Abstract
Purpose
Cellular therapy is an emerging cancer treatment modality, but its application to epithelial cancers has been limited. This clinical trial evaluated tumor-infiltrating lymphocyte (TIL) therapy for the treatment of patients with metastatic human papillomavirus (HPV)-associated carcinomas.
Experimental Design
The trial was a phase II design with two cohorts, cervical cancers and non-cervical cancers. Cell infusion was preceded by a lymphocyte-depleting conditioning regimen and followed by systemic high-dose aldesleukin.
Results
Objective tumor responses occurred in 5/18 (28%) patients in the cervical cancer cohort and 2/11 (18%) patients in the non-cervical cancer cohort. Two of the responses in cervical cancer were complete and are ongoing 67 and 53 months after treatment. Responses in the non-cervical cancer cohort were in anal cancer and oropharyngeal cancer. The HPV reactivity of the infused T cells correlated with clinical response. Peripheral blood repopulation with HPV-reactive T cells also correlated with clinical response.
Conclusions/Discussion
These findings support the concept that cellular therapy can mediate the regression of epithelial cancers, and they suggest the importance of predictive biomarkers and novel treatment platforms for more effective therapies.
Keywords: cellular therapy, immunotherapy, cancer, human papillomavirus, T cell
INTRODUCTION
Human papillomavirus (HPV)-associated cancers are common epithelial malignancies that account for approximately 5% of all cancers worldwide.1 They occur at varied anatomical sites including the uterine cervix, anus, vagina, vulva, penis, and oropharynx.2–5 Although it is hoped that this family of cancers will be eliminated by preventive HPV vaccines in the future, they currently cause more than 300,000 deaths each year globally and an estimated 12,500 deaths each year in the United States.6,7 Advanced-stage HPV-associated cancers are difficult to treat. Combination chemotherapy plus bevacizumab offers some clinical benefit,8 and anti-programmed death 1 receptor (PD-1) therapy has shown clinical activity,9–12 but these malignancies generally are incurable and better treatments are needed.
Adoptive T-cell therapy (ACT), the systemic infusion of therapeutic T cells, is an emerging cancer treatment modality that can induce complete tumor responses in some patients with B cell malignancies or metastatic melanoma13. We sought to test if ACT could mediate the regression of HPV-associated epithelial cancers. We established a method to generate independent tumor-infiltrating lymphocyte (TIL) cultures from fragments of a resected metastatic tumor deposit.14 Because HPV-associated cancers constitutively express the HPV E6 and E7 oncoproteins, immunologically foreign viral proteins that are attractive targets for immunotherapy,3 cultures with HPV-oncoprotein reactivity were selected preferentially for administration to patients. Here we present a completed clinical trial with long-term follow up, 18 patients with metastatic cervical cancer (including 9 patients reported previously),14 and 11 patients with other cancers. Additionally, we also report a predictive biomarker and immunological correlates for the clinical trial.
MATERIALS AND METHODS
Study design
The trial was a single-center, phase II study (ClinicalTrials.gov NCT01585428) that was designed to determine if HPV-TILs could mediate regression of advanced HPV-associated cancers. Patients were treated in two disease cohorts (cervical cancers and non-cervical cancers) between April 24, 2012 and August 1, 2016. The protocol was approved by the National Cancer Institute Institutional Review Board at the National Institutes of Health Clinical Center, and informed consent was obtained from all patients. Patients were treated with a nonmyeloablative chemotherapy conditioning regimen (cyclophosphamide 60 mg/kg IV daily for two days and fludarabine 25 mg/m2 daily for five days), followed by a single IV infusion of HPV-TILs. After cell infusion, aldesleukin was administered as an IV bolus at 720,000 IU/kg/dose every eight hours to tolerance or a maximum of 15 doses. Tumor responses were determined using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. The primary objective of the study was to determine the objective tumor response rate and duration in patients with metastatic HPV-associated cancers. The secondary objectives were to determine the toxicity of this treatment regimen and to study immunologic correlates associated with this therapy.
Patients
Patients ≥ 18 to 70 years of age with a pathologically confirmed diagnosis of metastatic HPV-associated cancer were eligible for screening and metastectomy for generation of TILs. All patients had received prior platinum-based chemotherapy or chemoradiotherapy. Patients with three or fewer brain metastases that were less than 1 cm in diameter and asymptomatic were permitted to participate. An Eastern Cooperative Oncology Group performance status of 0 or 1 was required.
Generation of HPV-TIL cell products
The HPV type of metastatic tumors was determined by real-time PCR with HPV-16 and HPV-18 type-specific primer-probe sets. TIL cultures were initiated from tumor fragments and expanded using interleukin-2 (IL-2)-containing culture media as previously described.14 Briefly, cultures with lymphocyte outgrowth were tested for reactivity against HPV-16 or HPV-18 E6 and E7, when applicable. Flow cytometric analysis of each culture was performed using antibodies specific for CD3, CD4, CD8, and CD56 (BD Biosciences, San Jose, CA). TIL cultures were selected for further expansion and administration to the patient based on HPV-oncoprotein reactivity, rapid growth rate, high T-cell frequency, and high CD8+ T-cell frequency. If HPV reactivity was absent or could not be assessed, the other criteria were used. After treatment of 22 patients (14 cervical cancer and 8 non-cervical cancer), the protocol was amended to only treat patients whose tumors were HPV-16+ or HPV-18+, and who also had three or more TIL cultures with HPV-oncoprotein reactivity (as measured by interferon-gamma (IFN-γ) release).
Immunological assays
Immunological assays were performed as previously described.14 Briefly, T cells were cocultured with autologous immature dendritic cells loaded with 1 µM peptide pools (15-mer peptides overlapping by 11 amino acids) spanning E6, E7 or glycoprotein 100 (gp100, negative control) (Miltenyi Biotec, Bergisch Gladbach, Germany). For peripheral blood T cell assays, CD3+ T cells were isolated using pan T cell isolation kit (Miltenyi Biotec) prior to coculture. Dendritic cells were generated from the adherent fraction of peripheral blood mononuclear cells (PBMC) or from CD14+ cells isolated from PBMC with CD14 magnetic beads (Miltenyi Biotec). For IFN-γ production assays, the concentration of IFN-γ in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Bio-Techne Corp, Minneapolis, MN or Thermo Fisher Scientific, Waltham, MA). IFN-γ enzyme-linked immunospot (ELISPOT) (Mabtech, Cincinnati, OH) assays were performed according to the manufacturer’s instructions. CD137 upregulation assays were performed by flow cytometric analysis. After overnight coculture, cells were stained with fluorescent antibodies against CD137, CD3, CD4, and CD8 (BD Biosciences and Biolegend) and counterstained with propidium iodide or 4',6-diamidino-2-phenylindole (BD Biosciences). Data were acquired with a FACSCanto II flow cytometer (BD Biosciences) or Novocyte (Acea BioSciences Inc, San Diego, CA) and analyzed with FlowJo software (Treestar Inc, Ashland, OR).
Statistical Considerations
For each of the two cohorts, the trial used a two-stage optimal design with alpha=0.05 (5% probability of accepting a poor therapy) and beta=0.10 (10% probability of rejecting a good therapy). Initial enrollment was planned at 18 patients per cohort. Expansion to 35 patients was planned if three of more of the first 18 patients experienced responses of longer than four months duration (this measure was intended to consider both response rate and durability). If fewer than 6 of 35 patients in a cohort had a clinical response, then the treatment was to be considered inadequate for further investigation. Under the null hypothesis (10% response rate), the probability of early termination was 73%. Accrual to the non-cervical cohort was terminated due to limitations in cell manufacturing.
The Mann-Whitney U test was used to test for correlations between HPV reactivity and clinical responses (Graphpad Prism 7, La Jolla, CA). Reported P values are two-tailed and not adjusted for multiple comparisons, and P < .05 were considered statistically significant.
Additional details on the Patients and Methods are presented in the data supplement.
RESULTS
Patient Characteristics
Twenty-nine patients with metastatic HPV-associated cancer were treated (Table 1 and Appendix Fig A1, online only). Eighteen patients were diagnosed with cervical cancer. Eleven patients were diagnosed with other HPV+ cancers (oropharyngeal cancer (n=5), anal cancer (n=5), and vaginal cancer (n=1)). The median cell dose was 89 × 109, range 9 to 152 × 109. The median number of systemic IL-2 doses was 4, range 0 to 9. Infusion products consisted of a median of 53% CD4+, range 6 to 97%; and 41% CD8+, range 2 to 94% T cells. The median age of patients was 50, range 30 to 63 years. Tumors were squamous cell carcinoma (n=21), adenocarcinoma (n=5), adenosquamous carcinoma (n=2), or neuroendocrine (n=1). Cervical cancers were associated with HPV type 18 (n=11), type 16 (n=5), or other types (n=2). Non-cervical cancers were associated with HPV-16 (n=11). Twenty-five patients had previously received combination chemotherapy regimens. Two patients had also previously received immune checkpoint blockade directed against cytotoxic T-lymphocyte-associated protein 4 or PD-1.
Table 1.
Characteristics of Patients and Administered T cells
| Within CD3+ (%) |
Response |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) / Gender | Histology | HPV Type | Sites of Disease | Prior Systemic Treatment | Cells (x 109) | CD4+ | CD8+ | No. of IL-2 Doses | Type | Duration or TTP (months) |
| Cervical cancer | |||||||||||
| 1 | 30/F | ASC | 18 | Iliac lymph nodes, lung, lung hilum, retroperitoneum, vaginal cuff | Cisplatin | 101.4 | 29 | 72 | 7 | PD | 1 |
| 2 | 53/F | SCC | 18 | Bone, liver, lung, lung hilum, mediastinum, pelvis | Cisplatin, carboplatin, paclitaxel, topotecan, ixabepilone, dimethane sulfonate | 126.0 | 10 | 90 | 3 | PR | 3 |
| 3 | 36/F | SCC | 16 | Iliac lymph nodes, lung hilum, mediastinum, retroperitoneum | Cisplatin, vincristine, bleomycin, gemcitabine, paclitaxel, topotecan | 152.0 | 21 | 83 | 2 | CR | 67+ |
| 4 | 55/F | SCC | 16 | Axilla, breast, liver, omentum, pleura, soft tissue | Cisplatin, carboplatin, paclitaxel, fluorouracil, irinotecan, dovitnib, pemetrexed | 80.1 | 23 | 76 | 7 | PD | 2 |
| 5 | 44/F | SCC | 18 | Brain, mediastinum, supraclavicular nodes | Cisplatin | 90.0 | 66 | 29 | 5 | PD | 2 |
| 6 | 36/F | AC | 18 | Abdominal wall, liver surface, paracolic, pelvis, retroperitoneum | Cisplatin | 74.7 | 14 | 86 | 8 | CR | 53+ |
| 7 | 59/F | AC | 18 | Abdominal wall, lung | Cisplatin, paclitaxel, carboplatin, bevacizumab | 33.4 | 36 | 58 | 8 | PD | 1 |
| 8 | 31/F | ASC | 18 | Pelvis, perihepatic mass | Cisplatin, paclitaxel | 46.1 | 64 | 29 | 9 | PD | 2 |
| 9 | 37/F | AC | 18 | Axilla, bone, lung, mediastinum, pelvis, retroperitoneum | Cisplatin, carboplatin, paclitaxel, ipilimumab | 70.2 | 33 | 59 | 6 | PD | 1 |
| 10 | 39/F | SCC | not 16/18 | Adrenal, retroperitoneum | Cisplatin, paclitaxel, bevacizumab | 100.0 | 8 | 92 | 5 | PD | 2 |
| 11 | 31/F | SCC | 16 | Cervix, iliac lymph nodes, retroperitoneum | Carboplatin, paclitaxel, bevacizumab | 77.0 | 57 | 41 | 1 | PD | 2 |
| 12 | 48/F | SCC | 16 | Bone, inguinal lymph nodes, lung, mediastinum, retroperitoneum | Cisplatin, paclitaxel, bevacizumab, listeria-based vaccine trial | 70.6 | 93 | 4 | 0 | PR | 3 |
| 13 | 30/F | SCC | 18 | Cervix, inguinal lymph nodes, lung, mediastinum | Cisplatin, brachytherapy | 101.3 | 67 | 27 | 3 | PD | 3 |
| 14 | 49/F | SCC | not 16/18 | Gastro-esophageal junction, mediastinum, retroperitoneum | Cisplatin, carboplatin, paclitaxel, topotecan, bevacizumab | 68.8 | 53 | 41 | 2 | PD | 2 |
| 15 | 61/F | AC | 16 | Bone, inguinal lymph nodes, lung | Carboplatin, docetaxel, cisplatin, topotecan, ifosfamide, adriamycin, etoposide | 73.9 | 84 | 16 | 1 | PD | 3 |
| 16 | 51/F | SCC | 18 | Liver, pelvic, peripancreatic, spleen | Cisplatin, gemcitabine, carboplatin, paclitaxel, bevacizumab | 115 | 71 | 29 | 0 | PD | 2 |
| 17 | 63/F | SCC | 18 | Lung, lung hilum | Cisplatin, carboplatin, paclitaxel, bevacizumab | 112.0 | 78 | 22 | 4 | PD | 5 |
| 18 | 35/F | NE | 18 | Lung, lung hilum, liver | Cisplatin, etoposide, topotecan, paclitaxel, bevacizumab | 9.0 | 67 | 34 | 3 | PR | 3 |
| Non-cervical cancer | |||||||||||
| 19 | 55/M | Tonsillar SCC | 16 | Axilla, lung, subcutaneous tissue | Cisplatin, fluorouracil, taxotere, carboplatin, cetuximab | 89.1 | 96 | 2 | 1 | PD | 2 |
| 20 | 60/M | Head/Neck SCC | 16 | Axilla, bone, liver, peripancreatic, periportal lymph node, pleura | Cisplatin, capecitabine, carboplatin | 150.0 | 29 | 64 | 6 | PD | 2 |
| 21 | 58/F | Anal SCC | 16 | Lung, mediastinum, pleura | Cisplatin, fluorouracil, carboplatin, paclitaxel, cetuximab, irinotecan | 31.5 | 47 | 50 | 2 | PD | 3 |
| 22 | 50/F | Anal SCC | 16 | Mediastinum, retroperitoneum | Cisplatin, fluorouracil, mitomycin C, paclitaxel, carboplatin | 69.0 | 75 | 16 | 5 | PD | 8 |
| 23 | 58/F | Anal SCC | 16 | Iliac lymph nodes, liver, retroperitoneum | Cisplatin, fluorouracil, mitomycin C | 47.5 | 86 | 9 | 1 | PD | 2 |
| 24 | 60/M | Tonsillar SCC | 16 | Lung, mediastinum | Cisplatin, docetaxel, bevacizumab, cetuximab, fluorouracil, gemcitabine | 130.9 | 41 | 51 | 3 | PR | 5 |
| 25 | 49/F | Anal SCC | 16 | Pleura | Cisplatin, fluorouracil, rigosertib, capecitabine | 133.0 | 67 | 20 | 5 | PD | 2 |
| 26 | 48/F | Anal SCC | 16 | Lung | Cisplatin, fluorouracil, mitomycin C, capecitabine | 18.4 | 47 | 50 | 5 | PR | 4 |
| 27 | 52/M | Head/Neck SCC | 16 | Axilla, chest wall, lung hilum, mediastinum, subcutaneous tissue | Cisplatin, fluorouracil, taxotere | 125.0 | 97 | 3 | 5 | PD | 2 |
| 28 | 60/M | Head/Neck SCC | 16 | Lung, lung hilum, mediastinum, para-aortic lymph node, pleura | Cisplatin, carboplatin, fluorouracil, cetuximab, pembrolizumab | 102.0 | 6 | 94 | 4 | PD | 3 |
| 29 | 56/F | Vaginal AC | 16 | Lung, lung hilum, scapula, para-spinal | Cisplatin, paclitaxel, carboplatin, pemetrexed | 107.0 | 46 | 55 | 5 | PD | 4 |
Abbreviations: AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; CR, complete response; F, female; M, male; NE, neuroendocrine; PD, progressive disease; PR, partial response; SCC, squamous cell carcinoma; TTP, time to progression.
Clinical Responses
Seven of 29 patients attained objective tumor responses, 5 of 18 (28%) patients with cervical cancer and 2 of 11 (18%) patients with other HPV+ cancers (Table 1 and Fig 1A). In the cervical cancer cohort, two responses were complete (CR) and are ongoing 67 (patient 3) and 53 (patient 6) months after treatment (Fig 1B); three responses were partial (PR) and of 3 months duration (patients 2, 12 and 18) (Table 1, and Figs 1A and1B). In the non-cervical cancer cohort, one patient with oropharyngeal cancer (patient 24) attained a PR of 5 months duration, and one patient with anal cancer (patient 26) attained a PR of 4 months duration (Table 1, and Figs 1A and 1B). The patient with oropharyngeal cancer was a 60-year old male who had previously received five systemic anti-cancer agents (Table 1). At the time of treatment, he had multiple thoracic metastases, that were progressing prior to TIL infusion (Fig 1C). Five months after treatment, he developed a new brain metastasis, which was resected (Table 1, and Fig 1B). However, he experienced complete regression of all other disease sites (Figs 1B and 1C), and is without evidence of disease 51 months after treatment (Figs 1B and 1C). The patient with anal cancer was a 48-year old female who had previously received two combination chemotherapy regimens (Table 1). At the time of treatment with HPV-TILs, she had progressing cancer involving both lungs (Table 1 and Fig 1D). Following treatment, she experienced a PR that lasted 4 months (Table 1, and Figs 1B and 1D).
Fig 1.
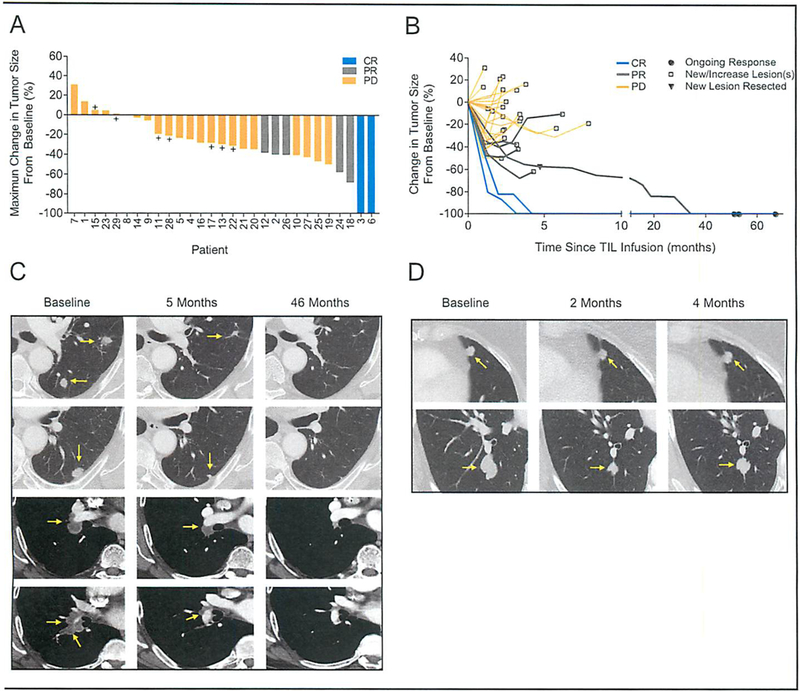
Clinical responses in patients after HPV-TIL therapy. (A) Waterfall plot of the maximum change in the sum of target lesions, as compared to baseline measurements, in 29 patients. CR, complete response; PR, partial response; PR, progressive disease; +, stable disease. (B) Spider plots of the change in the sum of target lesions from pretreatment baseline in 29 patients. Black circles (●) indicate ongoing responses, open squares (□) indicate progressive disease due to either a new lesion(s) or increasing target or non-target lesion(s). Black triangle (▼) indicates progressive disease in patient 24 with oropharyngeal cancer due to development of a new brain lesion after a PR of 5 months in duration. (C-D) Contrast-enhanced computed tomography scans obtained at baseline and after treatment for patient 24 with oropharyngeal cancer (C) and patient 26 with anal cancer (D). Tumors are marked by yellow arrows. Patient 24 had disease involving his left lung (first and second row) and mediastinum (third and fourth row). He experienced a PR of 5 months in duration due to a new brain lesion which was surgically excised. He was followed off-protocol, and his target lesions continued to regress, and there was no evidence of disease at most recent follow-up 51 months after treatment. Patient 26 had disease involving both lungs (first and second row). She experienced a PR of 4 months in duration due to increase in her target lesions.
Adverse events
There were no acute infusion-related toxicities and no autoimmune adverse events. The toxicity profile was consistent with the chemotherapy conditioning regimen and aldesleukin. The most common severe adverse events were the expected hematological toxicities of the conditioning regimen. Grade 3 and grade 4 adverse events are summarized in Table 2. No treatment related mortality occurred.
Table 2:
Adverse Events (grades 3 and 4)
| Adverse Event | No. of Patients (#) |
|---|---|
| Lymphopenia | 29 |
| Neutropenia | 29 |
| Thrombocytopenia | 29 |
| Anemia | 25 |
| Infection* | 17 |
| Febrile neutropenia | 12 |
| Metabolic disorders | 12 |
| Hypoxia | 8 |
| Nausea/vomiting | 6 |
| Dyspnea | 4 |
| Diarrhea | 3 |
| Fatigue | 3 |
| Hypotension | 3 |
| Cystitis | 2 |
| Hemorrhage† | 2 |
| Oliguria | 2 |
| Renal failure‡ | 2 |
| Syncope | 2 |
| Ureteral obstruction# | 2 |
| Dysphagia | 1 |
| Confusion | 1 |
Includes positive surveillance blood cultures
Associated with radiation colitis in one patient and with an in-situ cervical carcinoma in one patient
Associated with progressing pelvic tumor in one patient
Associated with progressing pelvic tumors in two patients
Immunological correlates of response
Post-hoc exploratory analyses were performed to test for immunological correlates of response. The frequency of infused T cells that responded to HPV E6 and E7 peptide stimulation was determined by flow cytometric measurement of the T-cell activation marker CD137 (Fig 2A). Cytokine production by infused T cells that responded to HPV E6 and E7 peptide stimulation was measured by IFN-γ production assays (Fig 2B). HPV-TILs displayed greater frequencies of HPV-reactive T cells (P = .0091) and a higher concentration of HPV-specific IFN-γ release (P = .0026) administered to responding versus non-responding patients (Appendix Fig A2, online only). No differences were detected between responding versus non-responding patients in the number of administered total T cells (P = .9999), CD4+ T cells (P = .1097), CD8+ T cells (P = .7086) or IL-2 doses (P = .4725) (Appendix Fig A3, online only). The frequency of HPV-reactive T cells in the peripheral blood before and following treatment was assessed by IFN-γ ELISPOT assay. Before treatment, minimal, if any, T-cell HPV reactivity was detected (Fig 2C). One month after treatment, 8 of 22 patients (all patients for whom samples were available) showed HPV reactivity (Fig 2C). The frequency of HPV-reactive T cells in peripheral blood one month after treatment correlated positively with clinical response (P = .0135, Appendix Fig A4, online only). No differences in the HPV reactivity of the infused T cells were detected between cervical and non-cervical cancer patients as measured by either immunological assay (P = .9515 for flow cytometry; P = .7627 for IFN-γ production) (Appendix Fig A5, online only). No significant differences were detected between cervical and non-cervical cancer patients in the number of administered total T cells (P = .5208), CD4+ T cells (P = .3869), CD8+ T cells (P = .3397) or IL-2 doses (P = .8852) (Appendix Fig A6, online only).
Fig 2.
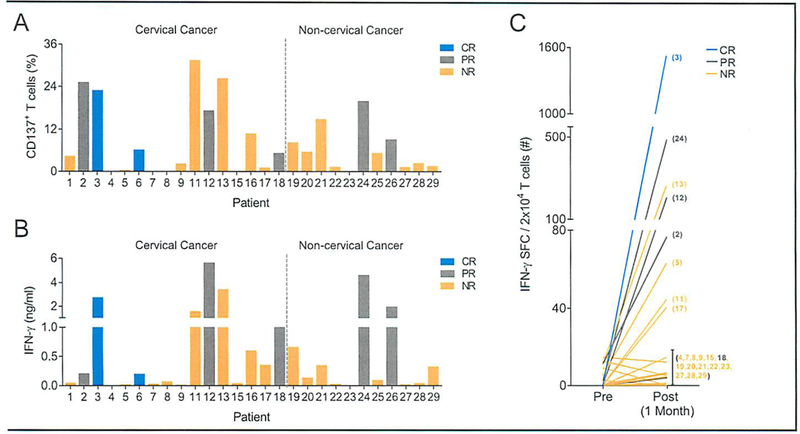
Human papillomavirus (HPV) reactivity of the infused T cells and peripheral blood T cells after infusion. (A-B) Infused HPV-TILs to 27 cervical and non-cervical cancer patients with HPV16+ or HPV18+ tumors were assessed for reactivity against HPV type– specific E6 and E7 oncoproteins using (A) CD137 upregulation by flow cytometry, and interferon gamma (IFN-γ) production assays. The HPV-type of each patients’ tumor is provided in Table 1. Patients 10 and 14 had non-HPV16+/18+ tumors and were not included in this analysis. Values shown represent sum of HPV-type specific E6 and E7 reactivity after background subtraction (gp100). CD137 upregulation is depicted for CD3+ T cells. Data are representative of two independent experiments for patients 11 to 13 and 15 to 29, and one experiment for patients 1 to 9 due to unavailability of samples. Peripheral blood (PB) T cells from before and one-month after treatment were assessed by IFN-γ enzyme-linked immunospot assay for reactivity against HPV-type specific E6 and E7 oncoproteins in 22 patients (5 responding and 17 nonresponding patients). Patient numbers are indicated in the parenthesis. Patients 1, 6, 16, 25 and 26 did not have available samples for this analysis. Patients 10 and 14 had non-HPV16+/18+ tumors and were not included in this analysis. Values shown represent sum of HPV-type specific E6 and E7 spot-forming cells (SFC) after background subtraction (gp100). Eight of 22 patients had discernable HPV reactivity (>20 SFC after background subtraction). Data are representative of two independent experiments patients 11 to 13, 15, 17 to 24 and 27 to 29, and one experiment for patients 2 to 5 and 7 to 9 due to unavailability of samples. CR, responding patient with a complete response, PR, responding patients with a partial response; NR, nonresponding patient.
CONCLUSIONS/DISCUSSION
Cellular therapy is effective for the treatment of certain B cell malignancies, but its study for the treatment of epithelial cancers has been limited.13 Here we report the treatment of patients with metastatic HPV-associated squamous cell carcinomas and adenocarcinomas with adoptive transfer of tumor-infiltrating T cells. Tumor responses occurred in patients with cervical cancer, anal cancer, and oropharyngeal cancer. Responses in two patients with cervical cancer were complete and are ongoing 67 and 53 months after treatment.
HPV-associated cancers are typical epithelial malignancies that are difficult to treat when metastatic. Chemotherapy generally consists of combinations of cytotoxic agents, often administered in conjunction with a biological agent.3, 15, 16 These regimens have limited clinical activity and substantial toxicity, and better treatments are needed. Immunotherapy works through different mechanisms than chemotherapy and has been a breakthrough for the treatment of certain malignancies.17 Although targeting HPV oncoproteins with antigen-specific immunotherapy using therapeutic vaccines has thus far been ineffective for metastatic disease, immunotherapy with PD-1-targeted agents has shown clinical activity in cervical,18 anal,10 and oropharyngeal9, 11, 12, 19 cancer.
The present trial represents a first step in the development of cellular immunotherapy for HPV-associated cancers and possibly other epithelial malignancies. It demonstrates the feasibility of the approach and provides evidence of clinical activity including durable, complete tumor regression in some patients. However, this clinical trial has important limitations. The response rate is not well defined due to the small sample size. While complete responses occurred, they were infrequent, and most patients did not appear to benefit from the therapy. In addition, the treatment requires surgery for procurement of a tumor from which to generate TILs, and the generation of high numbers of TILs takes approximately four to six weeks. In the present trial, 45 patients underwent surgery and 29 patients received treatment (Appendix Fig A1, online only). Another limitation is the patient-to-patient variability in the HPV reactivity of TILs and the infused cell product. Consistent with reports by others20, we that TILs from a number of patients possessed no HPV reactivity or low HPV reactivity (Fig 2A and 2B, Appendix Fig A1, online only). In the administered T cells, the frequency of HPV reactivity ranged from ≤0.1 to 31%, median 5% (Fig 2A) and the magnitude of IFN-γ release ranged from ≤0.1 to 5.6 ng/ml, median 0.2 ng/ml (Fig 2B). One strategy to circumvent surgery and generate a more consistent HPV oncoprotein-targeted cell product may be to administer HPV-specific T cells that are propagated and enriched ex vivo from peripheral blood.21 Another strategy may be to administer peripheral blood T cells that are genetically engineered ex vivo to target an HPV oncoprotein with a T cell receptor.13, 22–24 We are presently testing this strategy in an active clinical trial with gene engineered T cells that target HPV16 E7 (NCT02858310).
HPV-TIL therapy is a personalized treatment in which the characteristics of the adoptively transferred T cells themselves may serve as a mechanism-driven biomarker to predict treatment response and guide patient selection. Consistent with previous findings,14 the magnitude of HPV reactivity of the infused TILs was associated with clinical response. These findings may provide a biomarker of clinical response; however, they do not demonstrate a causal relationship between the targeting of HPV antigens and tumor regression. Indeed, the oncoprotein-reactive T cells represented a relatively small fraction of the infused polyclonal TILs (Fig 2A, range ≤0.1 to 31%, median 5%). Furthermore, HPV-associated cancers also harbor somatic gene mutations (mutated neoantigens) and epigenetically dysregulated genes (cancer germline antigens) that may be targeted by the TILs. Indeed, a global landscape analysis of tumor antigen targeting by TILs administered to cervical cancer patients who had complete responses revealed subdominant targeting of HPV antigens but immunodominant targeting of non-viral tumor antigens.25 For patient 3, the primary antigens targeted were mutated neoantigens, and for patient 6 the primary antigen targeted was a cancer germline antigen.25
The grade 3 and grade 4 hematological toxicities reported in this protocol were an expected consequence of the lymphodepleting conditioning regimen, which consisted of a single cycle of cyclophosphamide and fludarabine. It is uncertain whether the conditioning regimen may have direct anti-tumor activity in the chemotherapy-refractory cancers treated on this trial. While cyclophosphamide is not used in the treatment of advanced HPV-associated cancers, its analog ifosfamide has weak activity in platinum-experienced cervical cancer (response rate of 11%, 1.8 to 3.1 months duration)26 and in chemotherapy-experienced head and neck cancer (response rate of 10%)27. Fludarabine has no clinical activity in cervical cancer28 or head and neck cancer29.
Targeting of the oncogenic drivers of cancer with cellular immunotherapy is an attractive treatment strategy.13 HPV-associated cancers are driven by the E6 and E7 oncoproteins, and provide a potential model in which to explore this approach. A clinical trial of T cells genetically engineered with a T cell receptor that targets the E6 antigen has shown some clinical activity.23 A clinical trial with a higher avidity T cell receptor that targets the E7 antigen is actively accruing patients (NCT02858310).24
In summary, the present study reports perhaps the most substantial experience to date with cellular therapy in epithelial cancers. The overall response rate of 24 percent was modest and may reflect in part the manifold mechanisms of tumor resistance to immunotherapy that are emerging from other studies.30 However, the results support proof-of-principle that ACT can mediate regression of epithelial cancers, including durable complete regression that may be curative for some patients.
Supplementary Material
SIGNIFICANCE.
Here we report that cellular therapy can mediate the regression of HPV-associated cervical cancer, oropharyngeal cancer, and anal cancer, including durable, complete regression of cervical cancer. The findings support expanded research to discover and develop cellular therapy treatments for epithelial cancers.
ACKNOWLEDGEMENTS
We thank the Surgery Branch immunotherapy clinical fellows and nurses for their care of the patients, and the post baccalaureate students and research biologists for experimental assistance.
Research support for this study was provided by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health.
Conflict of interest statement:
J.C.Y and C.S.H. receive research funding from Kite Pharma, a Gilead Company, unrelated to this study. S.S., J.C.Y. and C.S.H. have patents or intellectual property interest (NIH institutional).
REFERENCES
- 1.de Martel C, Plummer M, Vignat J, et al. : Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 141:664–670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einstein MH, Schiller JT, Viscidi RP, et al. : Clinician’s guide to human papillomavirus immunology: knowns and unknowns. The Lancet Infectious Diseases 9:347–356, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Trimble CL, Frazer IH: Development of therapeutic HPV vaccines. The Lancet Oncology 10:975–980, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marur S, D’Souza G, Westra WH, et al. : HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11:781–789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosbie EJ, Einstein MH, Franceschi S, et al. : Human papillomavirus and cervical cancer. Lancet 382:889–899, 2013 [DOI] [PubMed] [Google Scholar]
- 6.CDC - HPV-Associated Cancer Statistics [Internet], 2018. [cited 2018 Jun 4] Available from: https://www.cdc.gov/cancer/hpv/statistics/index.htm
- 7.WHO | Human papillomavirus (HPV) [Internet] WHO; [cited 2018 Jul 30] Available from: http://www.who.int/immunization/topics/hpv/en/ [Google Scholar]
- 8.Tewari KS, Sill MW, Penson RT, et al. : Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390:1654–1663, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris RL, Blumenschein G, Fayette J, et al. : Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375:1856–1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris VK, Salem ME, Nimeiri H, et al. : Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 18:446–453, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiwert TY, Burtness B, Mehra R, et al. : Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 17:956–965, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Bauml J, Seiwert TY, Pfister DG, et al. : Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol 35:1542–1549, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinrichs CS: Molecular Pathways: Breaking the Epithelial Cancer Barrier for Chimeric Antigen Receptor and T-cell Receptor Gene Therapy. Clin Cancer Res 22:1559–1564, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevanović S, Draper LM, Langhan MM, et al. : Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 33:1543–1550, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermorken JB, Mesia R, Rivera F, et al. : Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Tewari KS, Sill MW, Long HJ, et al. : Improved survival with Bevacizumab in advanced cervical cancer. New England Journal of Medicine 370:734–743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A, Wolchok JD: Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenel J-S, Le Tourneau C, O’Neil B, et al. : Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J Clin Oncol 35:4035–4041, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Chow LQM, Haddad R, Gupta S, et al. : Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol 34:3838–3845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piersma SJ, Welters MJP, van der Hulst JM, et al. : Human papilloma virus specific T cells infiltrating cervical cancer and draining lymph nodes show remarkably frequent use of HLA-DQ and -DP as a restriction element. Int J Cancer 122:486–494, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Ramos CA, Narala N, Vyas GM, et al. : Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes for adoptive immunotherapy of HPV-associated malignancies. J Immunother 36:66–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draper LM, Kwong MLM, Gros A, et al. : Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin Cancer Res 21:4431–4439, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinrichs CS, Doran SL, Stevanovic S, et al. : A phase I/II clinical trial of E6 T-cell receptor gene therapy for human papillomavirus (HPV)-associated epithelial cancers. JCO 35:3009–3009, 2017 [Google Scholar]
- 24.Jin BY, Campbell TE, Draper LM, et al. : Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight In Press [DOI] [PMC free article] [PubMed]
- 25.Stevanović S, Pasetto A, Helman SR, et al. : Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 356:200–205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton GP, Blessing JA, Photopulos G, et al. : Gynecologic Oncology Group experience with ifosfamide. Semin Oncol 17:6–10, 1990 [PubMed] [Google Scholar]
- 27.Huber MH, Lippman SM, Benner SE, et al. : A phase II study of ifosfamide in recurrent squamous cell carcinoma of the head and neck. Am J Clin Oncol 19:379–382, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Von Hoff DD, Green S, Surwit EA, et al. : Phase II study of fludarabine phosphate (NSC 312887) in patients with advanced cervical cancer. A Southwest Oncology Group study. Am J Clin Oncol 13:433–435, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Mittelman A, Savona S, Puccio C, et al. : Phase II trial of fludarabine phosphate (F-Ara-AMP) in patients with advanced head and neck cancer. Invest New Drugs 8 Suppl 1:S65–67, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Hu-Lieskovan S, Wargo JA, et al. : Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168:707–723, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


