Abstract
RET fusions are oncogenic drivers of various tumors, including non-small cell lung cancers (NSCLCs). The safety and antitumor activity of the multikinase RET inhibitor RXDX-105 were explored in a phase I/Ib trial. A recommended phase 2 dose of 275 mg fed daily was identified. The most common treatment-related adverse events were fatigue (25%), diarrhea (24%), hypophosphatemia (18%), maculopapular rash (18%), and non-maculopapular rash (17%). In the phase 1b cohort of RET inhibitor-naïve patients with RET fusion-positive NSCLCs, the objective response rate (ORR) was 19% (95% CI 8%-38%, n=6/31). Interestingly, the ORR varied significantly by the gene fusion partner (p<0.001, Fisher’s exact test): 0% (95% CI 0% - 17%, n=0/20) with KIF5B (the most common upstream partner for RET fusion-positive NSCLC), and 67% (95% CI 30% - 93%, n=6/9) with non-KIF5B partners. The median duration of response in all RET fusion-positive NSCLCs was not reached (range 5 to 18+ months).
Keywords: RET fusion, RET rearrangement, KIF5B-RET
INTRODUCTION
RET (rearranged during transfection gene) is an established proto-oncogene (1). Genomic alterations involving RET, such as fusions and activating point mutations, are oncogenic drivers of a variety of tumors. RET fusions were initially identified in papillary thyroid carcinomas, and later in non-small cell lung cancers (NSCLCs) where these alterations are found in 1-2% of unselected cases (2). An increase in comprehensive molecular profiling subsequently resulted in the identification of RET fusions at lower frequencies in other solid tumors such as gastrointestinal malignancies, including colorectal cancer (CRC) (3, 4). Activating RET point mutations such as RETM918T are detected in medullary thyroid cancers (2).
RXDX-105 is an orally bioavailable, VEGFR-sparing, multikinase inhibitor with activity against RET. It inhibits wild-type RET, select mutant proteins (e.g. RETM918T), and chimeric oncoproteins generated by RET fusion (KIF5B-RET, CCDC6-RET, NCOA4-RET, PRKAR1A-RET). The drug is active in xenografts harboring the most common fusions in NSCLC (KIF5B-RET) and thyroid cancers (CCDC6-RET and NCOA4-RET). RXDX-105 is also active against wild-type BRAF and BRAFV600E, albeit to a lesser degree compared to RET (5).
RXDX-105 was selected for its potent preclinical activity against RET and its ability to spare VEGFR2/KDR and VEGFR1/FLT compared to other multikinase RET inhibitors such as cabozantinib, vandetanib, and lenvatinib (5–8). The inhibition of angiogenesis by other multikinase inhibitors with anti-RET activity can result in clinically significant side-effects that require dose modification and/or discontinuation, resulting in suboptimal RET target inhibition (1). The VEGFR-sparing nature of RXDX-105 was hypothesized to allow the clinical titration of the drug to a dose that would more optimally inhibit RET in comparison to other multikinase inhibitors, thus potentially improving outcomes for patients with RET fusion-positive or RET-mutant cancers.
This paper presents the results of a phase 1/1b trial of RXDX-105 and describes the safety and antitumor activity of this agent. While the drug was tested in a variety of solid tumors initially, including those harboring BRAF alterations, later-phase testing focused on RET fusion-positive lung cancers.
RESULTS
Patients.
A total of 152 patients were treated with RXDX-105; 55 were treated in the phase 1 dose-escalation portion of the study and 97 were treated in the phase 1b dose-expansion portion of the study (Table 1 and Figure S1). The median age was 63 (range 27-90) years and the majority (89%) of patients were pretreated and received one or more prior systemic therapies. The most common tumor type was NSCLC (54%), followed by colorectal cancer (18%), and thyroid cancer (11%). All patients had an ECOG performance status of 0 or 1 at study entry. Patient disposition is summarized in Table S1.
Table 1. Clinicopathologic and molecular features.
The demographics, tumor types, and number of prior therapies of all patients enrolled onto the phase 1 and phase 1b portions of this study are summarized. In addition, for RET tyrosine kinase inhibitor-naïve patients with RET fusion-positive lung cancers enrolled onto the phase 1b portion, the RET fusion type is shown.
| All patients in phase 1 and 1b, n=152 | n (%)* |
|---|---|
| Age | 63 years (27-90 years) |
| Sex | |
| Female | 79 (52%) |
| Male | 73 (48%) |
| Tumor type | |
| Non-small cell lung cancer | 81 (54%) |
| Non-squamous | 69 (46%) |
| Squamous | 12 (8%) |
| Gastrointestinal cancer | 39 (25%) |
| Colorectal | 28 (18%) |
| Other (Hepatocellular, Pancreas) | 11 (7%) |
| Thyroid cancer | 17 (11%) |
| Other cancers (head and neck, ovarian, primary brain tumor) | 15 (10%) |
| Number of prior systemic therapies | |
| 0 | 16 (11%) |
| 1-2 | 57 (36%) |
| 3 or more | 79 (53%) |
| Patients in phase 1b, n=97 | n (%) |
| Cohorts | |
| RET fusion-positive lung cancer, TKI-naïve | 31 (33%) |
| KIF5B-RET | 20 (65%) |
| CCDC6-RET | 6 (20%) |
| EML4-RET | 2 (6%) |
| PARD3-RET | 1 (3%) |
| Unknown (FISH-positive) | 2 (6%) |
| RET fusion-positive lung cancer, prior TKI | 9 (9%) |
| RET-altered solid tumor (non-lung), TKI-naïve | 1 (1%) |
| BRAF V600E-mutant lung cancer, TKI-naïve | 7 (7%) |
| BRAF V600E-mutant colorectal cancer, TKI-naïve | 9 (9%) |
| BRAF V600E-mutant cancer (non-lung, non-melanoma) | 8 (8%) |
| Squamous cell lung cancer | 9 (9%) |
| Other cancers | 23 (24%) |
except for age for which median and range are shown
In the dose-escalation phase of the trial, patients were treated in nine dose-level cohorts (Table S2). In the first seven cohorts, RXDX-105 was administered at doses that ranged from 20 mg daily up to a dose of 275 mg daily without dietary restrictions. In the last two cohorts, RXDX-105 was administered at 275 mg daily and 350 mg daily in the fed state.
Safety.
In patients treated with any dose of RXDX-105 (n=152), the most common treatment-related adverse events observed in more than 10% of patients were fatigue (25%), diarrhea (24%), hypophosphatemia (18%), maculopapular rash (18%), non-maculopapular rash (17%), nausea (15%), elevated alanine (14%) or aspartate (13%) aminotransferase, muscle spasms (13%), decreased appetite (11%), and vomiting (10%). These are summarized in Table 2.
Table 2. Drug-related adverse events.
The most common adverse events related to RXDX-105 therapy that were observed in greater than 10% of all patients are listed. The frequency of these toxicities is shown for all 152 patients who were treated with RXDX-105 at any dose, and in 74 patients who were treated at the recommended phase 2 dose (RP2D) of 275 mg fed. ALT - alanine aminotransferase, AST – aspartate aminotransferase.
| Adverse Event | All Doses n=152 | 275 mg Fed (RP2D) n=74 | ||||
|---|---|---|---|---|---|---|
| All Grades n (%) | Grade 1-2 n (%) | Grade 3-4 n (%) | All Grades n (%) | Grade 1-2 n (%) | Grade 3-4 n (%) | |
| Fatigue | 38 (25%) | 33 (22%) | 5 (3%) | 16 (22%) | 16 (22%) | - |
| Diarrhea | 37 (24%) | 29 (19%) | 8 (5%) | 16 (22%) | 13 (18%) | 3 (4%) |
| Hypophosphatemia | 27 (18%) | 14 (9%) | 13 (9%) | 12 (17%) | 7 (10%) | 5 (7%) |
| Rash, maculopapular | 27 (18%) | 16 (11%) | 11 (7%) | 12 (17%) | 5 (7%) | 7 (10%) |
| Rash, non-maculopapular | 26 (17%) | 24 (16%) | 2 (1%) | 16 (21%) | 15 (20%) | 1 (1%) |
| Nausea | 22 (15%) | 22 (15%) | - | 6 (8%) | 6 (8%) | - |
| Elevated ALT | 21 (14%) | 9 (6%) | 12 (8%) | 12 (16%) | 6 (8%) | 6 (8%) |
| Elevated AST | 20 (13%) | 12 (8%) | 8 (5%) | 12 (16%) | 8 (11%) | 4 (5%) |
| Muscle spasms | 19 (13%) | 19 (13%) | - | 5 (7%) | 5 (7%) | - |
| Decreased appetite | 17 (11%) | 17 (11%) | - | 8 (11%) | 8 (11%) | - |
| Vomiting | 16 (11%) | 16 (11%) | - | 6 (8%) | 6 (8%) | - |
The most common grade 3 or higher treatment-related adverse events observed in ≥ 5% of patients were as follows: hypophosphatemia (9%), elevated alanine aminotransferase (8%), maculopapular rash (7%), elevated aspartate aminotransferase (5%), and diarrhea (5%). No QT/QTc prolongation was observed. Drug-related toxicities commonly associated with VEGFR2/KDR inhibition such as hypertension (3%), and proteinuria (1%) of any grade were uncommon.
In the dose-escalation portion, four dose-limiting toxicities (DLTs) were reported. These included rash (grade 3, 200mg daily), fatigue (grade 3, 275 mg daily fasted), diarrhea (grade 3, 275 mg fed daily), and hyperbilirubinemia (grade 3, at 350 mg fed daily). These toxicities resolved after treatment interruption and dose reduction.
Dose reduction was required in 28% (n=43/152) of patients in the safety data set, and 31% (n=19/62) of patients who were treated at 275 mg daily in the phase 1b portion. The most common adverse events resulting in dose reduction in the safety data set of 152 patients were liver function test abnormalities (increased alanine/aspartate aminotransferase or bilirubin) in 9% (n=13/152), and cutaneous disorders (maculopapular rash, non-maculopapular rash, generalized rash, acneiform dermatitis, or skin discoloration) in 8% (n=12/152). Dose discontinuation secondary to a treatment-emergent adverse event occurred in 16% (n=25/152) of patients in the safety data set, and 13% (n=8/62) of patients treated at 275 mg daily in the phase 1b portion.
Hypersensitivity to RXDX-105.
Three cases of treatment-related cutaneous hypersensitivity to RXDX-105 were observed. All three had select features consistent with a differential diagnosis of DRESS (drug rash with eosinophilia and systemic symptoms) A 64-year-old woman with a metastatic RET fusion-positive lung cancer was previously treated with chemoradiation, durvalumab, stereotactic radiosurgery for brain metastases, pemetrexed and bevacizumab, and atezolizumab and an adenosine-A2A receptor antagonist. She developed a full body rash twelve days after the initiation of RXDX-105 at 275 mg daily. This required hospitalization and steroid administration. Her course was also marked by the development of a transaminitis. Peripheral eosinophilia was not noted. RXDX-105 was discontinued and the patient was taken off study. These side-effects thereafter resolved.
A 58-year-old woman with a BRAFD594G-mutant lung cancer was previously treated with cisplatin and pemetrexed, atezolizumab and cobimetinib, and gemcitabine and vinorelbine. She also developed a full body rash involving the conjunctiva bilaterally, facial swelling, fevers, hypotension, and thrombocytopenia twelve days after starting RXDX-105 treatment at 275mg daily. These adverse events likewise after study drug discontinuation and steroids.
A 71-year-old woman with a metastatic RET fusion-positive lung cancer was previously treated with carboplatin, pemetrexed, and bevacizumab, palliative radiation to the lung, rib, spine, and brain, and finally, pembrolizumab. Twelve days after RXDX-105 was initiated at 350 mg daily, she developed a full body erythematous maculopapular rash as is shown in Figure S2. This was accompanied by facial swelling, oral mucositis, and hoarseness from suspected vocal cord edema. She was hospitalized, study drug was held, and steroids were initiated.
Despite this, the patient developed fulminant multiorgan dysfunction with respiratory failure requiring intubation, kidney failure, transaminitis, pancytopenia, and atrial fibrillation. A bronchoscopy revealed bleeding consistent with diffuse alveolar hemorrhage. A skin biopsy revealed interface dermatitis, lymphocytic exocytosis, and perivascular eosinophils, although eosinophilia in the peripheral blood was not observed. She died secondary to these complications. In addition to RXDX-105, her medication list at study entry included drugs that have also been associated with DRESS (etoricoxib, pregabalin, esomeprazole and tramadol).
Pharmacokinetics.
The steady state pharmacokinetics of RXDX-105 following once daily dosing at various dose levels are depicted in Figure 1. Dose-dependent increases in RXDX-105 plasma exposures were observed. For the fed cohorts, patients were instructed to take RXDX-105 with breakfast (including solid food) or within 30 minutes after eating breakfast. Steady state exposures of RXDX-105 were only slightly higher in the fed versus the food-uncontrolled states suggesting the absence of a substantial food effect.
Figure 1. Pharmacokinetics of RXDX-105.
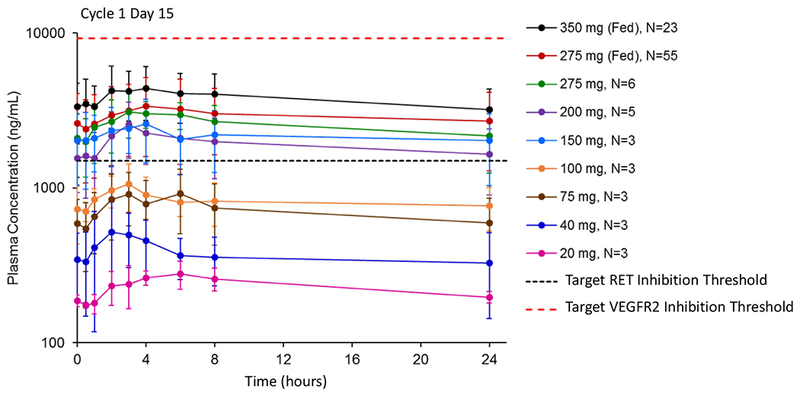
The mean steady state plasma concentration profiles of RXDX-105 at escalating dose levels on day 15 of cycle 1 were plotted following once-daily continuous dosing. For the two fed cohorts, patients were instructed to take RXDX-105 with breakfast (which included solid food) or within 30 minutes after eating breakfast. Instructions regarding food were not provided (food-uncontrolled) for all other cohorts. The estimated target RET inhibition was based on RXDX-105-induced tumor growth inhibition in a RET fusion-containing xenograft mouse model. The estimated target VEGFR2 inhibition was estimated based on the in vitro IC50 of RXDX-105 for VEGFR2 with correction for protein binding and tissue distribution. At the recommended phase 2 dose of 275 mg fed daily (red curve), plasma exposures exceeded RET target coverage, and a wide therapeutic window between calculated RET and VEGFR2 inhibition was observed.
Based on an analysis of the toxicity profile and pharmacokinetics of RXDX-105 at all dose levels, a recommended phase 2 dose (RP2D) of 275 mg daily in the fed state was chosen. At steady state levels (cycle 1, day 15) on this dose, the median Tmax was 4 hours. The estimated mean effective t1/2 was 45 hours. The mean Cmax was 3,890 ng/mL [47.7% coefficient of variation (CV)]. The AUC0-24 (day 15 AUC0-24/day 1 AUC0-24) was 69,600 ng·h/mL (47.7% CV). The accumulation ratio was 3.19 (54.6% CV).
At steady state levels, the mean plasma concentration at the RP2D was about 2-fold above the target threshold for effective RET inhibition (1,500 ng/mL, estimated based on data generated in RET fusion-containing patient-derived xenograft models). This calculated value was presumed to apply to both wild-type RET and select RET chimeric oncoproteins, extrapolating from previously generated biochemical data that showed similar activity for both (i.e. IC50s of 0.33 nM for wild-type RET and 0.33 nM for CCDC6-RET). Of note, this target threshold was not expected to effectively cover select RET mutations. Specifically, RXDX-105 was not substantially active against the V804M and V804L gatekeeper substitutions preclinically; the biochemical IC50s of 266 nM for RETV804M and 319 nM for RETV804L were approximately 1,000-fold higher than 0.33 nM for wild-type RET).
Steady state plasma concentrations were also above the calculated threshold for effective BRAF inhibition (>2,500 ng/mL, estimated from a nude mouse model bearing A375 human melanoma BRAFV600E-mutant tumor xenografts). Notably, the calculated threshold for BRAF inhibition was much higher than the calculated threshold for RET inhibition. This suggests that RXDX-105 was poised to more effectively target RET compared to BRAF in the clinic.
Finally, a wide therapeutic window between calculated RET inhibition and the much higher threshold for the inhibition of angiogenesis was observed. Plasma concentrations in patients were approximately one-third of the estimated pharmacokinetic threshold for VEGFR2/KDR inhibition (~10,000 ng/mL), estimated from the in vitro IC50 of RXDX-105 for VEGFR2/KDR with correction for protein binding and tissue distribution.
Efficacy.
Early signals of anti-tumor activity were observed during the phase 1 dose-escalation portion of the study. In the 55 patients treated with RXDX-105 in this phase, the best overall response to RXDX-105 was as follows: 0 (0%) complete responses, 2 (4%) partial responses, 20 (36%) stable disease, 22 (40%) progressive disease, and 11 (20%) unevaluable.
The two confirmed partial response were observed in a patient with medullary thyroid cancer with a RETM918T mutation (50% tumor regression) and in a patient with NSCLC with a KRASG12C mutation (40% tumor regression). The exact mechanism driving response in the latter patient whose tumor did not harbor a RET or BRAF alteration remains unclear. Tumor regression was also observed in a patient with an ovarian cancer harboring a BRAFV600E mutation (26% reduction) and in a patient with a NSCLC harboring a BRAFD594G mutation (28% reduction). Additionally, clinical benefit was noted in two of four patients with squamous NSCLC. Stable disease for more than 6 months was achieved in both cases, with a 27% reduction in tumor burden observed in one patient.
The phase 1b portion of this study was designed with this activity in mind (Figure S3). Eight cohorts of patients were treated with RXDX-105. Six cohorts were molecularly-enriched, and two were enriched by histology: (1) TKI-naïve RET fusion-positive lung cancers, (2) TKI pre-treated RET fusion-positive lung cancers, (3) TKI-naïve RET-altered non-lung solid tumors, (4) TKI-naïve BRAFV600E-mutant lung cancers, (5) TKI-naïve BRAFV600E-mutant colorectal cancers, (6) BRAFV600E-mutant non-lung and non-melanoma solid tumors, (7) squamous cell lung cancers, and (8) other cancers.
The activity of RXDX-105 in each of these cohorts in summarized in Table 3. In RET fusion-positive lung cancers, no responses were observed in 9 patients who previously received a RET inhibitor. The multikinase RET inhibitors that patients received prior to RXDX-105 included cabozantinib and vandetanib and are listed in Table S3. No patient received a selective RET inhibitor such as LOXO-292 or BLU-667 prior to RXDX-105. In all 9 patients, disease progression occurred within the first two to four treatment cycles. One complete response was achieved in a RET inhibitor-naïve patient with a colorectal cancer that harbored a CCDC6-RET fusion. This patient was treated in a separate cohort (the non-molecularly-enriched “other cancers” cohort). Response was not observed in BRAF-mutant cancers. This included 7 patients with BRAFV600E-mutant NSCLC and 9 patients with BRAFV600E-mutant colorectal cancers. No additional responses were observed in squamous cell lung cancers.
Table 3. Activity of RXDX-105.
In the phase 1b portion of this study, eight cohorts of patients were treated with RXDX-105. In the molecularly-enriched cohorts, RET fusion-positive non-small cell lung cancers (NSCLCs), RET-altered other solid tumors, and BRAFV600E-mutant NSCLCs, colorectal cancers (CRCs), and other non-melanoma solid tumors were accrued. The best objective response to therapy is listed for each cohort along with the objective response rate (ORR). With the exception of one complete response in a patient with a colorectal cancer harboring a CCDC6-RET fusion, response to RXDX-105 was only observed in tyrosine kinase inhibitor (TKI)-naïve patients with RET fusion-positive lung cancers.
| Alteration-Based Enrichment | Histology-Based Enrichment | |||||||
|---|---|---|---|---|---|---|---|---|
| Cohort | RET fusion-positive | RET-altered | BRAFV600E-mutant | squamous cell lung cancer (n=9) | other cancers (n=23) | |||
| NSCLC TKI-naïve (n=31) | NSCLC prior TKI (n=9) | other solid tumor TKI-naïve (n=1) | NSCLC TKI-naïve (n=7) | CRC TKI-naïve (n=9) | other non-melanoma solid tumor (n=8) | |||
| Complete response | - | - | - | - | - | - | - | 1 (4%) |
| Partial response | 6 (19%) | - | - | - | - | - | - | - |
| Stable disease | 12 (39%) | 3 (33%) | 1 (100%) | 3 (43%) | 4 (45%) | 3 (38%) | 3 (33%) | 9 (39%) |
| Progressive disease | 10 (32%) | 4 (45%) | - | 1 (14%) | 2 (22%) | 1 (12%) | 3 (33%) | 7 (30%) |
| Unevaluable | 3 (10%) | 2 (22%) | - | 3 (43%) | 3 (33%) | 4 (50%) | 3 (33%) | 6 (26%) |
| ORR (95% CI) | 19% (8-38%) | 0% (0-34%) | 0% (0-98%) | 0% (0-41%) | 0% (0-34%) | 0% (0-37%) | 0% (0-34%) | 4% (0-22%) |
RXDX-105 was most active in patients with RET inhibitor-naïve RET fusion-positive lung cancers. A total of 31 patients were treated in this cohort. The objective response rate (ORR) was 19% (95% CI 8%-38%, n=6/31). No complete responses were observed. Confirmed partial responses were observed in 6 patients (19%), stable disease in 12 patients (39%), and progression of disease in 10 patients (32%). Therapy was discontinued in 2 patients for drug-related toxicity and 1 patient had a non-complete response and non-progression (non-CR/non-PD). A waterfall plot of best objective response to RXDX-105 in 27 evaluable patients is shown in Figure 2. The median duration of response was not reached (range 5 to 18+ months); the median follow-up was 21.7 months. In all RET inhibitor-naïve patients with RET fusion-positive NSCLCs, response to therapy occurred early, after 4-8 weeks on treatment, and was durable in several patients, as shown in Figure 3.
Figure 2. Antitumor activity of RXDX-105 in patients with RET fusion-positive lung cancers.
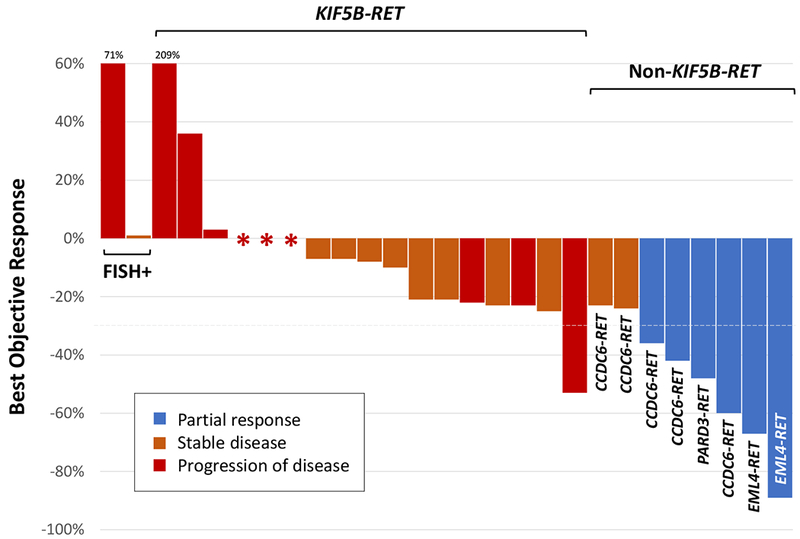
A waterfall plot of the best objective response to RXDX-105 in 27 evaluable patients with RET tyrosine kinase inhibitor-naïve RET fusion-positive non-small cell lung cancers is shown. Cases are grouped by upstream partner: KIF5B-RET, non-KIF5B-RET, and unknown (FISH-positive). Each bar represents the maximal percent change from baseline based on the sum of target lesions by RECIST version 1.1. A confirmed partial response, stable disease, and progressive disease are indicated by blue, orange, and red bars, respectively. The patient with a KIF5B-RET fusion-positive NSCLC who had a >50% reduction in target lesions had a best response of progressive disease due to the presence of new non-target lesions on follow-up imaging.
Figure 3. Duration of RXDX-105 therapy in patients with RET fusion-positive lung cancers.
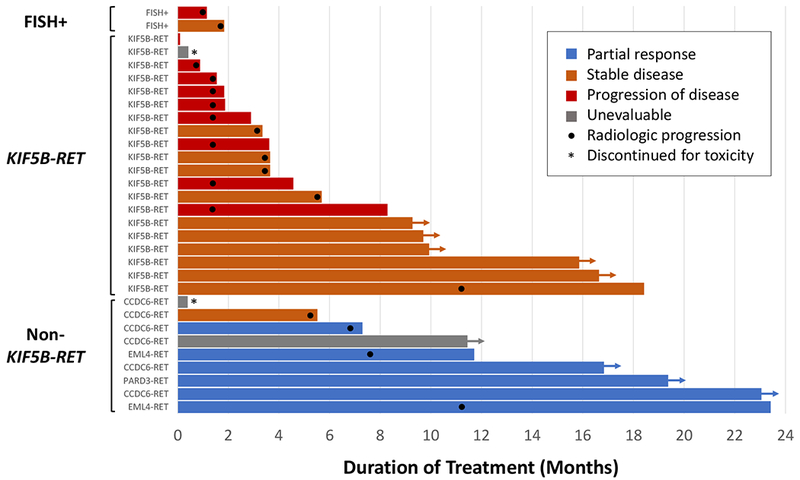
In this swimmer plot, each bar indicates the duration of RXDX-105 treatment. Arrows indicate patients who remained on treatment at the time of the data cut-off. Bars without arrows represent patients who had discontinued therapy. Black dots indicate the time at which radiologic progression occurred. A partial response, stable disease, progressive disease, and cases unevaluable for response are indicated by blue, orange, red, and gray bars, respectively. An asterisk indicates discontinuation secondary to toxicity. Cases are grouped by upstream partner: KIF5B-RET, non-KIF5B-RET, and unknown (FISH-positive).
While 14% (n=21/152) of patients in the safety data set had a history of brain metastases, these lesions were all previously treated (untreated brain metastases were exclusionary) and thus the intracranial outcomes of RXDX-105 could not be assessed. At the time of the data cut-off, of the 152 patients treated with RXDX-105 on this trial, 140 patients discontinued therapy for the following reasons: 96 for disease progression, 23 for toxicity, 11 after consent withdrawal, 5 for death, and 5 for other reasons (e.g. investigator discretion for protocol non-compliance). Twelve patients remained on therapy with RXDX-105.
Activity by RET Fusion Partner.
In an analysis of cancers that harbored known upstream partners, the ORR of RXDX-105 was 21% (95% CI 8%−40%, n=6/29). A significant difference in activity was noted between RET fusions involving a KIF5B versus a non-KIF5B upstream partner (p<0.001, Fisher’s exact test) as shown in Figure 2. While no objective responses were observed in KIF5B-RET-containing tumors, in tumors harboring RET fusions with a non-KIF5B partner, the ORR was 67% (95% CI 30% - 93%, n=6/9). Despite this difference in response, prolonged disease control was observed in select patients with cancers harboring both KIF5B-RET and non-KIF5B-RET fusions (Figure 3).
A similar pattern of decreased activity in KIF5B-RET-containing compared to non-KIF5B-RET-containing lung cancers was found when the activity of RXDX-105 was compared to that of the multikinase inhibitors cabozantinib, vandetanib, and lenvatinib by upstream partner as shown in Figure 4. Data on the activity of cabozantinib (6), vandetanib (7, 8), and lenvatinib (9) were derived from prospective trials of these agents in RET fusion-positive lung cancers. Within each of these trials, the objective response rates and/or median progression-free survival (when available) in KIF5B-RET-containing tumors were lower than in non-KIF5B-RET-containing tumors.
Figure 4. Differential activity of multikinase inhibition by upstream partner.
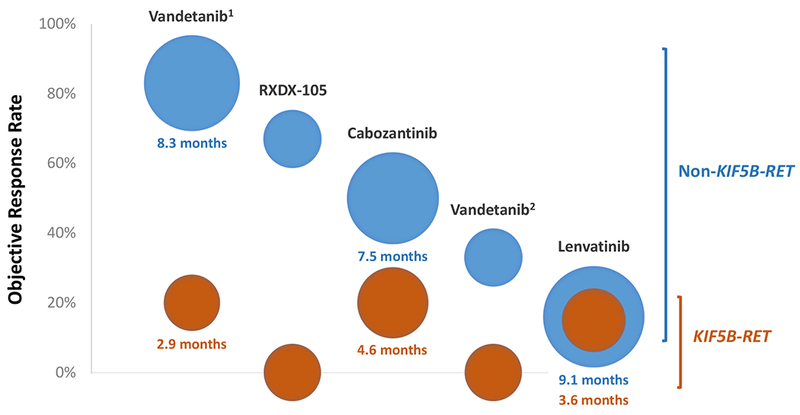
The activity of RXDX-105 in RET fusion-positive lung cancers is compared to that of other multikinase inhibitors. Data on the latter were derived from prospective trials of cabozantinib, vandetanib (results from two separate vandetanib trials are shown: 1 - a Japanese phase 2 trial, 2 - a South Korean phase 2 trial), and lenvatinib. Each column represents a single prospective trial showing the differential activity of each agent in tumors harboring KIF5B-RET (orange) versus non-KIF5B-RET (blue) fusions. The position of each bubble on the y-axis corresponds to the objective response rate (ORR). The size of each bubble represents the median progression-free survival (PFS), with larger bubbles indicating a longer median PFS. The value of the median PFS is also specified below each bubble when known. When the median PFS was not available or not reached, the size of each bubble was fixed; this corresponded to a median PFS of 3 months for reference. In general, the ORR and/or median PFS with RET-directed multikinase inhibition are numerically improved in tumors that contain non-KIF5B-RET fusions, recognizing that the latter represents a highly heterogenous group with a wide variety of upstream partners.
To investigate possible reasons for this discrepancy, in vitro data on the activity of RXDX-105 and other multikinase inhibitors (cabozantinib, vandetanib, sitravatinib, and alectinib) were generated. About a 2-fold increase in IC50s was observed in KIF5B-RET-containing compared to non-KIF5B-RET-containing Ba/F3 cell lines as shown in Figure S4. For RXDX-105, IC50s for CCDC6-RET and NCOA4-RET were 103 nM and 87 nM, respectively, compared to 190 nM for KIF5B-RET.
DISCUSSION
A number of multikinase inhibitors with activity against RET have been explored as treatments for RET fusion-positive solid tumors and RET-mutant thyroid cancers (1). These include cabozantinib, vandetanib, lenvatinib, ponatinib, sunitinib, and sorafenib. The goal of the RXDX-105 development program was to surpass the activity of these agents, considering that the relative sparing of VEGFR2/KDR by RXDX-105 might allow titration of the drug’s dose to a level where RET would be more optimally inhibited (5).
In this phase I/Ib trial, the activity of RXDX-105 was most notable in patients with RET fusion-positive lung cancers who had not previously received a RET inhibitor. Pharmacokinetic analyses revealed good calculated RET target coverage, and the frequency of VEGFR2/KDR-mediated adverse events was lower compared to other multikinase inhibitors with more potent anti-angiogenic activity (e.g. hypertension of any grade was 3% with RXDX-105 compared to 33% with cabozantinib (10)). Despite these factors, the overall activity of RXDX-105 did not differ substantially from the activity of other multikinase inhibitors in RET fusion-positive lung cancers. The ORR with RXDX-105 was 19% compared to an ORR of 16% to 53% with cabozantinib, vandetanib or lenvatinib (1).
While the activity of RXDX-105 in RET fusion-positive lung cancers was modest, a differential response to RXDX-105 was observed that seemed to be dictated by the gene fusion partner. Specifically, responses were only observed with non-KIF5B upstream partners. An analysis of prior clinical trials of other multikinase inhibitors also showed lower response rates and/or median progression-free survival when KIF5B-RET-containing tumors were compared to non-KIF5B-RET containing tumors, consistent with the results of this trial (1, 11).
The exact reasons for this discrepancy remain unclear, although several factors are potentially contributory. First, KIF5B-RET may be somewhat more challenging to target with multikinase inhibition in preclinical models. Early in the development of multikinase inhibitors for RET, cell lines containing KIF5B-RET were not widely available. While the preclinical activity of RXDX-105 did not substantially differ between select non-KIF5B-RET fusions that were initially tested (biochemical IC50s of 0.33 nM for CCDC6-RET, 0.41 nM for NCOA4-RET, and 0.81 nM for PRKR1A-RET; cell-free kinase assay platform), we subsequently tested RXDX-105 and other multikinase inhibitors in Ba/F3 cells harboring KIF5B-RET, CCDC6-RET, and NCOA4-RET. In these experiments, we observed at least a two-fold increase in cellular IC50 values in KIF5B-RET-containing cell lines compared to CCDC6-RET- or NCOA4-RET-containing cell lines with RXDX-105; a shift was likewise observed with other multikinase inhibitors.
It is unclear if this fold-change is responsible for the differential activity observed in the clinic, however, the plasma exposures of multikinase RET inhibitors have been shown to be suboptimal at effectively inhibiting RET, especially considering that dose modifications are frequent secondary to treatment-related toxicities with select drugs (1). If RET target coverage is already suboptimal, even a small window between the activity of multikinase inhibitors against KIF5B-RET and non-KIF5B-RET might meaningfully affect outcomes. Furthermore, while independent investigators have also shown suboptimal activity of RXDX-105 and other multikinase agents in KIF5B-RET-containing models (IC50s of 129 nM for RXDX-105 and 833 nM for vandetanib for the inhibition of RET autophosphorylation in Ba/F3 cells) (12), other series have not observed a difference compared to non-KIF5B-RET-containing models (13). This underscores the need to further explore the fusion-specific activity of various RET inhibitors in the laboratory.
Second, KIF5B is postulated to result in a high level of RET expression in KIF5B-RET-containing tumors (i.e. 30-fold higher RET expression compared to non-cancerous lung tissues (14)). In contrast, other upstream partners such as CCDC6 and NCOA4 are thought to result in lower levels of expression in RET fusion-positive tumors harboring these partners (15). This implies that KIF5B-RET-containing tumors may contain higher total levels of chimeric RET oncoproteins that need to be overcome by targeted therapy. Finally, signaling and functional differences between RET fusions of different upstream partner types have been identified. This was initially demonstrated in Drosophila models with CCDC6-RET and NCOA4-RET when these fusion genes were expressed in the epithelia during development (16). A study also using Drosophila models in addition to engineered human bronchial epithelial cells further revealed that the kinesin domain of KIF5B and the kinase domain of RET act together to form a multikinase (RET/EGFR/FGFR/SRC) signaling hub (17). This suggests that, rather than targeting RET alone, multiple kinase components of the KIF5B-RET signaling hub may need to be simultaneously targeted for optimal effect. In our Ba/F3 models, for example, single-agent MEK inhibition (trametinib) and single-agent pan-PI3K/mTOR inhibition (omipalisib) were active in KIF5B-RET-containing cells, raising the question of the utility of combinatorial therapy in RET fusion-positive lung cancers as is already being explored in the clinic (18).
Beyond the activity of RXDX-105, the drug’s safety profile is informative for the design and development of kinase inhibitors with activity against RET and BRAF. While the most common drug-related adverse events such as grade 1 or 2 rash, diarrhea, fatigue, and liver function test abnormalities were not uncharacteristic of inhibitors in this class, several other features were unique. In addition to the relative VEGFR sparing described above, drug-induced hypersensitivity syndrome with features of DRESS was identified as a rare but important adverse event for patients treated with RXDX-105. Notably, in all three cases presented in this series, rash occurred within the first two weeks of dosing and patients received prior treatment with an immune checkpoint inhibitor. DRESS was previously observed with other agents that inhibit BRAF (e.g. vemurafenib) especially after prior immunotherapy (19). This raises the possibility that this idiosyncratic reaction represents a class effect of BRAF inhibitors that can be potentiated by prior immune checkpoint inhibition. In general, an increase in drug-related toxicity has also been observed after immunotherapy with crizotinib in ALK fusion-positive cancers (20), and EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancers (21).
Finally, newer RET inhibitors, specifically those that are more selective for RET such as LOXO-292 (22) and BLU-667 (12) are currently in clinical testing. Preliminary data have shown that these drugs have increased activity and improved tolerability compared to multikinase inhibitors including RXDX-105 (23). While these drugs are likely to ultimately replace multikinase inhibition as the first line of targeted therapy in TKI-naïve patients, multikinase inhibitors remain viable treatment options after progression of disease on a selective RET inhibitor. Strategies to increase the activity of multikinase inhibitors should thus continue to be pursued, particularly for tumors harboring KIF5B-RET.
In conclusion, the multitarget, VEGFR-sparing RET inhibitor RXDX-105 is active in patients with RET inhibitor-naïve RET fusion-positive lung cancers, although overall clinical outcomes were not different from that of prior multikinase inhibitors. The activity of RXDX-105 was largely observed in non-KIF5B-RET-containing as opposed to KIF5B-RET-containing tumors, consistent with other trials of multikinase inhibitors. This exposes a potential biologic difference between chimeric RET oncoproteins that is dictated by the upstream fusion partner and requires further exploration. Finally, RXDX-105 has a unique safety profile that is characterized by a lower frequency of VEGFR2/KDR-related adverse events, and rare but substantial cases of cutaneous hypersensitivity that may be mediated by BRAF inhibition, especially in the wake of prior immunotherapy treatment.
METHODS
RXDX-105-01 (NCT01877811) was a first-in-human, multicenter, open-label, phase 1 dose-escalation and phase 1b dose expansion study of RXDX-105, an oral VEGFR-sparing multikinase inhibitor with potent activity against RET and BRAF. The phase 1 portion of the study enrolled patients with any solid tumor. The phase 1b portion was designed as a basket study and enrolled patients with solid tumors including those that harbored RET or BRAF alterations. Written informed consent was obtained from patients. This study was conducted in accordance with recognized ethical guidelines: the Declaration of Helsinki, CIOMS, the Belmont Report, and U.S. Common Rule. The protocol was approved by institutional review boards at each institution. Data were anonymized to protect the identities of patients involved in the research.
Study Design.
This phase 1 study followed a traditional 3+3 design for the dose-escalation portion. Dose levels were expanded on the basis of the occurrence of dose-limiting toxicities during the first cycle. The primary objective of this portion was to determine the maximum tolerated dose or recommended phase 2 dose of RXDX-105. The phase 1b portion was a dose expansion phase in patients with advanced solid tumors with specific molecular alterations of interest or histologies. Treatment in the phase 1b portion was administered at the RP2D.
Patients received once daily oral doses of RXDX-105 in 28-day treatment cycles until disease progression, unacceptable toxicity, withdrawal of consent, or protocol-specified parameters. Patients for whom no available approved and/or alternative therapy were allowed to continue treatment with RXDX-105 post-progression if the patient was felt to be deriving clinical benefit. Dose modification was permitted via a prescribed algorithm. In the phase 1 dose-escalation portion, patients who required dose reductions were treated at a prior dose cohort deemed to be safe. In the phase 1b portion, a maximum of two dose reductions (~25% decrease per dose reduction) was permitted.
Study Population.
In the phase 1 dose-escalation portion, all patients had histologically- or cytologically confirmed evidence of an advanced solid tumor for which curative therapy was not available, ECOG ≤ 1, any number of prior therapies, at least 18 years of age at screening and adequate bone marrow, liver, and renal function. In the phase 1b basket study, all patients had histologically- or cytologically-confirmed advanced solid tumors that harbored a RET fusion or mutation or BRAF mutation, detected by FISH or RNA/DNA-based methods (e.g. next generation sequencing) performed locally using a lab developed test or through third party commercial diagnostic labs in a CLIA environment or equivalent. A basket for patients whose tumors harbored squamous cell NSCLC was also included. Patients with untreated central nervous system metastases were ineligible.
Safety Assessments.
Safety assessments consisted of monitoring and recording adverse events, measurement of protocol-specified safety laboratory assessments, vital signs, and other protocol-specified tests were deemed critical to an evaluation of safety. Safety was assessed from the first dose until 30 days after last dose of RXDX-105 until resolution or stability of any drug-related toxicity (or until the patient was lost to follow up or withdrew consent). In the dose-escalation portion, clinical and laboratory assessments were performed at least once weekly during the first cycle of treatment, bi-weekly in each cycle, and at end of treatment. In the phase 1b portion, clinical and laboratory assessments were performed on day 1 of each cycle and at the end of treatment. Laboratory assessments included routine hematology and chemistry panels, cortisol analysis, and urinalysis. Twelve-lead single ECGs were performed at baseline, days 1 and 15 of cycles 1 and 2 (pre-dose and approximately 2 to 4 hours after study drug administration), day 1 of all subsequent cycles (pre-dose), and at the end of treatment. In the phase 1b portion, ECGs were performed pre-dose at baseline, day 1 of each cycle, and at the end of treatment. An ophthalmologic examination was performed at screening and at the end-of-treatment visit, and at any other times that the patient reported vision and/or ocular abnormalities.
Pharmacokinetics.
Serial blood samples for pharmacokinetic (PK) analyses were obtained for all patients at various timepoints throughout cycle 1. Samples were analyzed to determine RXDX-105 plasma concentrations. Full plasma PK profiles were generated using a validated assay based on high performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). PK analysis for all parameters was performed using Phoenix WinNonlin software version 6.4.0.768 (Pharsight Corp., CA). Parameters analyzed included maximum observed plasma drug concentration (Cmax), time of maximum observed plasma drug concentration (Tmax), area under the plasma drug concentration versus time curve from time 0 to the last measurable drug concentration (AUC0-t), and area under the plasma drug concentration versus time curve from time 0 to 24 hours after study drug administration (AUC0-24).
Tumor Assessments.
Computed tomography (CT) or magnetic resonance imaging of anatomic sites based on cancer type were performed at baseline, the end of cycle 2, and every 8 weeks thereafter. In the phase 1b portion, all patients had CT scans of the thorax and abdomen as part of tumor assessments. Imaging of the pelvis was also required for patients with colon cancer. Additionally, due to the high frequency of brain metastasis in NSCLC patients, brain imaging was performed during screening in all patients with NSCLC. Untreated brain metastases rendered patients ineligible. If treated brain metastases were present, brain imaging was performed at each tumor assessment. Brain imaging was not required in all patients, however.
Statistical Analysis.
Patients who received at least one dose of RXDX-105 were included in an analysis of safety. Demographics, baseline characteristics, adverse events, vital signs, and clinical laboratory evaluations were summarized with descriptive statistics. Patients with evidence of RET fusion-positive NSCLCs were analyzed as a subset of the safety population for efficacy. Best objective response was derived according to RECIST version 1.1 based on investigator assessment and classified as a complete response, partial response, stable disease, progressive disease, or unevaluable. A confirmed response was defined as a complete or partial response that was confirmed upon repeat imaging ≥ 4 weeks after initial documentation of response. Separately, the maximal response in measurable disease at any time on study was reported using waterfall plots. The data cutoff date for safety and efficacy analyses was May 2, 2018.
Cell Lines.
Ba/F3 cell lines were purchased from DSMZ (2016, German Collection of Microorganisms and Cell Culture). Ba/F3 cells were not authenticated. Cell lines were confirmed to be Mycoplasma-free (Biomiga) and were used between three and ten passages. The fusion genes, CCDC6(exon 1)-RET(exon12), NCOA4(exon6)-RET(exon12), and KIF5B(exon15)-RET(exon12), were synthesized at GenScript and cloned into pCDH-CMV-MCS-EF1-Puro plasmid (System Biosciences, Inc.). The corresponding cell lines were generated by transducing Ba/F3 cells lentivirus containing the desired fusion gene. In addition to RXDX-105, four other RET inhibitors (cabozantinib, vandetanib, sitravatinib, and alectinib), a MEK inhibitor (trametinib), and a pan-PI3K/mTOR inhibitor (omipalisib) were tested to determine IC50s against each fusion-containing cell line.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
While KIF5B-RET is the most common RET fusion in NSCLCs, RET inhibition with RXDX-105 resulted in responses only in non-KIF5B-RET-containing cancers. Novel approaches to targeting KIF5B-RET-containing tumors are needed, along with a deeper understanding of the biology that underlies the differential responses observed.
Acknowledgments
FINANCIAL SUPPORT
AD is supported by the National Institutes of Health award P30 CA008748. This study was sponsored by Ignyta.
Footnotes
DISCLOSURES
AD – Advisory board/honoraria Ignyta, Roche, Genentech, Exelixis, AstraZeneca, Pfizer, Takeda/Ariad, Loxo Oncology, Blueprint Medicines, Helsinn, Hengrui, Beigene; RCD – Advisory board and consulting Ignyta, AstraZeneca, Takeda, and Spectrum Pharmaceuticals; Sponsored Research Agreements: Ignyta and Loxo; Stock Ownership: Rain Therapeutics; Licensed Patents: Abbott Molecular and Rain Therapeutics; Licensed products: Ignyta and Ariad. KLR—Consulting: Loxo, Takeda, Tesaro, Euclises, Genentech, Guardant; AJO – Advisory board (honoraria to self): Takeda, BMS, Merck, Array and EMD Serono; Research (all money to FCCC): Amgen, Takeda, Immunocore, EMD Serono, Incyte, Kyowa, Lilly, Novartis, Pfizer, BMS, Kura, Checkmate, Targovax
REFERENCES
- 1.Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018;15:151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14:173–86. [DOI] [PubMed] [Google Scholar]
- 3.Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 2017;12:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrantonio F, Di Nicolantonio F, Schrock AB, Lee J, Morano F, Fuca G, et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol 2018; 6:1394–1401. [DOI] [PubMed] [Google Scholar]
- 5.Li GG, Somwar R, Joseph J, Smith RS, Hayashi T, Martin L, et al. Antitumor Activity of RXDX-105 in Multiple Cancer Types with RET Rearrangements or Mutations. Clin Cancer Res 2017;23:2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Lee JK, Ahn MJ, Kim DW, Sun JM, Keam B, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 2017;28:292–7. [DOI] [PubMed] [Google Scholar]
- 8.Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2016;1:42–50 [DOI] [PubMed] [Google Scholar]
- 9.Velcheti V, Hida T, Reckamp KL, Yang JC, Nokihara H, Sachdev P, et al. Phase 2 study of lenvatinib in patients with RET fusion-positive adenocarcinoma of the lung. Ann Oncol 2016;27:S178. [DOI] [PubMed] [Google Scholar]
- 10.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara R, Auger N, Auclin E, Besse B. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:27–45. [DOI] [PubMed] [Google Scholar]
- 12.Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov 2018;8:836–49. [DOI] [PubMed] [Google Scholar]
- 13.Schubert L, Le AT, Malkoski S, Nemenoff R, Doebele R. CRISPR/Cas9 generation of Ret and Ntrk1 fusion oncogenes and novel in vitro sgRNA screening method. Clin Cancer Res 2018;78:AB33. [Google Scholar]
- 14.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson DS, Gujral TS, Peng S, Asa SL, Mulligan LM. Transcript level modulates the inherent oncogenicity of RET/PTC oncoproteins. Cancer Res 2009;69:4861–9. [DOI] [PubMed] [Google Scholar]
- 16.Levinson S, Cagan RL. Drosophila Cancer Models Identify Functional Differences between Ret Fusions. Cell Rep 2016;16:3052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das TK, Cagan RL. KIF5B-RET Oncoprotein Signals through a Multi-kinase Signaling Hub. Cell Rep 2017;20:2368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbiah V, Berry J, Roxas M, Guha-Thakurta N, Subbiah IM, Ali SM, et al. Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases. Lung Cancer. 2015;89:76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding JJ, Pulitzer M, Chapman PB. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med 2012;366:866–8. [DOI] [PubMed] [Google Scholar]
- 20.Lin JJ, Chin E, Yeap BY, Ferris LA, Kamesan V, Lennes IT, et al. Brief Report: Increased Hepatotoxicity Associated with Sequential Immune Checkpoint Inhibitor and Crizotinib Therapy in Patients with Non-Small-Cell Lung Cancer. J Thorac Oncol 2018;18:33040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018;29:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drilon A, Subbiah V, Oxnard G, Bauer T, Velcheti V, Lakhani N, et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J Clin Oncol 2018;36:A102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


