell density (ECD) was evaluated by in vivo confocal microscopy (IVCM), and corneal thickness was measured by Fourier-domain optical coherence tomography (FD-OCT) and ultrasound pachymetry (USP).
Results:
Transcorneal freezing induced severe central corneal edema in dogs with restoration of transparency occurring within 4 weeks. Y27632 significantly decreased corneal thickness by FD-OCT and USP in the acute phase and significantly increased ECD at days 28 and 42 post-cryoinjury suggesting faster restoration of endothelial cell recovery.
Conclusion:
Canine corneal endothelial function recovers at a similar rate as NHPs but more slowly than rabbits following cryoinjury. Faster corneal endothelial wound healing was observed by IVCM and FD-OCT in dogs treated with Y27632 versus vehicle controls. Thus, a canine cryoinjury model may be a useful alternative to NHPs in detecting a response to therapies directed at endothelial regeneration.
Keywords: Dog, Corneal endothelium, Transcorneal freezing, Rho-associated kinase inhibitor, Y27632, Canine
INTRODUCTION
Corneal endothelial cells are essential to maintaining corneal transparency through preservation of stromal deturgescence. However, endothelial cell density (ECD) declines with age and corneal endothelial damage can result from trauma including intraocular surgeries, degenerative diseases such as Fuchs’ endothelial corneal dystrophy (FECD), uveitis, glaucoma and diabetes mellitus.1 Furthermore, with few exceptions, the corneal endothelium has a very low proliferative capacity in vivo, and typically fills any areas devoid of cells by migration and increased cell spreading.1 Consequently, when ECD decreases to a critical number, the pump function of the corneal endothelium cells is impaired resulting in concomitant corneal edema, termed bullous keratopathy. While corneal endothelial cells are capable of proliferation under certain conditions in vitro,2 the primary therapy for bullous keratopathy is corneal transplantation as there are no commercially available pharmaceutical interventions for endothelial regeneration.3 Endothelial dysfunction is common in human patients with FECD and pseudophakic bullous keratopathy accounting for 20–50% of the indications for corneal transplantation.4,5
Rho is a molecular switch that responds to messages from G-protein-coupled receptors and cell surface receptor-binding cytokines, growth factors and adhesion molecules.6 Rho-associated, coiled-coil containing protein kinase (ROCK) attaches to Rho and forms a complex that regulates multiple physiological functions, such as cell contraction, migration, proliferation, angiogenesis, and chemotaxis.7 Inhibition of ROCK stimulates corneal endothelial proliferation and adhesion in vitro and in vivo.8,9 In a landmark clinical study, cultured endothelial cells in combination with the ROCK inhibitor, Y27632, increased ECD and improved visual acuity in humans with bullous keratopathy.10 Various topical ROCK inhibitors can restore corneal transparency in animal models of endothelial injury as well as human patients.9,11
Due to lack of corneal donor tissue in many regions of the world, cell-based and pharmaceutical therapies for corneal endothelial regeneration are active areas of research. Consequently, animal models of endothelial injury are required for translation of promising lead therapeutic compounds in vitro to human clinical trials. Therefore, the purposes of this study were to describe a canine endothelial cryoinjury model and define its response to topical Y27632 therapy.
MATERIALS AND METHODS
Animals and Ocular Imaging
All aspects of the study were approved by the Institutional Animal Care and Use Committee of the University of California-Davis (#17798) and performed according to the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. Ambient temperature (21 ± 2 ºC) and light-to-dark cycle (14 hours of light to 10 hours of darkness) of the housing area were controlled. Dogs were housed separately in the same room and had ad libitum access to fresh water and a commercially prepared diet. A Student’s t-test power analysis showed that 3 dogs per group would allow us to detect a 10% difference in central corneal thickness (CCT) between eyes with a power of 0.8 and an alpha of 0.05; a previously reported CCT using ultrasound pachymetry (USP) in normal beagles was used for this analysis.12
Prior to and at days 3, 7, 14, 21, 28, 42 and 56 after cryoinjury, we performed the following procedures in this order to both eyes (oculus uterque, OU): Schirmer tear test (Schering-Plough Animal Health, Union NJ 07083) to measure tear production, color digital single lens reflex (SLR) photography with a Nikon D100 (Nikon Inc., Tokyo, Japan) camera attached to a Nikon autofocus micro Nikkor 105 mm (1:2.8 D) lens and a Nikon macro speedlight SB-29s ring flash, Cochet-Bonnet aesthesiometry (12/100 mm, Luneau Ophtalmologie, Prunayle-Gillon, France) to test corneal sensitivity, slit lamp biomicroscopy (SL-15 Slit-Lamp, Kowa Optimed, Inc., Torrance, CA, USA), binocular indirect ophthalmoscopy (Vantage Plus Wireless, Keeler Instruments Inc., Broomall, PA, USA) using a 28 D indirect lens (Volk Optical, Inc., Mentor, OH, USA), USP (Accupach VI; Accutome Ultrasound Inc., Malvern, PA, USA and Pachette 3; DGH Technology, Inc., Exton, PA, USA) to measure CCT and thickness of the superior, inferior, nasal, and temporal peripheral cornea after application of 0.5% proparacaine (Alcon, Inc., Fort Worth, TX, USA) as previously described,13 applanation tonometry (Tonopen XL; Medtronic Solan, Jacksonville, FL, USA) to measure intraocular pressure (IOP), Fourier-domain optical coherence tomography (FD-OCT; RTVue® 100, software version 6.1; Optovue Inc., Fremont, California, USA) to assess corneal thickness and morphology, in vivo confocal microscopy (IVCM; ConfoScan 4; Nidek Technologies, Gamagori, Japan) to assess corneal ECD and morphology, digital slit lamp photography (Imaging Module IM 900; Haag Streit, Koeniz, Switzerland), and fluorescein stain (HUB Pharmaceuticals, LLC, Rancho Cucamonga, CA, USA) to assess for epithelial injury. External photography was then performed after topical fluorescein stain application to measure epithelial wound area. Additionally, USP, applanation tonometry, and slit lamp biomicroscopy were performed at day 1, 2, 4, 5, 6, 10 and 17 after the surgery. Ophthalmic examinations and clinical assessment were performed using the semiquantitative preclinical ocular toxicology scoring (SPOTS) system at the aforementioned time points.14
Dogs were sedated with dexmedetomidine (3.0 μg/kg) intravenously (IV) for 30–45 minutes to facilitate IVCM, FD-OCT and digital slit lamp photography; an equal volume of atipamezole was administered intramuscularly (IM) to reverse the sedation at the completion of imaging. Dogs were then placed in sternal recumbency and an isotonic buffered ophthalmic solution (OcuSOFT Eyewash, Altaire Pharmaceuticals, Inc., Aquebogue, NY, USA) was applied to prevent corneal desiccation as needed throughout the sedation and subsequent procedures. FD-OCT imaging (26000 A scan/sec, 5 μm axial resolution, 840 nm superluminescent diode) of the central cornea was performed OU using previously described methods.12 The RTVue measuring tool was used to measure CCT and thickness of the epithelium, stroma and Descemet’s membrane (DM)-endothelium complex. The central cornea was imaged with IVCM with a ×40/0.75 objective lens and manual endothelial counts performed using the Confoscan 4 NAVIS imaging softwareas previously described.13
Cryoinjury and Treatment
Dogs were sedated with either dexmedetomidine (3.0 μg/kg) IV or hydromorphone (0.1 mg/kg) IM. General anesthesia was induced with isoflurane by mask (2–5% inhalation) or propofol (4 mg/kg) IV and midazolam (0.2 mg/kg) IV, and maintained with isoflurane (2–5% inhalation). At 5 and 1 minutes prior to transcorneal freezing, 0.05% proparacaine was administered for topical anesthesia. Transcorneal freezing was performed on the right eye (oculus dexter, OD) of all dogs (Figure 1A). An 8-mm diameter steel cryoprobe immersed in liquid nitrogen for 3 minutes to an approximate temperature of −196°C was applied to the cornea of OD for 20 seconds15; the left eye (oculus sinister, OS) remained unwounded. Then, both eyes were treated with 40 μl of 10 mM Y27632 (LC Laboratories, Woburn, MA, n = 3 dogs), a ROCK inhibitor, or phosphate buffered solution (PBS, GE Healthcare Life Sciences HyClone Laboratories, Logan, UT, USA, n = 3 dogs) 6 times daily for the initial 7 days after transcorneal freezing then 4 times for the remaining 49 days. Dogs were treated with topical 0.3% ofloxacin to prevent infection for days 0 to 14. Topical 1.0% atropine was used for days 0 to 21, and buprenorphine (0.01 mg/kg) IM was used once following the surgery and twice daily thereafter for days 0 to 7 for analgesia.
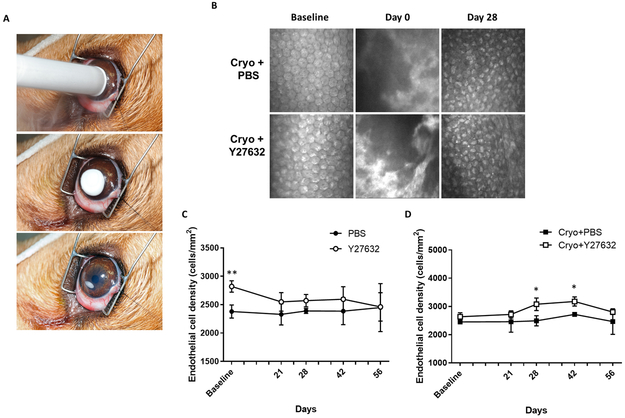
Figure 1. Topical administration of the ROCK inhibitor, Y27632, promoted the recovery of endothelial cell density (ECD) in a canine cryoinjury model.
(A) The central corneal endothelium was destroyed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. (B) Representative images of the corneal endothelium using IVCM show endothelial injury (day 0) then repair (day 28). A bare Descemet’s membrane without endothelium is observed on day 0 immediately post-cryoinjury. Subsequently, the endothelium could not be observed due to marked corneal edema until day 21. Restoration of the endothelial monolayer with small, irregularly shaped cells was observed at day 28 following reduction of corneal edema. (C, D) Confocal microscopy analysis revealed that ECD was significantly greater in the Cryo+Y27632 versus Cryo+PBS eyes at days 28 and 42. In eyes without cryoinjury, endothelial cell morphology and density did not differ over time or by treatment. The P values were determined by a Student’s t-test, **P < 0.01 and *P < 0.05.
Statistical Analysis
A Student’s t-test was used to compare ages and weights of the dogs receiving Y27632 or PBS. Four different treatment groups were evaluated since OD received cryoinjury while OS remained unwounded: PBS (no cryoinjury and PBS administration), Cryo+PBS (cryoinjury and PBS administration), Y27632 (no cryoinjury and Y27632 administration) and Cryo+Y27632 (cryoinjury and Y27632 administration). A two-way analysis of variance (ANOVA) was used to assess the effects of treatment and time on corneal thickness measured by USP or FD-OCT, corneal sensitivity and STT. A two-way ANOVA was used to assess the effects of time on corneal ECD and IOP. A Holm-Sidak’s multiple-comparison test was used for post-hoc analysis. A Student’s t-test was used to compare the effect of treatment on corneal ECD, epithelial wound area and IOP between 2 groups at each time point. A Mann-Whitney test was used to analyze the semiquantitative SPOTS scoring results.14 Significance was P < 0.05 for all analyses. Unless otherwise stated, data are presented as mean ± standard deviation (SD). Statistically significant differences are indicated as *P < 0.05, **P < 0.01 or ***P < 0.001 within figures unless stated otherwise.
RESULTS
Six sexually intact female beagles were included in this study; ages (2.36 ± 0.07 years; P = 0.42) and weights (8.63 ± 0.53 kg; P = 0.52) did not significantly differ between the 2 groups. All eyes were examined by slit lamp daily and scored using the SPOTS scoring system as previously described.14 Following cryoinjury OD, transient corneal opacity due to edema, corneal vascularization, conjunctival congestion, conjunctival chemosis, and mild anterior uveitis were observed. These abnormalities resolved at different timepoints during the observation period, and topical Y27632 did not affect these observations (data not shown). In the early phase of observation, the lens and vitreous could not be observed due to corneal edema; however, no abnormalities were observed in the lens, vitreous and retina following restoration of corneal transparency. Epithelial injury, as defined with fluorescein stain retention, was healed by a median (range) 3 (3–4) and 6 (5–14) days in Cryo+PBS and Cryo+Y27632 groups, respectively. At day 2 post-cryoinjury, Y27632 treated eyes had a significantly larger epithelial wound area versus PBS treated eyes (P = 0.044). However, no significant changes were observed between Y27632 and PBS treated groups at all other time points. One dog in the Y27632 treated group required an epithelial debridement to stimulate epithelial wound healing on day 5. At day 7, subtle stromal loss was observed in the central cornea and infectious keratitis was suspected. Therefore, 0.5% moxifloxacin hydrochloride 4 times daily was substituted for 0.3% ofloxacin; epithelial wound closure was observed on day 14 (Supplementary Figure 1). Corneal sensitivity and tear production as measured by Cochet-Bonnet aesthesiometry and STT, respectively, did not differ between Y27632 and PBS treated dogs (data not shown).
Endothelial Cell Density
In the present study, corneal endothelial cell regeneration was observed in all dogs at day 28 following cryoinjury (Figure 1B). Until day 21 post-cryoinjury, endothelial cells could not be clearly visualized OD in the central cornea by IVCM due to severe corneal edema. At days 28 to 56 post-cryoinjury, small, irregularly shaped endothelial cells were observed OD in the central cornea. The ECD OD was significantly higher in dogs receiving Cryo+Y27632 versus Cryo+PBS at days 28 (P = 0.024) and 42 (P = 0.037) (Figure 1D); Y27632 did not significantly alter ECD OS (Figure 1C).
Corneal Thickness
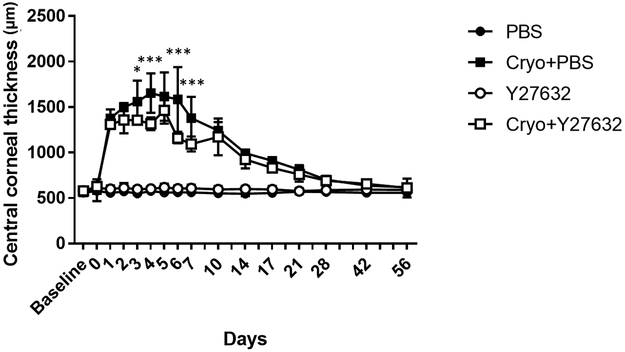
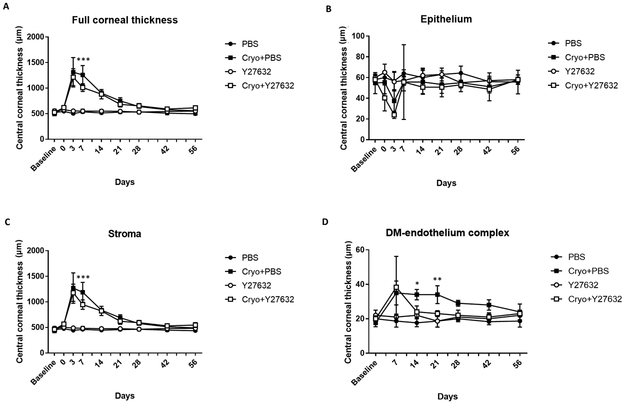
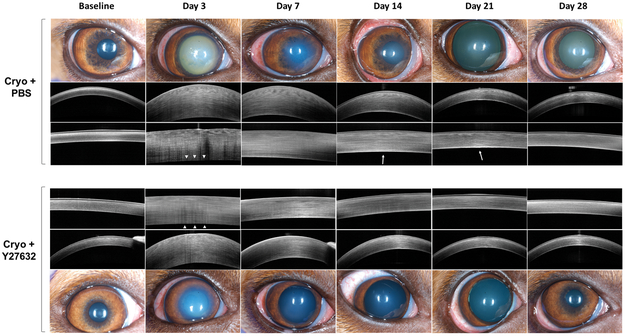
Transcorneal freezing OD resulted in a greater than 2-fold increase in corneal thickness from days 1 to 10 post-cryoinjury, and remained significantly increased from baseline to day 14 in Y27632 treated eyes and to day 21 post-cryoinjury in PBS treated eyes (Figure 2). Following day 5, a gradual decrease of CCT was observed through the observation period. Topical administration of Y27632 resulted in a significant decrease in CCT as measured by USP at days 3 (P = 0.016), 4, 6 and 7 (P < 0.001) post-cryoinjury (Figure 2). However, the peripheral cornea exhibited less marked changes in thickness following cryoinjury and thus more variability was observed between the Y27632 and PBS treated groups (Supplementary Figure 2). Manual pachymetry by FD-OCT demonstrated a significant decrease in CCT (P < 0.001) primarily due to a reduction in stromal edema (P < 0.001) in Y27632 versus PBS treated eyes at day 7 post-cryoinjury (Figures 3A and 3C). Furthermore, FD-OCT also demonstrated decreased reflectivity of DM-endothelium complex OD on day 3 post-cryoinjury with restoration of its normal reflectivity at day 7 in Y27632 treated eyes (Figure 4). Furthermore, DM-endothelium complex remained significantly increased in Cryo+PBS group at days 14 (P = 0.020) and 21 (P = 0.009) post-cryoinjury, while Y27632 promoted a return to normal DM-endothelial thickness at these same timepoints (Figures 3D and4).
Figure 2. Central corneal thickness (CCT) was significantly decreased following topical administration of the ROCK inhibitor, Y27632, in comparison to vehicle controls using a canine cryoinjury model.
The central corneal endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. Marked increase in CCT was observed immediately after cryoinjury in both groups but topical Y27632 significantly decreased CCT on days 3, 4, 6 and 7 post-injury. The P values were determined by Holm-Sidak’s multiple comparison test, ***P < 0.001 and *P < 0.05 for Cryo+PBS group versus Cryo+Y27632 group.
Figure 3. In the central cornea, full corneal thickness as well as stromal and DM-endothelial cell thickness as measured by FD-OCT differed between Y27632 and PBS treated eyes in a canine cryoinjury model.
The central corneal endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. (A, C) On day 7 following cryoinjury, Y27632 significantly decreased CCT and central stromal thickness versus vehicle treated controls. (B) Epithelial thickness showed no significant difference between groups throughout the observation period. (D) A significant increase in DM-endothelial thickness was observed in Cryo+PBS group on days 14 and 21 while Y27632 promoted a faster return to normal DM-endothelial thickness. The P values were determined by Holm-Sidak’s multiple comparison test, ***P < 0.001, **P < 0.01 and *P < 0.05 for Cryo+PBS group versus Cryo+Y27632 group.
Figure 4. The ROCK inhibitor Y27632 enhanced the recovery of corneal transparency and normal thickness following topical application 4 times daily in a canine cryoinjury model.
The central corneal endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. Corneal transparency and structure were assessed using color photography and FD-OCT, respectively, and representative images are shown over time. All eyes that underwent cryoinjury showed marked corneal edema with increased CCT with Y27632 promoting a faster restoration of normal corneal transparency and thickness. At day 3, the endothelial reflectivity was not observed in both Cryo+PBS and Cryo+Y27632 groups (white arrow heads), however was re-established by day 7 in both groups. Persistence of a thick DM-endothelial complex thickness was observed in the Cryo+PBS group at days 14 and 21 (white arrows).
Intraocular Pressure
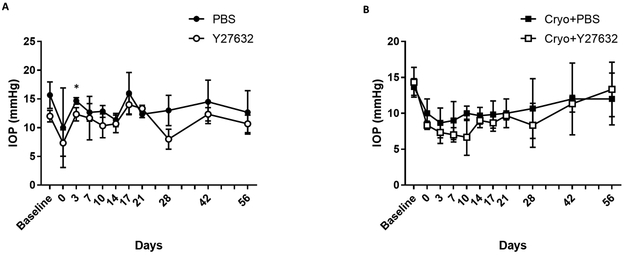
In the present study, a significant decrease in IOP from baseline was observed immediately after cryoinjury OD in the Y27632 treated groups at days 3, 7, and 10 (P = 0.013, P = 0.007 and P = 0.003, respectively, Figure 5B). On day 3, Y27632 treated eyes had a significantly lower IOP versus PBS treated eyes OS (P = 0.035, Figure 5A). However, no significant changes were observed between Y27632 and PBS treated groups at all other timepoints OS or at any timepoint for OD.
Figure 5. The ROCK inhibitor Y27632 and cryoinjury decreased IOP in the eyes of normal dogs.
The central corneal endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. (A) The Y27632 versus PBS treated eyes had a significantly lower IOP at day 3 in eyes without cryoinjury. (B) A significant decrease in IOP was observed immediately after cryoinjury OD in the Y27632 treated groups. The P values were determined by a Student’s t-test, *P < 0.05.
Comparison to Other Animal Models of Endothelial Cryoinjury
In the present study, a significant decrease in ECD was not observed following cryoinjury, and small, irregularly shaped endothelial cells were observed by IVCM once corneal edema resolved. This observation suggests that corneal endothelial cells of young dogs have a proliferative capacity similar to that of rabbits.16–19 However, recovery of endothelial cell function, as measured by a return to normal corneal transparency and thickness, was slower in dogs than rabbits and thus more similar to nonhuman primate (NHP) cryoinjury models where corneal edema and increased thickness remain for greater than 4 weeks post-injury.20 In aggregate, these data suggest that canine corneal endothelial morphology and function recovers at a similar rate as NHPs but more slowly than rabbits following transcorneal freezing.
DISCUSSION
Epithelial wound healing typically consists of cell migration, spreading and proliferation. However, corneal endothelial cells, also known as the posterior epithelium, generally do not proliferate following their initial formation of a monolayer. Consequently, cell enlargement and migration are the predominant courses of repair for the corneal endothelium. Therefore, excessive loss of endothelial cells without proliferation to restore cell density can lead to decompensation of its pump function with concomitant irreversible corneal edema.21 While corneal transplantation is the major treatment for corneal endothelial dysfunction, several challenges exist including shortage of donor tissue, intra- and post-operative complications such as infection, and allograft rejection with associated destruction of donor endothelial cells. Therefore, regenerative medical procedures have been investigated as alternative treatments for corneal endothelial dysfunction.
Numerous studies have demonstrated that ROCK inhibitors can increase the proliferation and migration of corneal endothelial cells while decreasing their apoptosis in vitro.8,9 Similarly ROCK inhibitors have demonstrated corneal endothelial cell regeneration in rabbit and NHP endothelial injury models as well as human patients with Y27632 being the most common ROCK inhibitor studied.11,15 Given that dogs experience a disease similar to FECD,13,23 we were interested in assessing the regenerative capacity of the normal canine endothelium following cryoinjury in vivo and in response to Y27632 treatment. The present study demonstrated that canine eyes treated with Y27632 showed higher corneal ECD than PBS treated eyes at day 28 and 42 post-cryoinjury whereas no significant difference was observed at day 56. We also observed that Y27632 significantly decreased CCT in the early phase of observation suggesting that Y27632 restored an adequate ECD and their critical pump function more quickly in comparison to dogs treated with PBS. This positive effect of the ROCK inhibitor on corneal endothelial cell proliferation was consistent with previous reports tested in NHPs and rabbits.9,15 In aggregate, these data suggest that this proliferative effect on corneal endothelial cells is independent of species or type of ROCK inhibitor studied. Recently, the efficacy of ROCK inhibitors on corneal endothelial wound healing in human patients has been reported after cryoinjury,15 following descemetorhexis without endothelial keratoplasty;24 and in combination with corneal endothelial cell therapy.10 Thus, spontaneous and inducible canine models of FECD may be useful in assessing ROCK inhibitors in combination with interventional procedures. 13,23
Transcorneal freezing is a well-established technique to experimentally injure the corneal endothelium.15–20,25,26 While a NHP endothelial cryoinjury model well represents human corneal endothelial cell behavior, this model is more expensive, less available and requires sedation for all assessments. By contrast, rabbits are frequently used to assess corneal endothelial wound healing; however, their corneal endothelial cells rapidly recover normal density, morphology and function post-cryoinjury.18 In the present study, canine corneal endothelial morphology and function recovered at a similar rate as NHPs but more slowly than rabbits following transcorneal freezing. Thus, the canine cryoinjury model would be ideal to assess cell- or gene-based therapies for corneal endothelial disorders where a longer time to see an effect may be required. Additionally, we highlight that 2 spontaneous canine models of FECD have been reported as well as their utility in assessing a novel surgical treatment for bullous keratopathy.13,23,27 In addition to many genetic traits, dogs share a similar environment and common stressors with humans. Thus, novel therapeutics for endothelial regeneration could be assessed initially for safety and efficacy using the canine cryoinjury model and further refined in preclinical trials using client owned dogs with spontaneous FECD-like disease. The use of spontaneous canine models of disease are likely to increase the success rate of clinical trials for these and other therapies in comparison to using experimentally induced disease in genetically and environmentally homogenous laboratory animals.
In conclusion, we demonstrated the utility of a canine transcorneal freezing model and the ability to identify differences between dogs receiving Y27632 versus vehicle control in a small number of dogs (n = 3/group). Furthermore, the canine cryoinjury model appears to be an excellent alternative to NHPs with a similar rate of recovery of corneal transparency albeit with a higher ECD post-injury. This study also adds to the body of literature that demonstrate that ROCK inhibitors such as Y27632 are potent enhancers of corneal endothelial regeneration in numerous species and hold promise in the treatment of dogs with corneal endothelial dysfunction as a preclinical model for FECD.
Supplementary Material
Supplementary Figure 1. The ROCK inhibitor Y27632 did not markedly impact corneal epithelial wound healing in canine eyes following cryoinjury. The central corneal epithelium and endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. At day 2 post-cryoinjury, Y27632 versus PBS treated eyes showed a significantly larger epithelial wound area following cryoinjury. However, no significant difference was observed between Y27632 treated and PBS treated eyes at any other time points. One dog in the Y27632 treated group required an epithelial debridement to stimulate healing on day 5. At day 7, subtle stromal loss was observed in the central cornea and infectious keratitis was suspected. Therefore, 0.5% moxifloxacin hydrochloride 4 times daily was substituted for 0.3% ofloxacin; epithelial wound closure was observed on day 14. The P values were determined by a Student’s t-test, *P < 0.05.
Supplementary Figure 2. In the peripheral cornea, changes in thickness were less marked following cryoinjury and more variability was observed between Y27632 versus PBS treated eyes. The central corneal endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. (A) In the superior cornea, Y27632 treated eyes a showed significant decrease in corneal thickness at day 3. (B) By contrast, PBS treated eyes showed significant decrease of corneal thickness between day 7 and 14 in the nasal cornea. (C, D) No significant effects by Y27632 were observed in the inferior and temporal regions. The P values were determined by Holm-Sidak’s multiple comparison test, **P < 0.01 and *P < 0.05 for Cryo+PBS group versus Cryo+Y27632 group.
ACKNOWLEDGMENTS
The authors thank Dr. Paul Russell for invaluable scientific discussion and Ms. Monica Motta, Ariana Marangakis, Alyssa Hoehn, Jasmyne Sermeno, Allison Calderon and Kristin Boswell for assisting with drug preparation, patient examinations and treatments and data analysis.
Conflicts of Interest and Source of Funding: This study was funded by grants from the National Institutes of Health K08 EY021142, R01 EY019970, R01 EY016134, and P30 EY12576, UC Davis Academic Federation Innovative Development Award, Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis, and an unrestricted grant from Research to Prevent Blindness.
REFERENCES
- 1.Tuft SJ, Coster DJ. The corneal endothelium. Eye (Lond) 1990;4 ( Pt 3):389–424. [DOI] [PubMed] [Google Scholar]
- 2.Nayak SK, Binder PS. The growth of endothelium from human corneal rims in tissue culture. Invest Ophthalmol Vis Sci 1984;25:1213–1216. [PubMed] [Google Scholar]
- 3.Tan DT, Dart JK, Holland EJ, et al. Corneal transplantation. Lancet 2012;379:1749–1761. [DOI] [PubMed] [Google Scholar]
- 4.Zhang AQ, Rubenstein D, Price AJ, et al. Evolving surgical techniques of and indications for corneal transplantation in Ontario: 2000 – 2012. Can J Ophthalmol 2013;48:153–159. [DOI] [PubMed] [Google Scholar]
- 5.Frigo AC, Fasolo A, Capuzzo C, et al. Corneal transplantation activity over 7 years: changing trends for indications, patient demographics and surgical techniques from the Corneal Transplant Epidemiological Study (CORTES). Transplant Proc 2015;47:528–535. [DOI] [PubMed] [Google Scholar]
- 6.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008;9:690–701. [DOI] [PubMed] [Google Scholar]
- 7.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003;4:446–456. [DOI] [PubMed] [Google Scholar]
- 8.Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci 2009;50:3680–3687. [DOI] [PubMed] [Google Scholar]
- 9.Okumura N, Okazaki Y, Inoue R, et al. Effect of the Rho-Associated Kinase Inhibitor Eye Drop (Ripasudil) on Corneal Endothelial Wound Healing. Invest Ophthalmol Vis Sci 2016;57:1284–1292. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita S, Koizumi N, Ueno M, et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N Engl J Med 2018;378:995–1003. [DOI] [PubMed] [Google Scholar]
- 11.Okumura N, Inoue R, Okazaki Y, et al. Effect of the Rho Kinase Inhibitor Y-27632 on Corneal Endothelial Wound Healing. Invest Ophthalmol Vis Sci 2015;56:6067–6074. [DOI] [PubMed] [Google Scholar]
- 12.Strom AR, Cortes DE, Rasmussen CA, et al. In vivo evaluation of the cornea and conjunctiva of the normal laboratory beagle using time- and Fourier-domain optical coherence tomography and ultrasound pachymetry. Vet Ophthalmol 2016;19:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomasy SM, Cortes DE, Hoehn AL, et al. In Vivo Imaging of Corneal Endothelial Dystrophy in Boston Terriers: A Spontaneous, Canine Model for Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci 2016;57:Oct495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton JS, Miller PE, Bentley E, et al. The SPOTS System: An Ocular Scoring System Optimized for Use in Modern Preclinical Drug Development and Toxicology. J Ocul Pharmacol Ther 2017;33:718–734. [DOI] [PubMed] [Google Scholar]
- 15.Okumura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci 2013;54:2493–2502. [DOI] [PubMed] [Google Scholar]
- 16.Khodadoust AA, Green K. Physiological function of regenerating endothelium. Invest Ophthalmol 1976;15:96–101. [PubMed] [Google Scholar]
- 17.Van Horn DL, Sendele DD, Seideman S, et al. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci 1977;16:597–613. [PubMed] [Google Scholar]
- 18.Minkowski JS, Bartels SP, Delori FC, et al. Corneal endothelial function and structure following cryo-injury in the rabbit. Invest Ophthalmol Vis Sci 1984;25:1416–1425. [PubMed] [Google Scholar]
- 19.Ichijima H, Petroll WM, Jester JV, et al. In vivo confocal microscopic studies of endothelial wound healing in rabbit cornea. Cornea 1993;12:369–378. [DOI] [PubMed] [Google Scholar]
- 20.Van Horn DL, Hyndiuk RA. Endothelial wound repair in primate cornea. Exp Eye Res 1975;21:113–124. [DOI] [PubMed] [Google Scholar]
- 21.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 2003;22:359–389. [DOI] [PubMed] [Google Scholar]
- 22.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol 2008;20:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shull OR, Reilly CM, Davis LB, et al. Phenotypic Characterization of Corneal Endothelial Dystrophy in German Shorthaired and Wirehaired Pointers Using In Vivo Advanced Corneal Imaging and Histopathology. Cornea 2018;37:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis Without Grafting for Fuchs Endothelial Dystrophy-Supplementation With Topical Ripasudil. Cornea 2017;36:642–648. [DOI] [PubMed] [Google Scholar]
- 25.Tuft SJ, Williams KA, Coster DJ. Endothelial repair in the rat cornea. Invest Ophthalmol Vis Sci 1986;27:1199–1204. [PubMed] [Google Scholar]
- 26.Han SB, Ang H, Balehosur D, et al. A mouse model of corneal endothelial decompensation using cryoinjury. Mol Vis 2013;19:1222–1230. [PMC free article] [PubMed] [Google Scholar]
- 27.Horikawa T, Thomasy SM, Stanley AA, et al. Superficial Keratectomy and Conjunctival Advancement Hood Flap (SKCAHF) for the Management of Bullous Keratopathy: Validation in Dogs With Spontaneous Disease. Cornea 2016;35:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eysteinsson T, Jonasson F, Sasaki H, et al. Central corneal thickness, radius of the corneal curvature and intraocular pressure in normal subjects using non-contact techniques: Reykjavik Eye Study. Acta Ophthalmol Scand 2002;80:11–15. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg RA, Raza S, Walford E, et al. Fuchs endothelial corneal dystrophy: clinical characteristics of surgical and nonsurgical patients. Clin Ophthalmol 2014;8:1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nautscher N, Bauer A, Steffl M, et al. Comparative morphological evaluation of domestic animal cornea. Vet Ophthalmol 2016;19:297–304. [DOI] [PubMed] [Google Scholar]
- 31.Thomasy SM, Raghunathan VK, Winkler M, et al. Elastic modulus and collagen organization of the rabbit cornea: epithelium to endothelium. Acta Biomater 2014;10:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res 2013;37:1–12. [DOI] [PubMed] [Google Scholar]
- 33.Whitlock NA, Harrison B, Mixon T, et al. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J Ocul Pharmacol Ther 2009;25:187–194. [DOI] [PubMed] [Google Scholar]
- 34.Toris CB, McLaughlin MA, Dworak DP, et al. Effects of Rho Kinase Inhibitors on Intraocular Pressure and Aqueous Humor Dynamics in Nonhuman Primates and Rabbits. J Ocul Pharmacol Ther 2016;32:355–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The ROCK inhibitor Y27632 did not markedly impact corneal epithelial wound healing in canine eyes following cryoinjury. The central corneal epithelium and endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. At day 2 post-cryoinjury, Y27632 versus PBS treated eyes showed a significantly larger epithelial wound area following cryoinjury. However, no significant difference was observed between Y27632 treated and PBS treated eyes at any other time points. One dog in the Y27632 treated group required an epithelial debridement to stimulate healing on day 5. At day 7, subtle stromal loss was observed in the central cornea and infectious keratitis was suspected. Therefore, 0.5% moxifloxacin hydrochloride 4 times daily was substituted for 0.3% ofloxacin; epithelial wound closure was observed on day 14. The P values were determined by a Student’s t-test, *P < 0.05.
Supplementary Figure 2. In the peripheral cornea, changes in thickness were less marked following cryoinjury and more variability was observed between Y27632 versus PBS treated eyes. The central corneal endothelium was removed by transcorneal freezing using an 8 mm steel probe immersed in liquid nitrogen then applied to the cornea of OD for 20 seconds (OS remained unwounded); 10 mM of ROCK inhibitor Y27632 (n = 3 dogs) or PBS control (n = 3 dogs) was applied topically to both eyes 6 times daily for 7 days after cryoinjury, then 4 times for the remaining 49 days. (A) In the superior cornea, Y27632 treated eyes a showed significant decrease in corneal thickness at day 3. (B) By contrast, PBS treated eyes showed significant decrease of corneal thickness between day 7 and 14 in the nasal cornea. (C, D) No significant effects by Y27632 were observed in the inferior and temporal regions. The P values were determined by Holm-Sidak’s multiple comparison test, **P < 0.01 and *P < 0.05 for Cryo+PBS group versus Cryo+Y27632 group.