Abstract
Background:
Preclinical models showed that blocking PD-1/PD-L1 pathways enhanced anti-leukemic responses. Azacitidine up-regulates PD-1 and interferon-gamma signaling.
Methods:
In this single arm trial, patients with relapsed/refractory (R/R) AML were treated with azacitidine 75mg/m2 Days 1–7 intravenously or subcutaneously with nivolumab 3mg/kg intravenously on Day 1 and 14, every 4–6 weeks.
Findings:
Seventy patients were treated. The median age was 70 years (range, 22–90). The median number of prior therapies received was 2 (range, 1–7). The overall response rate (ORR) was 33% including 15 (22%) complete remission (CR)/complete remission with insufficient recovery of counts (CRi), 1 partial response, and 7 patients with hematologic improvement (HI) maintained >6 months. Six patients (9%) had stable disease >6 months. The ORR was 58% and 22%, in HMA-naïve (n=25) and HMA pre-treated (n=45) patients, respectively. Grade 3–4 immune related adverse events occurred in 8 (11%) patients. Pretherapy bone marrow and peripheral blood CD3 and CD8 were significantly predictive for response on flow-cytometry. CTLA-4 was significantly up-regulated on CD4+Teff in non-responders after 2 and 4 doses of nivolumab.
Interpretation:
Azacitidine and nivolumab therapy produced an encouraging response rate and overall survival in patients with R/R AML, particularly in HMA-naïve and Salvage 1 patients. Pretherapy bone marrow aspirate and peripheral blood CD3 percentage may be biomarkers for patient selection.
Trial Registration ID:
Clinicaltrials.gov identifier: NCT02397720
Keywords: nivolumab, azacitidine, acute myeloid leukemia, checkpoint inhibitors
INTRODUCTION
Over the last decade, six PD-1, PD-L1, and CTLA-4 antibodies have been approved for over 25 indications in 10 tumor types in the United States (US) and Europe. High clinical efficacy with single agent PD-1 inhibition was seen in classical Hodgkin’s lymphoma.(1) In other hematologic malignancies, such as non-Hodgkin’s lymphoma, the benefits of immune checkpoint inhibitors (ICPI) were not evident with single-agent CPI therapy,(2) but with rationally designed combinations.(3)
T-cell population has been shown to be preserved in the bone marrow (BM) and peripheral blood (PB) of patients with AML and comparable with healthy donors.(4,5) In murine models the progression of AML was associated with increased PD-1 expression on circulating CD8 T cells,(6) resulting in decreased CD8 cytotoxic activity. This was partially reversible with murine PD-1 blockade.(7) Immune checkpoint receptors, most strikingly PD-1 and OX-40, were more frequently expressed on CD8 cells from BM aspirates (BMAs) in patients with relapsed AML compared with healthy donors.(5) Single agent anti-PD-1 antibodies have demonstrated minimal activity in patients with relapsed AML and high-risk MDS.(2,8)
Azacitidine is approved in the US and Europe for patients with MDS, and is approved in Europe and commonly used in the US to treat older patients with newly diagnosed AML.(9) Hypomethylating agents (HMAs), azacitidine and decitabine, promote anti-tumor immune signaling by up regulation of interferon-gamma pathway genes, increased expression of HLA class 1 antigens, and activation of viral defense pathways.(10) The HMAs concurrently dampen anti-tumor immunity by increasing the expression of PD-1 and PD-L1 in solid tumors(11) and in myelodysplastic syndrome (MDS)/AML.(12) Up regulation of these immune checkpoint molecules may be a mechanism of resistance to HMAs. This study was designed to assess whether the addition of nivolumab to azacitidine was safe and effective.
RESULTS
Patient Characteristics and Treatments
Seventy patients were treated. All received azacitidine 75mg/m2 Days 1–7 with nivolumab 3mg/kg Days 1 and 14. Patient characteristics are shown in Table 1. The median number of prior therapies for AML was 2 (range, 1 – 7). Prior exposure to HMA was allowed and 45 patients (65%) had received prior HMA-based therapy. The median duration on study for all patients was 3.5 months (range, 0.3 – 26.3). Study discontinuations were due to: primary refractory disease (n = 27), relapse after initial response (n = 19), ASCT in CR/CRi (n=3), death on study (n=16), and patient preference (n=3). No protocol discontinuations were due to myelosuppression or immune toxicities. Two patients died of toxicities possibly related to the CPI, discussed in more detail under “Toxicities”.
Table 1.
Patient characteristics for Azacitidine+Nivolumab patients (N=70) and for historic HMA-based clinical trial control (N=172).
| Characteristic | N (%); Median [Range] |
||
|---|---|---|---|
| Azacitidine+Nivolumab | Control | Pvalue | |
| Age, years | 70 [22 – 90] | 64 [18 – 90] | 0.004 |
| Age ≥ 60 years | 56 (80) | 103 (60) | |
| Diagnosis, n (%) | |||
| AML- de novo | 39 (56) | 112 (65) | 0.19 |
| Secondary AML | 31 (44) | 60 (35) | |
| Prior therapies | 2 [1 – 7] | 1 [1 – 6] | 0.76 |
| Prior therapiesᵻ | |||
| HMA-based | 45 (64) | 51 (30) | <0.0001 |
| HIDAC | 27 (39) | 99 (58) | 0.0073 |
| IDAC | 21 (30) | 5 (3) | <0.0001 |
| Targeted therapies* | 33 (47) | 15 (9) | <0.0001 |
| Prior allogeneic SCT | 13 (19) | 16 (9) | 0.0441 |
| BM blast | 35 [4 – 94] | 38 [7 – 98] | <0.0001 |
| White blood cell count (x109/L) | 2.7 [0.5 – 81] | 2.4 [0.2 – 232] | 0.9121 |
| Platelets (x109/L) | 28 [1–203] | 25 [1–816] | 0.2379 |
| Cytogenetics | |||
| Diploid | 9 (13) | 23 (13) | 0.9146 |
| Miscellaneous | 36 (51) | 27 (16) | |
| Not available | 0 (0) | 62 (36) | |
| Del 5/−7/complex | 25 (36) | 60 (35) | 0.9023 |
| Molecular mutational panel | Done on all 70 pts | Positive/Total tested | |
| TP53 | 16 (23) | 18/54 (33) | 0.1948 |
| DNMT3A | 12 (17) | 7/58 (12) | 0.4215 |
| TET2 | 11 (16) | 20/32 (63) | <0.0001 |
| ASXL1 | 11 (16) | 13/38 (34) | 0.0272 |
| CEBPA | 8 (11) | 9/81 (11) | 0.9509 |
| RAS | 9 (13) | 8/123 (7) | 0.1343 |
| IDH2 | 9 (13) | 5/62 (8) | 0.3721 |
| PTPN11 | 7 (10) | 1/27 (4) | 0.3123 |
| IDH1 | 6 (9) | 7/82 (9) | 0.9939 |
| JAK2 | 3 (4) | 9/62 (15) | 0.0413 |
| Treatment group | |||
| HMA single agent | 0 (0) | 64 (37) | |
| HMA+Immuntherapy | 70 (100) | 49 (29) | |
| HMA+others | 0 (0) | 59 (34) | |
This included IDH1/2 and FLT3-inhibitor, BCL-2 inhibitor, MEK inhibitor, histone deacetylase inhibitor, JAK2 inhibitor, and Grb-2 inhibitor based therapies.
Patients might have received multiple different type of targeted, HMA, HIDC or IDAC therapy. The number and percentage do represent patients, not a percentage from total prior therapy.
Abbreviations: N, number, HMA, hypomethylating agent, Ara-C, cytarabine, BM, bone marrow, Del, deletion, SCT: Stem Cell Transplant, HIDAC: High dose Ara-C based, IDAC: Intermediate dose Ara-C based.
The median number of azacitidine and nivolumab cycles received were 3 (range, 1 – 25). The median number of nivolumab doses received was 6 (range, 1 – 54). Dose interruptions of nivolumab occurred in 24 of 70 (34%) patients due to pneumonitis/colitis (n=13), liver enzyme elevation (n=2), cytokine release syndrome (n=1), bone pains (n=1), lung infections (n=3), hypothyroidism (n=1), creatinine elevation (n=2), and febrile neutropenia (n=1). Nine of 70 patients (13%) discontinued nivolumab and remained on azacitidine alone, due to pneumonitis (n=7), cytokine release syndrome and immune nephritis (1 each). Overall, 10 of 70 patients (14%) had to hold azacitidine at some point on study, due to cytopenias (n=7), infection (n=2), and elevated creatinine (n=1). Twelve of 70 (17%) patients required dose reductions of azacitidine, all due to cytopenias.
Responses
The ORR was 33% including 15 CR/CRi (22%) (4 CR and 11 CRi), 1 PR, and 7 HI (Table 2). The median number of cycles to response was 2 (range, 1–6). Additionally, 6 patients (9%) remained on study with SD >6 months. The remaining 41 patients (58%) had NR. The four- and eight-week mortalities were 3% and 11%, respectively. Three patients (4%) went to ASCT in CR/CRi. By univariate analysis the factors significantly associated with improved ORR included no prior HMA-based therapy, pretherapy BM blast <20%, circulating WBC </=10,000/μL, the presence of an ASXL1 mutation, and pretherapy BM aspirate CD3+ (Table 3). On multivariate analysis (done on the 47 patients who had pretherapy BM CD3+ flow cytometry data available) no factor was statistically significant, although no prior HMA (P=0.059), higher pretherapy BM aspirate CD3+ (P=0.065), and the presence of ASXL1 mutation (P=0.053) showed a trend for improved ORR [Supplemental Table 1]. A heat-map showing the relationship between pretherapy karyotype, mutation profile, and responses is shown in Supplemental Figure 1.
Table 2:
Best Response for Azacitidine+Nivolumab patients (N=70) and for historic HMA-based clinical trial control (N=172).
| Best response | N (%); Median [Range] | |
|---|---|---|
| Azacitidine/Nivolumab | Control | |
| Overall Response Rate | 23 (33) | 35 (20) |
| CR | 4 (6) | 17 (10) |
| CRi/CRp | 11 (16) | 15 (9) |
| PR | 1 (1) | 1 (1) |
| HI*(6 months+) | 7 (10) | 2 (1) |
| Stable disease (6 months+)@ | 6 (9) | NA |
| Non responders | 41 (58) | 131 (76) |
| Median cycles to response | 2 [1 – 13] | 2 [1 – 6] |
| Median follow up, in months | 13.3 [8.2 – 25.5] | 51 [0.1 – 64.8] |
Abbreviations: N, number, CR, complete remission, CRi, complete remission with incomplete count recovery, PR, partial response, HI, hematologic improvement
Hematologic improvement in one or more parameter maintained >6 months on study
Stable disease (SD) was defined as the absence of CR, CRi, PR, MLFS, HI without evidence of clinical deterioration or proliferative disease, maintained >6 months on study (see text for detailed definition).
Table 3:
Overall response rate (CR, CRi, PR, HI) by baseline characteristics.
| No Response (n=47) | Response (n=23) | p-value | |||
|---|---|---|---|---|---|
| N | % (mean) | N | % (mean) | ||
| Age | 0.79 | ||||
| <60 | 9 | 19 | 5 | 22 | |
| >/=60 | 38 | 81 | 18 | 78 | |
| Age | 0.67 | ||||
| <70 | 23 | 49 | 10 | 43 | |
| >/=70 | 24 | 51 | 13 | 57 | |
| Salvage status | 0.20 | ||||
| S1 | 19 | 40 | 13 | 57 | |
| >S1 | 28 | 60 | 10 | 43 | |
| Salvage status | 0.12 | ||||
| S1/S2 | 34 | 72 | 21 | 91 | |
| >S2 | 13 | 28 | 2 | 9 | |
| Diagnosis | 0.54 | ||||
| AML - de novo | 25 | 53 | 14 | 61 | |
| Secondary AML | 22 | 47 | 9 | 39 | |
| Prior ASCT | 0.52 | ||||
| Yes | 10 | 21 | 3 | 13 | |
| No | 37 | 79 | 20 | 87 | |
| Prior HMA | 0.01 | ||||
| Yes | 35 | 74 | 10 | 57 | |
| No | 12 | 26 | 13 | 43 | |
| Cytogenetic | 0.13 | ||||
| Diploid | 13 | 28 | 12 | 52 | |
| miscellaneous | 16 | 34 | 5 | 22 | |
| −5/−7/complex | 18 | 38 | 6 | 26 | |
| TP53 | 0.23 | ||||
| Negative | 34 | 72 | 20 | 87 | |
| Positive | 13 | 28 | 3 | 13 | |
| IDH1 | 0.99 | ||||
| Negative | 43 | 91 | 21 | 91 | |
| Positive | 4 | 9 | 2 | 9 | |
| IDH2 | 0.46 | ||||
| Negative | 42 | 89 | 19 | 83 | |
| Positive | 5 | 11 | 4 | 17 | |
| RAS | 0.14 | ||||
| Negative | 43 | 91 | 18 | 78 | |
| Positive | 4 | 9 | 5 | 22 | |
| ASXL1 | 0.03 | ||||
| Negative | 43 | 91 | 16 | 70 | |
| Positive | 4 | 9 | 7 | 30 | |
| BM BL>/=30 | |||||
| No | 19 | 40 | 12 | 52 | 0.35 |
| Yes | 28 | 60 | 11 | 48 | |
| BM BL>/=20 | 0.02 | ||||
| No | 8 | 17 | 10 | 43 | |
| Yes | 39 | 83 | 13 | 57 | |
| BM BL>/=10 | 0.10 | ||||
| No | 3 | 6 | 5 | 22 | |
| Yes | 44 | 94 | 18 | 78 | |
| WBC>10 | 0.03 | ||||
| No | 34 | 72 | 22 | 96 | |
| Yes | 13 | 28 | 1 | 4 | |
| PLT>50 | 0.43 | ||||
| No | 33 | 70 | 14 | 61 | |
| Yes | 14 | 30 | 9 | 39 | |
| BM pretherapy CD3+ | 23 | (17.56) | 19 | (32.47) | 0.042 |
| PB pretherapy CD3+ | 22 | (21.83) | 18 | (45.07) | 0.0058 |
Abbreviations: N: number, ORR: overall response rate, HMA: hypomethylating agent, SCT: Allogeneic stem cell transplant, BM: Bone marrow, PB: peripheral blood.
Survival
With a median follow up of 21.4 [95% CI 14.8 – not estimated] months, 57 (81%) of the patients have died. Figure 1 is a swimmers-plot of the 70 patients enrolled. Sixteen patients died on azacitidine and nivolumab therapy: 8-week mortality (n=8), relapsed/refractory AML (n=1), death in CR/CRi/PR/HI from sepsis (n=6), or hemorrhage (n=1). Forty-one patients died after discontinuation of azacitidine with nivolumab: progressive AML (n=8), pneumonia (n=5), post-ASCT complications (n=2), sepsis (n=13), cardiac arrest (n=1), transition to hospice (n=8), and unknown cause of death (n=4).
Figure 1.
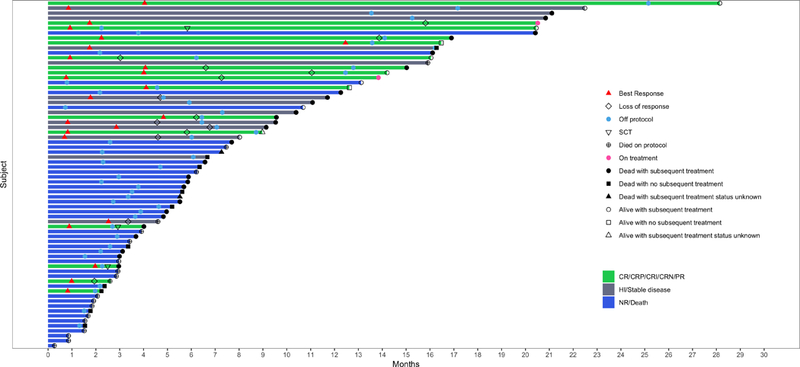
Swimmers plot illustrating the clinical course of study patients (N = 70). The best response, on or off study status, alive or dead status, and allogeneic stem cell status for the 70 patients enrolled on study is shown in this swimmers plot.
The median OS for the 70 patients was 6.3 months (Figure 2A). The median EFS among responders/stable disease (n=29) and DOR among responders (n=23) were 4·5 and 5.2 months, respectively (Figure 2B, 2C). Patients who achieved a CR/CRi/PR/HI/SD (n=29; 42%) had significantly improved OS compared with NRs (n=41; 58%), without censoring for ASCT (16·16 versus 4.1 months; p<0·0001; Figure 2D) and also after censoring for ASCT (p<0·001). OS was not significantly different in patients who achieved CR/CRi/PR versus HI versus SD (17·1 versus 11·9 versus 16·2; p=0·8; Figure 2E). By univariate analysis the factors significantly associated with improved OS were achievement of any response or SD to therapy, salvage 1 status, and the presence of an ASXL1 mutation (Supplemental Table 2, Supplemental Figure 2A, 2B and 2C).
Figure 2.
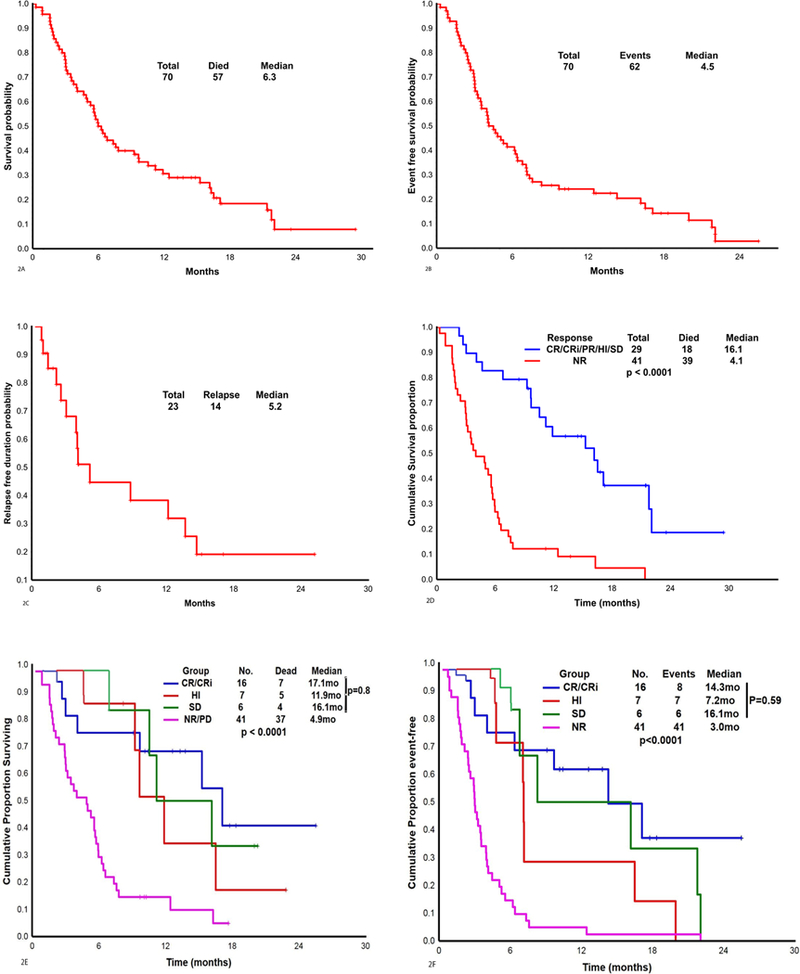
A. Overall survival in the 70 patients treated with azacitidine and nivolumab.
Figure 2B. Event free survival in the 70 patients treated with azacitidine and nivolumab.
Figure 2C. Duration of response among the 23 patients with a response (CR, CRi, PR, HI) on azacitidine with nivolumab.
Figure 2D. Overall survival in patients who had response/stable disease (CR, CRi, PR, HI, SD) versus patients who had no response with azacitidine with nivolumab (N = 70).
Figure 2E. Overall survival by the best response to therapy (N = 70) (P value <0.0001).
Figure 2F. Event free survival by the best response to therapy (N = 70) (P value <0.0001).
Three patients proceeded to ASCT in CR/CRi with matched unrelated (n=1) and umbilical cord donors (n=2): two of the three patients died from post-ASCT infections, after 0·8 and 1·3 months (both in CRi); the third is alive and in remission 6·5 months post-ASCT.
We identified a historical cohort of 172 patients with relapsed/refractory AML treated on HMA-based clinical trials (including single agent HMA and HMA-combinations) at our institution between 2005–2017 (N=172) [list of clinical trials provided in Supplemental Table 3]. The baseline characteristics in the study population of azacitidine with nivolumab (N=70) and the historical HMA-based clinical trial controls (n=172) are shown in Table 1. The historical controls were younger (P=0.004), were less frequently exposed to prior HMA-based therapies (P=<0.0001), and had a lower frequency of post-ASCT relapses (P=0.04). The ORR with azacitidine and nivolumab was 33% versus 20% with historical controls in the entire population, and 52% versus 22% in the prior HMA-naïve population (Table 2). The median OS with azacitidine with nivolumab (n=70) compared favorably to the historical cohort (n=172) both in the “all salvage” population (6·3 versus 4·6 months; n=70 versus 172; p=0·013) (Supplemental Figure 2D), but more prominently in the “first salvage” population (10.6 versus 5.3 months; n=32 versus 91; p=0.011) (Supplemental Figure 2A), with and without censoring for ASCT. Similarly, EFS was longer in patients treated with azacitidine and nivolumab than on historical HMA-based clinical trials in the “all salvage” (4·2 months versus 2.2 months; p<0·0001) and in the “first salvage” population (6.8 months versus 2.7 months, p<0.0001) (Supplemental Figure 3A, 3B).
Immune Profiling of Pretherapy and On Therapy BMAs by MFC and CyTOF
MFC was performed on pre- and post-therapy BMAs, after 2 doses (end of cycle 1 or EOC1) and 4 doses (EOC2) of nivolumab in 19 of 23 responders (CR/CRi/PR/HI) (83%) and 28 of 41 NRs (68%). Responders had a higher frequency of pretherapy BMA CD3+ cells compared with NRs (32.5% versus 17.5%; p=0.04) (Figure 3A) and a higher frequency of pretherapy PB CD3+ cells (45% versus 21.8%, p=0.0058). We also observed a trend towards higher frequency of CD4+Teff cells (15·6% versus 9·0%; p=0·08), and CD8+ T cells (13·1% versus 6·9%, p=0.09) in responders compared with NRs in the pretherapy BMAs. The higher frequency of total CD3+T cells and its subsets persisted in EOC1 and EOC2 BMA in responders (Figure 3A).
Figure 3.
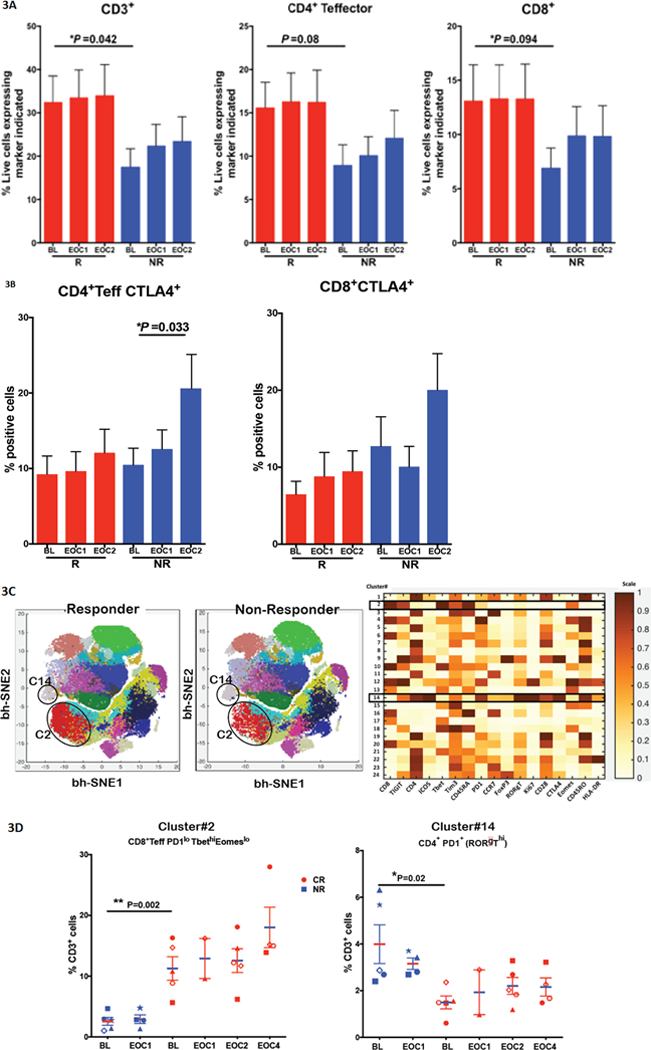
A and B. Bone marrow T-cell profile and checkpoint expression in responders versus non-responders. Bar graphs indicating frequency of CD3+, CD4+Teffector, and CD8+ T cells in total live cells (Fig 5A) and CTLA4+ CD4+Teffectors and CTLA4+CD8+ T cells (Fig 5B) in BMA of responders (CR/CRi/PR/HI) (n=19) and NRs (n=23) at pretherapy, EOC1 and EOC2 as analyzed by flow cytometry.
Figure 3C. Phenograph based clustering approach of T cell subsets by mass cytometry (CyTOF). t-SNE map of 10,000 randomly selected CD3+ cells colored by distinct clusters (1–24) in responders (Fig 5C left), NRs (Fig 5C middle) and heatmap showing normalized expression of different immune markers on CD3+ metaclusters including Cluster 2 (C2) and cluster 14 (C14) (Fig 5C right).
Figure 3D. CD45RA+PD1loTbethiEomeslo (C2) cells were significantly higher in the pretherapy BMAs of patients with CR/CRi (n=5) than NRs (n=5), and with a trend toward expansion in patients with CR/CRi but not in NRs particularly after 8 doses of nivolumab (EOC4), by mass cytometry (Fig 5D left). By contrast, CD4+PD1+(RORgThi) (C14) which was suggestive of Th17-like T-cell population is higher in NR compared to responders (4·0% versus 1·5%; p=0·02). Th17 cells were reported to negatively correlate with prognosis in AML. Each shape/structure in the plot represents an individual patient at baseline and followed over time for this analysis.
A significant increase in the BM CD4+Teffector subset expressing CTLA-4 was noted in the post-therapy samples, after 4 doses of nivolumab (EOC2), as compared to pretherapy samples (EOC2 20.5% versus pretherapy 10.4%; p=0.03) in NRs, while responders did not demonstrate these changes in post-therapy samples as compared to pretherapy samples (Figure 3B). Pretherapy PDL1 on gated AML blasts, CD3+ cells, or combination of AML blasts and CD3+ cells; and pretherapy PD1 on CD3+ cells did not predict for response. A comprehensive list of comparisons by pretherapy biomarkers for responders versus NRs, and by OS 1 versus > 1 year are shown in Supplemental Figure 4 and Supplemental Table 4A.
Optimal cut-offs for predicting responses were identified using the Yeodan index. CD3+ and CD8+ cells in pretherapy BMAs were identified to be the best predictors of response, with optimal cut-offs of 13.2% and 4.01%, respectively. The ORR was 56% in patients with pretherapy BM CD3+ ≥13.2% versus 23% in patients with CD3+ <13.2% (p=0.020). The cut-off CD3+ >13·2% in pretherapy BMA had a sensitivity of 74% and a specificity of 65% (p=0.029), for predicting response. The CD3+ was ≥13.2% in 26 of 47 patients (55%) who had an evaluable pretherapy BMA. Twenty-four of the 26 patients (92%) with pretherapy BM CD3+ >13.2% were Salvage 1 or 2 status, which may explain the higher response rates and improved OS seen in these patients compared with advance salvage patients. This suggests that T-lymphocyte depletion either from progressive AML related BM and PB T-cell depletion or from exposure to repeated rounds of AML directed therapy in advanced salvage patients, may abrogate the ability of these patients to achieve response to such therapies. Patients who had CD8+ >4·01% in pretherapy BMA had a sensitivity of 74% and a specificity of 65% for predicting response. Pretherapy PB CD3+ was also predictive for response with optimal cut-off of 20.5%. The ORR was 65% in patients with PB CD3+ ≥20.5% versus 25% in patients with CD3+ <20.5% (p=0.024). A comprehensive list of cut-offs by responders versus NRs and by OS < 1 versus > 1 year, for pretherapy BM and PB biomarkers, are shown in Supplemental Table 4B and 4C.
We performed 36 parameter CyTOF on pretherapy and post-therapy BMAs after 2 (EOC1), 4 (EOC2) and 8 (EOC4) doses of nivolumab, in 5 patients with CR/CRi and 5 NRs. PhenoGraph clustering of all CD3-gated cells revealed 24 meta-clusters of T cells (Figure 3C), of which 13 were CD4+ cell clusters and 9 were CD8+ clusters. One CD4+ cluster (cluster C14) co-expressed elevated levels of PD-1 and Ki-67 along with RORγT and ICOS (Figure 3C), suggesting a Th17-like T-cell population, with significantly different frequencies in the pretherapy BMAs of responders versus NRs (1·5% versus 4·0%; p=0·02). Previous studies showed that Th17 cells increase in AML, and this negatively correlated with prognosis(19,20). This appeared to be the case in our analysis, as Th17 was higher in non-responders compared to responders (Figure 3D). The frequency of an effector CD8+ T cell cluster (cluster C2) expressing CD45RA+PD1loTbethiEomeslo was significantly higher in the pretherapy BMAs of responders versus NRs (11·2% versus 2·5%; p=0·002), with a further trend for expansion of this population in responders but not in NRs after 8 doses of nivolumab (EOC4) (Figure 3D).
Immune profiling of pretherapy BMs by IHC
We were able to adequately perform IHC on both BM clots and BM biopsies (Supplemental Figure 5A). On BM IHC, the pretherapy CD3+ density was higher in patients who achieved CR/CRi/PR compared with NRs (p=0.036). A similar trend was seen for CD8+ cells (p=0.08) (Supplemental Figure 5B). This difference was lost when HI patients were included in the IHC analysis.
Toxicities
Treatment related non-hematologic toxicities all grades are in Table 4 and all grade toxicities irrespective of attribution are in Supplemental Table 5. Grade 3–4 and grade 2 immune related adverse events (irAEs) were observed in 8 (11%) and 8 (11%) patients, respectively. Of the 16 (23%) patients with grade 2–4 immune toxicities, 9 episodes were pneumonitis, 6 were nephritis, 3 were immune related skin rash, and 2 were transaminitis (some patients had more than 1 irAE). Fourteen of the 16 (88%) toxicities responded to steroids, and these 14 patients were safely rechallenged with nivolumab. In our study, a total of 13% had to discontinue nivolumab (all discontinuations were due to Grade 3/4 irAEs, no discontinuations due to Grade 2 irAEs) and maintained only on azacitidine. irAE related deaths occurred in 2 (3%) patients, both were refractory to steroids and subsequent infliximab therapy: from progressive pneumonia/pneumonitis (E. Coli infection with suspicion for a super-imposed immune pneumonitis) in one patient, and from hemophagocytosis lymphohistiocytosis in another. The time to onset of irAEs ranged from 4 days after the first dose of nivolumab to 3·5 months after the last dose of nivolumab, with the majority (12 of 16; 75%) occurring in the first 8 weeks after nivolumab initiation.
Table 4:
Non-hematologic treatment related toxicities (N = 70).
| Adverse event | Grade | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | Total | |
| Immune system disorders | 1 (1) | 1 (1) | ||||
| Alanine/aspartate transaminase elevation | 2 (3) | 2 (3) | ||||
| Colitis | 1 (1) | 1 (1) | ||||
| Cytokine release syndrome | 1 (1) | 1 (1) | 2 (3) | |||
| Autoimmune disorder | 1 (1) | 1 (1) | ||||
| Enterocolitis | 1 (1) | 1 (1) | ||||
| Erythema multiforme | 1 (1) | 1 (1) | ||||
| Elevated bilirubin | 1 (1) | 1 (1) | ||||
| Myositis | 1 (1) | 1 (1) | ||||
| Rash, acneiform | 1 (1) | 1 (1) | ||||
| Rash, maculo-papular | 1 (1) | 4 (6) | 5 (8) | |||
| Pneumonitis | 8 (11) | 1 (1) | 9 (13) | |||
| Pruritus | 2 (3) | 2 (3) | ||||
| Chest pain - cardiac | 1 (1) | 1 (1) | ||||
| Arthralgia | 1 (1) | 1 (1) | ||||
| Confusion | 1 (1) | 1 (1) | ||||
| Constipation | 15 (21) | 3 (4) | 18 (26) | |||
| Creatinine increased | 2 (3) | 1 (1) | 3 (4) | |||
| Diarrhoea | 14 (20) | 14 (20) | ||||
| Dizziness | 1 (1) | 1 (1) | ||||
| Dry skin | 3 (4) | 3 (4) | ||||
| Dysphagia | 1 (1) | 1 (1) | ||||
| Eye disorders | 1 (1) | 1 (1) | ||||
| Fatigue | 1 (1) | 1 (1) | 2 (3) | |||
| Gastrointestinal disorders | 1 (1) | 1 (1) | ||||
| Generalized muscle weakness | 2 (3) | 2 (3) | ||||
| Insomnia | 1 (1) | 1 (1) | ||||
| Vomiting | 6 (9) | 6 (9) | ||||
| Mucositis oral | 1 (1) | 1 (1) | ||||
| Nausea | 8 (11) | 8 (11) | ||||
| Sinus bradycardia | 4 (6) | 1 (1) | 5 (8) | |||
| Febrile neutropenia | 4 (6) | 4 (6) | ||||
| Lung infection | 5 (7) | 2 (3) | 7 (11) | |||
| Cough | 1 (1) | 1 (1) | 2 (3) | |||
| Skin and subcutaneous tissue disorders | 2 (3) | 1 (1) | 1 (1) | 4 (6) | ||
| Dyspnoea | 2 (3) | 2 (3) | ||||
| Sore throat | 1 (1) | 1 (1) | ||||
| Total AEs | 62 (89) | 29 (41) | 15 (21) | 0 (0) | 1 (1) | 107 |
| Immune related (irAEs) | 2 (3) | 8 (11) | 8 (11) | 0 (0) | 0 (0) | 18 (25) |
| All Infections | 6 (9) | 2 (3) | 6 (9) | 0 (0) | 2 (3) | 15 (23) |
Abbreviations: N, number, G, grade
DISCUSSION
Historical studies evaluating single agent HMA therapy in relapsed/refractory HMA naïve AML have reported ORRs of 10–20% with a CR/CRi rates of 10–16%.(21–23) Similarly, the ORR in a historical cohort of 172 patients with relapsed/refractory AML treated on HMA-based salvage clinical trials at our institution was 20%. The combination of azacitidine and nivolumab yielded an ORR of 33% (CR/CRi rate of 22%) with an additional 6 patients (9%) with meaningful stable disease in the entire study population. Most historical HMA-based studies including studies at our center have excluded patients exposed to any prior HMA based therapies, where as our study did not. A large proportion (64% of patients) enrolled on this study had received prior HMA-based therapies. The ORR in only the HMA naïve patients on our study was 52%. In our historical controls the ORR among the HMA-naïve patients was 19%. In a recent large multicenter analysis with HMA-based therapies in salvage (n=655) that included only prior HMA-naïve patients, Stahl et al noted an ORR of 25%. (23)
The median OS of 10.6 months in the salvage 1 patients treated with azacitidine and nivolumab was significantly better than the median OS of 5.2 months in the historical control Salvage 1 patients, treated on other HMA-based clinical trials at our institution between 2005–2017. This was noteworthy, especially considering that the patients in the historical cohort were younger, more likely to be prior HMA naïve, and less likely to have relapsed post-ASCT. Stahl et al noted that salvage 1 patients had a median OS of 6.7 months and 1- and 2-year survivals of 25% and 15%, respectively.(23) All of these patients were HMA naïve. In salvage 1 patients treated with azacitidine with nivolumab (including 47% who had received prior HMA-based therapies), 1- and 2-year survival rates were 50% and 25%, respectively. Over the last decade a number of HMA-based combinations have been evaluated. One of the most exciting combinations that has emerged is the combination of HMA with venetoclax, demonstrating CR/CRi rates >70% in frontline elderly AML patients.(24) However, in the salvage setting, the HMA with venetoclax combination had ORR of 25–30% and median OS of <5·0 months in two separate analyses.(25,26) Randomized studies are needed to make definitive conclusions, but, thus far, the response rates and OS, with azacitidine and nivolumab regimen appear encouraging, especially in previously HMA-naïve patients and in salvage 1 AML patients, respectively. Of note, among prior HMA-exposed patients the ORR was lower at 22%, but responses were achieved with azacitidine and nivolumab.
Higher response rates were observed among patients who were HMA naïve, had lower leukemia burden (<20% BM blasts), had an ASXL1 mutation, and higher pretherapy BMA CD3+ infiltrate. In multivariate analysis, no prior HMA, increased pretherapy BM CD3, and the presence of ASXL1 mutation had a trend to improved ORR. Patients who were Salvage 1, had ASXL1 mutations, or achieved any response or SD had improved OS. Patients with AML in advanced salvage have depleted BM CD3+, CD8+, and CD4+Teff populations (5) and this may be one reason they are less likely to benefit from T cell dependent therapies. This was noted in our analysis wherein patients with higher pretherapy BM CD3+ cells were more likely to respond, and such patients were more likely to be in the salvage 1 and 2 setting. Lower leukemic burden and early salvage status have similarly been shown to be associated with improved response rates with other T-cell harnessing therapies such as blinatumomab (27,28) and chimeric antigen receptor (CAR) therapies in patients with acute lymphoblastic leukemia (29). These data suggest that in both AML and ALL the T cell based therapies may be most effective when introduced early in the course of the disease, and possibly in a lower disease burden setting. Whether T-cell functionality is better preserved in patients who have inherently low burden disease or whether the disease burden is lower in these patients as they have more functional T-cells infiltrating the tumor environment requires further investigation. A recent report based on gene expression profiling of patients with wild-type or mutated ASXL1 suggested an upregulation of the immune response pathway in patients harboring the ASXL1 mutation (30). It is plausible that the immunogenicity of ASXL1 may have been a driver of better responses and OS seen in patients harboring this mutation in our study but this observation is based on small numbers and needs validation in a larger set.
Six patients did not achieve an IWG measurable response but had SD with median OS 16.1 months. The conventional Response Evaluation Criteria in Solid Tumors (RECIST) criteria, underestimated the benefit of ICPIs, requiring the development of specific immune response criteria for patients with solid tumors on immunotherapy trials. (31) Similarly, the achievement of SD with or without hematologic improvement with ICPI based approaches should be independently assessed and collected in ongoing and future ICPI trials in AML and MDS.
The non-immune toxicities with this combination were similar to other HMA-based salvage therapies.(23) Immune mediated grade 3/4 toxicities were observed in 11% of the patients. Solid tumor and lymphoma studies of single agent PD-1 inhibitors have demonstrated similar grade 3/4 irAE rates with ICPIs.(2,32) The irAEs frequently occurred within 8 week after ICPI initiation, similar to solid tumors.(32) All patients were treated with steroids,(32) and most (14 of 16, 88%) responded and could be rechallenged with nivolumab. The grade 2 irAEs in most cases did not result in hospitalization or treatment discontinuation and responded rapidly to steroid therapy.
Patients who achieved a response with azacitidine and nivolumab had higher CD3+, CD4+Teff, and CD8+ T cells in the pretherapy tumor environment (BMA in this case) compared with NRs. These are well-established biomarkers of response to ICPIs in other tumor types.(33,34) CD3+ cells in the pretherapy BMAs with a cut-off of 13·2%, had a sensitivity of 74% and a specificity of 65% for predicting response. In our study, a sizeable proportion (55%) of all evaluable patients (especially salvage 1 and 2) had a pretherapy CD3+> 13.2%. Similar PB CD3 was also predictive for response with optimal cut-off of 20.5%. These are relatively simple biomarkers and if validated in ongoing/future trials, may be important for selecting patients for future trials. In addition, the frequency of CTLA-4 expressing CD4+Teffector and CD8+ T cell populations increased on therapy in the BMAs of NRs but not in responders, highlighting CTLA4 up-regulation as a potential mechanism of resistance to PD-1 blockade in the NRs as has been in most solid tumors treated with ICPI therapies. Concomitant or sequential blockade of the inhibitory signals mediated by CTLA-4 may further enhance T cell responses. Furthermore, there may be a differential efficacy profile for PD-1 versus CTLA-4 inhibition in myeloid malignancies.(8,35) Studies evaluating concomitant PD-1 and CTLA-4 inhibition in patients with relapsed AML with or without azacitidine and as a maintenance post-ASCT in high-risk AML are ongoing (NCT02397720, NCT03600155).
In conclusion, azacitidine with nivolumab produced an encouraging response rate and OS, especially in HMA-naïve and Salvage 1 patients, respectively. Immune toxicities should be recognized and treated promptly. A randomized phase III and a randomized phase II study of azacitidine with or without PD-1 inhibitor in frontline elderly AML (NCT03092674, NCT02775903) and a randomized trial of PD-1 inhibitor for eradication of MRD in high-risk AML in remission (NCT02275533) have been initiated. Clinical and immune biomarker enriched trials are likely to yield further improved outcomes with HMA+ICPI therapies in AML and are strongly encouraged.
PATIENTS AND METHODS
Patient Eligibility
Patient’s ≥18 years of age who had failed prior therapy for AML (including prior therapy with HMAs) were eligible. Patients were required to have an Eastern Cooperative Oncology Group performance status ≤ 2; serum creatinine ≤ 2 × upper limit of normal range (ULN); serum bilirubin ≤ 2 × ULN or ≤ 3 × ULN if the bilirubin elevation was deemed related to leukemic involvement or Gilbert’s syndrome; serum transaminase ≤ 2.5 times the ULN or ≤ 5 times ULN if the transaminase elevation was deemed related to leukemic infiltration. Exclusion criteria included a known history of a systemic autoimmune condition, severe interstitial lung disease or active pneumonitis; prior solid organ allograft; symptomatic CNS leukemia; and any other uncontrolled disease. Patients with grade 1 or no graft versus host disease (GVHD), requiring ≤ 10 mg of prednisone without additional immunosuppressive therapies, who had allogeneic stem cell transplant (ASCT) >3 months prior to study entry were eligible. All patients signed an informed consent form approved by the Institutional Review Board (IRB). The study was conducted in accordance with the Declaration of Helsinki (ClinicalTrials.gov identifier: NCT02397720) (Full Protocol attached as Supplemental).
Study Design and Objectives
This was a single center; open-label, non-randomized phase II study. The study recruited patients between January 2015 and June 2017. The data cut-off was March 1, 2018. Primary study endpoints were safety and overall response rate (ORR) [ORR = CR, CR with incomplete recovery of peripheral counts (CRi), partial remission (PR), morphologic leukemia-free state (MLFS)(13), durable hematologic improvement (HI) (defined as improvement in one or more parameter of hemoglobin, platelets, neutrophils maintained ≥6 months)(14), captured as the best response achieved on study. Patients who achieved any of these responses were considered responders. Stable disease (SD) was defined as the absence of CR, CRi, PR, MLFS, HI, after exposure to treatment for a duration considered sufficiently suitable to achieve a response to therapy (≥6 months), but with no evidence of progressive BM disease (defined as more than 50% increase in BM blast or ≥15% in blasts when blast at baseline <30%), no increase in transfusion requirements and/or hospital admissions, the absence of new or progressive extramedullary or CNS disease, and no clinical deterioration in terms of functional status, weight/appetite, level of energy or limiting side effects. Patients who did not achieve CR, CRi, PR, HI, SD were considered non-responders (NRs). Secondary endpoints included overall survival (OS), event free survival (EFS), and the duration of response (DOR).
Treatment Regimen
Therapy consisted of azacitidine 75 mg/m2 Day 1–7 administered intravenously (IV) over 60–90 minutes or subcutaneously, and nivolumab 3 mg/kg administered as a 60–90 minutes IV infusion on Days 1 and 14 of each cycle. One cycle was 28 days. The first 6 patients were treated with nivolumab 3mg/kg and evaluated for dose-limiting toxicities (DLTs) for 28 days. The 3mg/kg dose of nivolumab was found to be safe and was established as the recommended phase 2 dose (RP2D) in combination with azacitidine (Supplemental Table 6).
Cycles were repeated every 4–6 weeks, depending on count recovery. Required BMAs were done at the end of cycles 1, 2, 4, 7, and 11. Dose reductions or interruptions of azacitidine and/or dose interruptions of nivolumab were allowed as specified in the protocol (Supplemental Table 7). Patients continue on therapy as long as they have evidence of clinical benefit.
Baseline Assessments
Pretreatment evaluations included a complete history and physical examination, complete blood count with differential, comprehensive chemistry panel, pregnancy test, thyroid hormones and cortisol, and bone marrow aspiration (BMA) for multiparametric flow-cytometry (MFC), karyotype, a 28-gene next generation sequencing (NGS), and immune profiling. MFC for minimal residual disease (MRD) was performed as previously described.(15) The NGS-based analysis for the detection of somatic mutations in the coding sequences of 28 genes was performed on DNA extracted from BMA (Supplemental Table 8), as previously described.(16)
Immunophenotyping of Lymphocytes and Blasts from BMAs and PB
We performed 17-color flow cytometry on pretherapy and post-therapy BMAs and peripheral blood mononuclear cells (PBMCs) obtained at protocol specific time-points, to evaluate the expression of inhibitory (PD1, CTLA4, LAG3, TIM3) and activating checkpoint receptors (GITR, OX40, 41BB, ICOS), on the following T-cell subsets: effector CD4 T cells (Teff) defined as CD3+CD4+CD127lo/+Foxp3-; regulatory CD4 T cells (Treg) defined as CD3+CD4+CD127-Foxp3+; and CD8 T cells (Supplemental Table 9A and Supplemental Figure 6A). AML blasts were assessed for ligands 41BBL, B7–1, B7–2, ICOSL, PD-L1, PD-L2 and OX40L (Supplemental Figure 6B). These analyses were performed on BMAs within 12 hours of collection by the UT/MDACC Department of Immunology, using flow-cytometry panels as previously published.(17)
Thirty-six parameter mass cytometry (CyTOF)(Supplemental Table 9B) was performed on pretherapy and post-therapy BMAs in a subset of the responders and NRs with available samples. Details of the technique and the time-points of BMA and PB collection are in Supplemental Methods and Supplemental Table 9C.
Immunohistochemistry Staining and Analysis
Immunohistochemistry (IHC) was performed on FFPE tissue sections. Density (absolute numbers of positive cells / mm2 area analyzed) calculation was done using Imagescope software (Aperio/Leica Technologies). Details of BM staining are in Supplemental Methods.
The MFC based immunophenotyping, mass cytometry, and IHC were performed as a part of correlative research on this study, and willing patients gave a separate IRB approved informed consent for these analysis.
Toxicity Assessment
Patients were monitored continuously for toxicity. Toxicity was defined as any clinically significant CTCAE version 4.03 grade 3 or 4 non-hematological toxic effects or death attributable to the study drug. Per predefined rules, we would stop the trial if there were more than 95% chance that the toxicity rate would be greater than 30%.
Statistical Methods
Futility and toxicity was monitored simultaneously using the Bayesian approach of Thall and Sung.(18) For futility monitoring, we would stop the trial if there was a less than 1% chance that the ORR of azacitidine with nivolumab was greater than the ORR of standard treatment by 15%. OS was calculated from start of therapy to death from any cause, and was censored at last follow up. DOR was calculated from time of documented response to loss of response or censored at last follow up or death if response was maintained on the drug combination. EFS was calculated from start of treatment to disease progression/death or censored at last follow up while on the drug.
Differences among groups were evaluated by the chi-square test (or Fisher’s exact test for cell frequencies <5) for categorical variables and t-test or Wilcoxon-Mann-Whitney test for continuous variables. Paired t-tests were applied to detect the changes in immune markers between pretherapy and on-therapy. Univariate logistic regression models were fitted to evaluate the relationships between the immune markers and clinical responses, and optimal cut-offs of markers for predicting responses were identified by maximizing the Youden Index. Survival distributions were estimated using the Kaplan–Meier method and compared using the log-rank test. All p-values were two-sided and p < 0.05 was considered statistically significant. Statistical analyses were carried out using Stata/SE version 13.1 statistical software (Stata Corp. LP, College Station, TX) and IBM SPSS Statistics 21 for Windows (SPSS Inc., Chicago, Illinois).
Supplementary Material
Statement of Significance.
Azacitidine in combination with nivolumab appeared to be a safe and effective therapy in patients with acute myeloid leukemia who were Salvage 1, prior hypomethylator naive, or had increased pretherapy CD3+ bone marrow infiltrate by flow-cytometry or immunohistochemistry. Bone marrow CD3 and CD8 are relatively simple assays that should be incorporated to select patients in future trials.
ACKNOWLEDGEMENTS
This work was supported by Bristol-Myers Squibb (BMS), the MD Anderson Cancer Centre Leukemia Support Grant CA016672, the MD Anderson Cancer Center Leukemia SPORE CA100632, the Dick Clark Immunotherapy Research Fund, and the MD Anderson Moon Shots Program.
Funding: Supported by Bristol-Myers Squibb (BMS), the MD Anderson Cancer Centre Leukemia Support Grant CA016672, the MD Anderson Cancer Center Leukemia SPORE CA100632, the Dick Clark Immunotherapy Research Fund, and the MD Anderson Moon Shots Program.
Footnotes
Conflicts of Interest Disclosure: ND, GGM, JC, FR, EJ, TK, JA, PS, and HK have received research funding from BMS. ND, GGM, JC, EJ, TK, JA, PS, and HK have served as consultants and/or received honoraria from BMS.
Prior presentations: Oral presentation ASH 2016, Oral presentation EHA 2017
REFERENCES
- 1.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372(4):311–9 doi 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008;14(10):3044–51 doi 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 3.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014;15(1):69–77 doi 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rifca Le Dieu DCT, Alan G. Ramsay, Richard Mitter, Faridah Miraki-Moud, Rewas Fatah, Lee Abigail M, Andrew Lister T, Gribben John G. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. . Blood 2009;114(18): p. 3909–16(18):3909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams P BS G-MG, Cortes JE, Ravandi F, Al-Hamal Zainab, et al. Checkpoint Expression By Acute Myeloid Leukemia (AML) and the Immune Microenvironment Suppresses Adaptive Immunity. American Society of Hematology, December 2017. (abstract) 2017. [Google Scholar]
- 6.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 2010;116(14):2484–93 doi 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009;114(8):1545–52 doi 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillermo Garcia-Manero NGD, Guillermo Montalban-Bravo, Elias J. Jabbour, Courtney D. DiNardo, Steven M. Kornblau, et al. A Phase II Study Evaluating the Combination of Nivolumab or Ipilimumab with Azacitidine in Patients with Previously Treated or Untreated Myelodysplastic Syndromes. American Society of Hematology, December 2016. (oral presentation). [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28(4):562–9 doi JCO.2009.23.8329 [pii] 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 10.Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia 2018;32(5):1094–105 doi 10.1038/s41375-018-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget 2013;4(11):2067–79 doi 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014;28(6):1280–8 doi 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003;21(24):4642–9 doi 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006;108(2):419–25 doi 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 15.Ravandi F, Jorgensen J, Borthakur G, Jabbour E, Kadia T, Pierce S, et al. Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer 2016. doi 10.1002/cncr.30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luthra R, Patel KP, Reddy NG, Haghshenas V, Routbort MJ, Harmon MA, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica 2014;99(3):465–73 doi 10.3324/haematol.2013.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23(6):1388–96 doi 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 1998;17(14):1563–80. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y, et al. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol 2009;158(2):199–204 doi 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and interleukin-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer Sci 2014;105(8):933–42 doi 10.1111/cas.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tawfik B, Sliesoraitis S, Lyerly S, Klepin HD, Lawrence J, Isom S, et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML). Annals of hematology 2014;93(1):47–55 doi 10.1007/s00277-013-1940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George TJ, Woolery JE, Wetzstein GA, Ho VQ, Lancet JE, List AF, et al. A Retrospective Study of Decitabine for the Treatment of Relapsed or Refractory Acute Myeloid Leukemia: Lack of Response Observed In a Heavily Pretreated Population. Blood 2010;116(21):2186-. [Google Scholar]
- 23.Stahl M, DeVeaux M, Montesinos P, Itzykson R, Ritchie EK, Sekeres MA, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv 2018;2(8):923–32 doi 10.1182/bloodadvances.2018016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018;19(2):216–28 doi 10.1016/S1470-2045(18)30010-X. [DOI] [PubMed] [Google Scholar]
- 25.DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 2018;93(3):401–7 doi 10.1002/ajh.25000. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg AD HT, Hsu M, Devlin SM, Cuello BM, Daley RJ. Venetoclax Combined with Either a Hypomethylating Agent or Low-Dose Cytarabine Shows Activity in Relapsed and Refractory Myeloid Malignancies. American Society of Hematology, December 2017. (abstract) 2017. [Google Scholar]
- 27.Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 2016;375(8):740–53 doi 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16(1):57–66 doi 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018;378(5):449–59 doi 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thol F, Friesen I, Damm F, Yun H, Weissinger EM, Krauter J, et al. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J Clin Oncol 2011;29(18):2499–506 doi 10.1200/JCO.2010.33.4938. [DOI] [PubMed] [Google Scholar]
- 31.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15(23):7412–20 doi 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 32.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378(2):158–68 doi 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 33.Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8+ T-cell Density-A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21(11):2635–43 doi 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 34.Badros A, Hyjek E, Ma N, Lesokhin A, Dogan A, Rapoport AP, et al. Pembrolizumab, pomalidomide and low dose dexamethasone for relapsed/refractory multiple myeloma. Blood 2017. doi 10.1182/blood-2017-03-775122. [DOI] [PubMed] [Google Scholar]
- 35.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med 2016;375(2):143–53 doi 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


