Abstract
12-lipoxygenase (12-LOX) is one of several enzyme isoforms responsible for the metabolism of arachidonic acid and other poly-unsaturated fatty acids to both pro- and anti-inflammatory lipid mediators. Mounting evidence has shown that 12-LOX plays a critical role in the modulation of inflammation at multiple checkpoints during diabetes development. Due to this, interventions to limit pro-inflammatory 12-LOX metabolites either by isoform-specific 12-LOX inhibition, or by providing specific fatty acid substrates via dietary intervention, has the potential to significantly and positively impact health outcomes of patients living with both type 1 and type 2 diabetes. To date, the development of truly specific and efficacious inhibitors has been hampered by homology of LOX family members; however, improvements in high throughput screening have improved the inhibitor landscape. Here, we describe the function and role of human 12-LOX, and mouse 12-LOX and 12/15-LOX, in the development of diabetes and diabetes-related complications, and describe promise in the development of strategies to limit pro-inflammatory metabolites, primarily via new small molecule 12-LOX inhibitors.
Keywords: lipoxygenase, type 1 diabetes, type 2 diabetes-related complications, inflammation, lipoxygenase inhibitors
1. Introduction:
The lipoxygenase (LOX) family of enzymes is represented across multiple species, from plants to mammals (Kuhn et al., 2015). In humans, 6 family members have been sequenced and characterized. Seven members have been described in mice. These enzymes are responsible for the metabolism of poly-unsaturated fatty acids (PUFAs) to form functionally active metabolites that act in an autocrine, paracrine, and endocrine manner. The diversity in the function across this family of enzymes (Table 1) has frequently led to confounding results, which are only recently beginning to be clarified. In this review, we will discuss the tissue specific roles of various lipid metabolites produced by 12-LOX (gene product of ALOX12), that contribute to the progression of diabetes and co-morbidities. We will also discuss the functional role of mouse 12/15-LOX (the gene product of mouse Alox15), in diabetes and complications related to diabetes, as various rodent models have been used to generate proof-of-concept evidence for pathogenicity of this pathway. Focused discussion on the effects of pro-inflammatory 12-LOX metabolites, such as 12-hydroxyeicosatetraenoic acid (12(S)-HETE) on beta cells, adipocytes, immune cells, vascular cells, and hepatocytes will highlight the functional complexities and opportunities for intervention. Understanding the impact of inflammatory 12-LOX metabolites on the intracellular events involved in the transition from functional to dysfunctional beta cells could offer new approaches to preserve beta cells exposed to inflammation. Similarly, interventions to limit pro-inflammatory effects in adipose tissue and liver, as well as the vasculature and kidney, could positively impact on outcomes for diabetes co-morbidities. Recent therapeutic opportunities using selective and potent 12-LOX isoform inhibitors will be critically discussed in the context of type 1 and type 2 diabetes onset and progression.
Table 1.
Description of LOX Isoforms Discussed in This Review
| Lipoxygenase Protein Name |
Human Gene |
Mouse Gene |
Products Forms from AA Metabolism |
Term Used in This Review |
|---|---|---|---|---|
| Leukocyte-type 12- LOX (15-LOX1 for human, 12/15-LOX for mouse |
ALOX15 | Alox15 | Human protein produces 10% 12(S)-HETE/90% 15(S)-HETE; Mouse protein produces 90% 12(S)-HETE/10% 15(S)- HETE |
12/15-LOX |
| Platelet-type 12- LOX |
ALOX 12 | Alox12 | Human and mouse proteins are functionally similar, and produce 12(S)-HETE |
12-LOX |
Biochemistry of 12-LOX
12-LOX is an oxygenase that generates a hydroperoxide product by inserting an oxygen molecule into a 1,4-diene of PUFAs (Kuhn et al., 2015; Yeung and Holinstat,2011), which is subsequently reduced to its alcohol by glutathione peroxidase 4 (Imai et al., 1998; Schneider et al., 2010). Numerous PUFAs serve as substrates for 12-LOX, including arachidonic acid (AA), dihomo-γ-linoleic acid, docosahexaenoic acid (DHA), and eicosapentaenoic acid (Ikei et al.. 2012). Notably, the promiscuity of 12-LOX activity results in the generation of oxylipin products from 12(S)-HETE to Maresin 1, with a wide breadth of biological activity (Serhan and Petasis, 2011). Another aspect of 12-LOX that is notable is its product selectivity. For example, with AA as the substrate, 12-LOX produces one stereo-isomer,12(S)-HETE; however, the related family member, 12/15-LOX (ALOX15 gene in humans), is less selective and produces 90% 15(S)-HETE and 10% 12(S)-HETE (Ivanov et al., 2010). Both 12- and 15- LOX metabolites can have pro-inflammatory effects in humans. To mechanistically dissociate the effects of the 12- and 15-LOX pathways and their respective metabolites is crucial to understanding how these pathways function in human disease. To further clarify the particular role of these products in disease, specific pharmacologic inhibitors for each of the LOX isoforms were developed, and will be discussed later in the review.
Species similarities: rodent versus human
The development pipeline for enzymatic inhibitors frequently uses mouse models to test safety and efficacy of potential inhibitors. Therefore, understanding the structural and functional relationships between human and mouse lipoxygenases is key in this process. Human and mouse platelet 12-LOXs share 85% sequence homology. More importantly, they are similar in their reactivity, with both producing only 12(S)-HETE. In comparing different human isoforms, however, human 15-LOX1 shares only 65% sequence homology to human 12-LOX. It should be emphasized that for lipoxygenases, specific active site residues have dramatic effects on product production, and therefore, whole protein sequence identity should be viewed with caution. For example, human ALOX15 product, 12/15-LOX, produces 90% 15(S)-HETE and 10% 12(S)-HETE, while mouse 12/15-LOX produces 90% 12(S)-HETE and 10% 15(S)-HETE, despite 73% sequence homology. Therefore, even though the two proteins originate from the homologous gene family, they do not produce the same oxylipins. These factors must be addressed during the development and selection of potential enzymatic inhibitors of lipoxygenase family members. Special emphasis based on recent data was on preventing production of 12(S)-HETE that was shown to have a multitude of pro-inflammatory effects in different cells and tissues, as discussed throughout this review.
2. Approaches for 12-LOX and 15-LOX inhibition
Rationale for 12-LOX and 15-LOX Inhibition
Lipoxygenase activation is an important component of inflammation, possibly having greater relevance for chronic inflammatory responses. In recent years, the contribution of chronic inflammation has been recognized as a significant component to the underlying pathology of several major diseases, including diabetes. Increased 12/15-LOX activity has been implicated as a contributing factor in the development of both atherosclerosis and diabetic neuropathies (Wen et al., 2007; Nagelin et al., 2008; Stavniichuk et al., 2010). Activation of macrophages by 12/15-LOX, and activation of downstream cytokine and chemokine pathways contribute to these chronic inflammatory processes. Thus, great interest has been applied to the identification of selective and potent inhibitors of lipoxygenase enzymes, specifically 12/15-LOX. Efforts have been based on classic quantitative high-throughput screening (HTS) supported by medicinal chemistry, computational chemistry and natural product isolation.
12-LOX and 15-LOX inhibitors – a historical perspective
In general, LOX inhibitors are relatively easy to discover, with numerous publications of new inhibitors being produced monthly. This is due, in part, to the fact that there are a variety of approaches to generate inhibitors of different classifications, such as redox, iron chelating, suicide, allosteric, and competitive. However, few of the numerous LOX inhibitors discovered are specific to a particular human LOX isozyme, and even fewer target human platelet 12-LOX. Specifically inhibiting 12-LOX, and not the other LOX isozymes, is important because each LOX isozyme has a specific biosynthetic role in human inflammatory responses (Serhan and Petasis, 2011), and therefore, utilizing non-specific LOX isozyme inhibitors would confound biological interpretation, and possibly result in drug safety consequences due to off-target effects. For example, baicalein, (Deschamps et al., 2006) caffeic acid (Cho et al., 1991; Doiron et al., 2017), and the caffeic acid derivative, Cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate (CDC) (Pergola et al., 2011), were initially thought of as a specific 12-LOX inhibitors, but were later proven to be non-specific. This creates a challenge for the biochemists trying to generate and characterize specific inhibitors.
Initially, the only 12-LOX selective inhibitors were obtained from natural sources. The most significant by far is hinokitiol, whose potency is sub-micromolar (IC50 = 0.1) and its 15-LOX-1/ 12-LOX (15/12) ratio is approximately 500 (Suzuki et al,. 2000). Unfortunately, hinokitiol was found to be cytotoxic and teratogeneic in living tissue (Inamori et al., 1993). Other natural products were also shown to be selective, such as bromophenols (15/12 ratio = 5) (Segraves, et al., 2004) and isoflavanones (15/12 ratio = 26) (Vasquez-Martinez et al., 2007), but they were not potent enough for drug discovery. HTS was also applied to finding potent/selective 12-LOX inhibitors with mixed results. Our initial screen found potent/selective molecules, but they did not fulfill the drug-like qualities (Deschamps et al., 2007). A subsequent HTS produced an 8-hydroxyquinoline (ML127), which was both potent (IC50 = 0.4 uM) and selective (100-fold less active against both other LOX and COX isozymes, Figure 1) (Kenyon et al., 2011). However, ML127 is metal chelator, which raises the specter of off-target effects, lowering its appeal as a drug candidate. From the same HTS, a benzenesulfonamide was discovered (ML355, Figure 1), whose structure was much more “drug-like.” Its potency was sub-micromolar (IC50 = 0.3 uM), and its selectivity was greater than 100-fold higher compared to both other LOX and COX isozymes (Luci et al., 2014). More importantly, ML355 showed cellular potency in human platelets and mouse bleed models (Tourdot and Holinstat, 2017). ML355 is currently the state-of-the-art for potent/selective 12-LOX inhibitors, and is an appropriate molecule for 12-LOX biological studies.
Figure 1. Selective human 12-LOX inhibitors and human 12/15-LOX dual inhibitor.
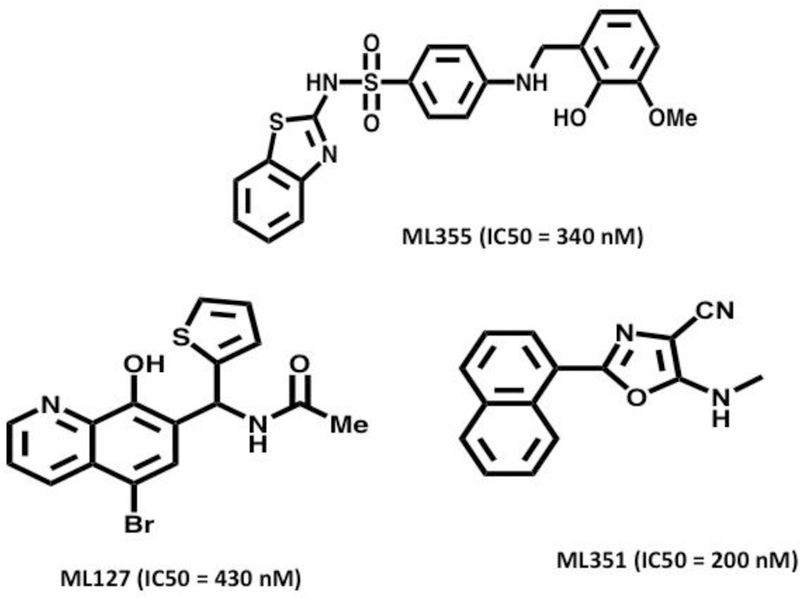
Shown are the structures of selective human 12-LOX inhibitors: 0-hydroxyquinoline-based ML127 and N-(benzo[d]thiazol-2-yl)-4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide ML355. Also shown is the 12/15-LOX dual inhibitor ML351, which is an amine oxazol.
A brief mention should be given to the specific human 12/15-LOX inhibitor ML351, which has the ability to inhibit mouse 12/15-LOX (Figure 1) (Rai et al., 2014). This is noteworthy because mouse 12/15-LOX (encoded by the Alox15 gene) produces primarily 12(S)-HETE, while human 15-LOX1 produces primarily 15-HETE, as discussed above. This result indicates their active sites are distinct; however, ML351 inhibits them both. As will be discussed later in this review, this ability to inhibit mouse 12/15-LOX is important in the pre-clinical study of diabetes due to the involvement of 12/15-LOX in mouse models of diabetes, but not in human diabetes. These differences in mouse versus human enzymatic activity have led to difficulties in testing inhibitors, and developing the translational pipeline. As will be discussed later in this review, the ability of inhibitors, such as ML351, to inhibit mouse 12/15-LOX is important for proof-of-concept mechanistic studies in mouse models of diabetes. In comparing ML355 and ML351 (Figure 1), one can observe their chemical structures are very distinct. ML351 is an amine oxazole, while ML355 is a benzenesulfonamide. These structural differences present distinct chemical surfaces that lead to their LOX selectivity.
3. 12-LOX and 12/15-LOX pathogenic roles and their inhibition and pancreatic beta cells
The relationship of 12/15-LOX activation to the development of diabetes has been validated in genetic deletion studies (Bleich et al., 1999; McDuffie et al., 2008), where global deletion of 12/15-LOX led to significant protection (>98%) from diabetes development in both the streptozotocin (STZ) and spontaneous non-obese diabetic (NOD) mouse models. The application of the selective human and mouse 12/15-LOX inhibitor, ML351, and the human and mouse 12-LOX inhibitor, ML355, has facilitated dissection of the contribution of these pathways to inflammation-mediated beta cell dysfunction. Exposure of beta cell lines and primary islets to pro-inflammatory cytokines (PIC; TNF-α, IL-1β, IFN-γ) results in compromised beta cell function and survival. The effects of PICs can be reproduced with direct addition of 12-HETE, (Ma et al., 2010). This implied role for 12-LOX and 12/15-LOX activation as a mediator of beta cell dysfunction was validated with the use of ML127/ML355 selective inhibitors. With inclusion of either ML127 or ML355, markers of beta cell function and survival were preserved in the presence of PICs (Luci et al., 2014; Taylor-Fishwick et al., 2015). Furthermore, protection with ML127/ML355 was seen in homogeneous beta cell lines and primary human islets (Ma et al., 2017; Taylor-Fishwick et al., 2015). Such a strategic approach would slow the progression of diabetes as mono-therapy, or reverse diabetes as a combinatorial therapeutic approach when coupled with an islet regeneration strategy. This could be utilized in either endogenous beta cell stimulation or exogenous repopulation (cell transplantation/encapsulation/xenotransplantation etc.).
12/15-LOX and oxidative stress in beta cells
As mentioned above, 12/15-LOX activation contributes to islet demise. Pro-inflammatory cytokines potently induce cellular reactive oxygen species (ROS). Relative to other cells, beta cells exhibit a lower activity of free-radical detoxifying enzymes, including catalase, superoxide dismutase, and glutathione peroxidase. Consequently, beta cells are predisposed to oxidative stress (Lenzen, 2008). Sources of ROS in the beta cell include induced mitochondrial stress (Newsholme et al., 2007), endoplasmic reticulum (ER) stress (Volchuk and Ron, 2010), and recently recognized NADPH oxidase activation (Taylor-Fishwick, 2013). Pro-inflammatory cytokines induce NADPH oxidase-1 (NOX-1) and elevate intracellular ROS in beta cells. Studies that modulate NOX-1 activity support an important role for NOX-1 in beta cell pathology. Selective inhibition of NOX-1 preserves beta cell function and islet survival (Weaver et al., 2015; Weaver and Taylor-Fishwick, 2017; Weaver et al., 2015a).
Lipid mediators have been linked with the activation of NOX. Two products of phospholipase A2 (PLA2) activity, arachidonic acid (AA) and lysophosphatidic acid (LPA) are involved in direct interaction with and activation of the NOX complex, principally the NADPH oxidase isoenzyme NOX-2 (Matono et al., 2014; Ha et al., 2018). While the importance of this interaction is clear, the exact activation pathways linking lipid mediators and NOX activation is far from resolved. Previous studies are often limited by data derived using non-specific inhibitors of NOX, and numerous downstream enzymes and bioactive metabolites in the eicosanoid pathway (cyclooxygenase, lipoxygenase, leukotrienes etc.) activate NOX in a variety of experimental cell systems (reviewed in Cho et al., 2011).
An association between the 12/15-LOX pathway and NOX-1 pathway has been investigated in beta cell lines and islets (Weaver et al., 2012). Direct addition of 12(S)-HETE to beta cells induced NOX-1 expression. Conversely, inhibition of 12/15-LOX activity blocked NOX-1 upregulation mediated by pro-inflammatory cytokines (Weaver et al., 2012). 12/15-LOX inhibition also reduced elevation of ROS in beta cells stimulated with pro-inflammatory cytokines. These data help integrate intracellular events in the beta cell exposed to inflammation.
12/15-LOX and interleukin-12 in pancreatic islets and beta cells
Beta cells are not only extremely susceptible to inflammatory damage, but they also have the ability to produce inflammatory cytokines. Identification of interleukin-12 (IL-12) production and function in beta cells presents a further integration of 12/15-LOX (Taylor-Fishwick et al., 2013). A consequence of PIC-stimulation in beta cells and primary islets is the production of IL-12, which facilitates an escalating loop of inflammation.
Beta cells, including human beta cells, express the receptor for the IL-12 ligand and are responsive to IL-12-ligand/IL-12-receptor ligation. Administration of exogenous IL-12 directly mediates beta cell dysfunction (Taylor-Fishwick et al., 2013). Exogenous IL-12 induces a dose dependent expression of IFN-γ in beta cell lines, suggesting a functional IL-12/STAT4/IFN-γ axis. Previous studies describe STAT4 signaling in islet beta cells (Yang et al., 2004; Yang et al., 2003). Additionally, selective inhibition of the IL-12/STAT4 axis in beta cells, utilizing multiple strategies of inhibition, preserves beta cell survival in the presence of PICs (Weaver et al., 2015b). Neutralization of IL-12 with an IL-12 antibody blocked beta cell dysfunction induced by PIC stimulation (Taylor-Fishwick et al., 2013), and functional defects in beta cells mediated by IL-12 correlate with those seen following PIC stimulation. Both receptor and ligand for IL-12 are upregulated in beta cells exposed to PIC stimulation. These studies suggest that pro-inflammatory cytokines induce local IL-12 expression that may be a mediator of PIC-induced beta cell dysfunction. Inhibitors of 12/15-LOX suppress the induction of IL-12 ligand in islets and beta cells exposed to PIC. Pro-inflammatory cytokine stimulation of 12/15-LOX activity is implied in the regulation of beta cell IL-12 production.
The mechanisms by which 12/15-LOX activation leads to IL-12 production will require additional investigation; however, ROS production has been linked to IL-12 production (Aramaki et al., 2002). In beta cells, activation of NOX-1, subsequent to 12/15-LOX, could be the intermediary step to the production of ROS that regulates IL-12 production in beta cells following exposure to PICs. Given the impact of 12/15-LOX activity on beta cells, it is not surprising that this enzyme plays a critical role in the development of both type 1 and type 2 diabetes (T1D and T2D, respectively), and also contributes to the development of diabetic complications. The development of potent and specific 12/15-LOX inhibitors has the potential to significantly alter the landscape of therapeutics in diabetes.
4. 12-LOX and 12/15-LOX in T1D
Role of 12/15-LOX in islet and immune cell types in T1D
Historically, T1D has been characterized as an autoimmune disease that is initiated and perpetuated by autoreactive T cells. Despite the historical emphasis on the T cell response, mounting evidence suggests that inflammatory damage to islets and innate immune cells also contribute to disease development (Maganti et al., 2014).
Since both islets and innate immune cells are capable of expressing activated 12/15-LOX, it is not unreasonable to think that these two cell types might interact to contribute to T1D development. A potential causative role for 12/15-LOX in T1D pathogenesis was first suggested nearly twenty years ago (Bleich et al., 1999), when it was shown that mice deficient for the gene encoding 12/15-LOX on the C57BL/6J background were protected from developing diabetes in the multiple low-dose STZ model of T1D. This research led to the subsequent development of NOD congenic mouse lines in which the Alox15 gene was deleted globally (McDuffie et al., 2008). In the absence of 12/15-LOX, NOD mice are significantly protected from spontaneous diabetes development, with only ~3% of mice developing disease by 30 weeks of age (compared to average rates of 60–80% spontaneous diabetes development in wild-type NOD mice). Notably, there was a significant decrease in the numbers of macrophages in the islets of mice at 4–5 weeks of age, a time point that precedes the development of T cell-mediated insulitis in the NOD strain. Further characterization of these mice showed that whereas both islets and macrophages produce 12/15-LOX, adaptive immune cells (B cells and T cells) do not. Characterization of both islets and macrophages from Alox15-deficient NOD mice demonstrated decreased pro-inflammatory phenotypes (Green-Mitchell et al., 2013). Both islets and macrophages from Alox15-deficient mice exhibit reduced expression of the genes encoding pro-inflammatory cytokines IFN-γ, IL-12, TNF-α, and IL-1β. Furthermore, islets in Alox15-deficient NOD mice have increased beta cell mass prior to the onset of insulitis. Taken together, these data suggest that 12/15-LOX likely contributes directly to both beta cell dysfunction and loss, as well as macrophage activation (Figure 2). 12/15-LOX deficiency does not appear to affect the general health of the mice, suggesting that targeting the activity of this enzyme may be a safe and viable therapeutic option.
Figure 2. Role of 12-LOX and metabolites in beta cell demise.
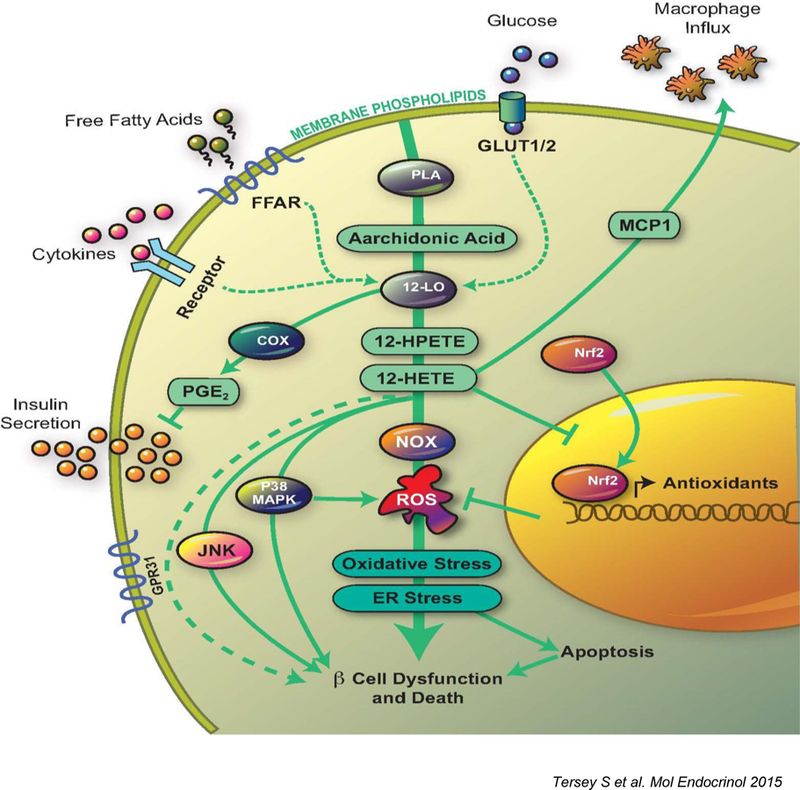
The figure depicts the pathways activated by 12-LOX in the pancreatic islet beta cell in response to elevated glucose levels, saturated free fatty acids, or PIC. 12-LOX activation leads to the production of pro-inflammatory lipid intermediates (12(S)-hydroperoxyeicosatetraenoic acid and 12(S)-HETE). These intermediates trigger subsequent inflammatory pathways mediated by c-Jun N-terminal kinase, p38-MAPK, and NOX. 12(S)-HETE prevents translocation of Nrf2 as well. Signaling through these pathways leads to increased ROS, oxidative stress, ER stress, which eventually cause beta cell dysfunction and death. Although not depicted in this diagram, other lipid mediators such as LPA and LPC contribute to MCP-1 mediated chemotaxy; ceramides contribute to beta-cell death, etc. FFAR, free fatty acid receptor; PLA, phospholipase A2; PGE2, prostaglandin E2; MCP1, monocyte chemoattractant protein 1.
To test more directly the role of 12/15-LOX in the pancreatic islet, a Cre-lox strategy was utlilized to delete Alox15 in islets using the Pdx1-Cre deleter strain on the C57BL/6J mouse background (Tersey et al., 2014). In this model, islet-specific deletion of 12/15-LOX led to protection from multiple low-dose STZ-induced diabetes, a phenotype that is virtually identical to the whole-body Alox15 knockout mice (Bleich et al., 1999). Recently, use of a 12/15-LOX inhibitor (ML351) in the NOD mouse model was shown to preserve beta cell mass and function, and to reduce insulitis and hyperglycemia (Hernandez-Perez et al., 2017). Interestingly, although these findings were attributed to the effects of 12/15-LOX inhibition in the beta cell, it was also observed that the nature of the remaining islet infiltrate was altered, with greater presence of alternatively activated (M2) macrophages. Therefore, these results suggest the possible contribution of 12/15-LOX to both beta cell function and inflammatory cell phenotypes. Studies to date have not fully resolved the contributions of the individual cell types. To address the specific role of 12/15-LOX in beta cells versus innate immune cells, targeted deletion Cre-lox strategies on the NOD background are being pursued.
Although adaptive immune cells do not express detectable levels of 12/15-LOX, natural killer (NK) cells express surprisingly high levels of 12/15-LOX (Semeraro et al., 2017). NK cells from congenic Alox15-deficient NOD mice display significantly higher levels of stimulatory receptors on their surface as compared to wild-type NOD NK cells (Semeraro et al., 2017), a finding that correlates with results seen in non-diabetic human NK cells as compared to those from donors with T1D (Qin et al., 2011). NK cells not only have the capacity to interact with macrophages, but also with pancreatic beta cells, giving them the potential to play a multi-faceted role in T1D development.
Role of 12-LOX in human T1D
Translationally, human 12-LOX has been implicated in the pathogenesis of human T1D. Recent studies (Hennessy et al., 2017) have shown that individuals with recent-onset T1D exhibit elevations in the circulation of the major pro-inflammatory product of 12-LOX, 12(S)-HETE, raising the possibility of a causative correlation between the 12-LOX pathway and T1D pathogenesis. Isolated human islets treated with PIC expressed elevated levels of 12-LOX (Ma et al., 2010). PIC treatment under these conditions is thought to mimic the response of islet beta cells to autoimmune attack in T1D (Figure 2). These results correlate well with the protein expression of 12-LOX in pancreatic sections obtained from human donors, which revealed that islets only express 12-LOX under systemic inflammatory conditions, including cases of pre-T1D (autoantibody positive), early T1D, and T2D (Grzesik et al., 2015). In this study, it was noted that expression of 12-LOX dissipates once endogenous insulin production is abolished, possibly as a result of beta cell dedifferentiation. Although convincing evidence points towards a key role for 12/15-LOX metabolites at the onset of T1D, there is no evidence showing how such metabolites may change during interventions designed to address the underlying pathology of T1D, apart from insulin treatment, such as anti-CD3, selective tyrosine kinase inhibitors (Gleevec), or CTLA4-Ig. Such studies using immunologic approaches should be a valuable source of information for revealing changes in the 12-LOX metabolites that may further support the contention that 12-LOX pathway is directly related to the immune-mediated mechanisms in type 1 diabetes.
5. 12-LOX and 12/15-LOX in T2D
Role of 12/15-LOX in islet dysfunction using mouse models of T2D
The first evidence that 12/15-LOX in pancreatic islets contributed to the development of T2D was obtained in a diet-induced obese (DIO) mouse model with a germline deletion of the 12/15-LOX (Alox15 gene) (Nunemaker et al., 2008). The Alox15 deficient mice on Western diet maintained glucose tolerance at the levels similar to wild type (WT) C57BL/6 mice fed a normal chow. Improved glucose tolerance in Western diet-fed Alox15-deficient mice compared to WT controls was due in part to reduced adipose tissue inflammation, but also likely due to improved islet function. 12(S)-HETE levels, were increased in the islets of Western diet-fed WT mice compared to Alox15-deficient mice. In addition, islets of Alox15 deficient mice showed higher glucose-stimulated insulin secretion and decreased apoptosis compared to islets from WT mice on Western diet (Nunemaker et al., 2008).
To further define the contribution of 12/15-LOX in the development of islet dysfunction in T2D, glucose homeostasis and islet function were assessed in high fat fed mice with Pdx1-Cre mediated deletion of Alox15 (pLOKO) (Tersey et al., 2014). Of note, the expression of Alox15 in exocrine pancreas is minimal, and therefore, pLOKO mice provide a means to elucidate the contribution of islet 12/15-LOX. pLOKO mice became insulin resistant on high fat diet (HFD), similar to their HFD-fed WT littermates; however, their glucose tolerance was better and insulin secretion was higher than in WT mice on HFD. In addition, pLOKO mice on HFD had increased beta cell area compared with WT mice on HFD, indicating a better compensatory increase in beta cell mass in the absence of islet 12/15-LOX. The islets from WT mice showed increase in markers of oxidative stress, ER stress, and beta cell death. In contrast, these parameters were lower in pLOKO mice. The upregulation of antioxidant genes (SOD, Gpx1, catalase) by the activation of the Nrf 2 transcription factor was implicated as a mechanism by which islets of pLOKO showed better adaptation to HFD (Tersey et al., 2014). In the db /db model of T2D, both forms of 12/15-LOX are expressed in islets (12-LOX and 15-LOX). The islet 12/15-LOX increase parallels the decline in beta cell mass (Dobrian et al., 2018). Collectively, studies conducted in mouse models of T2D strongly support the causal relationship between 12/15-LOX activity and beta cell dysfunction in T2D.
Role of 12-LOX in beta cell dysfunction in humanT2D
There is evidence that 12-LOX is elevated in human islets during the development of T2D. 12-LOX expression, tested by immunofluorescence, is increased in islets of some T2D donors, along with T1D and autoantibody positive donors (Grzesik et al., 2015). Furthermore, human islets isolated from donors with T2D also showed higher expression of 12-LOX than islets from non-diabetic donors (Butcher et al., 2014). When glucose stimulated insulin secretion was examined, T2D islets expressing high levels of ALOX12 mRNA had a reduced, but detectable response to glucose compared with islets from non-diabetic donors. In contrast, the expression of pro-inflammatory genes CCL2 and TNFA, but not ALOX12, were increased in T2D islets that showed blunted response in insulin secretion in response to glucose. Considering that 12-LOX disappears once endogenous insulin supplies are exhausted in T1D islets (Grzesik et al., 2015), the up-regulation of 12-LOX in islets with mild to moderate impairment in insulin secretion in T2D may reflect active involvement of 12-LOX in stressed beta cells prior to beta cell loss or dedifferentiation in T2D. Importantly, the inhibition of 12-LOX with ML-355 was shown to improve the function of human islets from type 2 diabetic donors (Ma et al., 2017). 24-hour treatment of human islets from type 2 diabetic donors with the 12-LOX inhibitor ML355 improved glucose-stimulated insulin secretion and oxygen consumption rate compared with vehicle treated islets from the same donors. Thus, the activation of 12-LOX appears to contribute to the reduction of functional beta cell mass in both T1 and T2D, and the inhibition of 12-LOX may prevent the progression of beta cell loss in both forms of diabetes.
Role of 12-LOX and 12/15-LOX in adipose tissue in obesity and T2D
Type 2 diabetes is associated with obesity and insulin resistance. These conditions impact heavily on the physiology of adipose tissue. In obesity, adipose tissue (AT) is characterized by a state of low grade inflammation that alters the metabolic performance of adipocytes, and can indirectly or directly impact on metabolism and inflammation in the liver and skeletal muscle. AT inflammation is now considered a hallmark of obesity and insulin resistance (Figure 3). The remarkable cellular complexity and plasticity of this tissue implies multi-faceted roles for various inflammatory pathways that are usually operational in more than one cell type, and are causatively associated with both persistence or resolution of inflammation (Cole et al., 2013). There is emerging evidence that the various 12/15-LOX isoforms in both white (WAT) and brown fat are expressed in multiple cell constituents of AT, and their metabolites may have either pro- or anti-inflammatory effects in a cell-specific and temporal manner (Lopategi et al., 2016).
Figure 3. The complex role of 12- and 15-LOX in exacerbation and resolution of adipose tissue inflammation.
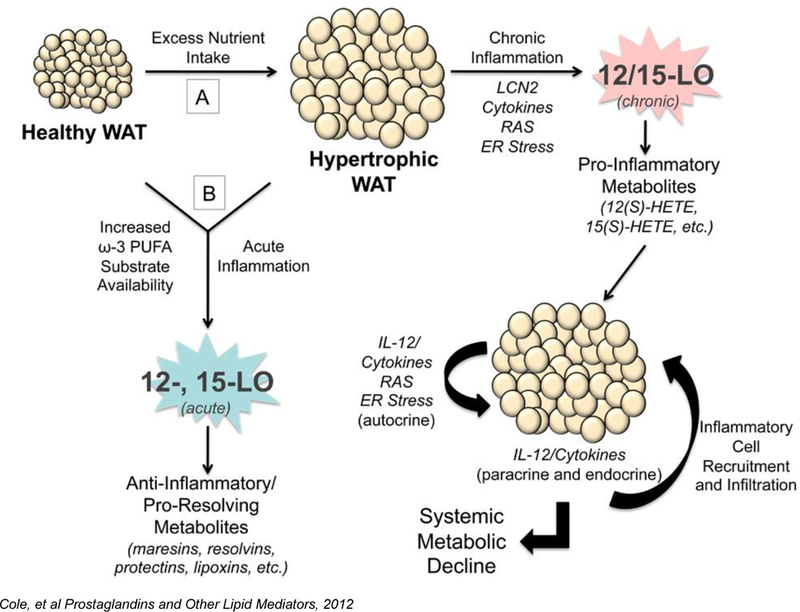
A) Excess consumption of energy demands an increased nutrient storage capacity of adipocytes in white adipose tissue (WAT). As a result, adipocytes become hypertrophic and stressed, leading to dysfunction marked by ensuing inflammation. Expression of pro-inflammatory cytokines, lipocalin-2 (LCN2), renin angiotensin system (RAS) markers, and ER stress markers by these stressed adipocytes and stromal vascular fraction (SVF) of the WAT leads to chronic leukocyte-type 12-LOX (12/15-LOX) activation in the adipocyte and SVF and subsequent generation of pro-inflammatory lipid metabolites, such as 12(S)-HETE and 15(S)-HETE. 12/15-LOX activity promotes further amplification of these pathways, in particular the interleukin-12 (IL-12) pathway. This inflamed fat promotes the recruitment of macrophages and other inflammatory cells into the fat bed, further propagating the inflammatory autocrine cascade. In addition, WAT exerts paracrine and endocrine pro-inflammatory effects through secreted cytokines on various organ systems, including the pancreas, liver, and vasculature, leading to metabolic decline. B) Diets enriched in ω−3 fatty acids or acute inflammatory responses acutely activate the 12- and 15-LOX enzymes in normal or inflamed hypertrophic WAT to generate anti-inflammatory or pro-resolving lipid metabolites, such as the maresins, resolvins, protectins, and lipoxins. Thereby targeting 12/15-LOX activity may be a novel therapeutic target in treating Local WAT and systemic metabolic dysfunction.
Evidence for the pro-inflammatory role of 12/15-LOX in adipocytes emerged from proof of concept pre-clinical models of genetic or diet-induced obesity (DIO), insulin resistance, and type2 diabetes. As described above, 12/15-LOX knockout mice are protected from developing insulin resistance in DIO (Nunemaker et al., 2008). In DIO C57Bl/6J mice, high fat diet is accompanied by increased 12/15-LOX expression and activity in adipose tissue and white epididymal adipocytes (Nunemaker et al., 2008; Sears et al., 2009; Chakrabarti et al., 2009). Zucker obese rats, a genetically-induced model of obesity and insulin resistance, also exhibit increased expression of 12/15-LOX in isolated white adipocytes compared to lean controls (Chakrabarti et al., 2011). Also, more recent data from our lab showed that both 12/15-LOX and 12-LOX are present in AT and adipocytes from db/db mice, and they undergo expressional increases that coincide with the metabolic decline (Dobrian, et al., 2018).
To confirm a direct role of 12/15-LOX activity in mediating inflammation and insulin resistance in adipocytes, the effect of 12(S)-HETE, the major 12/15-LOX metabolite in mouse, was examined in 3T3-L1 adipocytes by Chakrabarti et al.(Chakrabarti et al., 2009). Addition of both 12(S)-HETE and its precursor, 12(S)-hydroperoxyeicosatetraenoic acid, to fully differentiated 3T3-L1 adipocyte cultures significantly induced pro-inflammatory gene expression, and secretion of many pro-inflammatory cytokines, including TNF-α, MCP-1, IL-6, and IL-12p40. Adipocyte dysfunction is not only marked by chronic inflammation and insulin resistance of WAT, but also by ER stress (Boden, 2009). Evidence from our lab demonstrates that 12/15-LOX is a novel inflammatory pathway that mediates ER stress in adipocytes (Cole et al., 2012a). To further confirm that adipocyte-derived 12/15-LOX plays a key role in obesity-induced insulin resistance, our lab has generated a 12/15-LOX adipocyte-specific germline deficient mouse that displayed reduced AT inflammation and macrophage infiltration into the epididymal WAT (Cole et al., 2012b). Interestingly, inflammation in the pancreatic islet was also reduced in the high-fat diet-fed 12/15-LOX fat-specific-deficient mice compared to WT controls (Cole et al., 2012b). These data suggest a crosstalk between 12/15-LOX expression in WAT and inflammation in pancreatic tissue, revealing a considerable systemic impact of chronic 12/15-LOX activation in AT in DIO. Much less is known about the contribution of AT macrophage-derived 12/15-LOX.
Analysis of AT and the corresponding stromal vascular fraction (SVF) showed a significant increase of 12/15-LOX gene expression along with 12- and 15-HETEs in db/db mice compared to heterozygous controls. We found that out of the LOX metabolites measured, 12(S)-HETE was the most abundant one, and was selectively increased in db/db mice in parallel with the decline in glycemic control.
There is also evidence that the 12/15-LOX pathway may also generate metabolites that are key in the resolution of inflammation (Serhan and Chiang, 2008 Serhan et al., 2008a; Serhan et al., 2008b). In particular, 12/15-LOX may generate ω−6 PUFAs, such as lipoxins, or ω−3 PUFAs, such as maresins, resolvins, and protectins, in conjunction with the 5-lipoxygenase and COX enzymes (Chiang et al., 2005; Serhan et al., 2018) (Figure 3). AT dysfunction in obesity is likely the result of an inappropriate inflammatory response that remains uncontrolled due to intrinsic inability of the tissue for complete resolution of inflammation. The pro-resolving metabolites formed as a result of different LOXs may therefore play beneficial effects for limiting inflammation in the AT as a result of nutritional overload. A recent publication showed that resolvin D1 promotes resolution of AT inflammation in diet-induced obese mice by eliciting macrophage polarization toward an M2-like phenotype (Titos et al., 2013). Also, there is evidence to suggest that alternatively activated macrophages expressing 12/15-LOX act as a sink for distinct soluble receptors for apoptotic cells via the controlled phagocytosis by the resident tissue macrophages (Uderhardt et al., 2012). In addition, β3-adrenergic stimulation of AT macrophages led to adipocyte apoptosis via induction of 12/15-LOX that resulted in production of 9- and 15-hydroxyoctadecadienoic acid. This further stimulated PPARγ and adipogenesis (Kwon et al., 2016). A similar mechanism was described for de novo beige/brown adipogenesis in rodents (Lee et al., 2016).
Interestingly, AT is the major storage site for PUFAs in obese individuals (Lundbom et al., 2009). The 12-hydroxy docosahexaenoic acid metabolite, along with protectin D1 and resolvin D1, were identified in AT of obese ob/ob mice (Gonzalez-Periz et al., 2009). Recent data from our laboratory also showed a variety of both pro- and anti-inflammatory lipid mediators in the SVF of db/db mice, which showed a change in abundance as the mice became more glucose intolerant and insulin resistant (Dobrian et al., 2018).
There are important differences in the 12/15-LOX pathway between rodents and humans (Dobrian et al., 2010a). We were the first to report 12-LOX mRNA and protein expression in human AT, with exclusive localization in the SVF in both the subcutaneous (SC) and omental (OM) fat (Dobrian et al., 2010b). In rodents, adipocytes are the abundant source of 12/15-LOX (Chakrabarti et al., 2009; Chakrabarti et al., 2011). Increased expression of all 12/15-LOX enzyme isoforms in OM versus SC AT suggests that the pathway may contribute to the pro-inflammatory milieu prominently associated with visceral fat in obesity (Dobrian et al., 2010b). Interestingly, in patients with T2D, 12-LOX in particular was up regulated in OM adipose tissue suggesting a prominent role in T2D (Dobrian et al., 2010b).
Gene array analysis of AT showed that AA metabolism is the second most significantly upregulated pathway in human OM compared to SC AT in obese human subjects, with a 7.6-fold higher expression of 15-LOX-1 in omental fat (Gealekman et al., 2011). The recent discovery of novel metabolites with anti-inflammatory properties, such as DHA-derived fatty acid esters of hydroxyl fatty acids (Kuda et al., 2016) or newly characterized oxylipins (Barquissau et al., 2017), provides an important therapeutic opportunity to modulate 12/15-LOX activity through fatty acid drug discovery (Halade et al., 2018).
Role of 12/15-LOX in fatty liver development in obesity and T2D
Dysregulated adipose tissue function in obesity and T2D also has negative consequences in the liver, as both tissues have immediate access to an abundant vascular network and can communicate directly via the portal circulation. T2D and obesity are frequently associated with non-alcoholic fatty liver disease (NAFLD), as well as the more advanced stages of non-alcoholic steatohepatitis (NASH) characterized by irreversible fibrotic changes in the liver (Sanyal, 2005; Tilg and Moschen, 2008). A limited number of studies has investigated the role of 12/15-LOX and their metabolites in pathology of liver disease. Similar to other tissues, the substrate availability, the microenvironment, and the relative abundance of different LOX isoforms impacts the balance between pro-inflammatory and pro-resolving metabolites. In rodent models of DIO-induced steatosis, germline deletion of 12/15-LOX resulted in decreased hepatic lipid accumulation, decreased gene expression of TNF-α and IFN-γ, and decreased expression of chemokines (Lazic et al., 2014). Similarly, germline deletion of 12/15-LOX protected hyperlipidemic Apoe−/− mice from developing NAFLD (Martinez-Clemente et al., 2010). In a methionine/choline-deficient mouse model of NASH, serum metabolomics showed increased levels of 12(S)-HETE along with bile salts compared to a methionine/choline sufficient control (Matsubara et al., 2012). These studies suggest that 12/15-LOX contributes to the pathogenesis of NAFLD and NASH.
Pro-inflammatory metabolites of the LOX pathway may also play a pathogenic role in human liver disease. The pro-inflammatory metabolites 5-HETE, 11-HETE, and 15-HETE were increased in patients with NASH compared to healthy controls (Puri et al., 2009). Similar findings showed increased 15-HETE levels and higher inflammatory scores in histologic sections in the livers of NASH patients (Hall et al., 2017). Finally, a recent study showed an important role of 12-LOX/12(S)-HETE/GPR31 axis as a mediator of ischemic liver injury in ischemia-reperfusion across different species, from mice to rhesus macaques (Zhang et al., 2018).
Studies have also shown the beneficial role of the pro-resolving metabolites in liver disease. One recent study showed that protectin DX ameliorated hepatic steatosis in a DIO mouse model by suppressing ER stress (Jung et al., 2018). Also, resolvin D1 was shown to reduce ER stress-induced apoptosis and triglyceride accumulation in HepG2 cells (Jung et al., 2014). Available data suggests that either reducing the dietary ω6/ω3 ratio or inhibiting the enzymatic activity of 12/15-LOX may protect against Western diet-induced steatohepatitis.
6. 12-LOX and 12/15 LOX and Diabetes-related Complications
People with diabetes continue to suffer from high rates of microvascular complications such as retinopathy, nephropathy, neuropathy, and from atherosclerotic cardiovascular disease. In order to prevent or reduce the damaging effects of these complications, the identification of key pathways in disease etiology should be prioritized to foster the development of safe and efficacious medications. Evidence suggests that the 12/15-LOX pathway may contribute to these complications. Therefore, further understanding of human relevance of the role of the 12/15-LOX pathway in diabetes-related complications could lead to the opportunity to develop and test specific therapies that include enzyme inhibitors.
Diabetic retinopathy
Diabetic retinopathy (DR) is the leading cause of blindness among working age populations, despite the many current treatments to control glycemia in people with diabetes. Several new treatments have been developed primarily to target abnormal blood vessel development and bleeding (Wang et al., 2018). However, an improved understanding of the role of inflammation and relevant molecular pathways could help identify earlier targets for prevention and intervention (Wang et al., 2018). Studies have highlighted the role of lipid metabolites derived from 12/15-LOX in DR. 12-LOX expression and activity was documented in vitreous samples of people with DR (Al-Shabrawry et al., 2011), retina of diabetic mouse models (Othman et al., 2013), and human retinal endothelial cells treated with high glucose (Ibrahim et al., 2015a). Intravitreal injection of 12(S)-HETE compromises the endothelial barrier in the retina leading to inflammatory changes (Ibrahim et al., 2015b). Recent studies indicate that retinal endothelial cells, but not monocytes or macrophages, are key cells linked to 12/15-LOX-related retinal effects (Ibrahim et al., 2017). Not all of the effects leading to DR in the capillary bed are due to 12/15-LOX, since other studies also suggest a role of 5-LOX (Gubitosi-Klug et al., 2017) in the mouse. Additional studies are needed, particularly in human tissues, to clarify the particular role and relevance of each LOX isoform as a target for treatment.
Diabetic nephropathy
Diabetic nephropathy (DN) continues to be a major cause of end stage kidney disease, and affects people with type 1 or type 2 diabetes. While elevated glucose plays a key role, changes in blood pressure, dysregulation of the renin-angiotensin system (including increased activity of angiotensin Type 1 receptor), and dyslipidemia are also important factors. 12(S)-HETE levels are increased by high glucose and pro-fibrotic factors, such as TGF-β, in kidney mesangial cells in vitro (Kang et al., 2001; Reddy et al., 2002; Xu et al., 2006; Xu et al., 2009). In addition, 12/15-LOX activation leads to hypertrophy and expression of genes TGF-β and angiotensin Type 1 receptor, both of which are closely linked to development of DN (Reddy et al., 2002; Xu et al., 2008, Xu, et al. 2009; Zhu et al., 2007; Xu et al., 2016a). 12/15-LOX gene deficiency can attenuate TGF-β signaling in mesangial cells (Kim et al., 2003). Use of siRNA to reduce 12/15-LOX expression results in a reduction in glomerular dysfunction and in pro-fibrotic genes in diabetic mice (Xu et al., 2008; Yuan et al., 2008). A recent study postulates that 12/15-LOX-derived oxidized lipids modulate pro-fibrotic genes through epigenetic histone modifications (Yuan et al., 2016). Given that 12/15-LOX is highly expressed in the kidney and expression increases in type 1 or type 2 diabetic models (Kang et al., 2001; Reddy et al., 2002; Xu et al., 2009; Guo QY et al., 2011), such a pathway would be a promising target for treatment. Indeed, one small molecule inhibitor of 12/15-LOX was shown to reduce proteinuria and renal hypertrophy in a type 2 diabetic rat model (Xu et al., 2009). 12/15-LOX activation also can induce ROS superoxide anions and lipid peroxidation, which are also involved in advanced glycation end products (Kang et al., 2001; Reddy et al., 2002; Brownlee, 2001; Cyrus et al., 2001; Murea et al., 2010; Natarajan and Nadler, 2004). All of these factors are associated with DN. Interestingly, angiotensin receptor blockage, one of the treatments for DN, reduced 12/15-LOX activation and 12(S)-HETE levels in a diabetic rat model of DN (Xu et al., 2016b).
Diabetic peripheral neuropathy and other central nervous system disorders
Diabetic peripheral neuropathy (DPN) is a very common debilitating complication of T1D and T2D. DPN can lead to severe pain, and also is a leading cause of foot amputations. Currently, most approved therapies focus on symptom control, rather than actual disease modification. Therefore, a better understanding of the key pathways linked to development and progression of DPN are needed. There is likely a complex interplay between glucose levels, genetics, oxidative stress, and inflammation that contribute to DPN, which should be considered in the development of new therapeutics. There is evidence of increased 12/15-LOX expression and activity in tissues of experimental animal models of DPN (Stavniichuk et al., 2010; Obrosova et al., 2010; Stavniichuk et al., 2012). Pharmacologic inhibition of 12/15-LOX reduced p38 and ERK/MAPK activation in mouse sciatic nerve (Stavniichuk et al., 2013). In addition, inhibition of 12-LOX reduced glucose-mediated increase in phosphorylation of p38 and ERK/MAPK in human Schwann cells, thus suggesting a link between 12-LOX activation and DPN in humans (Stavniichuk et al., 2013). The use of the non-specific inhibitor, CDC, gives rise to caution in over-interpreting these results. Thus, new more specific and safe inhibitors are needed to move these promising pre-clinical studies forward.
The impact of 12/15-LOX is not limited to the peripheral nervous system, but also appears to play a strong role in the development of several central nervous system diseases. Interestingly, the 12/15-LOX pathway has been recently implicated in Alzheimer’s disease (Hur et al., 2018; Pratico et al., 2004; Lebeau et al., 2001; Lebeau et al., 2004). There is also compelling data for the involvement of 12/15-LOX in brain damage due to stroke (Jin et al., 2008; Liu et al., 2017; van Leyen et al., 2006; van Leyen et al., 2014; Yigitkanli et al., 2013; Yigitkanliet al., 2017).
Cardiovascular Disease (CVD)
People with diabetes continue to have an increased risk of atherosclerotic cardiovascular disease. Both inflammatory and anti-inflammatory mediators produced by 12/15-LOX activation play a complex role in atherosclerotic CVD. Under typical Western Diet conditions, it is likely that inflammatory lipids, such as 12(S)-HETE, prevail. The role of 12/15-LOX metabolites in vascular, renal pathology, and atherosclerosis has been reviewed earlier in great detail (Dobrian et al., 2010a; Imai et al., 2013). More recently, genetic deletion of 12/15-LOX in mice leads to post-myocardial infarction cardiac protection via augmented cytochrome p-450-generated, anti-inflammatory epoxyeicosatrienoic acids, and resolving neutrophil metabolites (Kain et al., 2018). In particular, reduced myofibroblast formation and collagen deposition led to reduced scarring (Kain et al., 2018). A recent report also has indicated that 12-LOX inhibition can be used as an adjuvant treatment with tissue plasminogen activator (Karatas et al., 2018). Platelet activation is a key mechanism in thrombotic disorders such as myocardial infarction and stroke. 12-LOX activation potentiates platelet activation and selective 12-LOX inhibition decreases thrombosis without prolonging hemostasis (Tourdot and Holinstat, 2017).
Thus, 12-LOX and12/15-LOX may be an attractive target to prevent or treat heart failure in people with diabetes. More studies using human samples are necessary to clarify the specific role of 12-LOX and related products in CVD associated with diabetes.
7. Conclusions
12/15-LOX isoforms clearly play a role in several key inflammatory aspects related to both T1D and T2D, from the initial pathogenic insults through the development of diabetes-related complications. Great strides have been made in generating new specific inhibitors that target individual LOX family members. Paired with the use of novel mouse strains in which 12/15-LOX is deleted in specific tissues, we are poised to significantly increase our understanding of the mechanisms underlying these inflammatory processes in diabetes. Furthermore, the development of specific inhibitors may prove useful in protecting patients from disease progression of diabetes and the accompanying complications.
The field of T1D is undergoing a renaissance, with questions being raised as to the etiology, pathophysiology, and pathology of the disease. In this context, the role of 12-LOX in human disease, with a special emphasis on translational mechanistic opportunities in rodent models, becomes especially important. For example, recent studies suggest a central role of viruses in the initiation of the disease, perhaps via signaling through type 1 interferons (Nyalwidhe et al., 2017). To date, however, the activation of 12/15-LOX through the interferons pathway is poorly understood, but clearly requires more study, since it remains possible that 12/15-LOX may initiate stress pathways within the beta cell that ultimately result in neo-epitope formation and autoimmunity. Similarly, a role for macrophages and innate immune cells in early T1D has been appreciated recently, and as such, the role of 12/15-LOX in macrophage polarization requires better characterization. The inhibition of human 12-LOX, by use of small molecule inhibitors, in early T1D may therefore represent a powerful approach to intervening in the early pathogenesis of the disease. A challenge in the development of next-generation small molecule inhibitors, however, lies in structural and functional differences between mouse and human enzymes. These differences limit testing of human-specific inhibitors in mouse preclinical models. Therefore, rather than inhibiting 12/15-LOX action directly, it may be more beneficial to block receptors, such as the G-protein-coupled receptor GPR31, which bind to pro-inflammatory ligands that are products of 12/15-LOX (Guo, Y et al., 2011; Zhang et al., 2018). This approach could represent a new avenue to overcome specificity effects of inhibitors targeting different 12/15-LOX isoforms. In addition, development of humanized mouse models in which the mouse 12/15-LOX is replaced by human 12-LOX would help test new small molecule 12-LOX inhibitors in mouse models of diabetes. Given that there are only limited concerns in treating with globally administered 12-LOX inhibitors, this appears to be a viable treatment target that should be pursued. We cannot conclude this chapter before fully acknowledging that, while we focused on the 12-LOX pathway on pathogenesis of diabetes and complications, other lipid mediators such as lysophosphatidic acids, diacylglycerols, ceramides, and sphingosines, play significant roles in inflammatory processes responsible for onset and progression of type 1 and type 2 diabetes. Concerted efforts and expertise from various investigators are therefore granted to fully understand the role of various lipid classes in inflammation and metabolic diseases and to find the most instrumental approaches for new therapeutic interventions.
Acknowledgements:
We gratefully acknowledge the work and effort of the many technicians, students, and postdoctoral fellows over the years. Animal experiments were performed in accordance with the guidelines set by the Institutional Animal Care and Use Committee at Eastern Virginia Medical School (EVMS, Norfolk, VA, USA) with its approvals. Human studies were approved by the Institutional Review Board at EVMS. Human islets were provided by Integrated Islet Distribution Program (IIDP). This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF). Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php.
This work was supported by the National Institutes of Health to Y.I. (R01-DK090490), National Institutes of Health to A.D.D. (R15H114062), National Institutes of Health to J.L.N. and R.M (R01-DK105588–01)
Abbreviations:
- LOX
lipoxygenase (12-LOX, 15-LOX, 12/15-LOX, etc., are isoforms of lipoxygenase)
- PUFA
poly-unsaturated fatty acids
- AA
arachidonic acid
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- 12(S)-HETE
12-S-Hydroxyeicosatetraenoic acid (also 15-S-HETE isoform)
- HTS
High throughput screening
- CDC
Cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate
- PIC
pro-inflammatory cytokines
- ROS
reactive oxygen species
- ER
Endoplasmic reticulum
- NOX-1
NADPH Oxidase-1
- IL-12
Interleukin-12
- IL-1β
Interleukin-1 beta
- TNF-α
Tumor necrosis factor-alpha
- IFN-γ
Interferon-gamma
- IL-6
Interleukin-6
- MCP-1
Monocyte chemoattractant protein-1
- TGF-β
Transforming growth factor-beta
- STAT4
Signal transducer and activator of transcription 4
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- STZ
streptozotocin
- NOD
Non-obese diabetic mouse
- Alox15
mouse Alox15 gene that encodes 12/15-LOX
- NK
Natural killer cell
- WT
Wild-type
- pLOKO
Pdx1-Cre-mediated deletion of Alox15
- Pdx1
Pancreatic and duodenal homeobox 1
- HFD
High Fat Diet
- CCL2
C-C Chemokine Ligand 2
- CTLA4-Ig
cytotoxic T-lymphocyte-associated protein 4- immunoglobulin
- AT
Adipose Tissue
- WAT
White Adipose Tissue
- DIO
Diet-induced Obesity
- SVF
Stromal vascular fraction
- DR
Diabetic retinopathy
- DN
Diabetic nephropathy
- DPN
Diabetic peripheral neuropathy
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- CVD
Cardiovascular disease
- SC
Subcutaneous fat
- OM
Omental fat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Patents have been filed for ML355 and ML127 by JLN, DTF, TH
References
- Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Sarthy EM, Nussbaum J, et al. (2011). Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implication in retinal neovascularization. Diabetes, 60, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki Y, Yotsumoto S, Watanabe H & Tsuchiya S (2002). NADPH-oxidase may contribute to IL-12 production in macrophages stimulated with CpG phosphorothioate oligodeoxynucleotides. Biological & Pharmaceutical Bulletin, 25, 351–355. [DOI] [PubMed] [Google Scholar]
- Barquissau V, Ghandour RA, Ailhaud G, Klingenspor M, Langin D, Amri EZ et al. (2017). Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie, 136, 3–11. [DOI] [PubMed] [Google Scholar]
- Bleich D, Chen S, Zipser B, Sun D, Funk CD & Nadler JL (1999). Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. Journal of Clinical Investigation, 103, 1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G (2009). Endoplasmic reticulum stress: another link between obesity and insulin resistance/inflammation? Diabetes, 58, 518–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee B (2001). Biochemistry and molecular cell biology of diabetic complications. Nature, 414, 813–820. [DOI] [PubMed] [Google Scholar]
- Butcher MJ, Hallinger D, Garcia E, Machida Y, Chakrabarti S, Nadler J, et al. (2014). Association of pro-inflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia, 57, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SK, Cole BK, Wen Y, Keller SR & Nadler JL (2009). 12/15-Lipoxygenase Products Induce Inflammation and Impair Insulin Signaling in 3T3-L1 Adipocytes. Obesity (Silver Spring) [DOI] [PMC free article] [PubMed]
- Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, et al. (2011). Evidence for Activation of Inflammatory Lipoxygenase Pathways in Visceral Adipose Tissue of Obese Zucker Rats. American Journal of Physiology: Endocrinology and Metabolism, 300, E175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Arita M & Serhan CN (2005). Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 73, 163–177. [DOI] [PubMed] [Google Scholar]
- Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, et al. (1991). Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. Journal of Medicinal Chemistry, 34, 1503–1505. [DOI] [PubMed] [Google Scholar]
- Cho K-J, Seo J-M & Kim J-H (2011). Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Molecules and Cells, 32, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BK, Kuhn NS, Green-Mitchell SM, Leone KA, Raab RM, Nadler JL et al. (2012a). 12/15-Lipoxygenase signaling in the endoplasmic reticulum stress response. American Journal of Physiology: Endocrinology and Metabolism, 302, E654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BK, Morris MA, Grzesik WJ, Leone KA, & Nadler JL (2012b). Adipose tissue-specific deletion of 12/15-lipoxygenase protects mice from the consequences of a high-fat diet. Mediators of Inflammation, 2012, 851798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BK, Lieb DC, Dobrian AD, & Nadler JL (2013) 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins & Other Lipid Mediators, 104–105, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, et al. (2001). Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation 103, 18 2277–2282. [DOI] [PubMed] [Google Scholar]
- Deschamps JD, Kenyon VA, & Holman TR (2006). Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorganic & Medicinal Chemistry 14, 4295–4301. [DOI] [PubMed] [Google Scholar]
- Deschamps JD, Gautschi JT, Whitman S, Johnson TA, Gassner NC, Crews P, et al. (2007). Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries. Bioorganic & Medicinal Chemistry 15, 6900–6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, & Nadler JL (2010a). Functional and pathological roles of the 12- and 15-lipoxygenases. Progress in Lipids Research, 50, 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Lieb DC, Ma Q, Lindsay JW, Cole BK, Ma K, et al. (2010b). Differential expression and localization of 12/15 lipoxygenases in adipose tissue in human obese subjects. Biochemical and Biophysical Research Communications, 403, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Huyck RW, Glenn L, Gottipati V, Haynes BA, Hansson GI, et al. (2018). Activation of the 12/15 lipoxygenase pathway accompanies metabolic decline in db/db pre-diabetic mice. Prostaglandins & Other Lipid Mediators 136, 23–32. [DOI] [PubMed] [Google Scholar]
- Doiron JA, Leblanc LM, Hebert MJ, Levesque NA, Pare AF, Jean-Francois J, et al. (2017). Structure-activity relationship of caffeic acid phenethyl ester analogs as new 5-lipoxygenase inhibitors. Chemical Biology Drug Design, 89, 514–528. [DOI] [PubMed] [Google Scholar]
- Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, , et al. (2011). Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation, 123, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, et al. (2009). Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB Journal, 23, 1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Mitchell SM, Tersey SA, Cole BK, Ma K, Kuhn NS, Cunningham TD, et al. (2013). Deletion of 12/15-lipoxygenase alters macrophage and islet function in NOD-Alox15(null) mice, leading to protection against type 1 diabetes development. PLoS One, 8, e56763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesik WJ, Nadler JL, Machida Y, Nadler JL, Imai Y, & Morris MA (2015). Expression pattern of 12-lipoxygenase in human islets with type 1 diabetes and type 2 diabetes. Journal of Clinical Endocrinology and Metabolism, 100, E387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, & Kern TS (2017). 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes, 57, 1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, et al. (2011). Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. Journal of Biological Chemistry, 286, 33832–33840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo QY, Miao LN, Li B, Ma FZ, Liu N, Cai L, et al. (2011). Role of 12-lipoxygenase in decreasing P-cadherin and increasing angiotensin II type 1 receptor expression according to glomerular size in type 2 diabetic rats. American Journal of Physiology: Endocrinology and Metabolism, 300, E708–16. [DOI] [PubMed] [Google Scholar]
- Ha JH, Radhakrishnan R, Jayaraman M, Yan M, Ward JD, Fung K-M, et al. (2018). Lysophosphatidic acid induces metabolic reprogramming in ovarian cancer via a pseudohypoxic response. Cancer Research, 78, 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Black LM & Verma MK (2018). Paradigm shift - Metabolic transformation of docosahexaenoic and eicosapentaenoic acids to bioactives exemplify the promise of fatty acid drug discovery. Biotechnology Advances, 36, 935–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z, Bond NJ, Ashmore T, Sanders F, Ament Z, Wang X, et al. (2017). Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology, 65, 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy E, Rakovac Tisdall A, Murphy N, Carroll A, O’Gorman D, Breen L, et al. (2017). Elevated 12-hydroxyeicosatetraenoic acid (12-HETE) levels in serum of individuals with newly diagnosed Type 1 diabetes. Diabetic Medicine, 34, 292–294. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perez M, Chopra G, Fine J, Conteh AM, Anderson RM, Linnemann AK, et al. (2017). Inhibition of 12/15-Lipoxygenase Protects Against beta-Cell Oxidative Stress and Glycemic Deterioration in Mouse Models of Type 1 Diabetes. Diabetes, 66, 2875–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Mateo V, Amalric N, Babiak M, Bereziat G, Kanony-Truc C, et al. (2018). Cerebrovascular β-amyLOXid Deposition and Associated Microhemorrhages in a Tg2576 Alzheimer Mouse Model are Reduced with a DHA-Enriched Diet. FASEB Journal, April 5, fj201800200R. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Elshafey S, Sellak H, Hussein KA, El-Sherbiny M, Abdelsaid M, et al. (2015a). A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase. Journal of Lipid Research, 56, 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Tawfik AM, Hussein KA, Elshafey S, Markland S, Rizk N, et al. (2015b). Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids. Biochimica et Biophysica Acta, 1851, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Saleh H, El-Shafey M, Hussein KA, El-Masry K, Baban B, et al. (2017). Targeting of 12/15-Lipoxygenase in retinal endothelial cells, but not in monocytes/macrophages, attenuates high glucose-induced retinal leukostasis. Biochimica et Biophysica Acta, 1826, 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR,et al. (2012). Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. Journal of Lipid Research,53, 2546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Narashima K, Arai M, Sakamoto H, Chiba N, & Nakagawa Y (1998). Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. Journal of Biological Chemistry, 273, 1990–1997. [DOI] [PubMed] [Google Scholar]
- Imai Y, Dobrian AD, Weaver JR, Butcher MJ, Cole BK, Galkina EV, et al. (2013). Interaction between cytokines and inflammatory cells in islet dysfunction, insulin resistance and vascular disease. Diabetes Obesity & Metabolism, 15 Suppl 3, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamori Y, Tsujibo H, Ohishi H, Ishii F, Mizugaki M, Aso H et al. (1993). Cytotoxic effect of hinokitiol and tropolone on the growth of mammalian cells and on blastogenesis of mouse splenic T cells. Biological & Pharmaceutical Bulletin, 16, 521–523. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H et al. (2010). Molecular enzymology of lipoxygenases. Archives of Biochemistry & Biophysics, 503, 161–174. [DOI] [PubMed] [Google Scholar]
- Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, et al. (2008). Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke, 39, 2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Hwang HJ, Hong HC, Choi HY, Yoo HJ, Baik SH et al. (2014). Resolvin D1 reduces ER stress-induced apoptosis and triglyceride accumulation through JNK pathway in HepG2 cells. Molecular and Cellular Endocrinology, 391, 30–40. [DOI] [PubMed] [Google Scholar]
- Jung TW, Kyung EJ, Kim HC, Shin YK, Lee SH, Park ES, et al. (2018). Protectin DX Ameliorates Hepatic Steatosis by Suppression of Endoplasmic Reticulum Stress via AMPK-Induced ORP150 Expression. The Journal of Pharmacology and Experimental Therapeutics, 365, 485–493. [DOI] [PubMed] [Google Scholar]
- Kain V, Ingle KA, Kabarowski J, Barnes S, Limdi NA, Prabhu SD et al. (2018). Genetic deletion of 12/15 lipoxygenase promotes effective resolution of inflammation following myocardial infarction. Journal of Molecular and Cellular Cardiology, 118, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Alder SG, Nast CC, Lapage J, Gu JL, Nadler JL, et al. (2001). 12-Lipoxygenase is increased in glucose-stimulated mesangial cells and in experiment diabetic nephropathy. Kidney International, 59, 1354–1362. [DOI] [PubMed] [Google Scholar]
- Karatas H, Jung JE, Lo EH, & van Leyen K (2018). Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models. Brain Research, 1678, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB 2nd, et al. (2011). Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. Journal of Medicinal Chemistry, 54, 5485–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Reddy MA, Lanting L, Adler SG, & Natarajan R (2003). Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney International, 64, 1702–1714. [DOI] [PubMed] [Google Scholar]
- Kuda O, Brezinova M, Rombaldova M, Slavikova B, Posta M, Beier P, et al. (2016). Docosahexaenoic Acid-Derived Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) With Anti-inflammatory Properties. Diabetes, 65, 2580–2590. [DOI] [PubMed] [Google Scholar]
- Kuhn H, Banthiya S & van Leyen K (2015). Mammalian lipoxygenases and their biological relevance. Biochimica et Biophysica Acta, 1851, 308–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Kim SN, Kim YA, & Lee YH (2016). The contribution of arachidonate 15-lipoxygenase in tissue macrophages to adipose tissue remodeling. Cell Death & Disease ,7, e2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic M, Inzaugarat ME, Povero D, Zhao IC, Chen M, Nalbandian M,et al. (2014). Reduced dietary omega-6 to omega-3 fatty acid ratio and 12/15-lipoxygenase deficiency are protective against chronic high fat diet-induced steatohepatitis. PLoS One, 9, e107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau A, Esclaire F, Rostene W, & Pelaprat D (2001). Baicalein protects cortical neurons from β -amyloid (25–35) induced toxicity. Neuroreport, 12, 2199–2202. [DOI] [PubMed] [Google Scholar]
- Lebeau A, Terro F, Rostene W, & Pelaprat D (2004). Blockage of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by β-amyLOXid peptide. Cell Death and Differentiation, 11, 875–884. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kim SN, Kwon HJ, Maddipati KR, & Granneman JG (2016). Adipogenic role of alternatively activated macrophages in beta-adrenergic remodeling of white adipose tissue. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology, 310, R55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S (2008). Oxidative stress: the vulnerable beta-cell. Biochemical Society Transactions, 36, 343–347. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng Y, Karatas H, Wang X, Foerch C, Lo EH, et al. (2017). 12/15-Lipoxygenase inhibition or knockout reduces warfarin-associated hemorrhagic transformation after experimental stroke. Stroke, 48, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopategi A, Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Titos E, et al. (2016). Role of bioactive lipid mediators in obese adipose tissue inflammation and endocrine dysfunction. Molecular and Cellular Endocrinology, 419, 44–59. [DOI] [PubMed] [Google Scholar]
- Luci DK, Jameson JB 2nd, Yasgar A, Diaz G, Joshi N, Kantz A, et al. (2014). Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. Journal of Medicinal Chemistry, 57, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbom J, Heikkinen S, Fielding B, Hakkarainen A, Taskinen MR, & Lundbom N (2009). PRESS echo time behavior of triglyceride resonances at 1.5T: detecting omega-3 fatty acids in adipose tissue in vivo. Journal of Magnetic Resonance, 201, 39–47. [DOI] [PubMed] [Google Scholar]
- Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, & Nadler JL (2010). 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. Journal of Clinical Endocrinoloy and Metabolism, 95, 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Xiao A, Park SH, Glenn L, Jackson L, Barot T,, et al. (2017). 12-lipoxygenase inhibitor improves functions of cytokine-treated human islets and type 2 diabetic islets. Journal of Clinical Endocrinology and Metabolism, 102, 2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganti A, Evans-Molina C, & Mirmira R (2014). From immunobiology to beta-cell biology: the changing perspective on type 1 diabetes. Islets, 6, e28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente M, Ferre N, Titos E, Horrillo R, Gonzalez-Periz A, Moran-Salvador E, et al. (2010). Disruption of the 12/15-lipoxygenase gene (Alox15) protects hyperlipidemic mice from nonalcoholic fatty liver disease. Hepatology, 52, 1980–1991. [DOI] [PubMed] [Google Scholar]
- Matono R, Miyano K, Kiyohara T & Sumimoto H (2014). Arachidonic acid induces direct interaction of the p67phox-Rac complex with the phagocyte oxidase Nox2, leading to superoxide production. Journal of Biological Chemistry, 289, 24874–24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Tanaka N, Krausz KW, Manna SK, Kang DW, Anderson ER, et al. (2012). Metabolomics identifies an inflammatory cascade involved in dioxin- and diet-induced steatohepatitis. Cell Metabolism, 16, 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, et al. (2008). Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes, 57, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, et al. (2010). Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney International, 78, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelin MH, Srinivasan S, Lee J, Nadler JL & Hedrick CC (2008). 12/15-Lipoxygenase activity increases the degradation of macrophage ATP-binding cassette transporter G1. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, & Nadler JL (2004). Lipid inflammatory mediators in diabetic vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 24, 1542–1548. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, et al. (2007). Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. Journal of Physiology, 583, 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, et al. (2008). 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. American Journal of Physiology: Endocrinology and Metabolism, 295, E1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalwidhe JO, Grzesik WJ, Burch TC, Semeraro ML, Waseem T, Gerling IC, et al. (2017). Comparative quantitative proteomic analysis of disease stratified laser captured microdissected human islets identifies proteins and pathways potentially related to type 1 diabetes. PLoS One, 12, e0183908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Nadler JL, et al. (2010). Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. American Journal of Pathology, 177, 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman A, Ahmad S, Megyerdi S, Mussell R, Choksi K, Maddipati KR, et al. (2013). 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS One, 8, e57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola C, Jazzar B, Rossi A, Buehring U, Luderer S, Dehm F, et al. (2011). Cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate is a potent inhibitor of 5-lipoxygenase. Journal of Pharmacology and Experimental Therapies, 338, 205–213. [DOI] [PubMed] [Google Scholar]
- Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, et al. (2004). 12/15 Lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. American Journal of Pathology, 164, 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, et al. (2009). The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology, 50, 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Lee IF, Panagiotopoulos C, Wang X, Chu AD, Utz PJ, et al. (2011). Natural killer cells from children with type 1 diabetes have defects in NKG2D-dependent function and signaling. Diabetes, 60, 857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai G, Joshi N, Jung JE, Liu Y, Schultz L, Yasgar A, et al. (2014). Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies. Journal of Medicinal Chemistry, 57, 4035–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Adler SG, Kim YS, Lanting L, Rossi J, Kang SW, et al. (2002). Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. American Journal of Physiology: Renal Physiology, 283, F985–F994. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ (2005). Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nature Clinical Practice. Gastroenterology & Hepatology, 2, 46–53. [DOI] [PubMed] [Google Scholar]
- Schneider M, Wortmann M, Mandal PK, Arpornchayanon W, Jannasch K, Alves F, et al. (2010). Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia, 12, 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, et al. (2009). 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One, 4, e7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves EN, Shah RR, Segraves NL, Johnson TA, Whitman S, Sui JK, et al. (2004). Probing the activity differences of simple and complex brominated aryl compounds against 15-soybean, 15-human, and 12-human lipoxygenase. Journal of Medicinal Chemistry, 47, 4060–4065. [DOI] [PubMed] [Google Scholar]
- Semeraro ML, Glenn LM, & Morris MA (2017) The Four-Way Stop Sign: Viruses, 12-Lipoxygenase, Islets, and Natural Killer Cells in Type 1 Diabetes Progression. Frontiers in Endocrinology, 8, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Chiang N (2008). Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. British Journal of Pharmacology, 153 Suppl 1, S200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, & Van Dyke TE (2008a). Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews. Immunology, 8, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, & Yang R (2008b). Anti-inflammatory and proresolving lipid mediators. Annual Review of Pathology, 3, 279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Petasis NA (2011). Resolvins and protectins in inflammation resolution. Chemical Reviews, 111, 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, & Levy BD (2018). Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. Journal of Clinical Investigation, May 14, pii: 97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Stevens MJ, Nadler JL et al. (2010). Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radical Biology & Medicine, 49, 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavniichuk R, Shevalye H, Hirooka H, Nadler JL, & Obrosova IG (2012). Interplay of Sorbitol Pathway of Glucose Metabolism, 12/15-Lipoxygenase and Mitogen-activated Protein Kinases in the Pathogenesis of Diabetic Peripheral Neuropathy. Biochemical Pharmacology, 83, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavniichuk R, Obrosov AA, Drel VR, Nadler JL, Obrosova IG, & Yorek MA (2013). 12/15-Lipoxygenase inhibition counteracts MAPK phosphorylation in mouse and cell culture models of diabetic peripheral neuropathy. Journal of Diabetes Mellitus, 3,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Ueda T, Juranek I, Yamamoto S, Katoh T, Node M, et al. (2000). Hinokitiol, a selective inhibitor of the platelet-type isozyme of arachidonate 12-lipoxygenase. Biochemical and Biophysical Research Communications, 275, 885–889. [DOI] [PubMed] [Google Scholar]
- Taylor-Fishwick DA (2013). NOX, NOX Who is There? The Contribution of NADPH Oxidase One to Beta Cell Dysfunction. Frontiers in Endocrinology, 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Weaver JR, Grzesik W, Chakrabarti S, Green-Mitchell S, Imai Y, et al. (2013). Production and function of IL-12 in islets and beta cells. Diabetologia, 56, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Weaver J, Glenn L, Kuhn N, Rai G, Jadhav A, et al. (2015). Selective inhibition of 12-lipoxygenase protects islets and beta cells from inflammatory cytokine-mediated beta cell dysfunction. Diabetologia, 58, 549–557. [DOI] [PubMed] [Google Scholar]
- Tersey SA, Maier B, Nishiki Y, Maganti AV, Nadler JL, & Mirmira RG (2014). 12-lipoxygenase promotes obesity-induced oxidative stress in pancreatic islets. Molecular and Cellular Biology, 34, 3735–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, & Moschen AR (2008). Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends in Endocrinology and Metabolism, 19, 371–379. [DOI] [PubMed] [Google Scholar]
- Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, et al. (2013). Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. Journal of Immunology, 187, 5408–5418. [DOI] [PubMed] [Google Scholar]
- Tourdot BE & Holinstat M (2017) Targeting 12-Lipoxygenase as a Potential Novel Antiplatelet Therapy. Trends in Pharmacological Sciences, 38, 1006–1015. [DOI] [PubMed] [Google Scholar]
- Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, et al. (2012). 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity, 36, 834–846. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Lee HY, Jin SR, Arai K, and Lo EH (2006). Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke, 37, 3014–4018. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Holman TR, & Maloney DJ (2014). The potential of 12/15-lipoxygenase inhibitors in stroke therapy. Future Medicinal Chemistry, 6, 1853–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Martinez Y, Ohri RV, Kenyon V, Holman TR, & Sepulveda-Boza S (2007). Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2. Bioorganic & Medicinal Chemistry, 15, 7408–7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk A & Ron D (2010). The endoplasmic reticulum stress response in the pancreatic beta-cell. Diabetes, Obesity, & Metabolism, 12 Suppl 2, 48–57. [DOI] [PubMed] [Google Scholar]
- Wang W & Lo AC-Y (2018). Diabetic Retinopathy: Pathophysiology and Treatments. International Journal of Molecular Sciences, 19, E1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JR, Holman TR, Imai Y, Jadhav A, Kenyon V, Maloney DJ, et al. (2012). Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Molecular and Cellular Endocrinology, 358, 88–95. [DOI] [PubMed] [Google Scholar]
- Weaver J & Taylor-Fishwick DA (2015). Role of NADPH Oxidase in Beta Cell Dysfunction. In Islam S (Ed.), Islets of Langerhans, 2nd Edition (pp 923–954). Dordrecht, Heidelberg, New York, London: Springer. [Google Scholar]
- Weaver JR, Grzesik W, & Taylor-Fishwick DA (2015a) Inhibition of NADPH oxidase-1 preserves beta cell function. Diabetologia, 58, 113–121. [DOI] [PubMed] [Google Scholar]
- Weaver JR, Nadler JL, & Taylor-Fishwick DA (2015b). Interleukin-12 (IL-12)/STAT4 Axis Is an Important Element for beta-Cell Dysfunction Induced by Inflammatory Cytokines. PLoS One, 10, e0142735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J & Taylor-Fishwick DA (2017). Relationship of NADPH Oxidase-1 expression to beta cell dysfunction induced by inflammatory cytokines. Biochemical and Biophysical Research Communications, 485, 290–294. [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, et al. (2007). The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology, 148, 1313–1322. [DOI] [PubMed] [Google Scholar]
- Xu HZ, Cheng YL, Wang WN, Zhang YY, Zang CS, & Xu ZG (2016a). 12-Lipoxygenase Inhibition on Microalbuminuria in Type-1 and Type-2 Diabetes Is Associated with Changes of Glomerular Angiotensin II Type 1 Receptor Related to Insulin Resistance. International Journal of Molecular Sciences, 17, 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HZ, Wang WN, Zhang YY, Cheng YL, & Xu ZG (2016b). Effect of angiotensin II type 1 receptor blocker on 12-lipoxygenase activity and slit diaphragm protein expression in type 2 diabetic rat glomeruli. Journal of Nephrology, 29, 775–782. [DOI] [PubMed] [Google Scholar]
- Xu ZG, Li SL, Lanting L, Kim YS, Shanmugam N, Reddy MA, et al. (2006). Relationship between 12/15-lipoxygenase and COX-2 in mesangial cells: potential role in diabetic nephropathy. Kidney International, 69, 512–519. [DOI] [PubMed] [Google Scholar]
- Xu ZG, Yuan H, Lanting L, Li SL, Wang M, Shanmugam N,et al. (2008). Products of 12/15-lipoxygenase upregulate the angiotensin II receptor. Journal of the American Society of Nephrology, 19, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZG, Miao LN, Cui YC, Jia Y, Huan H, & Wu M (2009). 12-lipoxygenase in high glucose-stimulated glomerular cells and type 2 diabetic glomeruli. Nephrology, Dialysis, Transplantation, 24, 1744–1752. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, & Nadler JL (2003). The novel anti-inflammtory compound, lisofylline, prevents diabetes in multiple low-dose streptozotocin-treated mice. Pancreas, 26, e99–104. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Ellett JD, Fialkow LB, Carter JD, McDuffie M et al. (2004). Autoimmune diabetes is blocked in Stat4-deficient mice. Journal of Autoimmunity, 22, 191–200. [DOI] [PubMed] [Google Scholar]
- Yeung J & Holinstat M (2011). 12-lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovascular & Hematological Agents in Medicinal Chemistry, 9, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, et al. (2013). Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of Neurology, 73, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigitkanli K, Zhen Y, Pekcec A, Lo EH, & van Leyen K (2017). Increased 12/15-lipoxygenase leads to widespread brain injury following global cerebral ischemia. Translational Stroke Research, 8, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Lanting L, Xu ZG, Li SL, Swiderski P, Putta S, et al. (2008). Effects of cholesterol-tagged small interfering RNAs targeting 12/15-lipoxygenase on parameters of diabetic nephropathy in a mouse model of type 1 diabetes. American Journal Physiology. Renal Physiology, 295, F605–F617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Reddy MA, Deshpande S, Jia Y, Park JT, Lanting LL, et al. (2016). Epigenetic Histone Modifications Involved in Profibrotic Gene Regulation by 12/15-Lipoxygenase and Its Oxidized Lipid Products in Diabetic Nephropathy. Antioxidants & Redox Signaling, 24, 361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, et al. (2018). An ALOX12–12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nature Medicine, 24, 73–83. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Usui HK, & Sharma K (2007). Regulation of transforming growth factor beta in diabetic nephropathy: implications for treatment. Seminars in Nephrology, 27, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]


