Summary
Immune checkpoint inhibitors induce durable responses in some advanced cancer patients, but may simultaneously trigger auto-inflammatory immune-related adverse events (irAEs). The pathogenesis of irAEs may relate to genetic predisposition, environmental insults, or tumor-host interactions. Elevated expression of certain cytokines may signal subclinical inflammation that evolves into severe irAEs with treatment.
Keywords: Immune checkpoint inhibitors, immune-related adverse events, nivolumab, pembrolizumab, ipilimumab, melanoma, toxicity
In this issue of Clinical Cancer Research, Lim and colleagues report that elevated expression of an 11-cytokine assay correlates with severe toxicity from immune checkpoint inhibitors (ICI) (1).
Since 2011, 7 different ICI agents have been approved in 15 distinct malignancies. These agents produce durable anti-tumor immune responses in a substantial fraction of treated patients, and introduce the possibility of long-term survival in some patients with metastatic disease. However, the removal of key nodes of immune self-tolerance may unleash autoimmune-like toxicities that may affect essentially every organ. Most commonly, immune-related adverse events (irAEs) present as colitis, dermatitis, endocrinopathies, pneumonitis, and hepatitis, although serious events may also involve the heart, brain, and peripheral nervous system (2). Glucocorticoids or other immunosuppressants are usually effective to manage these events. However, irAEs remain a significant clinical problem, as they may lead to premature therapy discontinuation, acute and chronic morbidity, and even death (3). The need to understand, predict, and manage irAEs effectively is heightened given the potential for long-term response in treated patients, and the increased use of these agents in the adjuvant or consolidative setting, when patients may already be cured of their disease by definitive local therapies.
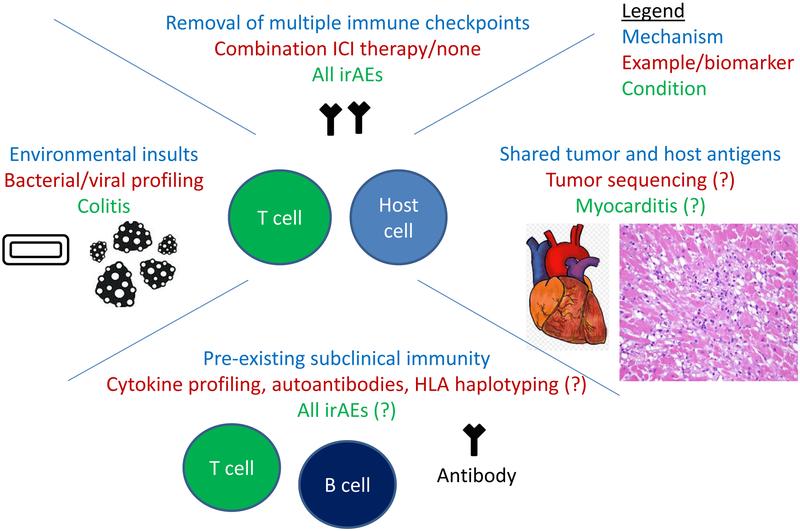
Development of reliable biomarkers that predict ICI toxicity would allow for improved treatment decision making and safety monitoring protocols, as well as provide insights into irAE pathogenesis, and facilitate drug development of more aggressive immunotherapy combinations (e.g. by assigning patients at low risk of irAEs to novel multi-drug regimens). However, an incomplete understanding of the pathogenesis of these events limits our ability to develop effective biomarkers. Potential mechanisms of irAEs may relate to antigens shared between the affected tissue and tumor, antibody dependent cytotoxicity (e.g. antibody deposition in the pituitary gland), pre-existing smoldering inflammation (triggered by the tumor or host), environmental insults, or pre-existing autoantibodies (Fig. 1) (4). Many of these proposed mechanisms relate to subclinical inflammation present in target organs prior to treatment, which is then unmasked with immune checkpoint blockade.
Potential mechanisms and biomarkers for irAEs
At this time, reliable clinical or molecular biomarkers to predict which patients will experience the most significant irAEs have not been identified. Although removal of self-tolerance appears to trigger irAEs, the occurrence of inflammation in certain organs in particular patients appears to arise in stochastic fashion. Even presumably obvious clinical risk factors (e.g. pre-existing autoimmune disorders, organ dysfunction) do not necessarily predict irAEs, as toxicity rates are largely similar in these putative high-risk populations as in unselected patients (5). The only clearly identified clinical risk factor is the use of dual immune checkpoint blockade, as combination PD-1 and CTLA-4 blockade substantially increases the risk of severe irAEs. Candidate molecular biomarkers have also been preliminarily studied. Several of these, including immune cell changes (B cell depletion and increased eosinophils) and T cell receptor expansion in the peripheral blood occurred early in treatment, rather than prior to therapy initiation, limiting their predictive utility in clinical decision making (6). Other studies have suggested that various bacterial species in the stool (specifically for colitis), pre-existing autoantibodies, and IL-17 levels prior to treatment may also predict for toxicity, although the predictive capacities of these markers are unclear. Other putative potential biomarkers could include polymorphisms in immune genes (e.g. those encoding PD-1/PD-L1 and CTLA-4), and particular mutations or proteins expressed by the tumor. While promising, each of the proposed biomarkers has relatively limited clinical data and/or lacks extensive validation.
In this study, Lim et al systematically obtained serum from patients with metastatic melanoma treated with ICI, either with single agent anti-PD-1 (cohort 1), or combination PD-1/CTLA-4 blockade (cohorts 2 and 3). They performed a broad analysis of cytokine expression in the peripheral blood prior to, early on, and later on treatment. Importantly, and somewhat surprisingly, they did not observe substantial intra-patient variation, suggesting that pre-treatment sample acquisition may be sufficient. They observed in their initial cohorts (1 [anti-PD-1] and 2 [anti-PD-1+anti-CTLA-4]), that an 11-cytokine signature prior to treatment and early on treatment strongly correlated with severe irAEs. This was replicated in an independent cohort (#3 [anti-PD-1 +anti-CTLA-4]). Importantly, these cytokine levels did not reproducibly correlate with anti-cancer outcomes (in terms of response to therapy or survival). The study is methodologically robust with appropriate inter-patient and intrapatient validation, and use of an independent validation cohort. Importantly, the presence of elevated cytokine levels prior to ICI treatment provides some support for the hypothesis that irAEs represent smoldering subclinical inflammation that is triggered by ICI administration. Alternatively, one could hypothesize that tumor-induced inflammation could explain the elevated cytokine levels and predispose patients to toxicities.
What are the implications and clinical significance of this study? As mentioned, reliable biomarkers of toxicity from ICI are urgently needed to identify patients at highest risk. While this study is an important first step, it may be premature to recommend routine cytokine profiling for patients in the clinic for two reasons. First, the test lacks sufficient accuracy to exclude potentially active therapies for patients. Second, despite the appropriate and well-designed study with an independent validation cohort, additional prospective validation is needed to confirm the results, particularly in pre-treatment samples. As is often the case in validation cohorts, reduced sensitivity and specificity was observed in external dataset analysis. Despite these limitations, this study provides an important building block to identify additional biomarkers of toxicity, and to integrate into clinical trials. Further, it provides insights into the mechanisms of irAEs, and potentially suggests therapeutic interventions to mitigate toxicities (e.g. cytokine antagonists), although additional preclinical validation of such approaches would be needed before clinical development.
In conclusion, biomarkers of immune toxicity are needed to assist in risk stratification, monitoring strategies, and development of immunotherapeutics. This study provides an important building block with a validated, 11 cytokine assay that can be determined both pre-treatment and early-on therapy.
Acknowledgments
Research Support: Douglas Johnson is supported by the National Institutes of Health (K23 CA204726) and the James C. Bradford Jr. Melanoma Fund.
Footnotes
Disclosures: D. B. Johnson reports receiving commercial research grants from Bristol-Myers Squibb and is a consultant/advisory board member for Array, Bristol-Myers Squibb, Genoptix, Incyte, Merck, and Novartis. J. M. Balko reports receiving commercial research grants from Genentech, Bristol-Myers Squibb, and Incyte, is an inventor on patents regarding immunotherapy response biomarkers, and has been a consultant/expert witness for Novartis. No other potential conflicts of interest were disclosed.
References
- 1.Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- 6.Patil PD, Burotto M, Velcheti V. Biomarkers for immune-related toxicities of checkpoint inhibitors: current progress and the road ahead. Expert Rev Mol Diagn. 2018;18:297–305. [DOI] [PubMed] [Google Scholar]