Abstract
Neuromyelitis optica spectrum disorder (NMOSD) attacks lead to incremental loss of function of the optic nerves and spinal cord. The standard of care for treatment of acute attacks to mitigate damage is high dose corticosteroids and, if needed, plasma exchange. Although the inclination among clinicians is to treat relapses as soon as they start, there is no previously published evidence to conclude that earlier treatment with corticosteroids is more effective in the long term. In this study, we correlated neurological outcomes from acute NMOSD relapses with delay to treatment, as well as demographic and clinical characteristics that influence prognosis.
Keywords: Neuromyelitis optica, Devic’s disease, Transverse myelitis, Optic neuritis, Outcomes
Introduction
Neuromyelitis Optica Spectrum Disorder (NMOSD) is a rare autoimmune disease that causes optic neuritis and longitudinally extensive transverse myelitis leading to blindness and paralysis (Oh and Levy, 2012). Long term disability in NMOSD is mediated by repeated inflammatory attacks, hence treatment of acute attacks is intended to mitigate disability (Abboud et al., 2016; Wingerchuk et al., 2007). Immunosuppression with high dose corticosteroids and/or plasma exchange (PLEX) is the current standard of care for the treatment of acute NMOSD relapses (Kessler et al., 2016). While there is a general consensus among clinicians to treat relapses as soon as possible, there is no natural history data in NMOSD to predict outcomes from acute relapses based on duration between onset of attack and start of treatment.
In this study, we correlated the degree of recovery after acute NMOSD relapse with treatment delay and hypothesized that patients treated sooner made better recoveries than if treatment were started later. We also looked for additional demographic and clinical factors that served as predictors of outcome after NMOSD attacks.
Methods
We retrospectively analyzed 137 distinct NMOSD relapses from 69 patients admitted to the Johns Hopkins Hospital. All patients met the clinical criteria of NMOSD from the 2015 International Panel of NMOSD Diagnosis (Wingerchuk et al., 2015). For this study, disease relapse was defined as a presentation of new or worsening physical symptoms corresponding with a change on neurological exam and attributed to a new T2 or contrast-enhancing lesion by MRI (Mealy et al., 2014). We only included patients whose relapse treatment was started with a five-day course of high dose corticosteroids (1000 mg methylprednisolone or equivalent). Among those with no or nominal improvement, as determined by the treating physician, treatment was escalated with plasma exchange at 1.0 – 1.5 plasma volumes as tolerated, every other day for a total of 5 exchanges (Bonnan and Cabre, 2012).
Severity and disability were measured as changes in the Extended Disability Status Scale (EDSS) score. Scores were calculated at four time points: at baseline before the presentation of new or worsening symptoms, at admission to the hospital, at discharge, and at follow-up of at least 30 days later. We classified the relapses into one of four categories based on how quickly treatment was received from the day of symptom onset: 0–3 days, 4–7 days, 8–14 days, >14 days. These time categories are based on the uncertainty inherent in determining the precise date and time of symptoms related to a new relapse. There were two issues that interfered with our ability to stamp a precise/date to every relapse: patient recall and relapse course. Patient recall was biased by chronic symptoms, fluctuation in symptoms due to medications (e.g., anti-epileptic drugs), and an incomplete understanding of which symptoms may be due to a new relapse. Relapse course in NMO attacks is variable with some relapses evolving slowly and mildly over weeks and others presenting to the nadir within hours.
Median EDSS scores were compared by Wilcoxon paired testing with p values < 0.05 considered statistically significant. Additionally, we used Pearson correlation to identify demographic, clinical, serological, and radiographical data that is associated with neurological outcomes. Outcomes versus age or severity were calculated by regression analysis using GraphPad Prism 7.0.
The Johns Hopkins Institutional Review Board approved this study.
Results
The demographic profile of the subjects included in this study is shown in Table 1. They represent a cross section of the NMOSD population seen at the Johns Hopkins Hospital between 2005 and 2016. Of the 137 relapse events, 55 were treated within 0–3 days of symptom onset, 38 treated within 4–7 days, 22 within 8–14 days, and 22 after 14 days. Of those who started treatment within three days from onset, the median baseline EDSS score of 3.5 worsened to 4.5 at presentation (p<0.0001), and then improved to 4.0 upon discharge (p=0.0014) after high dose corticosteroid treatment or corticosteroids plus plasma exchange. All time groups saw a similar trend, with the EDSS scores at discharge improved compared to presentation. This reinforced the notion that acute treatment with corticosteroids +/− PLEX not only prevents worsening of neurological disability, but on average also leads to improvement in function, regardless of when treatment is initiated (Figure 1).
Table 1.
Characteristics of NMOSD relapses.
| Relapse Characteristics | 0–3 days | 4–7 days | 8–14 days | > 14 days |
|---|---|---|---|---|
| Number of relapses | 55 | 38 | 22 | 22 |
| Median age at relapse | 44.9 | 47.0 | 47.2 | 48.8 |
| 25th IQR | 25.6 | 30.4 | 20.9 | 32.2 |
| 75th IQR | 60.6 | 53.3 | 56.3 | 54.2 |
| Sex (% female) | 91% | 87% | 90% | 86% |
| Race (% white) | 42% | 43% | 50% | 36% |
| AQP4 seropositive | 89% | 94% | 100% | 100% |
| Duration of disease | 7.2 | 3.8 | 4.3 | 4.1 |
| Baseline EDSS | 3.5 | 3.8 | 4.0 | 3.0 |
| Preventive immunotherapy | 79% | 84% | 72% | 74% |
| Relapse type (% myelitis) | 51% | 64% | 73% | 45% |
| Severity of attack (ΔEDSS) | 1.8 | 1.7 | 2.6 | 2.4 |
| Escalated to PLEX | 55% | 50% | 43% | 53% |
Figure 1.
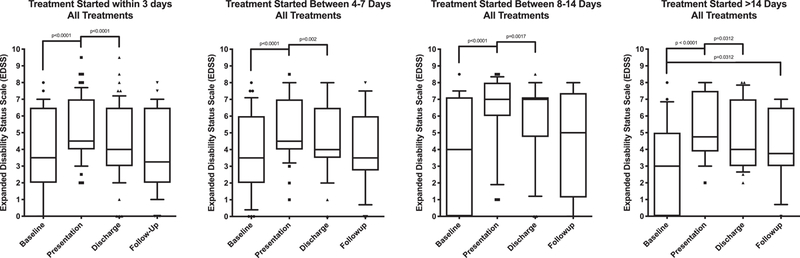
Changes in Expanded Disability Status Scale (EDSS) scores during acute NMOSD relapses by duration of onset of symptoms to treatment start: 0–3 days (A), 4–7 days (B), 8–14 days (C) and greater than 14 days (D). Within each time period, there was a significant difference in the EDSS scores at the time of presentation compared to baseline and there was a significant improvement at discharge compared to presentation. Treatment at any time point within 14 days generally led to good recovery by follow up with no statistical difference in the EDSS scores at baseline compared to follow-up from a single attack. In those who started treatment more than 14 days from onset (D), there was some improvement but the EDSS scores at follow up were significantly higher than baseline suggesting a permanent residual disability.
At follow-up after at least 6 months, those who received treatment within 14 days of symptom onset more often than not returned to baseline EDSS, i.e., there were no statistically significant differences between the average baseline EDSS scores and the follow-up EDSS scores. However, those who started treatment more than 14 days after symptom onset more often than not did not return to their EDSS baseline at follow-up— there was a significant difference between the baseline and follow-up EDSS scores indicating a permanent residual disability (median 3.0 vs. 3.75, p = 0.0312, Figure 1).
Although there was a clear advantage to earlier treatment on average, there were many fortunate good outcomes even when treatment was initiated more than 14 days after onset. Similarly, there were many unfortunate cases of poor outcome despite immediate presentation at onset. This variability led us to perform a post-hoc analysis to identify other predictors of outcome. Univariate analyses identified two factors that strongly correlated: age and severity at the time of presentation to the hospital.
The ages of the patients during acute NMOSD attack ranged from 4 to 78 years old. The severity of attack, defined as the difference in EDSS score between baseline and presentation to the hospital, is slightly higher in the younger age groups: the median severity at 20 years of age is 2.0, whereas at 60 it is 1.8, and there is a steady decline in median attack severity as the ages of the patients increase (Figure 2A). However, the degree in recovery is higher in younger age groups, despite having attacks which are slightly more severe (Figure 2B). At age 20, 85% recovery was seen on average, whereas at age 60, it was down to 60%. With increasing age, there was a significant decline in the degree of recovery (p=0.039) (Figure 2B).
Figure 2.
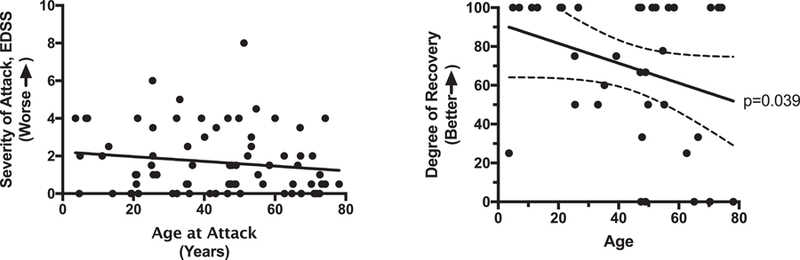
Younger age correlates with higher degree of recovery from any single attack. Although severity of attacks is slightly higher at the younger age groups (A), their degree of recovery is higher as well (B). There was a significant decline in degree of recovery with increasing age (p=0.039).
The degree of recovery at follow-up also correlated strongly with the severity of the attacks at presentation. Cases with attack severity at presentation of 2.5 or less (calculated as the difference between the presenting EDSS and the baseline EDSS) have a nearly 2-fold better chance of full recovery compared those with severity of 3.0 or higher (Fisher’s test, p=0.0067). Beyond an initial severity of 3.0, the degree of recovery drops significantly (Figure 3).
Figure 3.
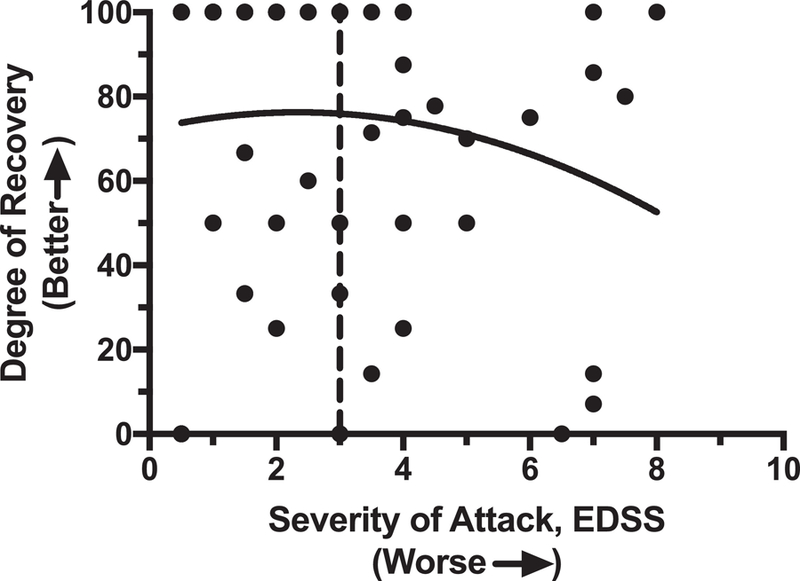
Degree of recovery is dependent on the severity of the attack at presentation. With higher attack severity, the degree of recovery declines. After the point when the EDSS change is greater than 3.0 (dashed line), there is an acute decline in the chance of recovery to baseline. (We refer to this dashed line as the “point of no return”.)
Discussion
Our results provide practical evidence for clinicians to treat NMOSD patients who are suffering from an acute relapse. The good news is that 69% of all relapses are treatable back to baseline neurological function – at least as measured by EDSS – using standard of care therapy with high dose corticosteroids with or without plasma exchange. In cases where steroid therapy alone leads to a complete resolution of the relapse, escalated therapy is not required. In cases where there is no improvement and in most cases of only partial improvement, our study is consistent with other studies supporting plasma exchange (Bonnan and Cabre, 2012; Lim et al., 2013; Merle et al., 2012). There is a meaningful benefit to treating within 14 days of onset of symptoms, and this study supports advocacy efforts to treat NMOSD relapses sooner rather than later to improve the odds of a good outcome. The bad news is that long term outcomes from relapses are strongly correlated with the severity of the attack at presentation regardless of treatment timing. In our study, there was no evidence that severity worsens with delay in treatment; rather, the severity of the attack appears to be random and not within control of patients or their treating physicians with the exception of the beneficial effect of preventive therapy. In our previous study, we found that the severity of relapses may be reduced by preventive therapy such as mycophenolate or rituximab (Abboud et al., 2016), but there are no placebo-controlled trials to formally test that observation. The sum of the evidence suggests that NMOSD patients are best served in the long term by preventive immunosuppressive therapy, and early intervention with high dose corticosteroids and/or plasma exchange in the event of a relapse (Bonnan et al., 2018; Kleiter et al., 2018; Kleiter et al., 2016).
The strong correlation between outcome and severity of attack “points to a mechanism of point of no return” in which crossing a threshold of damage in the spinal cord or optic nerves leads to permanent neurological disability. Two plausible explanations for this mechanism are: 1) The healing capacity in the central nervous system wanes with age, and 2) attack severity is influenced by vascular supply. Attacks that compromise vascular supply lead to damage seen in spinal cord strokes characterized by little, if any, healing. If vascular health of the central nervous system influences outcomes from NMOSD attacks, perhaps we should be paying more attention to general vascular risk factors. Hypertension, which is the most common risk factor associated with cerebrovascular disease, is notably the most common comorbidity in NMOSD (Ajmera et al., 2018). In addition to preventive therapies aimed at general vascular health, studies of spinal cord protection with spinal fluid drainage during relapses should also be considered in severe cases as this approach has been shown to be helpful in preventing spinal cord ischemia (Epstein, 2018).
The limitations of this study are the biases inherent in a single center NMOSD population and the retrospective study design. The small sample size of a single center limits the statistical power to compare differences across each time group. The severity score used in this study, the EDSS, is the most widely used severity scale in acute NMOSD studies because it combines optic nerve and spinal cord function; however, it is non-linear scale more heavily weighted towards spinal cord function and therefore likely influenced the outcome of this study to make transverse myelitis attacks more severe than optic neuritis attacks. Further studies are needed to mitigate CNS damage from relapses and improve long term outcomes in NMOSD patients.
Highlights.
Initiation of high dose corticosteroids for the treatment of acute relapses of neuromyelitis optica spectrum disorder within 14 days resulted in improved outcomes compared to treatment initiation 14 days after onset of symptoms.
Patients younger than 35 years of age were more likely to recover to baseline after a relapse compared to those older than 35 years of age.
Regardless of treatment initiation time, attacks that are milder at presentation have a better outcome compared to attacks that are more severe.
Acknowledgments
Role of Funding
The funding source played no role in the design of the manuscript, the content or style, or provide any input or revisions.
Funding: This study was funded by a grant from the National Institute of Allergy and Infectious Diseases, grant R01-AI130548 (ML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email mlevy@jhmi.edu.
Disclosures: Authors have no disclosures to report that are applicable to this study.
References.
- Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M, 2016. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler 22(2), 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmera MR, Boscoe A, Mauskopf J, Candrilli SD, Levy M, 2018. Evaluation of comorbidities and health care resource use among patients with highly active neuromyelitis optica. J Neurol Sci 384, 96–103. [DOI] [PubMed] [Google Scholar]
- Bonnan M, Cabre P, 2012. Plasma exchange in severe attacks of neuromyelitis optica. Mult Scler Int 2012, 787630. [DOI] [PMC free article] [PubMed]
- Bonnan M, Valentino R, Debeugny S, Merle H, Ferge JL, Mehdaoui H, Cabre P, 2018. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry 89(4), 346–351. [DOI] [PubMed] [Google Scholar]
- Epstein NE, 2018. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: A review. Surg Neurol Int 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RA, Mealy MA, Levy M, 2016. Treatment of Neuromyelitis Optica Spectrum Disorder: Acute, Preventive, and Symptomatic. Curr Treat Options Neurol 18(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Hellwig K, Pache F, Ruprecht K, Havla J, Kumpfel T, Aktas O, Hartung HP, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Stellmann JP, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Krumbholz M, Zeltner L, Ziemann U, Linker R, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Zettl UK, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C, Nemos, 2018. Apheresis therapies for NMOSD attacks: A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm 5(6), e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Wegner B, Hellwig K, Pache F, Ruprecht K, Havla J, Krumbholz M, Kumpfel T, Aktas O, Hartung HP, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Stellmann JP, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Zeltner L, Ziemann U, Linker R, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Winkelmann A, Marouf W, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C, Neuromyelitis Optica Study G, 2016. Neuromyelitis optica: Evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 79(2), 206–216. [DOI] [PubMed] [Google Scholar]
- Lim YM, Pyun SY, Kang BH, Kim J, Kim KK, 2013. Factors associated with the effectiveness of plasma exchange for the treatment of NMO-IgG-positive neuromyelitis optica spectrum disorders. Mult Scler 19(9), 1216–1218. [DOI] [PubMed] [Google Scholar]
- Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M, 2014. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol 71(3), 324–330. [DOI] [PubMed] [Google Scholar]
- Merle H, Olindo S, Jeannin S, Valentino R, Mehdaoui H, Cabot F, Donnio A, Hage R, Richer R, Smadja D, Cabre P, 2012. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol 130(7), 858–862. [DOI] [PubMed] [Google Scholar]
- Oh J, Levy M, 2012. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int 2012, 460825. [DOI] [PMC free article] [PubMed]
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG, International Panel for NMOD, 2015. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85(2), 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG, 2007. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 68(8), 603–605. [DOI] [PubMed] [Google Scholar]


