Abstract
Tau is a protein that is highly enriched in neurons and was originally defined by its ability to bind and stabilize microtubules. However, it is now becoming evident that the functions of tau extend beyond its ability to modulate microtubule dynamics. Tau plays a role in mediating axonal transport, synaptic structure and function, and neuronal signaling pathways. Although tau plays important physiological roles in neurons, its involvement in neurodegenerative diseases, and most prominently in the pathogenesis of Alzheimer disease (AD), has directed the majority of tau studies. However, a thorough knowledge of the physiological functions of tau and its post-translational modifications under normal conditions are necessary to provide the foundation for understanding its role in pathological settings. In this review, we will focus on human tau, summarizing tau structure and organization, as well as its posttranslational modifications associated with physiological processes. We will highlight possible mechanisms involved in mediating the turnover of tau and finally discuss newly elucidated tau functions in a physiological context.
Keywords: tau, microtubules, axonal transport, posttranslational modifications, dendrites
1. OVERVIEW.
It has been more than 40 years since a heat stable protein was first isolated from porcine brain that was essential for restoring normal features of in vitro microtubule assembly. In the original report it was noted that “we call this factor tau (τ) for its ability to induce tubule formation” (Weingarten et al., 1975). Given this ability to promote microtubule organization, it was postulated that the primary role of tau in the cell was to regulate microtubule assembly. This postulation guided the majority of the studies on the function of tau for decades. However, it is now evident that the capacity of tau to regulate microtubule dynamics in vitro may not be its unique function in neurons. For example, tau plays a role in protecting microtubules from severing (Jean and Baas, 2013), and regulates synaptic function (Kimura et al., 2013; Pooler et al., 2014), microtubule transport (Dixit et al., 2008) and the activation of MAP kinases (Leugers et al., 2013). These and other findings indicate that there is a need to re-evaluate the different roles of tau in the brain.
The first identified (Cleveland et al., 1977) and most thoroughly studied posttranslational modification of tau is phosphorylation. Initial studies provided evidence that global dephosphorylation of tau greatly enhanced its ability to bind and stabilize microtubules (Lindwall and Cole, 1984). Although the overall phosphorylation state of tau does impact its capacity to modulate microtubule dynamics, it is now evident that phosphorylation of specific sites is of more physiological relevance. Indeed, numerous studies have shown that the phosphorylation of specific sites differentially impacts the functions of tau (Mi and Johnson, 2006). In addition to phosphorylation, tau undergoes a number of other types of posttranslational modifications. Tau is glycosylated, and the glycosylated state of tau can regulate phosphorylation (Liu et al., 2004). Likewise, tau is acetylated, and previous findings propose that it may have intrinsic acetyltransferase activity and undergo self-acetylation (Cohen et al., 2013). In pathological conditions tau can be cleaved, glycated, nitrated and oxidized (Martin et al., 2011). Many, if not all these modifications impact the function of tau at some level, and therefore need to be considered when evaluating the implications of tau in normal conditions and in pathologic processes.
In addition to the physiological roles of tau in neurons, it plays a pivotal role in a group of neurodegenerative diseases known as tauopathies. Primary tauopathies are diseases in which tau plays the primary role. These include: frontotemporal lobar degeneration-tau (FTLD-tau), Progressive Supranuclear Palsy (PSP), Corticobasal degeneration (CBD), Sporadic Multiple System Tauopathy, and Pick’s disease. Secondary tauopathies are diseases in which tau is a significant contributor to disease pathogenesis but other factors are upstream or major factors in the disease process. These secondary tauopathies include: Chronic Traumatic Encephalopathy (CTE). Creutzfeldt-Jakob disease and Alzheimer’s disease (AD) (for reviews see: (Irwin et al., 2015; Kovacs, 2015). Of these tauopathies, the majority of studies have focused on AD.
In 1986, tau was first identified by several groups (Grundke-Iqbal et al., 1986; Kosik et al., 1986; Wood et al., 1986) as a component of the neurofibrillary tangles in AD brain using immunocytochemistry. This finding catapulted tau into the limelight and initiated an avalanche of tau-centric studies. However, with the discovery that mutations in Amyloid Precursor Protein (APP) (Goate et al., 1991) and presenilins (Campion et al., 1995; Levy-Lahad et al., 1995; Sherrington et al., 1995), both proteins involved in the processing and formation of Amyloid-β (Aβ), were causative in familial forms of AD, focus shifted away from tau, and tau pathology came to be viewed as a secondary hallmark for AD (Hardy and Selkoe, 2002). Although no tau mutations have been found to be associated with AD, in 1998 it was discovered that tau mutations lead to FTLD-tau (also known as FTDP-17) (Hutton et al., 1998; Poorkaj et al., 1998). These findings reinvigorated the tau field as they demonstrated that mutations in tau alone could result in neurodegenerative disease. Soon after the discovery that tau mutations were causative in FTLD-tau, it was demonstrated that tau was a mediator of Aβ-induced neurotoxicity. In 2002 it was shown that in the presence of tau in neurons sensitized them to Aβ-mediated damage (Rapoport et al., 2002). In subsequent studies using mouse models, it was demonstrated that the presence of tau was essential to promote Aβ-mediated neuronal dysfunction (Ittner et al., 2010; Roberson et al., 2007). During this time there was a growing awareness that the neurofibrillary tangles were likely not the primary neurotoxic entities (Santacruz et al., 2005), but rather soluble tau species either as post-translationally modified monomers or oligomers (Berger et al., 2007; Lasagna-Reeves et al., 2011; Leugers et al., 2013; Ward et al., 2012). However, the mechanisms by which pathological tau exerts its toxicity remain to be fully elucidated (Guo et al., 2017; Perez et al., 2018; Wang and Mandelkow, 2016).
In this review, we will start with a summary of tau, expression, structure and organization, as well as posttranslational modifications associated with normal functions of tau. We will then highlight possible mechanisms involved tau turnover followed by a discussion of different physiological functions of tau.
2. EXPRESSION AND DISTRIBUTION OF TAU
The tau gene in humans is situated on the long arm of chromosome 17 at 17q21 (Neve et al., 1986) with three different tau transcripts (Couchie et al., 1992; Georgieff et al., 1993; Goedert et al., 1989a; Goedert et al., 1989b; Wang et al., 1993). A 2kb tau transcript containing the entire tau coding region primarily encodes for tau that localizes in the nucleus (Wang et al., 1993). A 6kb transcript of tau is mainly found in the brain and is enriched in neurons (Goedert et al., 1989a; Goedert et al., 1989b), while the 8kb transcript is in the retina and peripheral nervous system (PNS) (Couchie et al., 1992; Georgieff et al., 1993). All these tau genes contain 16 exons, 8 of which are alternatively spliced. Exon 1 belongs to the promoter and is transcribed but not translated, and although exon 14 exists in mRNA, it does not encode for the protein. Exons 1, 4, 5, 7, 9, 11, 12 and 13 are normally expressed in adult human central nervous system (CNS) and PNS (Andreadis, 2005; Andreadis et al., 1992; Goedert et al., 1989a; Goedert et al., 1989b; Sawa et al., 1994). Exon 4a is constitutively expressed in the adult human PNS and the retina but is not expressed in the CNS (Couchie et al., 1992). Exons 2 and 3 are alternatively spliced, and adult human tau contains exon 2, exons 2 and 3, or neither. Exon 3 is only expressed in combination with exon 2 (Goedert et al., 1989a; Goedert et al., 1989b). Tau with exon 6 appears to be expressed in the human adult CNS and PNS, but in low abundance (Luo et al., 2004). In addition to the canonical exon 6, two alternative splice sites result in frameshifts and the introduction of stop codons and thus encode tau variants (6p and 6d) that cannot bind microtubules (Andreadis, 2005; Luo et al., 2004). Exon 8 was reported to be expressed in bovine tau (Chen et al., 1994; Himmler, 1989). Exons 9, 10, 11 and 12 span the microtubule binding domains of tau. From this group, only exon 10 is spliced out or in to encode for tau with 3 or 4 microtubule binding domains (Goedert et al., 1989a; Goedert et al., 1989b). Tau containing exon 10 is present in both the human CNS and PNS, although it is regulated (spliced in or out) in the CNS, while it is constitutively expressed in the PNS (Andreadis, 2005; Goedert et al., 1989b). For more extensive discussion of the regulation of tau splicing see (Andreadis, 2005; Luo et al., 2004). Within the tau gene an intronless gene encoding for the 128 amino acid protein saitohin has been identified (Conrad et al., 2002). This gene is located in the intron between exons 9 and 10 and little is known about its function, although polymorphisms have been associated with Parkinson’s disease (Lu et al., 2014; Wider et al., 2010) and late onset AD (Huang et al., 2017).
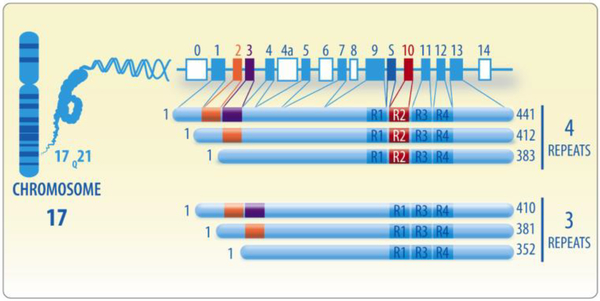
In the adult human brain, the expression of 6 predominant tau isoforms is observed (Goode and Feinstein, 1994; Gustke et al., 1994). These isoforms emerge from exons 2, 3 and 10 being alternatively spliced. Tau contains no N-terminal inserts, exon 2 (29 amino acids) or exons 2 and 3 (58-amino acids). Exon 10 can be spliced in (tau with 4 repeats) or out (tau with 3 repeats), encoding different microtubule binding sequences (Goedert et al., 1989a; Goedert et al., 1989b; Ittner and Gotz, 2011; Kolarova et al., 2012; Martin et al., 2011; Pedersen and Sigurdsson, 2015; Song et al., 2015). The results of this processing give the six tau isoforms: 0N/3R, 0N/4R, 1N/3R, 1N/4R, 2N/3R, and 2N/4R in which “R” indicates the number of microtubule repeats and “N” represent the number of Nterminal inserts. The 2N4R being the longest and 0N3R the shortest isoform comprising 441 and 352 amino acids, respectively (Pedersen and Sigurdsson, 2015). The tau protein is also defined by having specific domains (numbering is based on 441 amino acid 2N4R isoform). In the N-terminal region the first 44-amino acids contain a glycine-rich sequence, residues 45–102 encompass two highly acidic regions (N1 and N2), residues 151–243 contains two proline-rich regions (P1 and P2) and the remainder of the protein contains the four microtubule-binding domains (R1-R4) and a short C-terminal region (Pedersen and Sigurdsson, 2015). In mature human brain, the 3R and 4R tau isoforms are found in approximately equal molar ratios (1:1) (Goedert and Jakes, 1990; Hong et al., 1998), but the proportion of 1N (54%), 0N (37%), and 2N (9%) isoforms are expressed differentially (Goedert and Jakes, 1990; Hong et al., 1998). The expression of tau isoforms is also regulated during the development; in human fetal brain only tau without exons 2, 3, 4a and 10 is expressed (Goedert and Jakes, 1990; Kosik et al., 1989) (Figure 1).
Figure 1. Domains and alternative splicing of tau protein.
The MAPT (microtubule-associated protein tau) gene is situated on the long arm of chromosome 17 at band site 17q21 and encodes to six tau proteins as product of alternative splicing in the adult human brain. These isoforms are splicing variants of exons 2, 3 and 10. Tau may express no N-terminal inserts, just exon 2 or exons 2 and 3. Exon 10, which encodes a microtubule binding repeat sequence, can either be spliced in or out generating tau with 4 repeats or 3 repeats, respectively. S indicates a DNA sequence encoding the protein saithoin between exons 9 and 10.
Tau is mostly expressed in neurons with the original report indicating that it was only expressed in neurons (Binder et al., 1985). However, subsequent studies showed that it is also expressed in astrocytes of human brains (Shin et al., 1991) and oligodendrocytes of rat brains (LoPresti et al., 1995). In neurons, tau is predominantly in axons, although it is present in dendrites albeit at much lower levels (Ittner and Gotz, 2011). Moreover, tau is present in the synaptic compartment of healthy neurons and contributes to synapse physiology (for a recent review see: (Regan et al., 2016)). Posttranslational modifications of tau such as phosphorylation and acetylation have been shown to direct the localization of tau to these different compartments (Sohn et al., 2016; Tashiro et al., 1997; Xia et al., 2015). In addition, a recent study demonstrated that tau isoforms without exons 2 and 3 were efficiently sorted into the axon, while 2N4R tau was partially retained in the soma and dendrites (Zempel et al., 2017).
3. RELATIONSHIP BETWEEN TAU STRUCTURE AND FUNCTION
The ability of tau to bind microtubules is mediated by its microtubule-binding domains located in the C-terminus. The N-terminal extends from the microtubule region to facilitate its interaction with diverse components of the cytoskeleton or the plasma membrane (Gong et al., 2005; Maas et al., 2000). In each of the microtubule-binding domains there is a highly homologous 18 amino acid repeat region with inter-repeat sequences of 13–14 amino acids (R1-R2, R2-R3, R3-R4) (Goode and Feinstein, 1994; Gustke et al., 1994). Studies have provided evidence that 3R and 4R tau adopt different structures that are differentially regulated. The core microtubule binding domains of 3R and 4R tau display some similarities but also several differences, and thus the isoforms are clearly different in how they function. For example, the region immediately flanking the 3rd repeat region in 3R tau plays a significant role in facilitating its interaction with microtubules. In contrast, this region does not contribute considerably to the capacity of 4R tau to bind microtubules (Goode et al., 2000). There is also data suggesting that the regions immediately N-terminal and C-terminal to the microtubule-binding repeat domains may also regulate the targeting of tau to microtubules (Preuss et al., 1997). Further, 3R and 4R tau seem to impact microtubule motor function differentially. 4R tau reduces mitochondrial localization to axons to a greater extent than 3R tau. However, 3R tau is more efficient at increasing the percent of mitochondria moving in the retrograde direction compared to 4R tau (Stoothoff et al., 2009). Unexpectedly, in an in vitro kinesin-driven gliding assay the velocity of taxol-stabilized microtubules lacking tau, and 4R tau-stabilized microtubules was the same. In contrast, microtubules assembled with 3R tau glided at a significantly greater velocity than both taxol-stabilized and 4R tau stabilized microtubules. In all cases, the amount of tau used was in the physiological range (Peck et al., 2011). Additionally, monomeric 4R tau showed no changes on kinesin- and dynein-dependent fast axonal transport (FAT) rates (LaPointe et al., 2009). These and other studies demonstrate that the presence or absence of exon 10 affects not only the interactions of tau with microtubules but also affects other functions such as the speed of axonal transport. Also, it has been reported that 4R tau is prone to be phosphorylated in vitro and it is more likely to aggregate into filaments than 3R tau, a finding that could be relevant to the development of pathologies (Alonso Adel et al., 2004).
Although our understanding of the function of N-terminal regions encoded by exons 2 and 3 is still far from complete, studies have begun to shed light on possible functions of these domains. In the human adult brain the 2N isoform is significantly less abundant than the 1N isoform (Goedert and Jakes, 1990; Hong et al., 1998). Using isoform-specific antibodies tau expressing exon 2 was found to be highly enriched in nuclei in mouse brain, while tau expressing either no N-terminal inserts or both exons 2 and 3 was enriched in cell bodies and axons with minimal expression in the nucleus (Liu and Gotz, 2013). The different N-terminal inserts apparently do not affect microtubule assembly (Goedert and Jakes, 1990); but a recently study proposed that the N-terminal region could regulate microtubule stabilization. In this report tau truncated in the N-terminal region at Gln124 (absence of 1–124 amino acid in tau protein) bound more strongly to microtubules than full length tau and protected against depolymerization (Derisbourg et al., 2015). This last work, added to other studies under pathological conditions (Park et al., 2016), suggests that certain tau N-terminal isoforms (and in particular the 1N isoform) could favor tau aggregation and contribute to neurodegenerative processes (Park et al., 2016; Zhong et al., 2012). 0N tau isoforms are sorted efficiently into axons; however 2N4R tau is partially retained in cell bodies and dendrites, where it increased spine and dendrite growth (Zempel et al., 2017). 0N and 2N tau isoforms also bind specific proteins with differing affinities. For example, 0N4R tau was found to preferentially bind β-synuclein, while apolipoprotein A1 bound almost exclusively to 2N4R tau. Interestingly in this co-immunoprecipitation study almost half of the tau-interacting proteins identified were membrane-bound proteins; with mitochondrially localized proteins representing the largest portion of this group (Liu et al., 2016). This finding is actually not unexpected as tau has been shown to alter mitochondrial dynamics and function, mitophagy and mitochondrial transport (Hu et al., 2016; Jara et al., 2018; Li et al., 2016; Shahpasand et al., 2012). These and other findings demonstrate that the different N-terminal isoforms of tau play distinct roles in neurons.
4. POSTTRANSLATIONAL MODIFICATIONS OF TAU
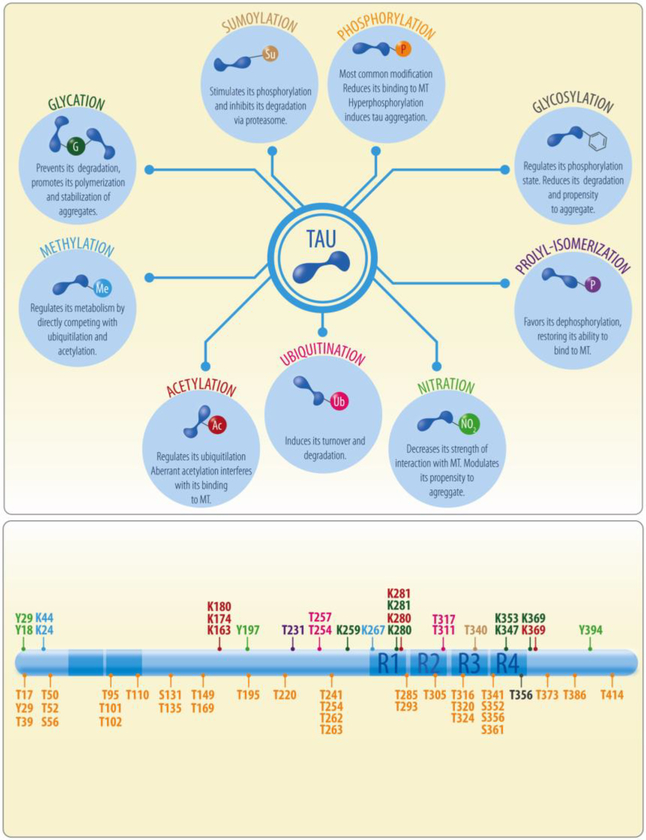
Numerous posttranslational modifications of tau have been identified and although studies often focus on pathological conditions, modifications also occur in physiological conditions. In this section, we will summarize tau modifications and how they may impact tau function (Figure 2).
Figure 2. Post-translational modifications of tau.
Tau exhibits many different post-translational modifications including phosphorylation, glycosylation, acetylation, nitration, methylation, prolyl-isomerization, ubiquitylation, sumoylation and glycation. Phosphorylation is the most recurrent post-translational modification reported; however all these modifications can regulate tau binding to microtubules (MT), its metabolism, turnover or aggregation. Lower panel shows the tau sites that undergo post-translational modifications under physiological conditions.
4.1. PHOSPHORYLATION
Phosphorylation is the most studied post-translational modification of tau (Bramblett et al., 1993; Mandelkow et al., 1995; Mi and Johnson, 2006). 2N4R tau from human brain has 85 potential phosphorylation sites (45 serines, 35 threonines, and 5 tyrosines) (Martin et al., 2011; Mietelska-Porowska et al., 2014). Using phosphorylation-dependent antibodies against tau as well as mass spectrometry and sequence analysis, more than 31 phosphorylation sites have been identified and associated with physiological functions (Table 1) (Hasegawa et al., 1998; Lovestone and Reynolds, 1997; Morishima-Kawashima et al., 1995; Paudel and Li, 1999; Roder et al., 1997; Takahashi et al., 1995). In addition, 16 other phosphorylation sites have been reported both in physiological and pathological conditions (Martin et al., 2011). Three class of protein kinases can phosphorylate tau: 1) proline-directed serine/threonine-protein kinases including GSK-3, Cdk5 and MAPK; 2) non-proline-directed serine/threonine-protein kinases, such as TTBK1/2, CK1, DYRK1A, MARK, Akt, PKA, PKC, AMPK and CaMKII, and 3) tyrosine kinases such as Src, Fyn, Abl, and Syk (Martin et al., 2011). Tau can be dephosphorylated by a number of phosphatases including PP1, PP5, PP2B and PP2A, with PP2A being the main phosphatase activity in the brain (Liu et al., 2005). The phosphorylation state of tau is regulated by a balance between the activities of tau kinases and phosphatases which thus regulate its function, with GSK-3β and PP2A playing prominent roles (Avila, 2008). Most sites on tau that are phosphorylated are outside the microtubule-binding domains, except Ser262 (R1), Ser285 (R1–R2 inter-repeat), Ser305 (R2–R3 inter-repeat), Ser324 (R3), Ser352 (R4) and Ser356 (R4) (Goedert et al., 1989b; Roder et al., 1997; Seubert et al., 1995). A large number of phosphorylated residues are Ser–Pro and Thr–Pro motifs; although sites on non-Ser/Thr–Pro have also been described, including Ser262 (Hasegawa et al., 1992; Morishima-Kawashima et al., 1995) (for more detail see Table 1 and 2). Although it is well established that tau hyperphosphorylation reduces its affinity for microtubules (Buee et al., 2000; Hanger et al., 2009; Sergeant et al., 2008), the phosphorylation of specific sites have differential effects on tau function. Tau phosphorylation at Ser262, Ser293, Ser324, and Ser356, localized in the KXGS motif of R1, R2, R3 and R4 domains respectively, reduced tau binding to microtubules (Dickey et al., 2007; Drewes et al., 1995). Tau phosphorylation at Thr231, Ser235, and Ser262 similarly affect the binding of tau from microtubules, dissociating 26%, 9% and 33% respectively (Sengupta et al., 1998).
Table 1.
Kinase phosphorylation sites of the Tau protein.
| Target Action | Kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GSK-3 | CdK5 | JNK | CK1 | Dyrk1A | AMPK | MARKs | PKA | TPKI & II | ||
| Serine (S) | 46 | [3] | - | - | - | - | - | - | - | - |
| 50 | [3] | - | - | - | - | - | - | - | - | |
| 113 | - | - | - | [3] | - | - | - | - | - | |
| 131 | - | - | - | [3] | - | - | - | - | - | |
| 178 | - | - | - | - | - | - | - | - | - | |
| 184 | - | - | - | A | - | - | - | - | - | |
| 185 | - | [4] | - | [3] | - | - | - | - | - | |
| 195 | [2] | [5] | - | - | - | - | - | - | - | |
| 198 | [2] | [5] | - | A | - | - | - | [3] | - | |
| 199 | [2] | [5] | [6] | [3] | [9];[10] | - | - | - | [15](I)e | |
| 202 | [1]; [2] | [4]; [5] | [6] | [7];[8]/T205b | [9];[10] | - | - | - | [16](II)f | |
| 208 | - | - | - | [3] | - | - | - | [3] | - | |
| 210 | [3] | - | - | - | - | - | - | - | - | |
| 214 | - | - | [6]/T212a | [3] | - | - | - | [3];[14] | - | |
| 235 | [2]; [3] | [5] | - | [3] | - | - | - | - | [16](II)f | |
| 237 | [2]; [3] | - | - | [3] | - | - | - | - | - | |
| 238 | [2] | - | - | [3] | - | - | - | - | - | |
| 239 | - | [5] | - | - | - | - | - | - | - | |
| 241 | [3] | - | - | A | - | - | - | - | - | |
| 258 | [3] | - | - | [3] | - | - | - | [3] | - | |
| 262 | [2] | - | - | [3] | - | - | [12];[13] | [14] | - | |
| 285 | [3] | - | [3] | - | - | - | - | - | ||
| 289 | [3] | - | - | [3] | - | - | - | - | - | |
| 293 | - | - | - | - | - | - | [12];[13] | - | - | |
| 296 | - | [5] | [6] | - | - | - | - | - | - | |
| 305 | [3] | - | - | [3] | - | - | - | [3] | ||
| 324 | [3] | - | - | - | - | - | [12];[13] | [3] | - | |
| 341 | - | - | - | [3] | - | - | - | - | - | |
| 352 | [3] | - | - | [3] | - | - | - | [3] | ||
| 356 | [2]; [3] | - | - | [3] | - | - | [3];[12];[13] | - | - | |
| 361 | - | - | - | [3] | - | - | - | - | - | |
| 396 | [1]; [2]; [3] | [4] | - | [7];[8]/S404c | [9];[10] | [11]/S404d | - | - | [15] (I)e | |
| 400 | [2]; [3] | [5] | - | [3] | [9];[10] | - | - | - | - | |
| 404 | [1];[2] | [4] | [6] | [7];[8]/S396c | [9];[10] | [11]/S396d | - | - | [16](II)f | |
| 409 | [3] | - | - | - | - | - | - | [3];[14] | - | |
| 412 | - | - | - | A | - | - | - | [3] | - | |
| 413 | - | - | - | [3] | - | - | - | [3] | [15](I)e | |
| 416 | - | - | - | [3] | - | - | - | - | - | |
| 422 | - | - | [6] | - | [9];[10] | - | - | - | - | |
| 433 | - | - | - | [3] | - | - | - | - | - | |
| 435 | - | - | - | [3] | - | - | - | [3] | - | |
| Threonine (T) | 17 | - | - | - | [3] | - | - | - | - | - |
| 95 | - | - | - | [3] | - | - | - | - | - | |
| 111 | - | [4] | - | - | - | - | - | - | - | |
| 149 | [3] | - | - | [3] | - | - | - | - | - | |
| 169 | - | - | - | [3] | - | - | - | - | - | |
| 175 | [3] | [4] | - | - | - | - | - | - | - | |
| 176 | - | - | - | - | - | - | - | - | - | |
| 181 | [2]; [3] | [4] | [6] | - | [9];[10] | - | - | - | - | |
| 205 | [2] | [5] | [6] | [7];[8]/S202b | [9];[10] | - | - | - | [16](II)f | |
| 212 | [2] | - | [6] | - | [9];[10] | - | - | - | - | |
| 217 | [2]; [3] | [5] | [6] | - | [9];[10] | - | - | - | - | |
| 220 | [3] | [5] | - | - | - | - | - | - | - | |
| 231 | [2]; [3] | [4] | [6] | - | [9];[10] | [11] | - | - | [15] (I)e | |
| 245 | [3] | - | - | - | - | - | - | [3] | ||
| 263 | - | - | - | [3] | - | - | - | - | - | |
| 361 | - | - | - | [3] | - | - | - | - | - | |
| 373 | [3] | - | - | [3] | - | - | - | - | - | |
| 386 | [3] | - | - | - | - | - | - | - | - | |
| 403 | - | [5] | - | - | - | - | - | - | - | |
T212 phosphorylation needed
Simultaneous phosphorylation by CK1
Simultaneous phosphorylation by CK1
Simultaneous phosphorylation by Dyrk1A
TPKI target
TPKII target
References Table 1
Lovestone, S., et al., Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol, 1994. 4(12): p. 1077–86.
Reynolds, C.H., et al., Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem, 2000. 74(4): p. 1587–95.
Hanger, D.P., et al., Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem, 2007. 282(32): p. 23645–54.
Noble, W., et al., Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron, 2003. 38(4): p. 555–65.
Cruz, J.C., et al., Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron, 2003. 40(3): p. 471–83.
Yoshida, H., et al., Phosphorylation of microtubule-associated protein tau by isoforms of c- Jun N-terminal kinase (JNK). J Neurochem, 2004. 90(2): p. 352–8.
Singh, T.J., I. Grundke-Iqbal, and K. Iqbal, Phosphorylation of tau protein by casein kinase-1 converts it to an abnormal Alzheimer-like state. J Neurochem, 1995. 64(3): p. 1420–3.
Li, G., H. Yin, and J. Kuret, Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J Biol Chem, 2004. 279(16): p. 15938–45.
Liu, F., et al., Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J, 2008. 22(9): p. 3224–33.
Wegiel, J., C.X. Gong, and Y.W. Hwang, The role of DYRK1A in neurodegenerative diseases. FEBS J, 2011. 278(2): p. 236–45.
Vingtdeux, V., et al., AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol, 2011. 121(3): p. 337–49.
Augustinack, J.C., et al., Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol, 2002. 103(1): p. 26–35.
Mocanu, M.M., et al., The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J Neurosci, 2008. 28(3): p. 737–48.
Liu, F., et al., Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci, 2007. 26(12): p. 3429–36.
Ishiguro, K., et al., Phosphorylation sites on tau by tau protein kinase I, a bovine derived kinase generating an epitope of paired helical filaments. Neurosci Lett, 1992. 148(1–2): p. 202–6.
Ishiguro, K., et al., A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett, 1991. 128(2): p. 195–8.
Table 2.
Phosphatase dephosphorylation sites of the Tau protein.
| Target Action | Phosphatase | ||||
|---|---|---|---|---|---|
| PP2B | PP2A | PP1 | PP5 | ||
| Serine (S) | 178 | - | - | - | - |
| 184 | - | - | - | - | |
| 185 | - | - | - | - | |
| 195 | - | - | - | - | |
| 198 | - | - | - | - | |
| 199 | [1] | - | - | [4](2nd)a | |
| 202 | - | [2]b | - | [4](2nd)a | |
| 210 | - | - | - | - | |
| 214 | - | - | - | [4](2nd)a | |
| 235 | - | - | - | - | |
| 237 | - | - | - | - | |
| 238 | - | - | - | - | |
| 239 | - | - | - | - | |
| 258 | - | - | - | - | |
| 262 | [1]a | - | [3](33%)c | [4](3th)a | |
| 289 | - | - | - | - | |
| 293 | - | - | - | - | |
| 296 | - | - | - | - | |
| 324 | - | - | - | - | |
| 356 | - | - | - | - | |
| 396 | [1]a | - | [3](12%)c | [4](2nd)a | |
| 400 | - | - | - | - | |
| 404 | - | - | - | [4](2nd)a | |
| 409 | - | - | - | [4](1st)a | |
| 413 | - | - | - | - | |
| 422 | [1] | - | [3](31%)c | - | |
| Threonine (T) | 69 | - | - | - | - |
| 111 | - | - | - | - | |
| 175 | - | - | - | - | |
| 176 | - | - | - | - | |
| 181 | - | - | - | - | |
| 205 | - | [2]b | - | [4](1st)a | |
| 212 | - | - | [3](40%)c | [4](1st)a | |
| 217 | [1] | - | [3](26%)c | - | |
| 220 | - | - | - | - | |
| 231 | - | - | - | - | |
| 403 | - | - | - | - | |
References Table 2
Rahman, A., I. Grundke-Iqbal, and K. Iqbal, PP2B isolated from human brain preferentially dephosphorylates Ser-262 and Ser-396 of the Alzheimer disease abnormally hyperphosphorylated tau. J Neural Transm (Vienna), 2006. 113(2): p. 219-30.
Landrieu, I., et al., Molecular implication of PP2A and Pin1 in the Alzheimer's disease specific hyperphosphorylation of Tau. PLoS One, 2011. 6(6): p. e21521.
Rahman, A., I. Grundke-Iqbal, and K. Iqbal, Phosphothreonine-212 of Alzheimer abnormally hyperphosphorylated tau is a preferred substrate of protein phosphatase-1. Neurochem Res, 2005. 30(2): p. 277-87.
Liu, F., et al., Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer's disease. J Biol Chem, 2005. 280(3): p. 1790-6.
Moreover, tau phosphorylation regulates the subcellular localization of tau. The antibody Tau1 was originally shown to preferentially label axons, and thus tau isoforms were tagged as “axonal proteins” (Binder et al., 1985). Subsequently it was found that Tau-1 preferentially recognizes tau when it is unphosphorylated between amino acids 189 and 207 (Biernat et al., 1992; Szendrei et al., 1993), and when brain sections were treated with phosphatase Tau-1 immunoreactivity was found in the soma and dendrites of neurons (Papasozomenos, 1997). Phosphorylation regulates tau’s localization to the plasma membrane (Pooler et al., 2012), nucleus (Sultan et al., 2011) and exocytotic vesicles (Tang et al., 2015), among other intracellular sites (Noble et al., 2013). Tau phosphorylation at Ser262 and Ser356 in the microtubule binding domains is necessary for the development of cell processes, while phosphorylation of Ser/Thr Pro motifs in the regions proximal to microtubule binding domains block neurite outgrowth (Biernat and Mandelkow, 1999). The phosphorylation of Ser262/356 is mediated by microtubule affinity-regulating kinases (MARK) (Biernat et al., 2002), decreasing taumicrotubule affinity, enhancing the dynamics of microtubules and finally leading to the development of neuronal processes and cell polarity (Biernat et al., 2002).
4.2. LYSINE POST-TRANSLATIONAL MODIFICATIONS ON TAU
More than 40 lysine residues are present in human tau protein, which are prone to different post-translational modifications, including acetylation, ubiquitylation, and SUMOylation. These modifications differentially regulate tau function, and in several cases with contradicitng effects.. For example, acetylation and SUMOylation can block ubiquitylation-dependent tau degradation (Figure 2).
Acetylation is controlled by two families of enzymes, histone acetyltransferases (HATs) that promote acetylation, and histone deacetylases (HDACs) which remove acetyl groups (Zhang et al., 2012). Tau can be acetylated at Lys163, Lys174, Lys280, and potentially Lys281 and Lys369 (Cohen et al., 2011; Min et al., 2010; Sohn et al., 2016). Tau acetylation may control the extent of tau ubiquitylation (Cohen et al., 2011), and neutralize charges in the microtubule binding domain, interfering with its ability to bind to microtubule, and leading to tau dysfunction (Kolarova et al., 2012; Sohn et al., 2016). Specifically, tau acetylation on K280 (located in the 275VQIINKK280 region) is one of three lysine residues of importance in the modulation of tau-microtubule association (Kolarova et al., 2012). This acetylation likely impairs the binding between tau and microtubules, generating an increase in cytosolic tau which could be considered an initiating step in tangle formation (Cohen et al., 2011; Kolarova et al., 2012), along with the acetylation-induced increases in the stability and half-life of tau (Min et al., 2010). In fact, lysine acetylation-deacetylation has been proposed as a possible regulatory modification for AD and other neurodegenerative diseases (Kolarova et al., 2012). Further, there is evidence that tau Lys280/Lys281 acetylation induces pathogenic hallmarks consistent with a “two-hit” mechanism, in which tau acetylation disengages tau from microtubules reducing their stability and also promotes tau aggregation (Trzeciakiewicz et al., 2017).
Ubiquitylation consists in the attachment of one or more ubiquitin subunits (a 76-amino acid protein) to lysine residues (Rape, 2017), which can result in protein monoubiquitylation or polyubiquitylation. Monoubiquitylation can regulate diverse processes, including DNA repair, histone function, gene expression and receptor endocytosis. Polyubiquitylation (addition of at least 4 Ubs) is necessary for degradation of proteins in an ATP-dependent manner via the ubiquitin–proteasome system (UPS) (Sadowski et al., 2012), or by autophagy (Yao, 2010). Monoubiquitinylated tau also can be subjected to lysosomal degradation (Avila et al., 2004). In pathological conditions tau is ubiquitylated at residues Lys254, Lys257, Lys311 and Lys317 (Morishima-Kawashima et al., 1993). In vitro, overexpression studies showed that the E3 ubiquitin ligase CHIP (C-terminus of HSC70-interacting protein) interacts with Hsp70/90 and promotes ubiquitylation of tau (Petrucelli et al., 2004). Similarly, the presence or absence of CHIP could regulate that ubiquitylation of tau and may favor the clearance of tau aggregates (Dickey et al., 2006). Studies in neuronal systems and under physiological conditions are necessary to validate these observations.
SUMOylation is the covalent addition of Small Ubiquitin-like Modifier Proteins (SUMO) to target lysines (Dorval and Fraser, 2006). This post-translational modification can affect protein-protein interactions, subcellular localization, and protein stability (Dorval and Fraser, 2006). Tau can be sumoylated at Lys340 in vitro by SUMO1 and to a lesser extent by SUMO2 and SUMO3 (Dorval and Fraser, 2006; Takahashi et al., 2008). The SUMOylation of tau at Lys340 promoted its hyperphosphorylation and reduced its ubiquitylation, suggesting that tau SUMOylation is a pathologic event potentially preventing degradation of tau and promoting its aggregation (Dorval and Fraser, 2006; Luo et al., 2014).
4.3. GLYCOSYLATION
Glycosylation is the covalent addition of oligosaccharides (mono- or polysaccharides) to proteins (Ohtsubo and Marth, 2006). In general, two types of glycosylation exist: N-glycosylation is when the oligosaccharide attachment is through an N-linkage to the N-terminal group of asparagine amino acid, and the second glycosylation, called O-glycosylation, is when the attachment is through the hydroxyl oxygen of serine, threonine, or tyrosine residues (Ohtsubo and Marth, 2006). Both glycosylation modifications have been reported in tau (Avila et al., 2004) and it has been suggested that N-glycosylation occurs on hyperphosphorylated tau, while unmodified tau can be O-glycosylated (Liu et al., 2002).
A special type of O-glycosylation of tau, O-GlcNAcylation (attachment of N-acetyl-glucosamine to serine or threonine residues), has been described (Arnold et al., 1996). This modification is somewhat analogous to phosphorylation (Marcus and Schachter, 2011), and tau contains 11 putative O-glycosylation sites and there are ~4 moles of O-GlcNAc per mole of bovine tau (Arnold et al., 1996). Glucosaminyl transferase (OGT) adds O-GlcNAc to proteins and hexosaminidase O-GlcNAcase (OGA) catalyzes its removal (Dias and Hart, 2007). Rats treated with inhibitors of OGA exhibited reduced tau phosphorylation in the cortex and hippocampus, indicating that GlcNAcylation may prevent phosphorylation (Yuzwa et al., 2008). Indeed, there is data to suggest that O-GlcNAcylation helps maintain tau association with microtubules by preventing its hyperphosphorylation and aggregation into NFTs (Marcus and Schachter, 2011). In vitro, O-GlcNAcylation of tau at Ser356 reduced tau’s susceptibility to aggregate by shifting the conformation of the R4 microtubule binding domain (Yu et al., 2008a). On the other hand, O-GlcNAcylated tau may be less efficiently degraded through the ubiquitin proteasome pathway (Han and Kudlow, 1997). Overall, it is clear that O-glycosylation of tau is a physiological process that likely helps maintain tau in its functional state and attenuate tau’s oligomerization and filament formation.
4.4. NITRATION
Nitration is the formation of 3-nitrotyrosine on specific tyrosine residues and mediated by reactive nitrogen species such as NO2, or ONOO- (Ischiropoulos, 2003). Tau can be nitrated in vitro, and there is also evidence that in vivo tau is nitrated, particularly in pathological conditions such as AD (Reynolds et al., 2006b). In vitro human tau is nitrated at four sites: Tyr18, Tyr29, Tyr197 and Tyr394 (Reynolds et al., 2005). Interestingly, the addition of a nitrotyrosine at Tyr29 or Tyr197 increases the formation of filamentous tau aggregates in vitro, while nitration of Tyr18 or Tyr394 decreases the susceptibility of tau to generate filamentous aggregates (Reynolds et al., 2005). Nitration decreases the strength with which tau and microtubules interact, thereby decreasing tubulin assembly (Reynolds et al., 2006a). To detect tau nitration, two monoclonal antibodies have been used, taunTyr197 and tau-nTyr394, recognizing human tau nitrated at Tyr197 and Tyr394, respectively (Reyes et al., 2011). These antibodies revealed that tau nitration at Tyr394 could not be detected in any pathological lesions characteristic of AD (Reyes et al., 2011). However, tau nitrated at Tyr197 is present in soluble tau fractions from control brain samples, as well as in insoluble paired helical filament (PHF) tau fractions from AD brain (Reyes et al., 2011); suggesting that nitration at Tyr197 may have normal biological functions (Reyes et al., 2011). Nitration may contribute to the development of tau aggregates by inducing filament formation (Cappelletti et al., 2004; Reynolds et al., 2006b). Moreover, nitrated tau may negatively affect cytoskeleton assembly in neurons, as nitrated tau binds microtubules with a lower affinity compared to non-nitrated tau (Pevalova et al., 2006).
4.5. METHYLATION
Methylation of tau can occur on arginine and lysine residues, a modification that is catalyzed by methyltransferases. Mono- or dimethylation represent the major physiological lysine modification on tau from normal human brain (Funk et al., 2014). Methylation may regulate tau metabolism by competing with ubiquitylation and acetylation because both post-translational modifications participate in directing tau turnover. Lysine methylation, both mono- and di-methyl, is a physiological tau post-translational modification in human brain which could protect against tau aggregation (Funk et al., 2014). However, seven lysine residues were also shown to be methylated in PHF-tau isolated from AD brain, but the direct effects of these methylation events on tau’s function are unknown (Funk et al., 2014; Thomas et al., 2012). Tau methylation sites were found distributed across both projection and assembly domains of tau (Funk et al., 2014). In the projection domain, Lys24 and Lys44 were found to be methylated and lie adjacent to putative caspase and calpain cleavage sites, respectively. In the microtubule-binding repeat region, where the majority of methylated Lys residues were identified, methylated sites overlapped with three out of five ubiquitylation sites on AD-derived filamentous tau indicating there is likely crosstalk between these two modifications of tau (Funk et al., 2014). Further, there also appears to be interactions between lysines that are methylated and nearby phosphorylation sites. For example, when Lys267 is methylated in PHF-tau, phosphorylation of Ser262 appears more frequently (Thomas et al., 2012). Although methylation seems to be mostly regulating physiological tau function, methylation of several sites such as Lys267 could also be associated with tau pathology.
4.6. PROLYL-ISOMERIZATION
Peptidyl-prolyl isomerases (PPIs) interconvert cis and trans isomers of peptide bonds N-terminal to proline residues in the protein. Cyclophilins and FKBPs are two major families of PPIases (Zhou et al., 2000). However, the PPI that predominantly acts on tau is Pin1 (Shen et al., 1998), which isomerizes only phospho-Ser/Thr-Pro motifs, and in particular binds to tau C-terminal to phosphoThr231 (Kolarova et al., 2012; Martin et al., 2011; Zhou et al., 2000). Moreover, tau prolyl isomerization occurs only at the Thr231 site (Bulbarelli et al., 2009; Zhou et al., 2000). The change from cis to trans in tau residues, leads to a spatial change of the peptide chain permitting PP2A access and subsequent dephosphorylation of tau at Thr231, thus allowing tau to once again bind to microtubules (Lu et al., 1999; Zhou et al., 2000). Pin1 also interacts with tau to prevent its pathological accumulation (Martin et al., 2011; Nakamura et al., 2013). However, in AD, modifications of tau may result in an impairment of Pin1 function (Balastik et al., 2007). Nakamura et al. developed cis and trans-specific antibodies against phosphorylated tau and subsequently determined that cis, but not trans phosphorylated tau is observed early in degenerating neurons in AD brains (Nakamura et al., 2012). Also, the cis isomer is unable to induce microtubule assembly and is less prone to dephosphorylation and degradation (Nakamura et al., 2012). Cis tau phosphorylation also occurs after traumatic brain injury (TBI) in humans and mice and impairs axonal microtubule networks and transport. Treatment of mice following traumatic brain injury with the Cis tau antibody inhibited the cis-specific phosphorylation of tau and prevented functional deficits in the microtubule structure and function (Kondo et al., 2015). In conclusion, cis isomerization of peptide bonds within tau may facilitate potential hyperphosphorylation, aggregation, and tauopathy.
4.7. GLYCATION
Glycation is the non-enzymatic covalent incorporation of carbohydrates (fructose, mannose, or glucose) into a protein most often on lysine residues, and is frequently observed in aged tissues (Kikuchi et al., 2003). This reaction results in the irreversible generation of advanced glycation end products (AGEs) (Kikuchi et al., 2003). AGEs are not efficiently eliminated or released from the neuron, and thus can accumulate over time with pathological consequences (Li et al., 2012). Glycation is not detected in normal tau protein (Pevalova et al., 2006). However, pathological tau can be glycated by D-glucose or D-ribose (Chen et al., 2009; Sasaki et al., 1998) at 12 different sites, 7 of which are situated in the microtubule binding regions (Martin et al., 2011). Tau glycation prevents degradation, and promotes polymerization, stabilization of aggregates, accumulation and neuronal cell death (Necula and Kuret, 2004). PHF-tau isolated from the frontal cortex of AD patients has higher AGEs content than tau protein isolated from the frontal cortex of normal patients, suggesting that tau glycation is a pathological event (Yan et al., 1994). More specifically, PHF-tau from AD patients seems to be glycated, whereas AD soluble tau or non-demented human tau is not (Gonzalez et al., 1998), indicating that glycated tau likely is more prone to aggregate (Ledesma et al., 1994). Glycated tau isolated from AD cases also promotes oxidative stress, activates inflammatory pathways such as NFκβ, and increases Aβ production (Yan et al., 1995). Therefore, glycation of tau not only seems to be a pathological result of upstream changes in modification with age, but it may also potentiate further pathology.
4.8. PROTEOLYTIC CLEAVAGE
The proteolytic cleavage of tau protein can be carried out by numerous proteases, including caspases, calpains (Arai et al., 2005; Gamblin et al., 2003; Jarero-Basulto et al., 2013), thrombins, cathepsins and aminopeptidases (Wang et al., 2010). Below the contribution of these different proteases to tau cleavage are briefly reviewed. For a more detailed discussion of tau proteolysis see (Chesser et al., 2013).
Caspases are cysteine proteases that have essential roles in apoptosis, but also in physiological processes (Hollville and Deshmukh, 2017). In vitro tau can be cleaved by caspases 1, 3, 6, 7 and 8 at Asp421 (Gamblin et al., 2003), with the majority of studies focusing on caspase 3 cleavage at this site (Jarero-Basulto et al., 2013; Rissman et al., 2004). Tau N-terminal fragments formed by caspase cleavage are more prone to accumulate and promote the aggregation and fibril formation of full-length tau (Jarero-Basulto et al., 2013). Human tau can also be cleaved in vitro after Asp13 by caspase 6, and as a consequence the N-terminal region interacts with the C-terminal tail, preserving tau solubility (Horowitz et al., 2006). Caspases 3-, 6-, 7- and 9-cleaved tau are markers for NFT formation in AD and correlate specifically with cognitive decline (Horowitz et al., 2004; Wai et al., 2009). Pseudo-phosphorylation of tau at Ser422 inhibits caspase-cleavage of tau Asp421 in vitro, suggesting that phosphorylation of this site may be protective, by inhibiting cleavage in vivo (Guillozet-Bongaarts et al., 2006). JNK3, as well as Dyrk1A (Liu et al., 2008), is able to phosphorylate tau at Ser422 (Yoshida et al., 2004), and therefore phosphorylation of this site by these kinases could prevent tau cleavage.
Calpains are calcium-dependent cysteine proteases, with 15 ubiquitous and tissue-specific isoforms in humans. Calpain isoforms 1, 2, 3, 5, and 10 are found in the CNS, but calpain 1 (μ-calpain) and calpain 2 (m-calpain), are the two major forms that are most studied (Ono et al., 2016). The calpain-induced proteolysis of tau was reported for first time by Johnson et al, 1989; they showed that tau that co-purified with microtubules was rapidly hydrolyzed by this protease (Johnson et al., 1989). In contrast, tau directly purified from the heat-stable fraction from the brain was resistant to degradation by calpain (Johnson et al., 1989). In addition, tau phosphorylated by cAMP-dependent protein kinase (PKA) results in increased resistance to hydrolysis by calpain; suggesting that phosphorylation may regulate its degradation by this protease (Litersky and Johnson, 1992). Calpain cleavage of tau generates a 17 kDa tau, and this tau fragment (45−230) (Canu et al., 1998) is mainly associated with AD, and in vitro can induce apoptosis (Park and Ferreira, 2005).
Thrombin is a serine protease, that can truncate tau in vitro at multiple arginine and lysine sites including Arg155-Gly156, Arg209-Ser210, Arg230-Thr231, Lys257-Ser258, and Lys340-Ser341, and this proteolysis is not suppressed by phosphorylation of tau at Thr212, Thr231, or Ser396/Ser404. Thrombin cleavage has been detected in NFTs of AD, suggesting that it could be involved in tau aggregation (Arai et al., 2005). Cathepsins are proteases which are predominantly found in lysosomes. Cathepsins A and G are serine proteases, cathepsins D and E are aspartyl proteases, and the rest of the cathepsins are cysteines proteases. Cathepsins D and B are present in senile plaques in AD samples, and both recombinant and human fetal tau were cleaved in vitro by cathepsin D, suggesting that cathepsin D is of particular importance in mediating tau turnover with regards to disease (Kenessey et al., 1997). PSA (puromycin-sensitive aminopeptidase) is a M1 class of metallopeptidases present only in neurons. Studies in Drosophila models of AD showed that PSA expression decreases tau protein and prevent tau-mediated neuronal damage, in contrast to PSA dysfunction that aggravated neuronal injury. In in vitro assays PSA may suppress tau pathology through modulating tau levels (Sengupta et al., 2006).
5. TAU TURNOVER
The half-life of a protein is determined by factors that promote its elimination and/or stabilization (Avila et al., 2004; Chesser et al., 2013). Previous studies that measured the half-life of tau have used doxycycline-regulatable expression of human tau in clonal cell or mouse models (Dolan and Johnson, 2010; Yamada et al., 2014; Yamada et al., 2015), However, in a recent elegant study Sato and colleagues (Sato et al., 2018) used neurons derived from human iPSCs and the stable isotope labeling kinetics (SILK) method to measure tau turnover rate and half-life. Using this approach they found that the half-life of tau was ~6.7 days and that 4R tau, as well as phosphorylated forms, exhibited a faster turnover rate. It was also found that in the human CNS the half-life of tau was approximately 23 days. Although these results are intriguing, the authors did note a few caveats. First, iPSC-derived human neurons exhibit an immature neuron profile of tau expression. These neurons express 4R tau at a fraction of the level of 3R tau, while in mature neurons the ratio of 4R to 3R is ~1:1. Further, given the methods used to measure tau turnover, the complex metabolism of tau in the human CNS is likely a contributing factor to the relatively long half-life of tau observed in vivo (Sato et al., 2018). Nonetheless, these studies provided important insights into tau turnover.
A primary determinant of protein half-life is the rate at which it is degraded. The two main protein degradation pathways in neurons are the Ubiquitin/Proteasome System (UPS) and the autophagy and lysosome pathway (ALP), and both pathways contribute to the turnover of tau. There is also data to suggest that tau is degraded through the endolysomal pathway (Vaz-Silva et al., 2018); these studies will be discussed at the end of the autophagy section.
5.1. UBIQUITIN-PROTEASOME SYSTEM
The UPS is an intracellular proteolytic pathway that facilitates the elimination of misfolded or damaged proteins (David et al., 2002; Han et al., 2014; Li and Chin, 2007), turnover of short-lived proteins (David et al., 2002), and regulation of differentiation, neurotransmission, and apoptosis (Li and Chin, 2007). For UPS-mediated degradation, substrate proteins are initially tagged by covalent linkage to various molecules of ubiquitin (Li and Chin, 2007) by the action of the enzymes E1, E2 and E3 (Eriksen, 2013). The E1 enzyme forms a thio-ester bound with ubiquitin at the expense of ATP, then E1 bound to activated ubiquitin recognizes E2 and transfers the ubiquitin. Finally E3 recognizes the substrate and transfers the ubiquitin from E2 to the client protein (Li and Chin, 2007). The 26S proteasome is formed of a central catalytic 20S core containing the active protease sites, and 19S regulatory complex caps at the ends of the 20S core (forming the 26S proteasome) (David et al., 2002; Eriksen, 2013; Han et al., 2014; Layfield et al., 2003; Li and Chin, 2007). Alternatively the 20S core can be capped with two 11S regulatory which gives rise to the immunoproteasome (David et al., 2002). The polyubiquitylated protein (with at ubiquitin chain of at least 4 molecules) is first recognized by the 19S complex where the attached target protein is unfolded before entry into 20S proteolytic core for its cleavage into small fragments (Eriksen, 2013; Layfield et al., 2003; Li and Chin, 2007). The polyubiquitin chain is removed from the substrate before entering the proteolytic core and is recycled to free ubiquitin by the action of deubiquitylating enzymes (DUBs) (Li and Chin, 2007). Proteasomes cleave their substrates to short peptides containing between six and ten amino acids, which are then degraded by different proteases to generate free amino acids (David et al., 2002).
A relationship between tau and the proteasome was initially demonstrated in vitro using lactacystin, (a specific inhibitor of the 20S core) demonstrating that the degradation of tau is blocked (David et al., 2002). Moreover, in vitro unfolded native tau was eliminated by the 20S proteasome in the absence of the addition of ubiquitin, indicating that a ubiquitin-independent pathway may mediate the degradation of unfolded tau by the proteasome (David et al., 2002; Grune et al., 2010; Keck et al., 2003). In vitro, 20S proteasome isolated from bovine pituitary, which constitutively expresses 3 different catalytic activities (chymotrypsin-like, trypsin-like and glutamyl), degraded tau approximately 10- fold slower than immunoproteasomes (which expresses inducible catalytic counterparts) isolated from bovine spleen (Cardozo and Michaud, 2002). 20S proteasomes activated by agonists (chlorpromazine or chemically manipulated forms of chlorpromazine) are able to degrade intrinsically disordered proteins such as tau, but not structured proteins, in vitro and in cell lines culture (Jones et al., 2017). Decreased proteasome function in brains of AD patients has been reported, showing distinct regional differences in proteasome activity that correlated with the presence of PHF-tau (Keck et al., 2003). Further studies have shown that normal tau is present in the pre- and post-synaptic regions and hyperphosphorylated tau can accumulate at both sites in human AD brains, and this synaptic accumulation of phospho-tau correlates with negative alterations in the function of the UPS (Tai et al., 2012). Therefore, the UPS could be involved in the accumulation/degradation of tau in both physiological and pathological conditions (Chesser et al., 2013; Wang and Mandelkow, 2012). Interestingly, data indicate that in cultured primary neurons proteasome inhibition does not lead to tau accumulation or aggregation, but in contrast promotes its degradation (Delobel et al., 2005) due to increased tau degradation by autophagy (Kruger et al., 2012).
5.2. AUTOPHAGY-LYSOSOME PATHWAY
Autophagy, is a catabolic process of “self-eating”, to degrade damaged components of the cell though lysosomes. Three different forms of autophagy are known: microautophagy, a non-selective lysosomal degradative process, characterized by direct engulfment of cellular components (Sahu et al., 2011); chaperone-mediated autophagy (CMA), in which chaperone complexes recognize the KFERQ motif present in the target protein (Kaushik and Cuervo, 2012); and macroautophagy, which requires initially the sequestration of the substrates in double-membrane vesicles called autophagosomes which subsequently fuse with lysosomes. Substrates targeted for degradation can be sequestered by the autophagosome through two different forms, selectively by autophagic adapter proteins that recognize the substrate, or through non-selective engulfment of cytoplasmic material (Nixon, 2013).
Studies indicate that tau can be eliminated by selective autophagy. An early study showed that tau degradation occurred in an acidic medium containing the lysosomal enzyme Cathepsin D, producing a reduction in full-length tau and increased levels of fragmented tau (Bednarski and Lynch, 1996). Later, other studies demonstrated that the inhibition of lysosomal function result in increased levels of tau in hippocampal slices (Bednarski and Lynch, 1996; Bendiske and Bahr, 2003). Interestingly, in rat primary neuronal cultures, proteasomal inhibition led to reduced total tau levels concomitant with increased LC3-II levels and autophagosome formation; these findings suggest that autophagy could act as a compensatory mechanism to eliminate tau (Kruger et al., 2012; Lei et al., 2015). In vivo studies have supported tau degradation though autophagy. In a Drosophila model that expresses the FTLD tau mutant R406W, deletion of cathepsin D exacerbated the neuronal toxicity and reduced the survival (Khurana et al., 2010); similar effects were observed in flies expressing 2N4R tau when the function of Atg6 (Beclin-1) was impaired (Ambegaokar and Jackson, 2011). In addition, knockout of Atg7, an essential autophagy gene, in mice resulted an increased presence of phosphorylated tau and neurodegeneration during aging (Inoue et al., 2012), indicating that autophagy-mediated tau degradation is a mechanism important for the prevention of neurodegenerative events in the neurons.
In starvation conditions, transcription factor EB (TFEB) translocates to the nucleus and promotes the expression of autophagy-associated genes (Settembre and Ballabio, 2011) and facilitates lysosomal biogenesis (Settembre et al., 2011; Settembre et al., 2012). In a transgenic tauopathy mouse model, AAV administration of TFEB gene reduced the levels of tau phosphorylation, decreasing tau toxicity (Polito et al., 2014). In neuronal cells, tau protein also can be degraded by selective autophagy through the adaptor protein NDP52, which is directly regulated by nuclear factor (erythroid-derived 2)-like 2 (Nrf2) that responds to cellular stress (Jo et al., 2014). Nonetheless, in 4 month-old Nrf2 knockout mice, total and phosphorylated tau levels are normal, along with the expression of autophagy genes. However, in 12 months-old Nrf2 knockout mice, there is a significant elevation of phospho-tau levels concomitant with a significant decrease in autophagy adaptors (NDP52, p62 and NBR1) and the co-chaperone BAG3. This is likely due to the fact that in control animals the autophagy adaptors and BAG3 increase with age, while in the Nrf2 knockout mice there is no age-dependent increase in these proteins (Tang et al., 2018). In primary neurons, activation of the Nrf2 pathway results in a reduction of phospho-tau levels, an effect that is not observed when NDP52 is knocked down (Jo et al., 2014). Therefore, considering that the autophagy rate in neurons is relatively fast (Boland et al., 2008) and that it is necessary for efficient tau degradation, impairment of autophagy processes may be a contributing factor to AD pathogenesis.
Pathological forms of tau can also be degraded by CMA and endosomal microautophagy. Tau with the P301L mutation is inefficiently degraded by all three autophagic pathways; whereas, the A152T tau mutant shows reduced degradation by endosomal microautophagy (Caballero et al., 2018). A similar result was observed when using a tau mutant containing deletion of Lys280 (hTau40 ΔK280) known to lead to tau aggregation. (Caballero et al., 2018). Interestingly, phosphorylation may also negatively affect autophagy as pseudo-phosphorylated tau (hTau40 AT8/AT100/PHF-1 and hTau40 4xKXGE [exchanging Ser262, Ser293, Ser324, and Ser356 for Glu]) is not efficiently degraded by CMA compared to wild type tau (hTau40). This phosphorylation-site specific processing could be due to two reasons: impaired binding to lysosomes or impaired translocation across the lysosomal membrane. hTau40 AT8/AT100/PHF 1 bound lysosomes inefficiently; whereas, hTau40 4xKXGE showed impaired lysosomal translocation (Caballero et al., 2018). Pseudo-phosphorylated forms of tau also impaired endosomal microautophagy, with reduced uptake/degradation, pseudo-phosphorylation in the microtubule binding domain resulting in the most pronounced impairment (Caballero et al., 2018).
In addition to autophagic mechanisms, tau can be degraded through endolysosomal pathways. In a recent study, it was demonstrated that through a Rab35 and endosomal sorting complex required for transport (ESCRT) machinery dependent process, tau is targeted to early endosomes and incorporated into multivesicular bodies (MVBs), which subsequently fuse with lysosomes resulting in tau degradation (Vaz-Silva et al., 2018). Further, extracellular tau that is taken up by neurons through macropinocytosis and dynamin-dependent endocytosis can also be targeted to the lysosome through this pathway (Evans et al., 2018).
6. PHYSIOLOGICAL FUNCTIONS OF TAU
As discussed above tau was initially identified as a neuronally enriched, microtubule –associated protein (MAP) that promoted microtubule polymerization and stability (Goedert and Jakes, 1990; Lindwall and Cole, 1984; Weingarten et al., 1975). However, currently tau is considered a multi-functional protein that plays a number of different roles in cells (See Figure 3). In the next sections, we will discuss the role of tau in various physiological process including stabilization of microtubules (Dubey et al., 2008; Iijima-Ando et al., 2012; Mietelska-Porowska et al., 2014), neuronal polarity, axonal stability (Dubey et al., 2008; Iijima-Ando et al., 2012),, axonal transport and synaptic function (Ittner and Gotz, 2011; Mietelska-Porowska et al., 2014; Reddy, 2011), neurite outgrowth, facilitation of enzyme anchoring (Reddy, 2011), and interaction with the membrane cytoskeleton (Ittner and Gotz, 2011; Maas et al., 2000).
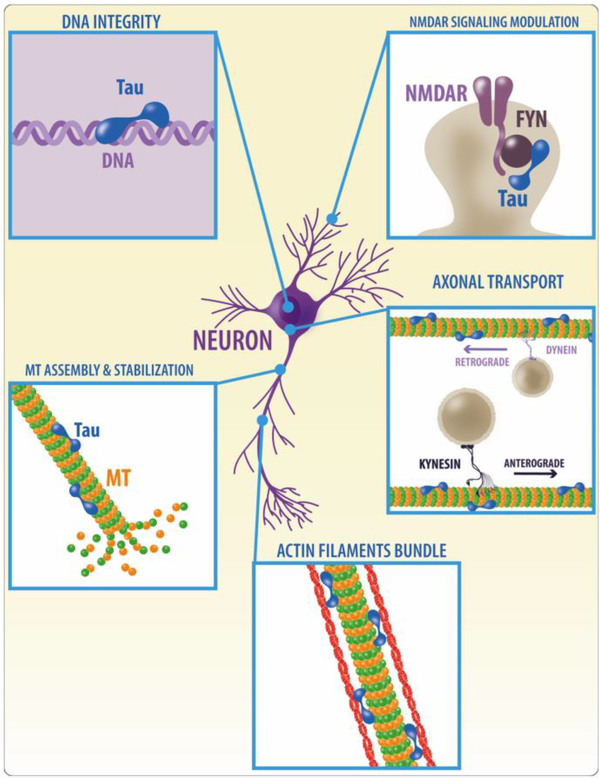
Figure 3. Physiological functions of tau.
Tau was initially identified as a protein that binds microtubules, and promotes microtubule polymerization and stability; however it is now evident that tau is a protein with multiple functions and that plays a number of different roles in cells including regulation of axonal transport, nuclear functions protecting DNA integrity; interactions with the actin cytoskeleton facilitating the formation of actin filaments; and regulation of NMDA receptor signaling through interactions with Fyn.
6.1. MICROTUBULE BINDING/STABILIZATION
The most studied role of tau protein is its binding to microtubules, where this interaction regulates microtubule assembly and stabilization (Gong et al., 2005; Marcus and Schachter, 2011; Morris et al., 2011). In this capacity, it contributes to the structural elements of axons and facilitates the regulation of the transport of organelles and biomolecules to and from synapses. Interestingly, the interactions between tau and microtubules can be both direct and indirect (Mietelska-Porowska et al., 2014). Tau has microtubule-binding and flanking domains that allow tau to bind both polymerized and unpolymerized tubulin which facilitates microtubule assembly and stability (Butner and Kirschner, 1991). In addition, tau can interact with other proteins, possibly in a manner independent of its microtubule binding domain and indirectly modulate microtubule dynamics (Mietelska-Porowska et al., 2014). For example, the inhibition of histone deacetylase-6 (HDAC6) by tau increases the levels of acetylated tubulin and might increase microtubule stability in absence of tau-microtubule binding (Morris et al., 2011). Tau drives tubulin assembly into microtubules which form the cytoskeletons within neurons and define neuronal morphology (Kolarova et al., 2012). The site of tau interaction is at the C-terminal end of tubulin (Gustke et al., 1994; Mietelska-Porowska et al., 2014). This interaction is modulated by the phosphorylation state of tau (Kolarova et al., 2012; Marcus and Schachter, 2011; Mietelska-Porowska et al., 2014). Tau phosphorylation can neutralize positive charges, affecting the conformation and detaching tau from microtubules (Mandelkow et al., 1995; Mietelska-Porowska et al., 2014). Physiologically, tau is phosphorylated at several residues that regulate its association with microtubules (Marcus and Schachter, 2011). However in pathological states, specific sites on tau are aberrantly phosphorylated which may decrease its association with microtubules and increase its propensity to self-associate and form toxic oligomeric species (Mandelkow and Mandelkow, 2012).
Numerous recent studies have provided significant insights into the mechanisms by which tau interacts with microtubules. Tau binds at the interface between α/β tubulin dimers and uses small groups of conserved residues with or immediately flanking the microtubule region. The sections of tau between the microtubule binding sites remain flexible when tau interacts with microtubules, which is reflective of the dynamic interaction between tau and microtubules (Janning et al., 2014; Kadavath et al., 2015). Furthermore, when tau is bound to microtubules it alters the repulsive forces between microtubules, with tau that expresses exon 2 or exon 2 and 3 being particularly efficient at conveying steric stabilization of single microtubules against bundling (Chung et al., 2015). A recent, cutting edge study used cryo-electron microscopy (cryo-EM) to create models of tau-tubulin interactions. The results of these studies confirmed the prediction that tau binds at the interface between tubulin heterodimers. Additonally, this work provided evidence that each microtubule binding repeat exhibits an extended conformation that spans both intra and interdimer interfaces (Kellogg et al., 2018). In an earlier study, tau was crosslinked only to residues in α tubulin (Kadavath et al., 2015); however, in the study by (Kellogg et al., 2018) the tau repeat regions were found to center on α tubulin and connect 3 tubulin monomers. Another key finding of this study was that Ser262, which is a prominent phosphorylation site, is involved in mediating tight contacts with tubulin at a polymerization interface. The location of this residue clearly indicates why phosphorylation of Ser262 plays a critical role in regulating tau’s ability to bind and stabilize microtubules. These and other studies have significantly increased our understanding of the mechanisms by which tau mediates microtubule dynamics.
6.2. REGULATION OF SRC KINASE FUNCTION
Fyn is a non-receptor tyrosine-protein kinase enzyme member of the Src family (Mietelska-Porowska et al., 2014). Tau can interact with members of Src-family such as Fyn and Lck, via its proline-rich region (Gong et al., 2005; Wolfe, 2012), and it seems that specific interactions between tau and Fyn may be necessary for controlling cell signaling in the neurons (Wolfe, 2012). Immunoprecipitation experiments in SH-SY5Y cells showed that a small portion of tau protein interacts with Fyn, and phosphorylation likely regulates this interaction. Moreover, co-transfections of tau and Fyn in COS cells showed that Fyn phosphorylates Tyr18, and this is the only site on tau that is phosphorylated by Fyn (Lee, 2005). Furthermore, phosphorylation of tau regulates its association with Fyn and this may be crucial for tau’s translocation into the plasma membrane where it could interact with membrane-associated protein complexes (Pooler et al., 2014). The tau-Fyn interaction may be crucial for targeting Fyn to the post-synapse (in dendritic spines), and this regulates NMDA receptor signaling by Fyn-mediated phosphorylation of NR2B subunits in dendrites (Ittner et al., 2010; Mietelska-Porowska et al., 2014; Pooler et al., 2014; Wolfe, 2012).
Tau exist at low concentrations in dendrites (Mietelska-Porowska et al., 2014; Wolfe, 2012), and it co-immunoprecipitates with Fyn and postsynaptic density 95 (PSD95) isolated from mouse brains (Morris et al., 2011), suggesting that tau is acting as a scaffolding protein. This interaction allows tau along with PSD95 to activate Fyn and facilitate the phosphorylation of NMDA receptors (Mietelska-Porowska et al., 2014; Wolfe, 2012). Tau can also facilitate c-Src-mediated actin reorganization after platelet-derived growth factor treatment (Mietelska-Porowska et al., 2014; Morris et al., 2011), and its absence affects Fyn trafficking toward the dendritic spines (Mietelska-Porowska et al., 2014; Morris et al., 2011).
6.3. REGULATION OF AXONAL TRANSPORT
Neurons need to transport organelles, proteins, and lipids from the soma into axon and dendrites and back again to maintain a normal functional state (Reddy, 2011). Microtubules act as conduits for both anterograde and retrograde transport of molecules (Duncan and Goldstein, 2006; Hollenbeck and Saxton, 2005; Sheng, 2014). This process is dependent on motor proteins; in the anterograde direction the kinesin family of proteins and in the retrograde direction, the dynein protein family (Duncan and Goldstein, 2006; Sheng, 2014). Tau has differential effects on dynein and kinesin motility. High concentrations of tau protein bind to microtubules and differentially inhibit both dynein and kinesin function. Dynein tends to reverse direction when it encounters microtubule-bound tau; whereas, kinesin detach at patches of microtubule-bound tau in a concentration- and isoform-dependent manner (Dixit et al., 2008). However, given the high concentrations of tau used in these studies, it is unclear whether these modulatory events occur in vivo in physiological conditions (see discussion below).
The phosphatase-activating domain (PAD) of tau is a functional motif comprised of amino acids 2–18 in the N-terminus (Kanaan et al., 2011). Alterations in the presentation of the PAD domain of tau due filament formation, truncation or hyperphosphorylation inhibit anterograde fast axonal transport (FAT) (Kanaan et al., 2011). This is due to the binding of protein phosphatase 1 (PP1) to the PAD domain and activation. PP1 subsequently dephosphorylates Ser9 on glycogen GSK-3β (or Ser21 on GSK3α) resulting in activation. It is then suggested that GSK3-mediated phosphorylation of kinesin light chains results in release of cargoes and thus FAT inhibition (Kanaan et al., 2012). Further, phosphorylation of tau at Tyr18 within the PAD by the tyrosine kinase Fyn, prevents inhibition of anterograde FAT (Kanaan et al., 2012). Therefore, it is suggested that the presence of phosphorylated sites at N-terminal domain could regulate tau-dependent inhibition of anterograde FAT in AD.
Tau-microtubule binding is a rapid, reversible interaction; therefore, although tau binds to microtubules in the physiological setting, it is unlikely that binding affects microtubule-dependent transport in axons (Janning et al., 2014). Indeed, it is only after drastic tau overexpression that alterations in axonal transport have been described (Dubey et al., 2008; Ishihara et al., 2001; Spittaels et al., 1999). In contrast, ablation of tau does not alter axonal transport in primary neuronal culture or in vivo (Morris et al., 2011). The explanation for this phenomenon may be due to the finding that tau dwells on a microtubule for a very short time period of approximately 40 ms, and then tau “hops” to the next microtubule (Janning et al., 2014). This process has been described as the “kiss-and-hop” mechanism by which tau induces microtubule assembly in neurons (Janning et al., 2014). Therefore, when tau is expressed within the physiological range it does not block axonal transport. In another work, it was demonstrated that tau isoform variation has little influence on tau-microtubule binding (Niewidok et al., 2016). However, this binding is drastically improved when a conserved C-terminal pseudo repeat region (PRR) in tau is present. Pathological mutations in the PRR (K369I, G389R) increases tau–microtubule dynamicity; in contrast, anomalous pseudo-phosphorylation of tau strongly reduces tau–microtubule interactions without affecting tau-microtubule dissociation rates (Niewidok et al., 2016). These data indicate that tau-microtubule binding is influenced by the occurrence of the PRR and tau hyperphosphorylation (Niewidok et al., 2016).
Axonal transport of mitochondria is essential for correct synaptic function, and the presence of motile mitochondria at presynaptic sites can directly impact synaptic vesicle release, and pathological changes to tau may impair this transport (Pooler et al., 2014). Several studies using cellular and animal AD models, and AD brain, have shown that mitochondria localization is reduced in the axon (Iijima-Ando et al., 2012). For example, in primary mouse neuronal cultures, expression of phosphorylated mutant forms of human tau reduced mitochondrial mobility, and changes in tau phosphorylation also affect mitochondrial transport in neurons (Pooler et al., 2014). Overexpression of normal or phosphorylated tau negatively affect the transport of organelles through the axon, including mitochondria (Reddy, 2011). Using hippocampal neurons from wild type mice, Vossel et al. showed that Aβ inhibited transport of mitochondria and neurotrophin receptor TrkA along axons.. Complete or partial reduction in tau expression prevented these defects, suggesting Aβ requires tau to impair axonal transport (Vossel et al., 2010). Moreover, Aβ oligomers impair axonal motility of cargoes through NMDA receptor signaling, activation of GSK3β and CK2, and tau may interact directly or indirectly with these pathways or enhance the effects independently by competing with motor proteins for microtubule access (Vossel et al., 2010).
6.4. SCAFFOLD FUNCTION AND INTERACTIONS WITH CYTOSKELETON
The cytoskeleton of a cell is made up of microtubules, actin filaments, intermediate filaments and associated proteins, and plays crucial roles in almost all cellular processes. (Kevenaar and Hoogenraad, 2015; Mandelkow and Mandelkow, 2012). Spectrin is a cytoskeletal protein that regulates the stability and structure of the cell membrane and the shape of a cell (Zhang et al., 2013). Tau can directly interact with spectrin and actin filaments (He et al., 2009; Yu and Rasenick, 2006), and this can stabilize microtubules and facilitate the interaction with neurofilaments to limit the movement of the microtubule lattices (Farias et al., 2002). Also, tau can bundle actin filaments which is primarily mediated by its microtubule binding domain and helped by the adjacent proline-rich domain (Morris et al., 2011). Moreover, in cortical neurons tau can associate with plasma membrane proteins by its N-terminal non-microtubule-binding domain, indicating that tau could act as a scaffold between microtubules and other axonal components (Shahani and Brandt, 2002). Additionally, tau can bind phosphatidylinositol bisphosphate (PIP2) which is a precursor for diacylglycerol and inositol trisphosphate and controls the function of others actin-binding proteins that participate in the assembly of the actin cytoskeleton (Shahani and Brandt, 2002). Further studies using cells null for filamin expression (a major actin-binding protein) showed membrane blebbing, and the presence of tau or Microtubule-associated protein 2 (MAP2) inhibits this effect restoring a normal phenotype. Moreover, deletion of tau in primary neuronal culture reduces growth cone area, filopodia amount and alters the distribution of actin filaments (Shahani and Brandt, 2002).
In neurons, there are several microtubule-severing proteins, including P60-katanin, spastin, fidgetin and fidgetin-like proteins (Roll-Mecak and McNally, 2010). These proteins contribute to axonal branch development. Studies showed that axons lacking tau are more prone to branch independent of the presence of spastin (Yu et al., 2008b); and that tau protect microtubules against microtubule severing by proteins including spastin and katanin (Qiang et al., 2006; Yu et al., 2008b).
In oligodendrocytes, the tau/Fyn interaction promotes process outgrowth, and the tubulin/Fyn interaction can recruit components of the microtubule network, stabilizing the cytoskeleton and facilitating transport processes (Seiberlich et al., 2015). Tau downregulation in oligodendrocytes reduces myelin basic protein expression, outgrowth and transport processes, oligodendrocyte differentiation, neuron-glia contact formation and the early myelination processes (Seiberlich et al., 2015). However the loss of tau is not associated with alterations in Fyn activity; instead it affects the tubulin/Fyn interaction interfering with oligodendroglia differentiation as well as tubulin assembly and bundling (Seiberlich et al., 2015).
Studies suggest that tau might modulate neuronal signal transduction by influencing the targeting and function of synaptic mitochondria because presynaptic mitochondria are involved in regulation of intracellular calcium and ATP levels which are critical for vesicle release during neurotransmission (Pooler et al., 2014). Moreover, in response to NGF treatment tau is re-localized to the ends of cellular extensions and interacts with actin in a microtubule-independent manner, facilitating NGF and EGF receptor dependent signaling and increasing the activity of MAPK through Ras-MAPK pathway (Mietelska-Porowska et al., 2014). Also, tau can directly interact with the SH3 domain in the p85alpha subunit of phosphatidylinositol 3-kinase, phospholipase C gamma 1, Grb2, Fyn, cSrc, and Fgr. The tau-SH3 binding is prevented when tau is hyperphosphorylated. The interaction with SH3 domains in diverse cell signaling molecules suggests other roles of tau in cytoskeletal structure and assembly regulated by phosphorylation (Reynolds et al., 2008).
6.5. NUCLEAR FUNCTIONS
Neurons require efficient mechanisms to protect DNA and RNA integrity and ensure their functionality and longevity (Violet et al., 2014). Tau can interact with DNA in vitro and in situ, and is found inside the nucleus (Camero et al., 2014). In vitro assays showed that tau binds preferentially to AT-rich DNA regions in contrast to GC-rich DNA sequences (Sultan et al., 2011). Moreover, tau binds double- and single-strands of DNA, interactions that are dependent on the aggregation and phosphorylation state of tau. This process protects DNA from denaturation in vitro (Sjoberg et al., 2006), and tau could be important in nucleolar conformation and heterochromatinization of ribosomal genes in stress situations by the presence of phosphorylated tau that modulates its translocation to the nucleus (Camero et al., 2014).
In the nucleus, tau is found at nucleolar organizer regions (NORs), in acrocentric chromosomes containing rDNA juxtaposed to pericentromeric structures where tau localizes (Brady et al., 1995; Sjoberg et al., 2006). In human fibroblasts, lymphoblasts and HeLa cells, nucleolar tau was found in in the internal periphery of nucleoli, indicating that tau may participate in nucleolar assembly and heterochromatinization of ribosomal genes (Sjoberg et al., 2006). Further studies in neuronal nuclei of embryonic and adult neurons showed that oxidative and heat stress promotes the appearance of dephosphorylated tau (Sultan et al., 2011). Moreover, neurons exposed to heat shock show increased interactions between endogenous tau and neuronal DNA, protecting DNA from damage. In addition, nuclear overexpression of human tau protects DNA from damage in cells lacking tau expression. These studies indicate that in stressful situations tau translocates to the nucleus and may protect neuronal DNA (Sultan et al., 2011). Also using an in vivo mouse model of hyperthermia/heat stress with wild-type and tau-deficient mice, it was found that tau deletion results in similar DNA damage under physiological conditions, but increased DNA breaks were observed in tau knockout mice under heat stress conditions (Violet et al., 2014). Further studies in HEK 293 transfected with tau evaluated the equilibrium constant and the enthalpy and entropy changes related to the Tau-DNA complex formation (Camero et al., 2014). These experiments showed that hydrophobicity might control tau location and DNA interaction, and tau hyperphosphorylation may impair this interaction contributing to AD pathogenesis (Camero et al., 2014).
6.6. TAU TRANSMISSION
It is well established that in AD the development of tau pathology exhibits a hierarchical pattern of accumulation that starts in layer II of entorhinal cortex (EC) and spreads through anatomically connected neuronal populations (Braak and Braak, 1991; Hyman et al., 1984). These findings strongly indicated that tau was being transmitted through synaptically connected neuronal populations, and in 2012 two groups demonstrated that this was the case. For their studies, they used transgenic mouse models in which P301L human tau was selectively expressed in the EC to demonstrate that, in a time-dependent manner, the mutant human tau appeared sequentially through the synaptically connected circuits (de Calignon et al., 2012; Liu et al., 2012).
Although these and other data have established that tau can be transmitted from cell to cell (for a recent review see: (Gibbons et al., 2018), the mechanisms of release and uptake have still not been completely elucidated. There are data indicating that tau transmission occurs via exosomes. Interestingly, tau contained in exosomes is not extensively phosphorylated, suggesting that exosomal tau transmission could be a physiological mechanism dependent on neuronal activity. In fact, depolarization of neurons promotes release of tau into exosomes. These exosomes are taken up by neurons and microglia, but not by astrocytes (Asai et al., 2015; Wang et al., 2017). Studies using exosomes obtained from cerebrospinal fluid (CSF) of AD and normal patients reveal that exosomal tau from AD samples can promote tau aggregation in vitro. Therefore, is possible that tau transmission could occur in three steps: 1) tau-containing exosomes are released into extracellular space and taken up by other neurons, 2) in the adjacent neuron, the internalized exosomes are transported into the axon and finally are released at the pre-synaptic site, and (3), the released exosomes are taken up by the neuron in synapses, resulting in the distribution of tau in all neurons via synaptic activity (Wang et al., 2017).
In addition to exosome mediated release and uptake, tau can be released in microvesicles or ectosomes which form from the plasma membrane. In one study, tau was found to be secreted predominantly in ectosomes and it was suggested that when tau accumulates, the exosome pathway is activated (Dujardin et al., 2014). Tau may also be released by a chaperone-dependent, non-classical exocytosis (Fontaine et al., 2016), or transferred from cell to cell through tunneling nanotubes (Tardivel et al., 2016). In another study using mouse N2A cells expressing human tau as well as rat primary cortical neurons, it was demonstrated that phosphorylated oligomeric forms of tau were directly released/transferred out of the cell via a novel, non-vesicular mechanism (Merezhko et al., 2018). Intriguingly, several studies have reported that most extracellular tau is not in vesicles (Wegmann et al., 2016; Yan et al., 2016). Non-vesicular, “free” tau is likely taken up through endocytotic processes as well as macropinocytosis. Monomeric tau is rapidly taken up by normal human neurons via endocytosis and also by micropinocytosis, which is a slower process (Evans et al., 2018). Aggregated tau is also taken up by human neurons via dynamin-dependent endocytosis, but not by macropinocytosis. Thus, it can be suggested that tau entry into human neurons is a physiological event that occurs normally, and could also be necessary for unknown tau functions. Overall, it is likely that numerous mechanisms are responsible for tau release from and uptake by neurons. These mechanisms, as well as the forms of tau present, likely differ in physiological and pathological conditions, as well as the forms of tau present. Future studies are needed to delineate the importance of tau transmission in neuronal communication.
6.7. ROLE OF TAU IN THE DENDRITIC/POST-SYNAPTIC COMPARTMENT
Previous reports showed that tau has a dendritic role in the postsynaptic targeting of the Fyn kinase Fyn as discussed above (see section 6.2). These studies suggested that tau regulates Fyn-mediated interactions with the NMDA receptor and PSD-95 and thus, mediated Aβ toxicity in the context of AD (Ittner and Ittner, 2018; Ittner et al., 2010). Tau interacts with Fyn through Pro-X-X-Pro (PXXP) motifs in the proline-rich region of tau promoting Fyn recruitment to NMDAR (Ittner et al., 2010; Lau et al., 2016). This targeting of tau to the postsynapse may be necessary to mediate synaptic plasticity. In fact, tau reduction or depletion (tau +/− or tau knockout mice) lead to reduced LTD formation, but normal LTP (Kimura et al., 2013); both processes are fundamental to memory formation (Nabavi et al., 2014). In addition, studies suggested that tau is involved in post-synaptic microtubule dynamics perhaps due dendritic tau accumulation, which disrupts the synaptic cytoskeleton (Zempel et al., 2017). Other studies using overexpression of mutant pathological human tau indicated that post-synaptic tau localization and dendritic distribution are directly modulated by phosphorylation at sites T231/S235, S262/S356, or S396/S404 (Xia et al., 2015). Although these findings are very intriguing, additional studies are required to understand the role of tau at the post-synapse and which mechanisms are governing post-synaptic tau localization (Ittner and Ittner, 2018).
6.8. TAU DELETION
The first tau knockout mouse was made by (Harada et al., 1994), with another one being made in 2001 (Dawson et al., 2001). Interestingly, these mice appear phenotypically normal, although subtle effects were observed such as a delay in neuronal maturation (Dawson et al., 2001). In addition, MAP1A expression is increased which likely represents a compensatory response to the loss of tau expression (Dawson et al., 2001). These mice have been crossed with AD mouse models to understand the role of tau in disease pathogenesis. For example, mice expressing human APP with the Swedish (K670M/N671L) and Indiana (V717F) familial AD mutations were crossed with tau knockout mice. Although Aβ pathology remained essentially unchanged, removing tau significantly improved behavior and decreased seizures in response to excitoxins (Roberson et al., 2007). Deletion of tau also decreased excitotoxic brain damage and attenuated neurological deficits following stroke (Bi et al., 2017). Knocking out tau also reduced stress-induced behaviors, and protected mice against stress-induced atrophy of hippocampal dendrites and deficits in hippocampal connectivity (Lopes et al., 2016). Tau knockout mice have also been used to demonstrate that tau plays a role in the maturation of newborn hippocampal granule neurons and the stress-induced cell death of immature granule neurons. Knocking out tau protects newborn neurons from the stress-induced dendritic atrophy and loss of PSDs. However, newborn granule neurons from tau knockout mice did not exhibit the stimulatory effect of environmental enrichment on dendritic development or the formation of PSDs. These data indicate that tau plays a key role in regulating the number of and maturation of granule neurons (Pallas-Bazarra et al., 2016). Additional studies on the effects of tau deletion on pathogenic processes have been reviewed in (Ke et al., 2012).
Although no significant, overt deficits were observed in young tau knockout mice, older mice have been reported to exhibit abnormalities. Older tau knockout mice show significant motor deficits and reduction in activity in the open field test (Lei et al., 2014). Tau-knockout mice have been reported to exhibit age-dependent brain atrophy, iron accumulation and substantia nigra neuronal loss which coincided with cognitive deficits and motor dysfunction (Lei et al., 2012). Deletion of tau resulted in an impaired hippocampal response to insulin along with alterations in energy metabolism (Marciniak et al., 2017). In addition tau deletion results in the loss of hippocampal synapses in older animals and deficiencies in the Morris Water Maze (Ma et al., 2014). Overall, it is evident that, in neuropathological processes, tau often plays a deleterious role; though, in physiological conditions, especially in older animals, the presence of tau is beneficial.
7. TAU IN DISEASE
Although this review is primarily focused on physiological aspects of tau, the role of tau in neurodegenerative diseases, especially AD, is of critical importance. Therefore, in this section, we will briefly discuss the use of tau as a biomarker, tau-based therapeutic strategies for AD, and finally we will highlight several recent findings that broaden our perspective in terms of the roles tau may play in disease processes.
7.1. TAU AS A BIOMARKER
Measure of CSF tau (total, pThr181, pThr231) in conjunction with Aβ42 are considered to be part of an accurate diagnosis of AD. It has also been suggested that these measures are useful for prodromal AD patients with MCI (Blennow, 2010). However, a more recent report, based on a literature review that evaluated data from 1282 individuals with MCI suggested that there was a degree of uncertainty in terms of the usefulness of CSF testing for tau, phospho-tau and phospho-tau/Aβ conversion to AD. Part of the problem was that in many of the studies “were of poor methodological quality” (Ritchie et al., 2017). Clearly there is a need for better controlled studies to definitively demonstrate the usefulness of CSF tau measures in MCI patients.
There has been a growing interest in using plasma tau levels as a possible biomarker for AD. In a prospective study following cases from the Alzheimer Disease Neuroimaging Initative (ADNI) found that plasma tau levels showed a large overlap between AD and aged matched controls. These findings did not support the use of plasma tau as biomarker (Mattsson et al., 2016). However, more recently there was a study that suggested that higher plasma levels of specific truncated forms of tau may actually be associated with a lower risk of AD and dementia (Neergaard et al., 2018). In another recent study, plasma pThr181-tau levels were elevated in AD cases and associated with Aβ and tau positron emission tomography (PET) readouts (Mielke et al., 2018).
PET imaging is being developed to assess the regional distribution and severity of tau pathology. Tau specific tracers are being developed and initial results are very promising, it is clear that more work is needed to establish their specific binding properties and their potential usefulness as a biomarker for AD (Hall et al., 2017; Saint-Aubert et al., 2017). One recent study showed that the tau PET ligand 18F-AV-1451 might be useful as a marker of “disease stage” as it increased with advancing clinical stages of AD. However, retention of 18F-AV-1451 was normal in preclinical AD cases that showed elevated CSF tau levels (Mattsson et al., 2016).
7.2. TAU BASED THERAPIES
Given the lack of success developing Aβ-directed therapies, more effort has been directed towards developing tau based therapies. Many different approaches have been taken to attenuate tau toxicity in the context of AD. One significant therapeutic approach that is being explored is tau immunotherapy. Although Aβ immunotherapies have proved unsuccessful to this point (Mullane and Williams, 2018), there is hope that reducing pathological tau species may be beneficial even when initiated after AD is diagnosed. The first demonstration that both active and passive immunization against a phosphotau epitope in a mouse model that expresses human P301L tau (JNPL3 line) reduces tau pathology was published more than 10 years ago (Asuni et al., 2007). Since this initial report, there have been numerous studies extending and improving this initial approach. Indeed, there are currently a number of ongoing Phase I/II clinical trials using both active and passive approaches with some encouraging results. For example, the results were recently published from a phase I trial for AADvac1, which is KLH-conjugated peptide of amino acids 294 to 305 (KDNIKHVPGGGS), and these results demonstrated that AADvac1 had a “benign safety profile” and that there was a tendency towards slower hippocampal atrophy based on MRI evaluation that correlated with higher IgG titers (Novak et al., 2018). Phase II studies for AADvac1 (NCT02579252) are now in progress.
Tau hyperphosphorylation is a key feature of pathological tau and, thus, approaches that inhibit tau protein kinases or up-regulate tau-targeting phosphatases have been considered. Mematine functions as an NMDA antagonist and is an FDA approved drug for the treatment of AD. In addition to being a weak NMDA receptor antagonist, it also increases PP2A activity which may contribute to its beneficial effects in AD patients (Cohan et al., 2004). In terms of tau kinases, significant efforts have been directed towards developing GSK3 inhibitors, e.g., Tideglusib (which is an irreversible inhibitor) and lithium. Tideglusib has been shown safe in humans with AD, but current clinical trials have not revealed significant clinical benefit (Lovestone et al., 2015). Lithium chloride which has long been used to treat bipolar disorder is also a GSK3 inhibitor. In MCI patients lithium treatment improved cognition, and in a cohort of AD patients stabilized cognitive measures (Forlenza et al., 2011; Nunes et al., 2013). A phase II trial for treatment of FTLD-tau with lithium is currently ongoing (NCT02862210).
Blocking tau aggregation is another therapeutic approach that is being explored. Methylene blue blocks tau aggregation very effectively in vitro (Wischik et al., 1996), and prevents tau aggregation when given prophylactically to mice that are expressing full-length pro-aggregant human tau (Hochgrafe et al., 2015). Although results from clinical trials with methylene blue derivatives have been variable, a recent study suggests that it may be therapeutic in mild AD cases, Further studies on the usefulness of methylene blue derivatives in treating AD and other tauopathies are needed (Wilcock et al., 2018).
Overall, it is clear that many tau-based strategies have been tested or are being tested for the treatment of AD and other tauopathies. Indeed, there are numerous tau-based therapies that are currently in clinical trials (see clinicaltrials.gov). For a more complete review of tau based therapeutics see (Congdon and Sigurdsson, 2018).
Reducing tau levels by increasing tau degradation through non-immune mechanisms is another treatment strategy that is being considered, although these approaches have not yet been tested in humans. For example, increasing autophagy by trehalose treatment in a mouse model that overexpresses human P301S tau caused a reduction in tau pathology and neurodegeneration (Schaeffer et al., 2012). Although these results are encouraging, many more studies are needed to determine feasibility of this approach as a treatment strategy for AD. Another novel approach being considered is the use of PROTACs (PROteolysis TArgeting Chimeras) which are heterobiofunctional peptides that bind an E3 ligase and a target protein resulting in ubiquitylation and degradation by the 26S proteasome (Neklesa et al., 2017). In a recent study a PROTAC peptide that recruits the Keap1-Cul3 ubiquitin ligase complex to tau, was shown to enter cells and induce tau degradation in overexpression models (Lu et al., 2018). These preliminary data are intriguing, but clearly further research is required to determine if PROTACs could be used to facilitate tau clearance as a therapeutic strategy for AD.
7.3. RECENT UPDATES ON THE ROLE OF TAU IN DISEASE PROCESSES
In this section, we present several recent, cutting edge studies on the role of tau in disease pathogenesis. For more comprehensive reviews on the role of tau in AD and other tauopathies see (Bakota and Brandt, 2016; Bloom, 2014; Morris et al., 2011) among others.
Nucleocytoplasmic transport is essential for the maintenance of cell health and defects in the functioning of the nuclear pore complex have been reported to be contributing factors to the pathogenesis of amytrophic lateral sclerosis (ALS) and several other neurodegenerative diseases (Kim and Taylor, 2017). A recent study from the Hyman lab (Eftekharzadeh et al., 2018) showed that phosphotau species interact with nucleoporins in AD brain and the nucleoporin Nup98 mislocalizes to the cytoplasm. Further, in in vitro studies Nup98 induced tau aggregation and increased the rate of tau fibril formation. Nuclear pore complex function is compromised in the tg4510 mice that dramatically overexpress human P301L tau, and suppressing tau levels by administering doxycycline ameliorated these deficits. Taken together, these data suggest that neurotoxicity of pathological tau species maybe due in part to impaired nucleocytoplasmic transport (Eftekharzadeh et al., 2018).
Although the presence of tau filaments characterize a number of neurodegenerative diseases, they do not all exhibit the same morphology. However, until recently, high resolution structures of these different tau filaments were not available. This all changed when cryo-EM was used to provide a detailed description of the structure of straight filaments and PHFs from an AD case (Fitzpatrick et al., 2017). The core structure of PHFs and straight filaments is composed primarily of R3–R4 of tau, with eight β-sheets along the length of the protofilament, Further, PHFs and straight filaments are ultrastructural polymorphs that differ in their inter-protofilament packing. This polymorphism was found to be due to differences in the lateral contacts between the two protofilaments (Fitzpatrick et al., 2017). In a subsequent study, tau filaments from a Pick’s disease case were analyzed using cryo-EM. Filaments from the AD case contained both 3R and 4R tau; however, the Pick’s disease tau filaments only contained 3R tau (Falcon et al., 2018; Fitzpatrick et al., 2017). The core of the tau filaments from the AD case compromised residues 254–378 of tau, while the tau filament core from the Pick’s disease case consisted of residues 306–378 and folded differently (Falcon et al., 2018). It was suggested that this novel fold in the Pick’s disease tau filaments may explain why 3R tau is enriched in Pick bodies, as well as the differential tau phosphorylation between AD and Pick’s disease (Falcon et al., 2018; Fitzpatrick et al., 2017).
It is well-established that neuroinflammation is a key factor in the pathogenesis of AD (Heneka et al., 2015), but the upstream mechanisms have not been fully defined. In a recent study using a mouse model in which human P301S tau is overexpressed, it was demonstrated that neurons with tau inclusions aberrantly expose phosphatidylserine on their plasma membranes resulting in recognition and phagocytosis by microglia (Brelstaff et al., 2018). This response is likely part of a homeostatic response which removes compromised cells to maintain the integrity of the tissue. However, in the context of neurodegenerative diseases, the removal of living neurons becomes a detrimental process and thus interventions that prevent this phagocytosis may be part of therapeutic treatment strategy for AD (Brelstaff et al., 2018).
8. FUTURE DIRECTIONS.
Over the past few years’ new directions in tau research have been opened and are yielding exciting and novel findings. With the opening of these novel vistas in tau biology, many new questions arise, a few of which are highlighted here. For example, there is a developing awareness that the release and uptake of tau is likely a physiological process, however the function of this transmitted tau is completely unknown. It is also apparent that there is localization of tau into dendrites in both physiological and pathological conditions. Although there have been initial studies on the targeting of specific tau species to dendrites and the functioning of tau in this neuronal subcompartment, more extensive studies are needed to fully understand the localization and function of tau in dendrites. These are especially important given the findings that in vivo tau plays a role in dendritic development and remodeling in response to different signals such as environmental enrichment and stress. In different model systems tau has been shown to be cleared through a number of different pathways; however, what is not known is how these different degradative mechanisms cooperate to maintain the appropriate levels of tau in vivo, and how the turnover of tau is regulated in the different cellular domains of a neurons (soma, axon, dendrites, synaptic compartments).Finally, a number of studies have begun to shed light on the role of the different N terminal domains of tau (0N, 1N and 2N), but further research is needed to fully understand the functions of these different splice variants of tau.
9. CONCLUSIONs
In summary, tau plays many key roles in the structure and function of brain cells, and in particular neurons. The functions highlighted in this review indicate that tau plays important regulatory roles in axons but also has other significant functions in dendrites and in the nucleus. Interestingly many, if not all tau functions are influenced by the different posttranslational modifications; and if dysregulated could lead to a loss of their physiological function, giving rise to pathological conditions. Therefore, maintaining the physiological state and correct conformation of tau protein mediated by an appropriate balance of posttranslational modifications is necessary for the maintenance of healthy, functional nervous system.
10. ACKNOWLEDGEMENTS
This work was supported by FONDECYT #1140968, FONDECYT #1170441 and Anillo ACT1411 to RAQ; FONDECYT N°11170546 and CONICYT PAI N°77170091 to CTR; and NS076789 and NS098769 to GVWJ. The authors declare that they have no competing interests.
Abbreviations.
- CNS
central nervous system
- PNS
peripheral nervous system
- AD
Alzheimer disease
- FTLD-tau
frontotemporal lobar degeneration-tau
- PSP
Progressive Supranuclear Palsy
- CBD
Corticobasal degeneration, DM1, myotonic dystrophy type 1
- Aβ
Amyloid-β
- HATs
histone acetyltransferases
- HDACs
histone deacetylases
- CHIP
C-terminus of HSC70-interacting protein
- UPS
ubiquitin– proteasome system
- HSC70
heat-shock cognate 70
- PHFs
Paired Helical Filaments
- OGT
Glucosaminyl transferase
- OGA
hexosaminidase O-GlcNAcase
- PPIs
Peptidyl-prolyl isomerases
- PP2A
Protein phosphatases 2A
- SUMO
small ubiquitin-like modifier proteins
- AGEs
advanced glycation end products
- PKA
cAMP-dependent protein kinase
- PSA
puromycin-sensitive aminopeptidase
- CMA
chaperone-mediated autophagy
- HDAC6
histone deacetylase-6
- PAD
Phosphatase-activating domain
- FAT
fast axonal transport
- PP1
protein phosphatase 1
- PRR
pseudo repeat region
- CHO
Chinese hamster ovary, PIP2, phosphatidylinositol bisphosphate
- GSK-3β
glycogen synthase kinase 3β
- NORs
nucleolar organizer regions
- CA1
Cornu Ammonis 1
- DG
dentate gyrus
- MVBs
multivesicular bodies
- ESCRT
endosomal sorting complex required for transport
Footnotes
11. CONFLICT OF INTERERST
The authors declare not conflict of interest regarding this work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. REFERENCES
- Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, and Iqbal K (2004). Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 279, 34873–34881. [DOI] [PubMed] [Google Scholar]
- Ambegaokar SS, and Jackson GR (2011). Functional genomic screen and network analysis reveal novel modifiers of tauopathy dissociated from tau phosphorylation. Hum Mol Genet 20, 4947–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis A (2005). Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta 1739, 91–103. [DOI] [PubMed] [Google Scholar]
- Andreadis A, Brown WM, and Kosik KS (1992). Structure and novel exons of the human tau gene. Biochemistry 31, 10626–10633. [DOI] [PubMed] [Google Scholar]
- Arai T, Guo JP, and McGeer PL (2005). Proteolysis of non-phosphorylated and phosphorylated tau by thrombin. J Biol Chem 280, 5145–5153. [DOI] [PubMed] [Google Scholar]
- Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, and Hart GW (1996). The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem 271, 28741–28744. [DOI] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, and Ikezu T (2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18, 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, and Sigurdsson EM (2007). Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27, 9115–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J (2008). Tau kinases and phosphatases. J Cell Mol Med 12, 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Lucas JJ, Perez M, and Hernandez F (2004). Role of tau protein in both physiological and pathological conditions. Physiol Rev 84, 361–384. [DOI] [PubMed] [Google Scholar]
- Bakota L, and Brandt R (2016). Tau Biology and Tau-Directed Therapies for Alzheimer’s Disease. Drugs 76, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balastik M, Lim J, Pastorino L, and Lu KP (2007). Pin1 in Alzheimer’s disease: multiple substrates, one regulatory mechanism? Biochim Biophys Acta 1772, 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski E, and Lynch G (1996). Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. Journal of neurochemistry 67, 1846–1855. [DOI] [PubMed] [Google Scholar]
- Bendiske J, and Bahr BA (2003). Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis--an approach for slowing Alzheimer disease? Journal of neuropathology and experimental neurology 62, 451–463. [DOI] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, et al. (2007). Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci 27, 3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi M, Gladbach A, van Eersel J, Ittner A, Przybyla M, van Hummel A, Chua SW, van der Hoven J, Lee WS, Muller J, et al. (2017). Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat Commun 8, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, and Mandelkow EM (1999). The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Mol Biol Cell 10, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, Mandelkow EM, Schroter C, Lichtenberg-Kraag B, Steiner B, Berling B, Meyer H, Mercken M, Vandermeeren A, Goedert M, et al. (1992). The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J 11, 1593–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, and Mandelkow EM (2002). Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell 13, 4013–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, and Rebhun LI (1985). The distribution of tau in the mammalian central nervous system. J Cell Biol 101, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K (2010). Biomarkers in Alzheimer’s disease drug development. Nat Med 16, 1218–1222. [DOI] [PubMed] [Google Scholar]
- Bloom GS (2014). Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71, 505–508. [DOI] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, and Nixon RA (2008). Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 6926–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, and Braak E (1991). Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol 1, 213–216. [DOI] [PubMed] [Google Scholar]
- Brady RM, Zinkowski RP, and Binder LI (1995). Presence of tau in isolated nuclei from human brain. Neurobiol Aging 16, 479–486. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, and Lee VM (1993). Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10, 1089–1099. [DOI] [PubMed] [Google Scholar]
- Brelstaff J, Tolkovsky AM, Ghetti B, Goedert M, and Spillantini MG (2018). Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep 24, 1939–1948 e1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, and Hof PR (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33, 95–130. [DOI] [PubMed] [Google Scholar]
- Bulbarelli A, Lonati E, Cazzaniga E, Gregori M, and Masserini M (2009). Pin1 affects Tau phosphorylation in response to Abeta oligomers. Mol Cell Neurosci 42, 75–80. [DOI] [PubMed] [Google Scholar]
- Butner KA, and Kirschner MW (1991). Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol 115, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero B, Wang Y, Diaz A, Tasset I, Juste YR, Stiller B, Mandelkow EM, Mandelkow E, and Cuervo AM (2018). Interplay of pathogenic forms of human tau with different autophagic pathways. Aging cell 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camero S, Benitez MJ, Cuadros R, Hernandez F, Avila J, and Jimenez JS (2014). Thermodynamics of the interaction between Alzheimer’s disease related tau protein and DNA. PLoS One 9, e104690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D, Flaman JM, Brice A, Hannequin D, Dubois B, Martin C, Moreau V, Charbonnier F, Didierjean O, Tardieu S, et al. (1995). Mutations of the presenilin I gene in families with early-onset Alzheimer’s disease. Hum Mol Genet 4, 2373–2377. [DOI] [PubMed] [Google Scholar]
- Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A, and Calissano P (1998). Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci 18, 7061–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti G, Tedeschi G, Maggioni MG, Negri A, Nonnis S, and Maci R (2004). The nitration of tau protein in neurone-like PC12 cells. FEBS Lett 562, 35–39. [DOI] [PubMed] [Google Scholar]
- Cardozo C, and Michaud C (2002). Proteasome-mediated degradation of tau proteins occurs independently of the chymotrypsin-like activity by a nonprocessive pathway. Arch Biochem Biophys 408, 103–110. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Hwo SY, and Kirschner MW (1977). Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 116, 227–247. [DOI] [PubMed] [Google Scholar]
- Cohen TJ, Friedmann D, Hwang AW, Marmorstein R, and Lee VM (2013). The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat Struct Mol Biol 20, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, and Lee VM (2011). The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun 2, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon EE, and Sigurdsson EM (2018). Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 14, 399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Vianna C, Freeman M, and Davies P (2002). A polymorphic gene nested within an intron of the tau gene: implications for Alzheimer’s disease. Proc Natl Acad Sci U S A 99, 7751–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchie D, Mavilia C, Georgieff IS, Liem RK, Shelanski ML, and Nunez J (1992). Primary structure of high molecular weight tau present in the peripheral nervous system. Proc Natl Acad Sci U S A 89, 4378–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wei Y, Wang X, and He R (2009). D-Ribosylated Tau forms globular aggregates with high cytotoxicity. Cell Mol Life Sci 66, 2559–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT, Liu WK, and Yen SH (1994). Expression of tau exon 8 in different species. Neurosci Lett 172, 167–170. [DOI] [PubMed] [Google Scholar]
- Chesser AS, Pritchard SM, and Johnson GV (2013). Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol 4, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung PJ, Choi MC, Miller HP, Feinstein HE, Raviv U, Li Y, Wilson L, Feinstein SC, and Safinya CR (2015). Direct force measurements reveal that protein Tau confers short-range attractions and isoformdependent steric stabilization to microtubules. Proc Natl Acad Sci U S A 112, E6416–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC, Layfield R, Serpell L, Narain Y, Goedert M, and Spillantini MG (2002). Proteasomal degradation of tau protein. Journal of neurochemistry 83, 176–185. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, and Vitek MP (2001). Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci 114, 1179–1187. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, et al. (2012). Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel P, Leroy O, Hamdane M, Sambo AV, Delacourte A, and Buee L (2005). Proteasome inhibition and Tau proteolysis: an unexpected regulation. FEBS Lett 579, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derisbourg M, Leghay C, Chiappetta G, Fernandez-Gomez FJ, Laurent C, Demeyer D, Carrier S, BueeScherrer V, Blum D, Vinh J, et al. (2015). Role of the Tau N-terminal region in microtubule stabilization revealed by new endogenous truncated forms. Sci Rep 5, 9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias WB, and Hart GW (2007). O-GlcNAc modification in diabetes and Alzheimer’s disease. Mol Biosyst 3, 766–772. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, et al. (2007). The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117, 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Yue M, Lin WL, Dickson DW, Dunmore JH, Lee WC, Zehr C, West G, Cao S, Clark AM, et al. (2006). Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci 26, 6985–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, and Holzbaur EL (2008). Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan PJ, and Johnson GV (2010). The role of tau kinases in Alzheimer’s disease. Curr Opin Drug Discov Devel 13, 595–603. [PMC free article] [PubMed] [Google Scholar]
- Dorval V, and Fraser PE (2006). Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem 281, 9919–9924. [DOI] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, and Mandelkow E (1995). Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem 270, 7679–7688. [DOI] [PubMed] [Google Scholar]
- Dubey M, Chaudhury P, Kabiru H, and Shea TB (2008). Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate taumediated neurite instability. Cell Motil Cytoskeleton 65, 89–99. [DOI] [PubMed] [Google Scholar]
- Dujardin S, Begard S, Caillierez R, Lachaud C, Delattre L, Carrier S, Loyens A, Galas MC, Bousset L, Melki R, et al. (2014). Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One 9, e100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JE, and Goldstein LS (2006). The genetics of axonal transport and axonal transport disorders. PLoS Genet 2, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekharzadeh B, Daigle JG, Kapinos LE, Coyne A, Schiantarelli J, Carlomagno Y, Cook C, Miller SJ, Dujardin S, Amaral AS, et al. (2018). Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer’s Disease. Neuron 99, 925–940 e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JL (2013). Therapeutic Targets in the Ubiquitin-proteasome System for Alzheimer’s Disease. Curr Enz Inhib 9:, 46–54. [Google Scholar]
- Evans LD, Wassmer T, Fraser G, Smith J, Perkinton M, Billinton A, and Livesey FJ (2018). Extracellular Monomeric and Aggregated Tau Efficiently Enter Human Neurons through Overlapping but Distinct Pathways. Cell Rep 22, 3612–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, Crowther RA, Ghetti B, Scheres SHW, and Goedert M (2018). Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561, 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GA, Munoz JP, Garrido J, and Maccioni RB (2002). Tubulin, actin, and tau protein interactions and the study of their macromolecular assemblies. J Cell Biochem 85, 315–324. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, and Scheres SHW (2017). Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine SN, Zheng D, Sabbagh JJ, Martin MD, Chaput D, Darling A, Trotter JH, Stothert AR, Nordhues BA, Lussier A, et al. (2016). DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J 35, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, and Gattaz WF (2011). Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry 198, 351–356. [DOI] [PubMed] [Google Scholar]
- Funk KE, Thomas SN, Schafer KN, Cooper GL, Liao Z, Clark DJ, Yang AJ, and Kuret J (2014). Lysine methylation is an endogenous post-translational modification of tau protein in human brain and a modulator of aggregation propensity. Biochem J 462, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, et al. (2003). Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci U S A 100, 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff IS, Liem RK, Couchie D, Mavilia C, Nunez J, and Shelanski ML (1993). Expression of high molecular weight tau in the central and peripheral nervous systems. J Cell Sci 105 ( Pt 3), 729–737. [DOI] [PubMed] [Google Scholar]
- Gibbons GS, Lee VMY, and Trojanowski JQ (2018). Mechanisms of Cell-to-Cell Transmission of Pathological Tau: A Review. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. (1991). Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706. [DOI] [PubMed] [Google Scholar]
- Goedert M, and Jakes R (1990). Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 9, 4225–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, and Crowther RA (1989a). Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–526. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, and Crowther RA (1989b). Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 8, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, and Iqbal K (2005). Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm (Vienna) 112, 813–838. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Farias G, and Maccioni RB (1998). Modification of tau to an Alzheimer’s type protein interferes with its interaction with microtubules. Cell Mol Biol (Noisy-le-grand) 44, 1117–1127. [PubMed] [Google Scholar]
- Goode BL, Chau M, Denis PE, and Feinstein SC (2000). Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J Biol Chem 275, 38182–38189. [DOI] [PubMed] [Google Scholar]
- Goode BL, and Feinstein SC (1994). Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol 124, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, and Binder LI (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 83, 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Botzen D, Engels M, Voss P, Kaiser B, Jung T, Grimm S, Ermak G, and Davies KJ (2010). Tau protein degradation is catalyzed by the ATP/ubiquitin-independent 20S proteasome under normal cell conditions. Archives of biochemistry and biophysics 500, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, and Binder LI (2006). Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem 97, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Guo T, Noble W, and Hanger DP (2017). Roles of tau protein in health and disease. Acta Neuropathol 133, 665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N, Trinczek B, Biernat J, Mandelkow EM, and Mandelkow E (1994). Domains of tau protein and interactions with microtubules. Biochemistry 33, 9511–9522. [DOI] [PubMed] [Google Scholar]
- Hall B, Mak E, Cervenka S, Aigbirhio FI, Rowe JB, and O’Brien JT (2017). In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Res Rev 36, 50–63. [DOI] [PubMed] [Google Scholar]
- Han DH, Na HK, Choi WH, Lee JH, Kim YK, Won C, Lee SH, Kim KP, Kuret J, Min DH, et al. (2014). Direct cellular delivery of human proteasomes to delay tau aggregation. Nat Commun 5, 5633. [DOI] [PubMed] [Google Scholar]
- Han I, and Kudlow JE (1997). Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol 17, 2550–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger DP, Anderton BH, and Noble W (2009). Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 15, 112–119. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, and Hirokawa N (1994). Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369, 488–491. [DOI] [PubMed] [Google Scholar]
- Hardy J, and Selkoe D (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Morishima-Kawashima M, Takio K, Suzuki M, Titani K, and Ihara Y (1992). Protein sequence and mass spectrometric analyses of tau in the Alzheimer’s disease brain. J Biol Chem 267, 17047–17054. [PubMed] [Google Scholar]
- Hasegawa M, Smith MJ, and Goedert M (1998). Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 437, 207–210. [DOI] [PubMed] [Google Scholar]
- He HJ, Wang XS, Pan R, Wang DL, Liu MN, and He RQ (2009). The proline-rich domain of tau plays a role in interactions with actin. BMC Cell Biol 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, WyssCoray T, Vitorica J, Ransohoff RM, et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A (1989). Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol 9, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgrafe K, Sydow A, Matenia D, Cadinu D, Konen S, Petrova O, Pickhardt M, Goll P, Morellini F, Mandelkow E, et al. (2015). Preventive methylene blue treatment preserves cognition in mice expressing fulllength pro-aggregant human Tau. Acta Neuropathol Commun 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, and Saxton WM (2005). The axonal transport of mitochondria. J Cell Sci 118, 5411–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollville E, and Deshmukh M (2017). Physiological functions of non-apoptotic caspase activity in the nervous system. Semin Cell Dev Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, et al. (1998). Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282, 1914–1917. [DOI] [PubMed] [Google Scholar]
- Horowitz PM, LaPointe N, Guillozet-Bongaarts AL, Berry RW, and Binder LI (2006). N-terminal fragments of tau inhibit full-length tau polymerization in vitro. Biochemistry 45, 12859–12866. [DOI] [PubMed] [Google Scholar]
- Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, and Binder LI (2004). Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci 24, 7895–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li XC, Wang ZH, Luo Y, Zhang X, Liu XP, Feng Q, Wang Q, Yue Z, Chen Z, et al. (2016). Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin. Oncotarget 7, 17356–17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Tian S, Cai R, Sun J, Xia W, Dong X, Shen Y, and Wang S (2017). Saitohin Q7R polymorphism is associated with late-onset Alzheimer’s disease susceptibility among caucasian populations: a meta-analysis. J Cell Mol Med 21, 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. (1998). Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, and Barnes CL (1984). Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225, 1168–1170. [DOI] [PubMed] [Google Scholar]
- Iijima-Ando K, Sekiya M, Maruko-Otake A, Ohtake Y, Suzuki E, Lu B, and Iijima KM (2012). Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer’s disease-related tau phosphorylation via PAR-1. PLoS Genet 8, e1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Rispoli J, Kaphzan H, Klann E, Chen EI, Kim J, Komatsu M, and Abeliovich A (2012). Macroautophagy deficiency mediates age-dependent neurodegeneration through a phospho-tau pathway. Molecular neurodegeneration 7, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VM, and Trojanowski JQ (2015). Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 129, 469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H (2003). Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun 305, 776–783. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Higuchi M, Zhang B, Yoshiyama Y, Hong M, Trojanowski JQ, and Lee VM (2001). Attenuated neurodegenerative disease phenotype in tau transgenic mouse lacking neurofilaments. J Neurosci 21, 6026–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner A, and Ittner LM (2018). Dendritic Tau in Alzheimer’s Disease. Neuron 99, 13–27. [DOI] [PubMed] [Google Scholar]
- Ittner LM, and Gotz J (2011). Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12, 65–72. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, et al. (2010). Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397. [DOI] [PubMed] [Google Scholar]
- Janning D, Igaev M, Sundermann F, Bruhmann J, Beutel O, Heinisch JJ, Bakota L, Piehler J, Junge W, and Brandt R (2014). Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons. Mol Biol Cell 25, 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara C, Aranguiz A, Cerpa W, Tapia-Rojas C, and Quintanilla RA (2018). Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol 18, 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarero-Basulto JJ, Luna-Munoz J, Mena R, Kristofikova Z, Ripova D, Perry G, Binder LI, and GarciaSierra F (2013). Proteolytic cleavage of polymeric tau protein by caspase-3: implications for Alzheimer disease. J Neuropathol Exp Neurol 72, 1145–1161. [DOI] [PubMed] [Google Scholar]
- Jean DC, and Baas PW (2013). It cuts two ways: microtubule loss during Alzheimer disease. EMBO J 32, 2900–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, and Johnson GV (2014). Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun 5, 3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GV, Jope RS, and Binder LI (1989). Proteolysis of tau by calpain. Biochem Biophys Res Commun 163, 1505–1511. [DOI] [PubMed] [Google Scholar]
- Jones CL, Njomen E, Sjogren B, Dexheimer TS, and Tepe JJ (2017). Small Molecule Enhancement of 20S Proteasome Activity Targets Intrinsically Disordered Proteins. ACS chemical biology 12, 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, and Zweckstetter M (2015). Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci U S A 112, 7501–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, Leitman E, Binder LI, and Brady ST (2012). Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging 33, 826 e815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, and Binder LI (2011). Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci 31, 9858–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, and Cuervo AM (2012). Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 22, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, and Ittner LM (2012). Lessons from tau-deficient mice. Int J Alzheimers Dis 2012, 873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck S, Nitsch R, Grune T, and Ullrich O (2003). Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem 85, 115–122. [DOI] [PubMed] [Google Scholar]
- Kellogg EH, Hejab NMA, Poepsel S, Downing KH, DiMaio F, and Nogales E (2018). Near-atomic model of microtubule-tau interactions. Science 360, 1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenessey A, Nacharaju P, Ko LW, and Yen SH (1997). Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J Neurochem 69, 2026–2038. [DOI] [PubMed] [Google Scholar]
- Kevenaar JT, and Hoogenraad CC (2015). The axonal cytoskeleton: from organization to function. Front Mol Neurosci 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, Mulkearns E, Tyynela J, Scherzer CR, and Feany MB (2010). Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS genetics 6, e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Shinpo K, Takeuchi M, Yamagishi S, Makita Z, Sasaki N, and Tashiro K (2003). Glycation--a sweet tempter for neuronal death. Brain Res Brain Res Rev 41, 306–323. [DOI] [PubMed] [Google Scholar]
- Kim HJ, and Taylor JP (2017). Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases. Neuron 96, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, Brown C, Hashikawa T, Murayama M, Seok H, et al. (2013). Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci 369, 20130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, and Ripova D (2012). Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis 2012, 731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, et al. (2015). Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, and Selkoe DJ (1986). Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A 83, 4044–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Bakalis S, and Neve RL (1989). Developmentally regulated expression of specific tau sequences. Neuron 2, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Kovacs GG (2015). Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41, 3–23. [DOI] [PubMed] [Google Scholar]
- Kruger U, Wang Y, Kumar S, and Mandelkow EM (2012). Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiology of aging 33, 2291–2305. [DOI] [PubMed] [Google Scholar]
- LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, and Brady ST (2009). The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res 87, 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, and Kayed R (2011). Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau DH, Hogseth M, Phillips EC, O’Neill MJ, Pooler AM, Noble W, and Hanger DP (2016). Critical residues involved in tau binding to fyn: implications for tau phosphorylation in Alzheimer’s disease. Acta Neuropathol Commun 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layfield R, Cavey JR, and Lowe J (2003). Role of ubiquitin-mediated proteolysis in the pathogenesis of neurodegenerative disorders. Ageing Res Rev 2, 343–356. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Bonay P, Colaco C, and Avila J (1994). Analysis of microtubule-associated protein tau glycation in paired helical filaments. J Biol Chem 269, 21614–21619. [PubMed] [Google Scholar]
- Lee G (2005). Tau and src family tyrosine kinases. Biochim Biophys Acta 1739, 323–330. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, et al. (2012). Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18, 291–295. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Moon S, Zhang Q, Volitakis I, Finkelstein DI, and Bush AI (2014). Motor and cognitive deficits in aged tau knockout mice in two background strains. Mol Neurodegener 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Brizzee C, and Johnson GV (2015). BAG3 facilitates the clearance of endogenous tau in primary neurons. Neurobiology of aging 36, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leugers CJ, Koh JY, Hong W, and Lee G (2013). Tau in MAPK activation. Front Neurol 4, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. (1995). Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269, 973–977. [DOI] [PubMed] [Google Scholar]
- Li J, Liu D, Sun L, Lu Y, and Zhang Z (2012). Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci 317, 1–5. [DOI] [PubMed] [Google Scholar]
- Li L, and Chin LS (2007). Impairment of the ubiquitin-proteosome system: a common pathogenic mechanism in neurodegenerative disorders In Di Napoli M, Wojcik C, editors: The Ubiquitin Proteasome System in the Central Nervous System: From Physiology to Pathology, New York: Nova Science Pub Inc, pp 553–577. [Google Scholar]
- Li XC, Hu Y, Wang ZH, Luo Y, Zhang Y, Liu XP, Feng Q, Wang Q, Ye K, Liu GP, et al. (2016). Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci Rep 6, 24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G, and Cole RD (1984). Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259, 5301–5305. [PubMed] [Google Scholar]
- Litersky JM, and Johnson GV (1992). Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem 267, 1563–1568. [PubMed] [Google Scholar]
- Liu C, and Gotz J (2013). Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PLoS One 8, e84849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Song X, Nisbet R, and Gotz J (2016). Co-immunoprecipitation with Tau Isoform-specific Antibodies Reveals Distinct Protein Interactions and Highlights a Putative Role for 2N Tau in Disease. J Biol Chem 291, 8173–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, and Gong CX (2005). Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22, 1942–1950. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, and Gong CX (2004). O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci U S A 101, 10804–10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Merkle RK, and Gong CX (2002). Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett 512, 101–106. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, and Duff K (2012). Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes S, Vaz-Silva J, Pinto V, Dalla C, Kokras N, Bedenk B, Mack N, Czisch M, Almeida OF, Sousa N, et al. (2016). Tau protein is essential for stress-induced brain pathology. Proc Natl Acad Sci U S A 113, E3755–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, and Binder LI (1995). Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci U S A 92, 10369–10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Boada M, Dubois B, Hull M, Rinne JO, Huppertz HJ, Calero M, Andres MV, GomezCarrillo B, Leon T, et al. (2015). A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimers Dis 45, 75–88. [DOI] [PubMed] [Google Scholar]
- Lovestone S, and Reynolds CH (1997). The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience 78, 309–324. [DOI] [PubMed] [Google Scholar]
- Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y, You Q, and Jiang Z (2018). Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur J Med Chem 146, 251–259. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Wulf G, Zhou XZ, Davies P, and Lu KP (1999). The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399, 784–788. [DOI] [PubMed] [Google Scholar]
- Lu SS, Gong FF, Feng F, Hu CY, Qian ZZ, Wu YL, Yang HY, and Sun YH (2014). Association of microtubule associated protein tau/Saitohin (MAPT/STH) MAPT_238bp/STH Q7R polymorphisms and Parkinson’s disease: A meta-analysis. Biochem Biophys Res Commun 453, 653–661. [DOI] [PubMed] [Google Scholar]
- Luo HB, Xia YY, Shu XJ, Liu ZC, Feng Y, Liu XH, Yu G, Yin G, Xiong YS, Zeng K, et al. (2014). SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc Natl Acad Sci U S A 111, 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MH, Leski ML, and Andreadis A (2004). Tau isoforms which contain the domain encoded by exon 6 and their role in neurite elongation. J Cell Biochem 91, 880–895. [DOI] [PubMed] [Google Scholar]
- Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, Kiosea NC, Nazari S, Chen PP, Nothias F, et al. (2014). Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris Water Maze with aging. J Neurosci 34, 7124–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas T, Eidenmuller J, and Brandt R (2000). Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J Biol Chem 275, 15733–15740. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, and Mandelkow E (1995). Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging 16, 355–362; discussion 362–353. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, and Mandelkow E (2012). Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med 2, a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, Issad T, Gerhardt E, Pagesy P, Vileno M, et al. (2017). Tau deletion promotes brain insulin resistance. J Exp Med 214, 2257–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, and Schachter J (2011). Targeting post-translational modifications on tau as a therapeutic strategy for Alzheimer’s disease. J Neurogenet 25, 127–133. [DOI] [PubMed] [Google Scholar]
- Martin L, Latypova X, and Terro F (2011). Post-translational modifications of tau protein: implications for Alzheimer’s disease. Neurochem Int 58, 458–471. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, Baker D, Tan Hehir CA, Jeromin A, et al. (2016). Plasma tau in Alzheimer disease. Neurology 87, 1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merezhko M, Brunello CA, Yan X, Vihinen H, Jokitalo E, Uronen RL, and Huttunen HJ (2018). Secretion of Tau via an Unconventional Non-vesicular Mechanism. Cell Rep 25, 2027–2035 e2024. [DOI] [PubMed] [Google Scholar]
- Mi K, and Johnson GV (2006). The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 3, 449–463. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, Airey DC, Knopman DS, Roberts RO, Machulda MM, et al. (2018). Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 14, 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietelska-Porowska A, Wasik U, Goras M, Filipek A, and Niewiadomska G (2014). Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15, 4671–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, et al. (2010). Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Titani K, and Ihara Y (1993). Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron 10, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, and Ihara Y (1995). Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 270, 823–829. [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, and Mucke L (2011). The many faces of tau. Neuron 70, 410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K, and Williams M (2018). Alzheimer’s disease (AD) therapeutics - 1: Repeated clinical failures continue to question the amyloid hypothesis of AD and the current understanding of AD causality. Biochem Pharmacol 158, 359–375. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, and Malinow R (2014). Engineering a memory with LTD and LTP. Nature 511, 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Greenwood A, Binder L, Bigio EH, Denial S, Nicholson L, Zhou XZ, and Lu KP (2012). Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer’s disease. Cell 149, 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Zhen Zhou X, and Ping Lu K (2013). Cis phosphorylated tau as the earliest detectable pathogenic conformation in Alzheimer disease, offering novel diagnostic and therapeutic strategies. Prion 7, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necula M, and Kuret J (2004). Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem 279, 49694–49703. [DOI] [PubMed] [Google Scholar]
- Neergaard JS, Dragsbaek K, Christiansen C, Karsdal MA, Brix S, and Henriksen K (2018). Two novel blood-based biomarker candidates measuring degradation of tau are associated with dementia: A prospective study. PLoS One 13, e0194802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neklesa TK, Winkler JD, and Crews CM (2017). Targeted protein degradation by PROTACs. Pharmacol Ther 174, 138–144. [DOI] [PubMed] [Google Scholar]
- Neve RL, Harris P, Kosik KS, Kurnit DM, and Donlon TA (1986). Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 387, 271–280. [DOI] [PubMed] [Google Scholar]
- Niewidok B, Igaev M, Sundermann F, Janning D, Bakota L, and Brandt R (2016). Presence of a carboxy-terminal pseudorepeat and disease-like pseudohyperphosphorylation critically influence tau’s interaction with microtubules in axon-like processes. Mol Biol Cell 27, 3537–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA (2013). The role of autophagy in neurodegenerative disease. Nature medicine 19, 983–997. [DOI] [PubMed] [Google Scholar]
- Noble W, Hanger DP, Miller CC, and Lovestone S (2013). The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 4, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, et al. (2018). FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res Ther 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MA, Viel TA, and Buck HS (2013). Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res 10, 104–107. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, and Marth JD (2006). Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867. [DOI] [PubMed] [Google Scholar]
- Ono Y, Saido TC, and Sorimachi H (2016). Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov 15, 854–876. [DOI] [PubMed] [Google Scholar]
- Pallas-Bazarra N, Jurado-Arjona J, Navarrete M, Esteban JA, Hernandez F, Avila J, and Llorens-Martin M (2016). Novel function of Tau in regulating the effects of external stimuli on adult hippocampal neurogenesis. EMBO J 35, 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos SC (1997). The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implications for Alzheimer disease. Proc Natl Acad Sci U S A 94, 6612–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SA, Ahn SI, and Gallo JM (2016). Tau mis-splicing in the pathogenesis of neurodegenerative disorders. BMB Rep 49, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, and Ferreira A (2005). The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci 25, 5365–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel HK, and Li W (1999). Heparin-induced conformational change in microtubule-associated protein Tau as detected by chemical cross-linking and phosphopeptide mapping. J Biol Chem 274, 8029–8038. [DOI] [PubMed] [Google Scholar]
- Peck A, Sargin ME, LaPointe NE, Rose K, Manjunath BS, Feinstein SC, and Wilson L (2011). Tau isoform-specific modulation of kinesin-driven microtubule gliding rates and trajectories as determined with tau-stabilized microtubules. Cytoskeleton (Hoboken) 68, 44–55. [DOI] [PubMed] [Google Scholar]
- Pedersen JT, and Sigurdsson EM (2015). Tau immunotherapy for Alzheimer’s disease. Trends Mol Med 21, 394–402. [DOI] [PubMed] [Google Scholar]
- Perez MJ, Jara C, and Quintanilla RA (2018). Contribution of Tau Pathology to Mitochondrial Impairment in Neurodegeneration. Front Neurosci 12, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, et al. (2004). CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Human molecular genetics 13, 703–714. [DOI] [PubMed] [Google Scholar]
- Pevalova M, Filipcik P, Novak M, Avila J, and Iqbal K (2006). Post-translational modifications of tau protein. Bratisl Lek Listy 107, 346–353. [PubMed] [Google Scholar]
- Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, Swartzlander DB, Palmieri M, di Ronza A, Lee VM, et al. (2014). Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med 6, 1142–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler AM, Noble W, and Hanger DP (2014). A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 76 Pt A, 1–8. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Usardi A, Evans CJ, Philpott KL, Noble W, and Hanger DP (2012). Dynamic association of tau with neuronal membranes is regulated by phosphorylation. Neurobiol Aging 33, 431 e427–438. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, and Schellenberg GD (1998). Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43, 815–825. [DOI] [PubMed] [Google Scholar]
- Preuss U, Biernat J, Mandelkow EM, and Mandelkow E (1997). The ‘jaws’ model of tau-microtubule interaction examined in CHO cells. J Cell Sci 110 ( Pt 6), 789–800. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, and Baas PW (2006). Tau protects microtubules in the axon from severing by katanin. J Neurosci 26, 3120–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape M (2017). Ubiquitylation at the crossroads of development and disease. Nat Rev Mol Cell Biol. [DOI] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, and Ferreira A (2002). Tau is essential to beta -amyloidinduced neurotoxicity. Proc Natl Acad Sci U S A 99, 6364–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH (2011). Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res 1415, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan P, Whitcomb DJ, and Cho K (2016). Physiological and Pathophysiological Implications of Synaptic Tau. Neuroscientist. [DOI] [PubMed] [Google Scholar]
- Reyes JF, Fu Y, Vana L, Kanaan NM, and Binder LI (2011). Tyrosine nitration within the proline-rich region of Tau in Alzheimer’s disease. Am J Pathol 178, 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CH, Garwood CJ, Wray S, Price C, Kellie S, Perera T, Zvelebil M, Yang A, Sheppard PW, Varndell IM, et al. (2008). Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. J Biol Chem 283, 18177–18186. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Berry RW, and Binder LI (2005). Site-specific nitration differentially influences tau assembly in vitro. Biochemistry 44, 13997–14009. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Lukas TJ, Berry RW, and Binder LI (2006a). Peroxynitrite-mediated tau modifications stabilize preformed filaments and destabilize microtubules through distinct mechanisms. Biochemistry 45, 4314–4326. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Reyes JF, Fu Y, Bigio EH, Guillozet-Bongaarts AL, Berry RW, and Binder LI (2006b). Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer’s disease and other tauopathies. J Neurosci 26, 10636–10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, and Cotman CW (2004). Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, and Martin S (2017). CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 3, CD010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, and Mucke L (2007). Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754. [DOI] [PubMed] [Google Scholar]
- Roder HM, Fracasso RP, Hoffman FJ, Witowsky JA, Davis G, and Pellegrino CB (1997). Phosphorylation-dependent monoclonal Tau antibodies do not reliably report phosphorylation by extracellular signal-regulated kinase 2 at specific sites. J Biol Chem 272, 4509–4515. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, and McNally FJ (2010). Microtubule-severing enzymes. Curr Opin Cell Biol 22, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Suryadinata R, Tan AR, Roesley SN, and Sarcevic B (2012). Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life 64, 136–142. [DOI] [PubMed] [Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, and Santambrogio L (2011). Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, and Nordberg A (2017). Tau PET imaging: present and future directions. Mol Neurodegener 12, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, et al. (2005). Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, Koike T, Wakayama I, Yanagihara R, Garruto R, et al. (1998). Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol 153, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, Sullivan M, Crisp MJ, Kasten T, Kirmess KM, et al. (2018). Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 97, 1284–1298 e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A, Oyama F, Matsushita M, and Ihara Y (1994). Molecular diversity at the carboxyl terminus of human and rat tau. Brain Res Mol Brain Res 27, 111–117. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Lavenir I, Ozcelik S, Tolnay M, Winkler DT, and Goedert M (2012). Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 135, 2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiberlich V, Bauer NG, Schwarz L, Ffrench-Constant C, Goldbaum O, and Richter-Landsberg C (2015). Downregulation of the microtubule associated protein tau impairs process outgrowth and myelin basic protein mRNA transport in oligodendrocytes. Glia 63, 1621–1635. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqbal I, and Iqbal K (1998). Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch Biochem Biophys 357, 299–309. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Horowitz PM, Karsten SL, Jackson GR, Geschwind DH, Fu Y, Berry RW, and Binder LI (2006). Degradation of tau protein by puromycin-sensitive aminopeptidase in vitro. Biochemistry 45, 15111–15119. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Bretteville A, Hamdane M, Caillet-Boudin ML, Grognet P, Bombois S, Blum D, Delacourte A, Pasquier F, Vanmechelen E, et al. (2008). Biochemistry of Tau in Alzheimer’s disease and related neurological disorders. Expert Rev Proteomics 5, 207–224. [DOI] [PubMed] [Google Scholar]
- Settembre C, and Ballabio A (2011). TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy 7, 1379–1381. [DOI] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, et al. (2012). A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31, 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GV, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ, et al. (1995). Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem 270, 18917–18922. [DOI] [PubMed] [Google Scholar]
- Shahani N, and Brandt R (2002). Functions and malfunctions of the tau proteins. Cell Mol Life Sci 59, 1668–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahpasand K, Uemura I, Saito T, Asano T, Hata K, Shibata K, Toyoshima Y, Hasegawa M, and Hisanaga S (2012). Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease. J Neurosci 32, 2430–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Stukenberg PT, Kirschner MW, and Lu KP (1998). The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev 12, 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng ZH (2014). Mitochondrial trafficking and anchoring in neurons: New insight and implications. J Cell Biol 204, 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. (1995). Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375, 754–760. [DOI] [PubMed] [Google Scholar]
- Shin RW, Iwaki T, Kitamoto T, and Tateishi J (1991). Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab Invest 64, 693–702. [PubMed] [Google Scholar]
- Sjoberg MK, Shestakova E, Mansuroglu Z, Maccioni RB, and Bonnefoy E (2006). Tau protein binds to pericentromeric DNA: a putative role for nuclear tau in nucleolar organization. J Cell Sci 119, 2025–2034. [DOI] [PubMed] [Google Scholar]
- Sohn PD, Tracy TE, Son HI, Zhou Y, Leite RE, Miller BL, Seeley WW, Grinberg LT, and Gan L (2016). Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Mol Neurodegener 11, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Lu SX, Ouyang X, Melchor J, Lee J, Terracina G, Wang X, Hyde L, Hess JF, Parker EM, et al. (2015). Analysis of tau post-translational modifications in rTg4510 mice, a model of tau pathology. Mol Neurodegener 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittaels K, Van den Haute C, Van Dorpe J, Bruynseels K, Vandezande K, Laenen I, Geerts H, Mercken M, Sciot R, Van Lommel A, et al. (1999). Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol 155, 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoothoff W, Jones PB, Spires-Jones TL, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, et al. (2009). Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem 111, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A, Nesslany F, Violet M, Begard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, et al. (2011). Nuclear tau, a key player in neuronal DNA protection. J Biol Chem 286, 4566–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendrei GI, Lee VM, and Otvos L Jr. (1993). Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res 34, 243–249. [DOI] [PubMed] [Google Scholar]
- Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, and Hyman BT (2012). The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol 181, 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ishida M, Komano H, and Takahashi H (2008). SUMO-1 immunoreactivity co-localizes with phospho-Tau in APP transgenic mice but not in mutant Tau transgenic mice. Neurosci Lett 441, 90–93. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tomizawa K, Ishiguro K, Takamatsu M, Fujita SC, and Imahori K (1995). Involvement of tau protein kinase I in paired helical filament-like phosphorylation of the juvenile tau in rat brain. J Neurochem 64, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Tang M, Ji C, Pallo S, Rahman I, and Johnson GVW (2018). Nrf2 mediates the expression of BAG3 and autophagy cargo adaptor proteins and tau clearance in an age-dependent manner. Neurobiol Aging 63, 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Ioja E, Bereczki E, Hultenby K, Li C, Guan Z, Winblad B, and Pei JJ (2015). mTor mediates tau localization and secretion: Implication for Alzheimer’s disease. Biochim Biophys Acta 1853, 1646–1657. [DOI] [PubMed] [Google Scholar]
- Tardivel M, Begard S, Bousset L, Dujardin S, Coens A, Melki R, Buee L, and Colin M (2016). Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun 4, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K, Hasegawa M, Ihara Y, and Iwatsubo T (1997). Somatodendritic localization of phosphorylated tau in neonatal and adult rat cerebral cortex. Neuroreport 8, 2797–2801. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Funk KE, Wan Y, Liao Z, Davies P, Kuret J, and Yang AJ (2012). Dual modification of Alzheimer’s disease PHF-tau protein by lysine methylation and ubiquitylation: a mass spectrometry approach. Acta Neuropathol 123, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciakiewicz H, Tseng JH, Wander CM, Madden V, Tripathy A, Yuan CX, and Cohen TJ (2017). A Dual Pathogenic Mechanism Links Tau Acetylation to Sporadic Tauopathy. Sci Rep 7, 44102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz-Silva J, Gomes P, Jin Q, Zhu M, Zhuravleva V, Quintremil S, Meira T, Silva J, Dioli C, SoaresCunha C, et al. (2018). Endolysosomal degradation of Tau and its role in glucocorticoid-driven hippocampal malfunction. EMBO J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, Talahari S, Nesslany F, Lefebvre B, Bonnefoy E, et al. (2014). A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, and Mucke L (2010). Tau reduction prevents Abeta-induced defects in axonal transport. Science 330, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai MS, Liang Y, Shi C, Cho EY, Kung HF, and Yew DT (2009). Co-localization of hyperphosphorylated tau and caspases in the brainstem of Alzheimer’s disease patients. Biogerontology 10, 457–469. [DOI] [PubMed] [Google Scholar]
- Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, et al. (2017). The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Garg S, Mandelkow EM, and Mandelkow E (2010). Proteolytic processing of tau. Biochemical Society transactions 38, 955–961. [DOI] [PubMed] [Google Scholar]
- Wang Y, Loomis PA, Zinkowski RP, and Binder LI (1993). A novel tau transcript in cultured human neuroblastoma cells expressing nuclear tau. J Cell Biol 121, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, and Mandelkow E (2012). Degradation of tau protein by autophagy and proteasomal pathways. Biochem Soc Trans 40, 644–652. [DOI] [PubMed] [Google Scholar]
- Wang Y, and Mandelkow E (2016). Tau in physiology and pathology. Nat Rev Neurosci 17, 5–21. [DOI] [PubMed] [Google Scholar]
- Ward SM, Himmelstein DS, Lancia JK, and Binder LI (2012). Tau oligomers and tau toxicity in neurodegenerative disease. Biochem Soc Trans 40, 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S, Nicholls S, Takeda S, Fan Z, and Hyman BT (2016). Formation, release, and internalization of stable tau oligomers in cells. J Neurochem 139, 1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, and Kirschner MW (1975). A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 72, 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C, Vilarino-Guell C, Jasinska-Myga B, Heckman MG, Soto-Ortolaza AI, Cobb SA, Aasly JO, Gibson JM, Lynch T, Uitti RJ, et al. (2010). Association of the MAPT locus with Parkinson’s disease. Eur J Neurol 17, 483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK, Gauthier S, Frisoni GB, Jia J, Hardlund JH, Moebius HJ, Bentham P, Kook KA, Schelter BO, Wischik DJ, et al. (2018). Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J Alzheimers Dis 61, 435–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Edwards PC, Lai RY, Roth M, and Harrington CR (1996). Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A 93, 11213–11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS (2012). The Role of Tau in Neurodegenerative Diseases and Its Potential as a Therapeutic Target. Scientifica Article ID 796024, 20 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Mirra SS, Pollock NJ, and Binder LI (1986). Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc Natl Acad Sci U S A 83, 4040–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Li C, and Gotz J (2015). Pseudophosphorylation of Tau at distinct epitopes or the presence of the P301L mutation targets the microtubule-associated protein Tau to dendritic spines. Biochim Biophys Acta 1852, 913–924. [DOI] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, Cirrito JR, Patel TK, Hochgrafe K, Mandelkow EM, et al. (2014). Neuronal activity regulates extracellular tau in vivo. J Exp Med 211, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Patel TK, Hochgrafe K, Mahan TE, Jiang H, Stewart FR, Mandelkow EM, and Holtzman DM (2015). Analysis of in vivo turnover of tau in a mouse model of tauopathy. Mol Neurodegener 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA, et al. (1994). Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A 91, 7787–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, et al. (1995). Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med 1, 693–699. [DOI] [PubMed] [Google Scholar]
- Yan X, Nykanen NP, Brunello CA, Haapasalo A, Hiltunen M, Uronen RL, and Huttunen HJ (2016). FRMD4A-cytohesin signaling modulates the cellular release of tau. J Cell Sci 129, 2003–2015. [DOI] [PubMed] [Google Scholar]
- Yao TP (2010). The role of ubiquitin in autophagy-dependent protein aggregate processing. Genes Cancer 1, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hastie CJ, McLauchlan H, Cohen P, and Goedert M (2004). Phosphorylation of microtubuleassociated protein tau by isoforms of c-Jun N-terminal kinase (JNK). J Neurochem 90, 352–358. [DOI] [PubMed] [Google Scholar]
- Yu CH, Si T, Wu WH, Hu J, Du JT, Zhao YF, and Li YM (2008a). O-GlcNAcylation modulates the selfaggregation ability of the fourth microtubule-binding repeat of tau. Biochem Biophys Res Commun 375, 59–62. [DOI] [PubMed] [Google Scholar]
- Yu JZ, and Rasenick MM (2006). Tau associates with actin in differentiating PC12 cells. FASEB J 20, 1452–1461. [DOI] [PubMed] [Google Scholar]
- Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, and Baas PW (2008b). The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol Biol Cell 19, 1485–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, et al. (2008). A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4, 483–490. [DOI] [PubMed] [Google Scholar]
- Zempel H, Dennissen FJA, Kumar Y, Luedtke J, Biernat J, Mandelkow EM, and Mandelkow E (2017). Axodendritic sorting and pathological missorting of Tau are isoform-specific and determined by axon initial segment architecture. J Biol Chem 292, 12192–12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang C, Zhao Q, and Li D (2013). Spectrin: structure, function and disease. Sci China Life Sci 56, 1076–1085. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wen H, and Shi X (2012). Lysine methylation: beyond histones. Acta Biochim Biophys Sin (Shanghai) 44, 14–27. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Congdon EE, Nagaraja HN, and Kuret J (2012). Tau isoform composition influences rate and extent of filament formation. J Biol Chem 287, 20711–20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Kullertz G, Stark M, Fischer G, and Lu KP (2000). Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 6, 873–883. [DOI] [PubMed] [Google Scholar]