Abstract
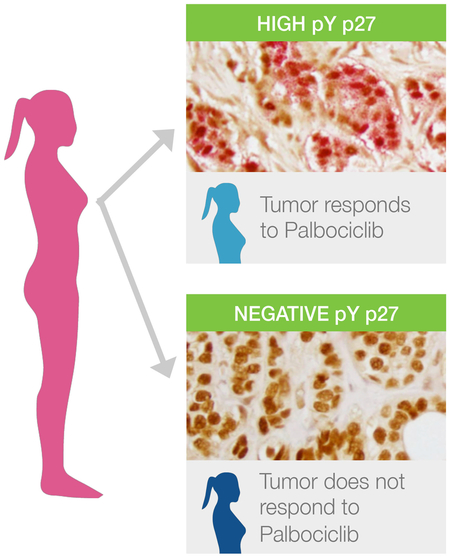
Cdk4 targeting drugs, such as Palbociclib, are approved for metastatic ER/PR+, Her2− breast cancer. However, other than loss of Rb, which is very rare in this subset, there are no biomarkers to predict response. Cyclin D or cdk4 levels are not by themselves indicative, since p27Kip1 is required for cyclin D-cdk4 complex activation. Tyrosine phosphorylation of p27, including modification on residue Y88 (pY88), activates DK4-p27, and the pY88 level correlates with Palbociclib responsiveness in cell lines. We developed dual immunohistochemical staining for p27 and pY88, and found that benign breast epithelium was negative, while breast cancer biopsies (of varied hormonal status) could be stratified for pY88 status. Lack of pY88 suggested that DK4 was inactive, and that these samples would not have the target required for Palbociclib response. Tumor resection material was grown in explant culture, treated with Palbociclib, and stained with Ki67 as a marker of response. Explants from the no pY88 group were non-responsive, while explants from the low or high pY88 group responded to drug.
Keywords: p27Kip1, tyrosine phosphorylation, breast cancer, cdk4 inhibitors, Palbociclib
Graphical Abstract
Introduction
Cyclin D-cdk4/6 complexes are critical for the G1-S phase transition and subsequent cell cycle progression [1]. This complex is stabilized by the assembly factor p27Kip1, and then activated by tyrosine phosphorylation of p27 on residue Y88 (or Y89). [2]. pY88 p27-cyclin D-cdk4 is the active or open complex, competent to phosphorylate the G1 gate keeper protein, Retinoblastoma (Rb), and cause passage into S phase. p27 is critical, but inhibitory, for the activity of another cell cycle cyclin-dependent kinase, cdk2, a molecule normally required for full passage into S phase. Although both Y phosphorylated and non-phosphorylated p27 interact with cdk2 and inhibit this kinase, the pY88 form is preferentially degraded, leading to p27-free cdk2 and cdk2 activation [3]. Thus, p27 plays a pivotal role at several steps in the cell cycle: Y phosphorylated p27 directly activates cdk4 and indirectly increases cdk2 activation.
Cyclin D-cdk4/6 is activated in many human cancers, either by overexpression of cyclin D, or loss of the CDKN2A tumor suppressor gene, which encodes p16Ink4A, the cdk4/6 inhibitor [1]. These observations prompted development of specific cdk4 inhibitors (cdk4i), such as Palbociclib, Abemaciclib and Ribociclib, which have been approved in combination with Estrogen modulation therapy for patients with metastatic, relapsed or drug refractory ER/PR+, Her2− breast cancer [1, 4, 5]. However, data from clinical trials suggest that ~20% of patients exhibit primary resistance to these drugs, and that even initial responders will develop secondary resistance within 2 years of treatment, resulting in the clinical observation of increased progression-free but no overall survival benefit [6]. Unlike most other targeted therapies, there are no clinically approved companion diagnostic markers to identify patients who have the active cdk4 target and thus might respond to this therapy. While loss of Rb expression correlates with Palbociclib non-responsiveness in tissue culture cells, this is a relatively rare event in ER+ breast cancer and has not been validated clinically. Several other cell cycle proteins, such as cyclin E, cdk2, and p16 have also been implicated, but again none have shown clinical utility. Previous work suggested that compensatory activity of cdk2 may explain acquired secondary resistance to Palbociclib, while the activity of the cyclin D-cdk4 complex itself may predict primary responsiveness [7].
Whereas overall cdk4 or cyclin D levels have been shown not to be predictive, we showed that the level of pY88-p27 correlates with cdk4 activity and Palbociclib responsiveness in tissue culture cells [3]. We hypothesized that the level of phosphorylated p27 would correlate with active cdk4 and therefore could be used to predict responsiveness to Palbociclib in patients. By double staining for p27 and pY88 (with an antibody specific for Y88) using immunohistochemistry, we show that both breast cancer cell lines and human breast cancer biopsy material, can be divided into groups based on the level of pY88 staining and that this grouping corresponded with Palbociclib responsiveness in ex vivo short term cultures of fresh tumor. We suggest that pY88 staining can be used as a marker to identify tumors with the active cdk4 target and to predict response to cdk4 inhibitors.
Materials and Methods
Archival Breast Tissue:
Archival tissue blocks were obtained, with IRB approval, from the files of SUNY Downstate Medical Center, University Hospital of Brooklyn. Breast specimens, both benign and malignant, were obtained by core needle biopsy. Hormone status of tumors was determined as part of clinical workup.
Explant culture:
Patients were consented prior to surgery in accordance with SUNY DMC IRB requirements. Fresh breast tumor tissue samples (6mm × 1 mm × 1 mm) were obtained within 20 minutes of removal of tumor from patients and placed into 3 ml of complete culture media (RPMI-1640 with 10% Fetal Bovine Serum, 1% Penicillin-Streptomycin, 10 μg/ml insulin and 1 mg/100ml hydrocortisone). Samples were divided into ~1mm3 pieces, and placed (one specimen per sponge) onto hemostatic gelatin dental sponges (Vetsponge, Elanco), which had been previously hydrated for 2 h in 12-well cell culture dishes in 1.5 ml complete media at 37°C and 5% CO2. Explants were maintained in complete media for 60 h prior to treatment. Media was removed and 1.5 ml fresh media containing 100 nM and 500 nM Palbociclib, or an equal volume of DMSO, was added to each well. Culture plates were incubated for 48 h. Each tumor specimen was treated in duplicate per condition. Samples were then removed from sponges, placed into embedding cassettes and fixed for 24 h in 10% neutral buffered formalin followed by paraffin-embedding.
Immunohistochemistry:
Antibodies: Ki-67 (SC-23900, Santa Cruz Biotechnology); p27-Kip1 (BD Biosciences, #610242), Rb #93095 RbSer780 #9307, and cdk2T160, #25615 (Cell Signaling). The p27 pY88 antibody and its specificity have been previously described [8]. Paraffin-embedded tumor sections or cell blocks were cut into 5 μM sections. MCF7-ALT cells, which express ALT in the presence of Doxycycline (Clontech) were grown and treated +/−Dox as described [3]. Cell blocks were used as a control in Cdk2T160 IHC. Ki67 staining was performed in either the automated stainer (Ventana Medical Systems, Inc.) for the archival material or manually as described below for the explant material. Slides were incubated for 30 min at 65°C, deparaffinized and rehydrated. Endogenous peroxide activity was quenched by incubation with peroxidase solution for 30 min. at room temperature. Slides were washed 3 times in 1x TBST, followed by antigen retrieval in 1x target retrieval solution (DAKO, S-1699) for 30 min. in 100°C water bath. Following antigen retrieval, slides were washed in 1x PBS, incubated with protein block for 1 h. (DAKO, 090930-2), incubated overnight at 4°C with the respective antibodies and developed using the Multiview IHC Kit (ENZO, ADI-950-101-0001). p27/pY88 dual staining assay: Slides were incubated overnight with pY88 antibody. The next day, sections were washed in 1xTBST and then subjected to antigen retrieval as described above, followed by incubation with p27-Kip1 antibody overnight at 4°C and developed as described.
Microscopic Evaluation:
Analysis of H&E stained sections including grading according to Modified Bloom Richardson (MBR) score was carried out blindly by two independent pathologists. For immunohistochemical stains, on patient material, four to six such high power fields (400x) were evaluated where possible and then averaged for a total percent positive/tumor sample. For explant samples with fewer viable tumor cells, total viable nuclei over the entire slide were counted and percent positive was reported. Samples were generally run three separate times for each stain and replicate reading were performed blindly at different times. Any discrepancies were reviewed jointly and resolved. Positive and negative controls were analyzed with each staining.
Statistics:
In Fig. 2-3, means and standard error were calculated using Excel and plotted. In Fig. 3C, right panel, raw Ki67 values from the 1, 2 pY88+ group and the 0 pY88+ group were analyzed using a box and whisker plot using GraphPad Prism. A two-sided paired T test was performed in Fig. 3 to compare the Ki67 scores from the pY88 group 1, 2 and pY88 group 0 patient populations. We used the 0.05 level of significance.
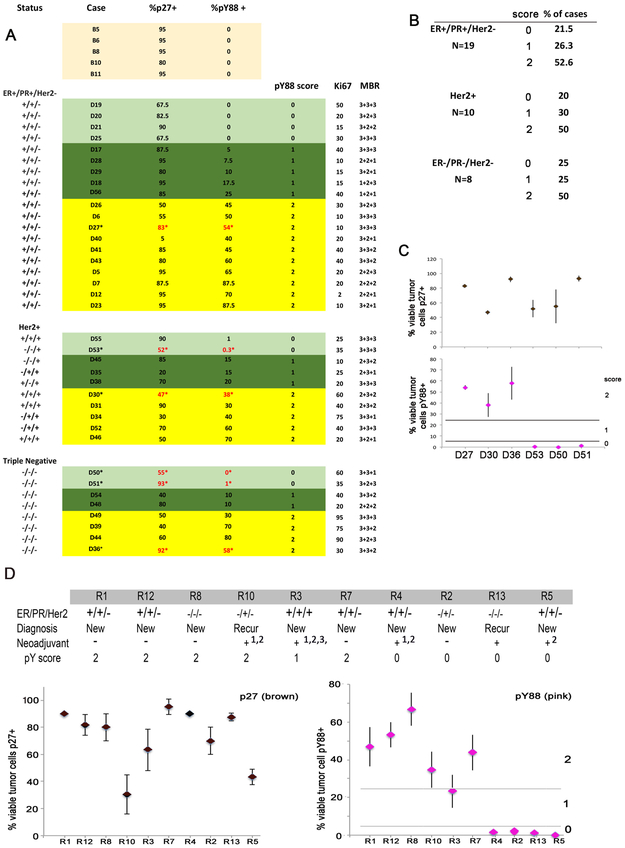
Figure 2.
A) Analysis of archival material from 37 breast cancer patients from all subgroups (ER+/PR+/Her2-: top, Her2+: middle, ER-/PR-/Her2-: bottom), stained with the pY88/p27 dual assay, and then grouped into different pY88 scores (light green=0, dark green=1, yellow=2). Samples were also scored for Ki67, and MBR (tubular + nuclear + mitotic rate, respectively, defined by the WHO). B) Summary of subgroup cohorts. C) Six samples from A were stained in triplicate as independent runs and read independently to determine reproducibility of the pY88/p27 stain and marked with * in A. Top: p27 staining, Bottom: pY88 staining. Data: Mean +/−SE. D) Patient cohort with pY88/p27. Neoadjuvant therapy: 1= Adriamycin Cytoxan 4 cycles; 2=Taxol 4 cycles; 3= Pertuzimab, Tratuzamab, 4 cycles. Biopsies from this cohort were analyzed in the pY88/p27 IHC assay (N=>3 independent stains). Data are mean +/− SE. Left: p27 staining. Right: pY88 staining. Line depicts break between pY88 subgroup 1 and 2.
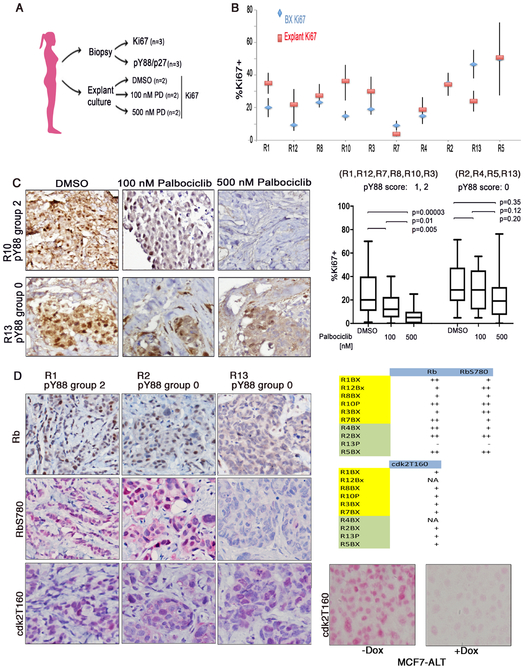
Figure 3:
A) Procedure for explant experiment. B) Patient cohort analysis with Ki67 staining. Biopsy material (blue); Fresh material grown in explant culture treated with DMSO (red). Data are mean +/− SE, N>=3. C) Left: Representative patient material (R10, R13) grown in explant culture treated with DMSO, 100 nM, or 500 nM Palbociclib for 48 h, formalin-fixed, paraffin-embedded and stained with Ki67. Right: Box and whisker plot of the Ki67 staining for the combined different treatment groups for the pY88 0 (R2, R4, R13, R5) and R1, R12, R7, R8, R10, R3) subgroups. P values for each comparison based on paired t-test. D) Left, Representative biopsy material from R1, R2, R13 stained with Rb, RbSer780, Cdk2T160 staining. Right top: Data of total Rb, RbSer780, Cdk2T160 staining. NA= tissue non-longer available. Yellow box: pY88 group 1,2; Green box: pY88 group 0. Rb and RbSer780 staining: +: <10%, ++: >10%. Cdk2T160 staining: +: >5% staining. N=2. Right, bottom: MCF7-ALT cells treated +/− Dox to induce ALT and inhibit cdk2, used as a control for cdk2T160 staining. +Dox condition, cells are arrested (data not shown).
Results
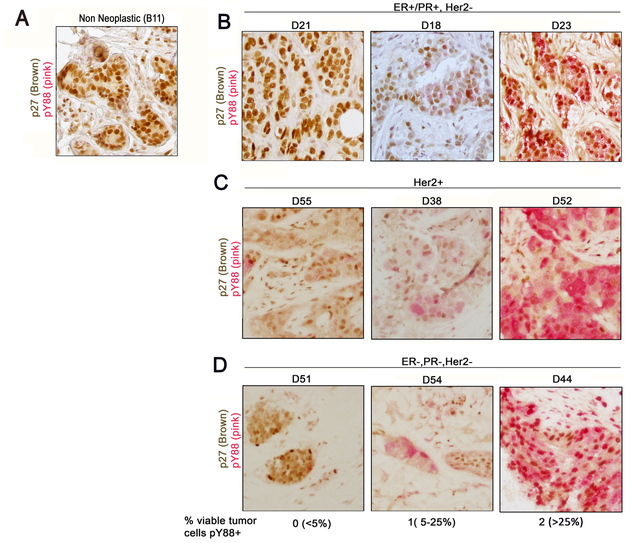
We had previously shown that the level of pY88 p27 measured by immunoblot analysis correlated with the level of active cdk4 measured by in vitro kinase assay and Palbociclib responsiveness [3]. The MCF7 breast cancer cell line, which responds to low concentrations of Palbociclib (IC50=200 nM), had low, but detectable levels of pY88 and active cdk4, while HCC1954 cells had more pY88, more active cdk4 and required a higher concentration of Palbociclib to reach IC50 (1000 nM) [3]. We developed a dual pY88/p27 IHC assay, where pY88 stained pink and p27 stained brown, for use with formalin fixed, paraffin-embedded archival material from patients at University Hospital (Fig. 1, 2). Benign material obtained from stereotactic core breast biopsies in patients without risk factors for breast cancer served as non-neoplastic control (Fig. 1A, Fig. 2A) and was compared with core needle biopsies from patients with invasive ductal carcinoma, not otherwise specified (NOS) (Fig. 1B-D, 2A). For the three breast cancer subgroups [ER/PR+/Her2-, Her2+ and triple negative (TN)], Ki67 positivity ranged from 10-50% and grading ranged from I to III using Modified Bloom Richardson score with 50% being Grade II (Fig. 2A). We scored for the % of viable tumor cells that were pY88+. While strong p27 staining (brown) was detected in normal epithelial cells, all benign epithelium was negative for pY88 (pink staining) (Fig. 1A, 2A), consistent with observations that cdk4 is inactive and benign mammary cells are not in cycle. The breast cancer samples, with the exception of D40, all had high p27 expression (> 20% cells positive), consistent with rare loss of this protein in breast cancer (Fig. 2A). Using the criteria of % pY88+ cells, three groups were identified in the breast cancer samples. In Group 0, <5% pY88+ cells were detected, with staining resembling benign material (Fig. 1B, representative D21, Fig. 2A, light green box). This suggests that this group does not detectable levels of active cdk4 and might not respond to Palbociclib treatment. The rest of the samples had cells that stained with pY88. In Group 1, between 5-25% of cells stained for pY88 (Fig. 1C, representative D18; Fig.2A, dark green box). In Group 2, >25% of cells stained pY88+ (Fig. 1C, representative D23; Fig. 2A, yellow box). When the data was grouped according to pY88 staining subgroups in Fig. 2A, we found that stratification was independent of overall MBR or Ki67 status (Fig. 2A). Roughly similar percentages of pY88 group 0, 1, 2 were seen in ER/PR+/Her2-, Her2+, and TN samples: 20-25% scored as 0 (no pY88+ cells), 25-30% scored as 1, and 50% scored as 2 (high % of pY88+ cells) (Fig. 2B). This suggests that 20-25% of patients across subgroups did not have detectable levels of the active cdk4 target and would never respond to Palbociclib treatment. To validate the reproducibility of the pY88/p27 staining protocol, we stained material from 6 patients three independent times, and the mean +/− SE is plotted (Fig. 2C). We found that whereas the intensity of the stain did vary (data not shown), the percentage of pY88+ cells was consistent across the replicates, with relatively small SE. Additionally, in all repeated cases, the pY88 groupings (Groups 0, 1, or 2) did not change during replicate testing (Fig. 2C).
Figure 1:
Dual pY88/p27 IHC assay with formalin-fixed paraffin-embedded archival human breast tissue. A) Benign breast. B) ER+/PR+/Her2− breast cancer; C) Her2+ breast cancer; D) ER−/PR−/Her2− (TN) breast cancer. All were invasive ductal breast carcinoma. Material was stained to determine % viable tumor cells positive. Score: 0=0-5%+, 1=5-25%+, 2=>25%+.
At the time of this study, few patients at University Hospital had been treated with Palbociclib, and outcome data was not yet available. We therefore identified patients scheduled to undergo mastectomy or lumpectomy (Fig. 2D). Following informed consent, we analyzed their corresponding archival biopsy material. Three patients from the cohort were excluded because there was only in situ carcinoma (R6, R9, R11). ER/PR+/Her2-, Her2+ and TN subgroups are all represented in this cohort (Fig. 2D, top). In 8/10 cases, initial biopsies were analyzed. The other two patients (R10 and R13) were recurrences. For patient R10, a core biopsy, which documented the recurrence, was used for immunohistochemistry and for R13, the resection material itself served this purpose as biopsy material was not available. All sections were stained with the dual pY88/p27 IHC assay to determine the % of pY88+ cells (Fig. 2D, bottom, N=>3). All patients had high p27 expression (>20% of cells were p27+) (Fig. 2D, bottom left). Using the scoring system described above, biopsies from patients R4, R2, R13, R5 did not have significant pY88 positivity (pY88 group 0), while R1, R12, R8, R10, R7 had a very high percentage of pY88+ tumor cells (pY88 group 2) (Fig. 2D, bottom right). R3 had a lower percentage of pY88+ cells (23%), slightly below the cutoff established previously and was assigned a score of 1.Thus, our cohort had 6 patients with pY88 scores of 1 or 2 and 4 patients with a score of 0.
Following surgery, fresh tumor material harvested from these patients was immediately grown in explant culture (Fig. 3A). Explants were first permitted to recover for 60 h and then DMSO, 100 nM or 500 nM Palbociclib was added to the media. Using a similar explant culture system, Dean, et al. found that all RB+ explants were inhibited by 500 nM Palbociclib [9]. We choose to examine Palbociclib response at 500 nM and at a lower, more pharmacologically relevant concentration of 100 nM. After 48 h of treatment, the explants were removed from culture, formalin-fixed and paraffin-embedded. In total, 6 explants were grown per patient (duplicates for each of the three conditions) and each explant was stained for Ki67 as a marker of proliferation at least 2 times, to provide N=>4 per condition, which were read blindly on different days (Fig. 3B). The exceptions to this were due to tissue loss during processing or the presence of too few tumor cells in the individual explant piece, which precluded accurate analysis. In all cases, the explant material was compared with the biopsy material for morphological similarities.
The Ki67 status of the biopsy material was compared to the Ki67 staining seen in the DMSO-treated explants to determine whether similar proliferation rates were seen in tumor removed from the patient and immediately fixed and that grown for several days in explant culture (Fig. 3B). Ki67 levels varied across the patient cohort but in general, the level in the biopsy was comparable to the level seen in the DMSO-treated explants, suggesting that the explants resembled the parent tumor. For example, the explant showing the lowest level of Ki67 (R7) was from the same patient showing the lowest Ki67 staining in biopsy material (Fig. 3C, right). Representative Ki67 staining for explant samples from R10 (Group 2) and R13 (Group 0) are shown (Fig. 3C). Ki67+ staining decreased for R10 as the Palbociclib concentration increased, while staining did not vary for R13. All of the Ki67 values for each treatment condition (DMSO, 100 and 500 nM Palbociclib) for all of the 1, 2 pY88 scoring explants (R1, R12, R8, R10, R7, R3) or the 0 pY88 scoring explants (R4, R2, R13, R5) were combined and analyzed using a box and whisker plot (Fig. 3C, right). Each box represents up to 36 data points for pY88 score 1, 2 patients or up to 24 for pY88 score 0 patients). Decreases seen in Ki67 staining after Palbociclib treatment in the 1, 2 pY88 scoring group were statistically significantly, while the smaller changes seen with the 4 explants from group 0 were not statistically different.
Thus, pY88 status correlated with Palbociclib response in the explant culture assay as determined by Ki67 level changes. It has been suggested that several other cell cycle proteins might serve as markers of Palbociclib response, including cyclin E, cdk2, or RB [10]. We stained the biopsy material from our cohort with antibodies against total RB, RbSer780 (phosphorylated RB), or cdk2T160 (the catalytically active form of cdk2), and a representative staining is shown (Fig. 3D). With the exception of one patient (R13), all of the samples were positive for RB and Rb phosphorylation, although the % of RB and phosphorylation varied among patients (Fig. 3D, right). R13 had a pY88 score of 0, and the corresponding explant material was not inhibited by Palbociclib, consistent with the Rb− status of this biopsy. Significantly, R5, R2 and R4, which were RB and RbSer780 positive), also had pY88 scores of 0 and were non-responsive to Palbociclib in the explant assay. As these patients were ER/PR+/Her2-, they would normally be clinical candidates for Palbociclib if they progressed to the metastatic setting due to their Rb+ status. Our results suggest that they would not be responsive to Palbociclib, and that pY88 staining can be used to predict this nonresponsiveness.
All of the samples were also stained with cdk2T160. (Representative, Fig. 3D). The cdk2T160 antibody was shown to be specific for active cdk2 by staining cell blocks generated from MCF7-ALT cells grown +/− Dox (Fig. 3D, right, bottom). In the presence of Dox and ALT induction, we had previously shown that cdk2 was inactive by in vitro kinase assay and cdk2T160 was not detected by immunoblot analysis [3] and IHC results were consistent. Although staining levels varied, no correlation between the amount of cdk2 activity seen in biopsy material and Palbociclib response could be seen. Both explants that responded to Palbociclib and those that did not had active cdk2.
Discussion
pY88 status correlated with Palbociclib response in the short term explant culture assay as determined by Ki67 level changes, suggesting that analysis of pY88 status could predict the presence of the active cdk4 target, which is required for Palbociclib response. Examination of active Cdk2, Rb or Rb phosphorylation by IHC did not correlate with Palbociclib response, suggesting that pY88 status was more predictive or that IHC was not sensitive enough to separate differences. TN and Her2+ patients, two populations in which Palbociclib is not currently used, were present in this cohort and responded similarly, suggesting that some patients in these subgroups do have the active cdk4 target and might show response to drug. Palbociclib should inhibit any cdk4-dependent tumor of any primary site, especially those with strong oncogenic signaling, such as Her2 overexpression, and use of a biomarker could identify patients who might be responsive. Gong, et al. identified that cancer cell lines that expressed cyclin D activating features were more sensitive to Abemaciclib inhibition [11]. These features include cyclin D1, 2, 3 alterations that should increase cyclin D stability and/or expression and ultimately cyclin D-cdk4/6 activity, and we would predict many or most of these cell lines might also be positive in the pY88 assay. Interestingly, they identified these alterations in cell lines from multiple types of cancer where cdk4i has not been extensively studied, consistent with the idea that having a biomarker for cdk4i response would permit use of cdk4i outside of the breast cancer space. Unlike most other targeted therapies used clinically, cdk4 inhibitors, like Palbociclib, are prescribed without any indication that the target of the therapy (active cdk4) is present in the patient. While our study suggests that pY88 may be a marker for the active cdk4 target, the same way Her2 staining identifies tumors that may respond to Tratuzamab, we did not address whether it would be predictive for response in metastatic breast cancer patients receiving combination therapy of Palbociclib and Letrozole. The presence of the target and long-term clinical response to the drug are two separate, but linked, issues, and this current study only addresses the former. We use the pY88 biomarker to identify active cdk4 in the explants and then demonstrate that this information is sufficient to predict response to the drug. Comparison of pY88 status in biopsy material with outcome data from patients treated clinically with cdk4i will be required to determine whether pY88 can also serve as a clinical biomarker of cdk4i response.
Implications:
Use of the pY88 biomarker, as a surrogate for cdk4 activity, may identify patients responsive to Cdk4 targeting drugs and expand use of this therapy.
Acknowledgements
We thank Khurram Shafique and Mengru Li for expert technical assistance. This work was supported by NIH R01CA201536 to S.W.B. This work was independently verified by the SUNY Compliance Management Committee.
Financial support: This work was supported by NIH R01CA201536.
Footnotes
Conflict of Interest Statement: SWB is the co-founder of Concarlo Holdings, LLC, which has licensed technology described in this report. PP is a current employee of Concarlo Holdings, LLC. This work was independently verified by the SUNY Compliance Management Committee.
References:
- 1.Sherr CJ, Beach D, and Shapiro GI, Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov, 2016. 6(4): p. 353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blain SW, Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle, 2008. 7(7): p. 892–8. [DOI] [PubMed] [Google Scholar]
- 3.Patel P, et al. , Dual Inhibition of CDK4 and CDK2 via Targeting p27 Tyrosine Phosphorylation Induces a Potent and Durable Response in Breast Cancer Cells. 2018. Mol Cancer Res, 6(3): 361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn RS, et al. , The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol, 2015. 16(1): p. 25–35. [DOI] [PubMed] [Google Scholar]
- 5.Ingham M and Schwartz GK, Cell-Cycle Therapeutics Come of Age. J Clin Oncol, 2017. 35 (25): p. 2949–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarducci C, et al. , Mechanisms of Resistance to CDK4/6 Inhibitors in Breast Cancer and Potential Biomarkers of Response. Breast Care (Basel)., 2017. 12(5): p. 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrera-Abreu MT, et al. , Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res, 2016. 76(8): p. 2301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, et al. , Brk/Protein tyrosine kinase 6 phosphorylates p27KIP1, regulating the activity of cyclin D-cyclin-dependent kinase 4. Mol Cell Biol, 2015. 35(9): p. 1506–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean JL, et al. , Therapeutic response to CDK$/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle, 2012. 11: p. 2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asghar U, et al. , Single-cell dynamics determines response to CDK4/6 inhibition in triple negative breast cancer. Clin Cancer Res. 2017. 23(18): p. 5561–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong X, et al. , Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell, 2017. 32: p. 761–776. [DOI] [PubMed] [Google Scholar]