Abstract
Background:
Epileptiform activity is common after cardiac arrest, although intensity of electroencephalographic (EEG) monitoring may affect detection rates. Prior work has grouped these patterns together as “malignant,” without considering discrete subtypes. We describe the incidence of distinct patterns in the ictal interictal spectrum at two centers and their association with outcomes.
Methods:
We analyzed a retrospective cohort of comatose post-arrest patients admitted at two academic centers from January 2011 to October 2014. One center uses routine continuous EEG, the other acquires “spot” EEG at the treating physicians’ discretion. We reviewed all available EEG data and classified epileptiform patterns. We abstracted antiepileptic drugs (AEDs) administrations from the electronic medical record. We compared apparent incidence of each pattern between centers, and compared outcomes (awakening from coma, survival to discharge, discharge modified Rankin Scale (mRS) 0–2) across EEG patterns and number of AEDs administered.
Results:
We included 818 patients. Routine continuous EEG was associated with a higher apparent incidence of polyspike burst-suppression (25% vs 13% P <0.001). Frequency of other epileptiform findings did not differ. Among patients with any epileptiform pattern, only 2/258 (1%, 95%CI 0–3%) were discharged with mRS 0–2, although 24/258 (9%, 95%CI 6–14%) awakened and 36/258 (14%, 95%CI 10–19%) survived. The proportions that awakened and survived decreased in a stepwise manner with progressively worse EEG patterns (range 38% to 2% and 32% to 7%, respectively). Among patients receiving ≥3 AEDs, only 5/80 (6%, 95%CI 2–14%) awakened and 1/80 (1%, 95%CI 0–7%) had a mRS 0–2.
Conclusion:
We found high rates of epileptiform EEG findings, regardless of intensity of EEG monitoring. The association of distinct ictal-interictal EEG findings with outcome was variable.
Keywords: Cardiac arrest, seizure, outcome, electroencephalography, antiepileptic, anticonvulsant, brain injury
Introduction
More than 125,000 Americans are treated in the hospital after successful resuscitation from cardiac arrest (CA) annually.[1] Brain injury is the major determinant of outcomes in this cohort: most non-survivors die after life-sustaining therapy is withdrawn because of perceived poor neurological prognosis.[2, 3] Among survivors, neurological disability is common and associated with long-term mortality and reduced quality of life.[4, 5] Electroencephalographic (EEG) findings on the ictal-interictal spectrum develop in up to one-third of comatose post-arrest patients and are associated with worse outcomes.[6–8] These pathological EEG findings range from convulsive seizures to non-periodic epilepitiform discharges, and likely vary in both the severity of the preexisting brain injury they reflect and their potential to cause secondary brain injury.[9]
The differential association of distinct patterns on the ictal-interictal spectrum, such as periodic or polyspike-wave discharges, with outcomes has not been rigorously explored. Most previous literature has aggregated any epileptiform discharges together as “malignant” (or epileptiform),[10–15] “highly-malignant,”[12, 13, 16] or considered well-defined subtypes of epilepitiform activity such as generalized periodic discharges or burst suppression with identical bursts.[17–19] Guidelines describing the prognostic role of EEG have focused on characteristics of the EEG background, such as suppression, reactivity and continuity, and development of frank seizures.[20] The significance of other potentially less ominous patterns is uncertain.
There are several common approaches to EEG monitoring, potentially limiting the generalizability of single center reports of post-arrest EEG. EEG can be monitored continuously (cEEG) or in brief 20–30 minute “spot” recordings. Further, EEG may be applied routinely to all high-risk patients above some pre-specified prevalence threshold (e.g. all those who are comatose after resuscitation) or obtained only based on clinical suspicion for seizure. Intensity of monitoring may affect the apparent incidence of epileptiform patterns. We describe the incidence of patterns in the ictal-interictal spectrum identified at two centers using different, clinically reasonable EEG monitoring strategies: 1) routine cEEG and 2) clinician-driven 30-minute spot EEG. We describe the association between EEG patterns and survival, awakening from coma, and functional impairment at time discharge from the hospital.
Methods
Study Population and Setting
The University of Pittsburgh Institutional Review Board approved all aspects of this study with a waiver of informed consent. We performed a retrospective cohort analysis of comatose CA survivors admitted to intensive care units (ICU) at two academic centers from January 2011 to October 2014.
At Center 1, the Pittsburgh Post-Cardiac Arrest Service coordinates a comprehensive bundle of post-arrest care, as has been previously described in detail, which includes routine cEEG monitoring and standardized AED treatment of EEG patterns on the ictal-interictal spectrum.[4, 21] We administered AEDs sequentially to suppress these patterns, except for rare or occasional non-periodic epileptiform discharges, which we did not treat.[21, 22] Details of cEEG acquisition, interpretation and AED treatment at Center 1 during the study period can be found in [21]. We continued antiepileptics being administered antecedent to cardiac arrest (for example, home medications) after arrest regardless of EEG observations. Typically, we wean anesthetic infusions around day 3 to facilitate multimodal neurological prognostication without pharmacological confounders. During the study period, we treated patients with targeted temperature management to 33oC for 24 hours, followed by slow rewarming at 0.25oC/hr to normothermia.
Center 2 is a tertiary referral care center that also treats a high volume of post-arrest patients, but at which cEEG monitoring was not performed during the study period. Intensivists at Center 2 obtain spot EEG if there is clinical suspicion for seizures, but spot EEG can typically be obtained at most once per day per patient. AEDs are administered at the discretion of the treating clinician and are sometimes given based on clinical observations (for example, myoclonic jerks) rather than based on EEG. The same critical care physician group staffs both Centers’ ICUs.
Data Collection
We maintain a prospective registry for the purposes of quality improvement and clinical research at both centers. From this, we obtained patient demographics including age, sex, shockable initial rhythm, arrest location (out-of-hospital cardiac arrest (OHCA) or in-hospital cardiac arrest (IHCA)), and early post-arrest illness severity (Pittsburgh Cardiac Arrest Category, PCAC). The PCAC is a validated clinical prediction tool that stratifies post-arrest patients into four levels based on neurological examination and cardiopulmonary dysfunction in the first 6 hours following CA.[23, 24] PCAC I patients are by definition awake and do not undergo EEG monitoring at either center, and were therefore excluded from our analysis. Outcomes included (1) whether or not the patient awakened from coma, which we defined as the ability to consistently follow verbal commands, (2) survival to hospital discharge, hospital length of stay, and (3) modified Rankin Scale score (mRS) of 0 to 2 at hospital discharge.[25]
We reviewed each patient’s available EEG data and classified the worst observed pattern on the ictal-interictal spectrum for each day. We based the ordering of these patterns on previously published schema that considered the association of various epileptiform transients with secondary injury and mortality in brain injured patients.[9, 26] Based on previous studies of EEG for prognostication after cardiac arrest and American Clinical Neurophysiology Society (ACNS) consensus terminology, we categorized EEGs as: nothing epileptiform; non-periodic epileptiform discharges; periodic discharges (both generalized and other); polyspike-wave discharges; or seizures.[14, 19, 21, 22, 27] We abstracted from the electronic medical record all AEDs and sedative medications that were administered, including medication name, dosage, route, and timestamp(s) of administration. In our main analysis, we did not consider propofol or midazolam to be AEDs, since in our setting these are routinely administered for routine sedation. However, since they have well-documented antiepileptic properties, in a preplanned secondary analysis, we reanalyzed our data with these agents included.
Statistical analysis
We used descriptive statistics to summarize population characteristics and outcomes. We used Fisher’s exact tests to test the overall association of each EEG finding and total number of AEDs administered with patient outcomes (awakening from coma, survival to discharge, and discharge with mRS 0–2), and calculated binomial confidence intervals around each proportion. We performed sensitivity analyses testing the association between AED administration and outcome after adding propofol, midazolam, then both to the total AED count. We used logistic regression models to test for an interaction between treating center with the predictors and outcomes of interest described above. We used Stata version 14.2 (StataCorp, College Station, TX) for all analyses.
Results
A total of 818 comatose post-arrest patients were admitted during the study period (513 at Center 1 and 305 at Center 2). Patients at Center 1 were younger and more likely to have arrested out-of-hospital, while Center 2 subjects had a higher initial illness severity (Table 1). At Center 1, a higher proportion of patients survived, awakened from coma, and had mRS 0–2 at time of discharge. Overall, 229 subjects (28%) awakened from coma after a median of 2 [interquartile range (IQR) 1 to 5] days, 120 (15% overall, 53% of survivors) were discharged to home or acute rehabilitation, and 54 (7% overall, 24% of survivors) were discharged with mRS 0–2. Patients treated at Center 1, where cEEG is obtained routinely, were more intensively monitored (median [IQR] 2 [0 – 3] days of cEEG versus 0 [0 – 1] days of spot EEGs at Center 2) (Table 2). In parallel, Center 1 detected more patterns on the ictal-interictal spectrum (P <0.001) (Figure 1), driven primarily by increased detection of polyspike bursts with or without associated myoclonic jerks (P <0.001). Among patients where EEG was never checked, only 34 (21%) survived to discharge at Center 2 while 82 (55%) survived to discharge at Center 1. At Center 1, reasons why cEEG was not obtained were non-survivable cerebral edema or herniation on initial brain imaging (65 subjects, 44% of cases with no cEEG), rapid awakening (52 subjects (21%)), with the remaining 52 cases (10% of overall cohort) not obtained because of delayed transfer to our facility, prior advanced directives, or lack of cEEG equipment availability. Patients at Center 1 received more intensive AED treatment (Figure 2, Table 2). Table 3 depicts the number of AEDs used for each category of ictal pattern, which is further stratified by Center in Supplemental Table 3.
Table 1:
Cohort characteristics and outcomes stratified by treating center
| Characteristic | Overall (n = 818) | Center 1 (n = 513) | Center 2 (n = 305) |
|---|---|---|---|
| Age, years | 61 [50 – 72] | 58 [48 – 71] | 64 [53 – 74] |
| Female sex | 327 (40) | 193 (38) | 134 (44) |
| Out-of-hospital arrest | 528 (65) | 382 (74) | 146 (48) |
| Shockable initial rhythm | 218 (27) | 143 (28) | 75 (25) |
| Pittsburgh Cardiac Arrest Category | |||
| II | 117 (22) | 131 (26) | 46 (15) |
| III | 137 (17) | 79 (15) | 58 (19) |
| IV | 504 (62) | 303 (59) | 201 (66) |
| Survived to discharge | 225 (28) | 160 (31) | 65 (21) |
| Awakened from coma | 229 (28) | 155 (30) | 74 (24) |
| Days from arrest to awakening | 2 [1 – 5] | 2 [1–4] | 2 [1–5] |
| Discharge to home or acute rehabilitation | 120 (15) | 84 (16) | 36 (12) |
| Discharge mRS 0–2 | 54 (7) | 40 (8) | 14 (5) |
| Hospital length of stay, days | |||
| Survivors | 17 [11 – 26] | 17 [11 – 26] | 17 [14 – 26] |
| Non-survivors | 3 [1 – 5] | 3 [2 – 5] | 3 [1 – 5] |
| Proximate cause of death* | |||
| Rearrest, shock, or multi-system organ failure | 113 (24) | 71 (23) | 42 (28) |
| Brain death | 41 (9) | 30 (10) | 11 (7) |
| Withdrawal for non-neurological prognosis | 39 (8) | 13 (4) | 26 (17) |
| Withdrawal for perceived poor neurologic prognosis | 269 (58) | 196 (63) | 48 (48) |
Data are presented as raw number with corresponding percentage or median with associated interquartile range.
Abbreviations: mRS – modified Rankin Scale score
Percentages are presented as proportion of non-survivors
Table 2:
Electroencephalographic monitoring and antiepileptic drug use stratified by center
| Characteristic | Center 1 (n=513) | Center 2 (n=305) | P value |
|---|---|---|---|
| Number of days with EEG checked | 2 [0–3] | 0 [0–1] | <0.001 |
| 0 | 149 (29) | 160 (52) | |
| 1 | 92 (18) | 86 (28) | |
| 2 | 97 (19) | 48 (16) | <0.001 |
| 3 | 75 (15) | 9 (3) | |
| 4 | 49 (10) | 1 (0) | |
| 5 | 51 (10) | 1 (0) | |
| Worst EEG pattern detected | Overall P <0.001 | ||
| Never checked | 149 (29) | 160 (52) | <0.001 |
| Nothing epileptiform | 168 (33) | 83 (27) | 0.10 |
| Non-periodic epilepitiform discharges | 25 (5) | 9 (3) | 0.18 |
| Periodic discharges | 30 (6) | 11 (4) | 0.16 |
| Seizures | 11 (2) | 2 (1) | 0.10 |
| Polyspike bursts with or without myoclonus | 130 (25) | 40 (13) | <0.001 |
| Antiepileptic drug use | |||
| Levetiracetam | 189 (37) | 77 (25) | <0.001 |
| Valproic acid | 138 (27) | 31 (10) | <0.001 |
| Phenytoin | 92 (18) | 19 (6) | <0.001 |
| Lacosamide | 20 (4) | 0 (0) | <0.001 |
| Phenobarbital | 14 (3) | 2 (1) | 0.04 |
| Carbamazepine | 1 (0) | 1 (0) | 0.71 |
| Propofol | 316 (71) | 132 (29) | <0.001 |
| Midazolam | 146 (34) | 288 (66) | 0.02 |
| Total number of intermittent antiepileptic drugs | 0 [0–2] | 0 [0–1] | <0.001 |
Data are presented as raw number with corresponding percentage or median with associated
Figure 1:
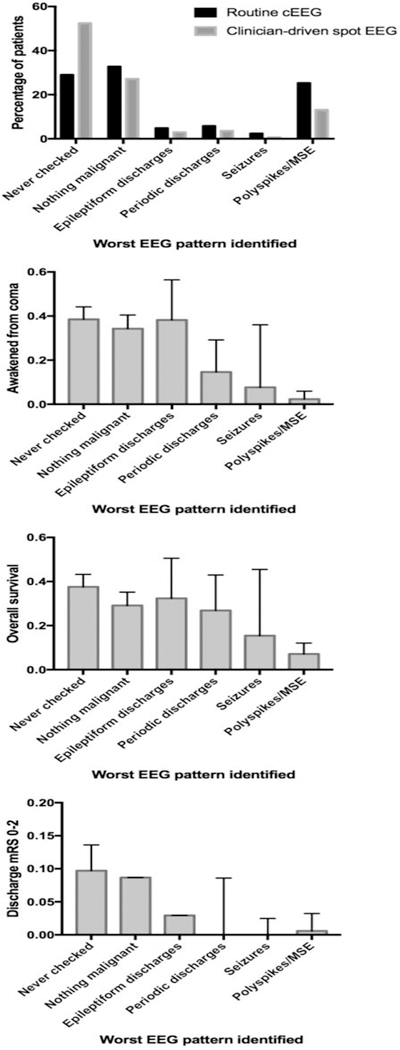
The rate at which patterns on the ictal-interictal spectrum are detected differs by monitoring strategy, however the association of these patterns with outcomes does not.
Figure 2:
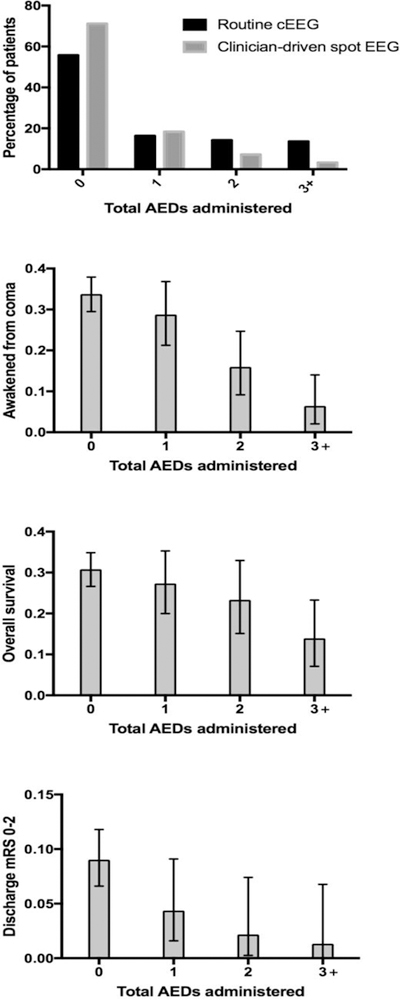
Electroencephalographic monitoring strategy is associated with the total number of antiepileptic drugs (AEDs) administered. Patients receiving more AEDs experienced worse outcomes.
Table 3:
Total number of intermittent antiepileptic drugs administered, not including propofol and midazolam, stratified by worst EEG pattern detected
| Number of antiepileptic drugs (AEDs) administered | ||||
|---|---|---|---|---|
| Worst EEG pattern detected | 0 AED (n = 503) | 1 AED (n = 140) | 2 AEDs (n = 95) | 3+ AEDs (n = 80) |
| Never checked (n = 309) | 279 (90) | 18 (6) | 8 (3) | 4 (1) |
| Nothing epileptiform (n = 251) | 182 (73) | 54 (22) | 14 (6) | 1 (0) |
| Non-periodic discharges (n = 34) | 6 (18) | 21 (62) | 3 (9) | 4 (12) |
| Periodic discharges (n = 41) | 7 (17) | 11 (27) | 8 (20) | 15 (37) |
| Seizures (n = 13) | 0 (0) | 1 (8) | 6 (46) | 6 (46) |
| Polyspike burst (n = 170) | 29 (17) | 35 (21) | 56 (33) | 50 (29) |
Data are presented as raw number with corresponding row percentages.
Worst EEG pattern over 5 days and number of AEDs administered were associated with awakening from coma, survival to discharge and functional outcome at discharge (Figures 1 and 2, Supplemental Tables 1 and 2). Among patients with any detected pattern on the ictal-interictal spectrum, only 2/258 (1%, 95%CI 0 – 3%) had mRS 0–2 at discharge, although 24/258 (9%, 95%CI 6 – 14%) awakened from coma and 36/258 (14%, 95%CI 10 – 19%) survived to hospital discharge. These proportions were lower than rates of awakening, survival and favorable discharge mRS in the whole cohort (P <0.01). The proportion of patients that awakened from coma and the proportion surviving to hospital discharge decreased in a stepwise manner with progressively worse EEG patterns (Figure 1, Supplemental Table 1). Four patients with polyspike-wave discharges awakened from coma. In review of these recordings, these patients all exhibited polyspikes superimposed on continuous EEG background activity. By contrast, the remaining patients had polyspike discharges superimposed on a suppressed background (burst suppression with identical bursts[18, 19]) and did not recover from coma. Center did not modify the relationship between worst EEG pattern and outcome.
Total number of AEDs was negatively associated with awakening, survival and mRS 0–2 at discharge (Figure 2). Among patients who received three or more AEDs, only 5/80 (6%, 95% confidence interval (CI) 2 – 14%) awakened from coma and only 1/80 (1%, 95%CI 0 – 7%) was discharged with mRS 0–2. Center did not modify the relationship between number of AEDs administered and outcome. Adding propofol and/or midazolam to the total AED did not change the overall pattern of the relationship (Supplemental Table 2).
Discussion
Our major finding is that post-arrest patient outcomes differ substantially based on severity of ictal-interictal patterns identified by EEG. Epileptiform patterns are detected commonly in this population, regardless of monitoring strategy used. Refractoriness to therapy, as approximated by the number of AEDs administered, portends worse outcomes. These findings were consistent across two centers with very different approaches to EEG monitoring and AED treatment, supporting the generalizability of our results to other settings.
We do note a difference in the frequency of epileptiform EEG patterns detected depending on the monitoring strategy that was used. Among the critically ill, most patterns on the ictal-interictal spectrum have no clinical correlate, necessitating EEG monitoring for detection.[28] Since these patterns may be intermittent, spot EEG monitoring is insensitive compared to cEEG.[29] The largest difference we observed was in detection of polyspike discharges. Approximately half of patients who have polyspike bursts of activity on a suppressed background have associated myoclonic jerks,[19] so it may be that at Center 2 these patients were diagnosed based on physical exam rather than EEG. However, outcome from post-anoxic myoclonus varies substantially by underlying EEG pattern and some patients enjoy favorable recoveries.[19] Because awakening after Lance-Adams variant post-anoxic myoclonus tends to be delayed for several weeks post-arrest, expectant management based on physical examination alone many not be sufficient to guide care.[19] Thus, even in the subgroup of patients with myoclonus, identification of specific patterns may alter treatment or prognostication. More generally, the differential detection rates we found across the two Centers supports that cEEG is more sensitive for detection of epileptiform transients. The variable association of these patterns with outcome suggests that their observation may inform prognostication, although the clinical importance of treating such patterns remains uncertain and is not addressed by our results.
We found that rates of awakening and functionally favorable recovery are near zero in the cohort of patients requiring treatment with three or more anticonvulsants. Although we included over 800 subjects, the number of patients receiving multiple AEDs was insufficient to make precise or stratified estimates of outcome (for example, association of AED treatment intensity with outcomes within subtypes of EEG patterns). Rates of favorable recovery in patients requiring three or more AEDs are low, potentially tempering enthusiasm to embark on a course of aggressive suppression of these patterns. It is unknown whether treating these patterns improves the chances of survival or quality of recovery. Post-anoxic generalized periodic discharges (GPDs), for example, are common in comatose post-arrest patients. But, it is unclear whether GPDs worsen secondary brain injury or if they are simply epiphenomena of injury,[9, 30, 31] though higher frequency GPDs have been associated with brain tissue hypoxia similar to that observed during seizures.[32] It is important to emphasize that our work simply demonstrates the association of number of AEDs administered with outcome. We presume that AEDs were given based on clinical need and describe this association as an estimate of refractoriness of patients’ EEGs to treatment. Our data do not address whether AED treatment or cEEG monitoring improves care or patient outcomes, a question that can only be resolved through a clinical trial. Collinearity between center and treatment strategy raises the potential for unmeasured confounders to have influenced care and outcomes in unmeasured ways. We and others have observed long-term good functional recovery after cardiac arrest complicated by refractory status epilepticus requiring multiple AEDs to control, and caution against therapeutic nihilism. Faced with difficult-to-control patterns on the ictal-interictal spectrum our data can support a more informed risk-benefit discussion with patients and providers and selection of a treatment plan consistent with the individual patient’s values and preferences.[33]
A large proportion of patients receiving AEDs had no EEG monitoring or no epileptiform activity on EEG (51% of those receiving one AED, 23% of those receiving 2 AEDs, and 6% of those receiving 3+ AEDs). In some cases, patients were receiving AEDs at the time of the arrest and the agent was simply continued. However, a number of patients at Center 2 received AEDs based on clinical suspicion for seizure (e.g. clinically observed myoclonus) despite no evidence of epileptiform activity on prior EEGs. The value of AEDs in this situation is uncertain. Likewise, AEDs were commonly administered to patients with polyspikes, the majority of whom had burst suppression with identical bursts. This EEG pattern is usually refractory to AED treatment, as reflected by the fact that patients with polyspike bursts comprised 65% of cases where 3+ AEDs were administered.[19] Data are needed about which EEG patterns and clinical correlates may benefit and which patterns are futile or inappropriate to treat.
While seizure suppression may prevent or minimize some types of secondary neurological injury, AEDs also have well characterized toxicities that may include hypotension, bradyarrhythmias, metabolic encephalopathy and depressed mental status that could prolong mechanical ventilation or increase ICU length of stay.[34–36] Although newer AEDs such as levetiracetam and lacosamine are better tolerated by critically ill patients, brain-injured patients treated with multiple AEDs may still develop a depressed level of consciousness.[37, 38] Whether treatment of epileptiform EEG patterns after cardiac arrest improves outcomes can only be tested in a clinical trial, and our results suggest that inclusion in such a trial should be limited to, or stratified by, specific patterns which have distinct physiological and prognostic significance. Nevertheless, our data may inform clinicians and families in discussions to choose the appropriate aggressiveness of care in these patients.
Our study has several important limitations. Our primary outcomes were survival, awakening, and functional outcome at hospital discharge. Because clinical providers recommending withdrawal of life-sustaining therapy based on perceived poor prognosis were not blinded to EEG results, self-fulfilling prophecies may have developed. Specifically, because prior literature has suggested that certain EEG patterns are particularly ominous after cardiac arrest (for example, polyspike bursts associated with myoclonus or electroencephalographic seizures), observation of these patterns may have prompted limitations of care that artificially increase mortality in these subgroups. In the absence of blinded neurological prognostication or data derived from countries or cultures where these limitations in care are rare or prohibited, all associations of clinical data with outcome must be interpreted with caution.
Because neurological and physical recovery continues after hospital discharge, the proportion of patients with functionally favorable recovery outcomes at discharge likely underestimates the proportion that will ultimately enjoy favorable recovery after rehabilitation and time.[39] Indeed, the proportion of patients discharged with poor mRS is higher than that described in large prospective studies.[40] In our local system of care, patients are typically discharged from their acute hospitalization when they are able to ambulate and provide self-care with assistance, if not sooner. Because discharge is timed in part based on the degree of observed functional recovery, this outcome must be interpreted with caution. Although cEEG provides around-the-clock monitoring of cortical activity, records may not be interpreted until after the EEG has been completed, resulting in the potential for AED administration to be delayed. Consequently, the impact of cEEG on outcomes from certain subgroups of patients with treatable abnormal EEG patterns may not reflect the maximum benefits of real-time monitoring. Although based on previously published literature,[9, 26] our classification system of “worse” EEG pattern by day may not be the most appropriate way to summarize these data. In the critical care population and after cardiac arrest specifically, EEG changes dynamically over time.[14, 21, 41] Multiple epileptiform transients may be present on any single day, and not just EEG pattern but also the overall burden of each pattern, treatment intervention, and underlying organic substrate may be clinically relevant.[9, 42] Current methods to classify these complex, temporally dynamic phenomena, including our own, are likely imperfect and this area well-suited to ongoing research.
In conclusion, we find high rates of epileptiform EEG findings after cardiac arrest, regardless of intensity of EEG monitoring the association of electroencephalographically distinct patterns on the ictal-interictal spectrum is variable. Continuous monitoring may facilitate more aggressive AED therapy, but whether AEDs improve patients’ outcomes cannot be determined from observational data. Refractory epileptiform activity, as indicated by treatment with three or more AEDs is associated with poor outcome. Our results suggest that both nuanced EEG interpretation and consideration of intensity of AED therapy are important indicators that can be used to inform prognostication.
Supplementary Material
Acknowledgments
Disclosures: Dr. Elmer’s research time is supported by the NIH through grants 5K12HL109068 and 1K23NS097629.
Dr. Rittenberger had an unrestricted grant from the Laerdal Foundation to evaluate a point of care EEG device.
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Callaway CW, Schmicker R, Kampmeyer M, Powell J, Rea TD, Daya MR, et al. Receiving hospital characteristics associated with survival after out-of-hospital cardiac arrest. Resuscitation 2010;81:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Elmer J, Rittenberger JC, Coppler PJ, Guyette FX, Doshi AA, Callaway CW, et al. Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation 2016;108:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wachelder EM, Moulaert VR, van Heugten C, Verbunt JA, Bekkers SC, Wade DT. Life after survival: long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation 2009;80:517–22. [DOI] [PubMed] [Google Scholar]
- [6].Knight WA, Hart KW, Adeoye OM, Bonomo JB, Keegan SP, Ficker DM, et al. The incidence of seizures in patients undergoing therapeutic hypothermia after resuscitation from cardiac arrest. Epilepsy Res 2013;106:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocritical care 2012;16:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Youn CS, Callaway CW, Rittenberger JC, Post Cardiac Arrest S. Combination of initial neurologic examination, quantitative brain imaging and electroencephalography to predict outcome after cardiac arrest. Resuscitation 2017;110:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 2005;22:79–91. [DOI] [PubMed] [Google Scholar]
- [10].Youn CS, Callaway CW, Rittenberger JC, Post Cardiac Arrest S. Combination of initial neurologic examination and continuous EEG to predict survival after cardiac arrest. Resuscitation 2015;94:73–9. [DOI] [PubMed] [Google Scholar]
- [11].Amorim E, Rittenberger JC, Baldwin ME, Callaway CW, Popescu A, Post Cardiac Arrest S. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation 2015;90:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Westhall E, Rossetti AO, van Rootselaar AF, Wesenberg Kjaer T, Horn J, Ullen S, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 2016;86:1482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Beuchat I, Solari D, Novy J, Oddo M, Rossetti AO. Standardized EEG interpretation in patients after cardiac arrest: Correlation with other prognostic predictors. Resuscitation 2018;126:143–6. [DOI] [PubMed] [Google Scholar]
- [14].Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med 2012;40:2867–75. [DOI] [PubMed] [Google Scholar]
- [15].Westhall E, Rosen I, Rundgren M, Bro-Jeppesen J, Kjaergaard J, Hassager C, et al. Time to epileptiform activity and EEG background recovery are independent predictors after cardiac arrest. Clin Neurophysiol 2018;129:1660–8. [DOI] [PubMed] [Google Scholar]
- [16].Backman S, Cronberg T, Friberg H, Ullen S, Horn J, Kjaergaard J, et al. Highly malignant routine EEG predicts poor prognosis after cardiac arrest in the Target Temperature Management trial. Resuscitation 2018;131:24–8. [DOI] [PubMed] [Google Scholar]
- [17].Ribeiro A, Singh R, Brunnhuber F. Clinical outcome of generalized periodic epileptiform discharges on first EEG in patients with hypoxic encephalopathy postcardiac arrest. Epilepsy Behav 2015;49:268–72. [DOI] [PubMed] [Google Scholar]
- [18].Hofmeijer J, Tjepkema-Cloostermans MC, van Putten MJ. Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2014;125:947–54. [DOI] [PubMed] [Google Scholar]
- [19].Elmer J, Rittenberger JC, Faro J, Molyneaux BJ, Popescu A, Callaway CW, et al. Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Annals of neurology 2016;80:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sandroni C, Cavallaro F, Callaway CW, D’Arrigo S, Sanna T, Kuiper MA, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation 2013;84:1324–38. [DOI] [PubMed] [Google Scholar]
- [21].Elmer J, Gianakas JJ, Rittenberger JC, Baldwin ME, Faro J, Plummer C, et al. Group-Based Trajectory Modeling of Suppression Ratio After Cardiac Arrest. Neurocritical care 2016;25:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- [23].Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation 2011;82:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coppler PJ, Elmer J, Calderon L, Sabedra A, Doshi AA, Callaway CW, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation 2015;89:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation 2011;82:1036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Johnson EL, Kaplan PW. Population of the ictal-interictal zone: The significance of periodic and rhythmic activity. Clinical Neurophysiology Practice 2017;2:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Oh SH, Park KN, Kim YM, Kim HJ, Youn CS, Kim SH, et al. The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation 2013;84:200–5. [DOI] [PubMed] [Google Scholar]
- [28].Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–8. [DOI] [PubMed] [Google Scholar]
- [29].Struck AF, Osman G, Rampal N, Biswal S, Legros B, Hirsch LJ, et al. Time-dependent risk of seizures in critically ill patients on continuous electroencephalogram. Annals of neurology 2017;82:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bauer G, Trinka E, Kaplan PW. EEG patterns in hypoxic encephalopathies (post-cardiac arrest syndrome): fluctuations, transitions, and reactions. J Clin Neurophysiol 2013;30:477–89. [DOI] [PubMed] [Google Scholar]
- [31].Sreedharan J, Gourlay E, Evans MR, Koutroumanidis M. Falsely pessimistic prognosis by EEG in post-anoxic coma after cardiac arrest: the borderland of nonconvulsive status epilepticus. Epileptic disorders : international epilepsy journal with videotape 2012;14:340–4. [DOI] [PubMed] [Google Scholar]
- [32].Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, et al. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol 2017;74:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology 2009;72:744–9. [DOI] [PubMed] [Google Scholar]
- [34].Varelas PN, Spanaki M. Management of seizures in the critically ill. The neurologist 2006;12:127–39. [DOI] [PubMed] [Google Scholar]
- [35].Riker RR, Gagnon DJ, Hatton C, May T, Seder DB, Stokem K, et al. Valproate Protein Binding Is Highly Variable in ICU Patients and Not Predicted by Total Serum Concentrations: A Case Series and Literature Review. Pharmacotherapy 2017;37:500–8. [DOI] [PubMed] [Google Scholar]
- [36].Luk ME, Tatum WO, Patel AV, Nau KM, Freeman WD. The safety of lacosamide for treatment of seizures and seizure prophylaxis in adult hospitalized patients. Neurohospitalist 2012;2:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fuller KL, Wang YY, Cook MJ, Murphy MA, D’Souza WJ. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: a prospective randomized study. Epilepsia 2013;54:45–57. [DOI] [PubMed] [Google Scholar]
- [38].Farrokh S, Tahsili-Fahadan P, Ritzl EK, Lewin JJ 3rd, Mirski MA Antiepileptic drugs in critically ill patients. Crit Care 2018;22:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Raina KD, Rittenberger JC, Holm MB, Callaway CW. Functional Outcomes: One Year after a Cardiac Arrest. BioMed research international 2015;2015:283608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197–206. [DOI] [PubMed] [Google Scholar]
- [41].Oh SH, Park KN, Shon YM, Kim YM, Kim HJ, Youn CS, et al. Continuous Amplitude-Integrated Electroencephalographic Monitoring Is a Useful Prognostic Tool for Hypothermia-Treated Cardiac Arrest Patients. Circulation 2015;132:1094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].De Marchis GM, Pugin D, Meyers E, Velasquez A, Suwatcharangkoon S, Park S, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology 2016;86:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


