Abstract
Due to the restrictions of liver transplantation, complication-guided pharmacological therapy has become the mainstay of long-term management of cirrhosis. This article aims to provide a complete overview of pharmacotherapy options that may be commenced in the outpatient setting which are available for managing cirrhosis and its complications, together with discussion of current controversies and potential future directions. PubMed/Medline/Cochrane Library were electronically searched up to December 2018 to identify studies evaluating safety, efficacy and therapeutic mechanisms of pharmacological agents in cirrhotic adults and animal models of cirrhosis. Non-selective beta-blockers effectively reduce variceal re-bleeding risk in cirrhotic patients with moderate/large varices, but appear ineffective for primary prevention of variceal development and may compromise renal function and haemodynamic stability in advanced decompensation. Recent observational studies suggest protective, haemodynamically-independent effects of beta-blockers relating to reduced bacterial translocation. The gut-selective antibiotic rifaximin is effective for secondary prophylaxis of hepatic encephalopathy; recent small trials also indicate its potential superiority to norfloxacin for secondary prevention of spontaneous bacterial peritonitis. Diuretics remain the mainstay of uncomplicated ascites treatment, and early trials suggest alpha-adrenergic receptor agonists may improve diuretic response in refractory ascites. Vaptans have not demonstrated clinical effectiveness in treating refractory ascites and may cause detrimental complications. Despite initial hepatotoxicity concerns, safety of statin administration has been demonstrated in compensated cirrhosis. Furthermore, statins are suggested to have protective effects upon fibrosis progression, decompensation and mortality. Evidence as to whether proton pump inhibitors cause gut-liver-brain axis dysfunction is conflicting. Emerging evidence indicates that anticoagulation therapy reduces incidence and increases recanalisation rates of non-malignant portal vein thrombosis, and may impede hepatic fibrogenesis and decompensation. Pharmacotherapy for cirrhosis should be implemented in accordance with up-to-date guidelines and in conjunction with aetiology management, nutritional optimisation and patient education.
Keywords: Cirrhosis, Beta-blockers, Rifaximin, Diuretics, Statins, Proton pump inhibitors, Pharmacology
Core tip: Pharmacological therapy is central to the management of cirrhosis and its complications. Whilst there has been recent debate about the safety of beta-blockade in patients with ascites, conversely there is growing interest in potential benefits relating to a reduction in gut bacterial translocation and hepatocellular carcinoma risk. In addition to its well-established role in treating hepatic encephalopathy, rifaximin may also have a key role in preventing secondary infections. In this review, we summarise these and other uncertainties, controversies and novel developments related to pharmacotherapy in the clinical management of chronic liver disease.
INTRODUCTION
Decompensation and mortality in cirrhosis are predominantly due to the compli-cations of portal hypertension, and prognosis in cirrhosis progressively deteriorates with the cumulative occurrence of ascites, variceal haemorrhage, hepatic encephalopathy (HE) and spontaneous bacterial peritonitis (SBP)[1]. While both cirrhosis incidence and prevalence have risen in recent decades, uptake of liver transplantation – the only definitive treatment option for cirrhosis - has stagnated at around 5500/year in Europe, primarily due to organ shortages[2]. Consequently, standardised cirrhosis mortality rates globally, and in the United Kingdom particularly, have increased significantly[3]. This development led to the United Kingdom-wide introduction of specialist cirrhosis clinics which integrate multidisciplinary services and aim to optimise supportive cirrhosis management by forestalling decompensation and facilitating recompensation. In the specialist clinic setting, one factor which has gained importance in chronic cirrhosis management is long-term, complication-guided pharmacological therapy. Whilst previous articles have addressed individual pharmacological agents and their role in treating specific complications of cirrhosis, the present article aims to provide an overview of the complete pharmacotherapy currently available for the long-term management of cirrhotic outpatients as well as an insight into emerging and future directions.
METHODS
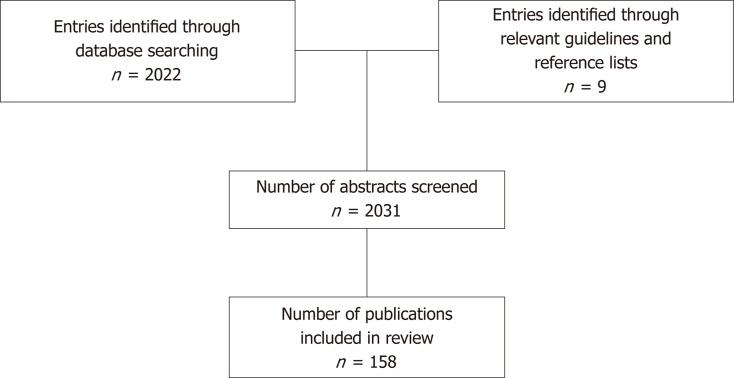
A search of the existing literature up to December 2018 was conducted using the electronic databases PubMed, Medline and the Cochrane library, as well as relevant guidelines and reference lists. Titles and abstracts were searched for the following key terms: “Cirrhosis” and (“beta-blockers” or “lactulose” or “rifaximin” or “L-ornithine L-aspartate (LOLA)” or “acarbose” or “diuretics” or “midodrine” or “clonidine” or “vaptans” or “human serum albumin” or “anti-coagulation” or “caffeine” or “faecal microbiota transplant”). A separate search was performed for international guidelines to cirrhosis management by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL). Additionally, reference lists of included articles were manually screened for further relevant publications. The abstracts of 2031 publications were identified and screened for studies evaluating the safety, efficacy and therapeutic mechanism of pharmacological agents in cirrhotic adults and animal models of cirrhosis. 158 publications were considered relevant to the key question and included in the present review (Figure 1). Only articles published in English were included.
Figure 1.
Flow diagram of study inclusion.
CURRENTLY-USED MEDICATIONS
Beta-blockers
Presently, non-selective beta-blockers (NSBB) are the only drug class endorsed for the long-term treatment of portal hypertension[4,5]. Along with endoscopic band ligation, NSBBs are employed for primary and secondary prophylaxis against variceal haemorrhage, as they combat the hyperkinetic portal-hypertensive syndrome by decreasing cardiac output and portal inflow (β-1 receptor blockade) and by achieving splanchnic vasoconstriction and reducing azygos blood flow (β-2 receptor blockade)[1,6-8]. Thus they efficaciously lower the risk of variceal bleeding and re-bleeding as evidenced by several randomized controlled trials (RCT)[9-11].
Recent concerns about beta-blockers: These trials, however, predominantly excluded patients with advanced decompensation, and recent studies have voiced concerns over the safety of beta-blockers in decompensated patients with refractory ascites or SBP and in cirrhotic patients with alcoholic hepatitis[12-14]. In a prospective cohort study of 151 patients with refractory ascites by Sersté et al[12], 1-year survival was significantly lower in patients who received propranolol (19%) compared to patients not on beta-blocker therapy (64%). In another retrospective cohort study by Sersté et al[13] including 139 cirrhotic patients with severe alcoholic hepatitis, cumulative incidence of acute kidney injury (AKI) was significantly higher in the group receiving beta-blockers (89.6%) vs the group not receiving beta-blockers (50.4%). In a retrospective analysis of 607 cirrhotic patients by Mandorfer et al[14], NSBB therapy was associated with increased transplant-free survival in patients without SBP, but with decreased transplant-free survival, increased length of non-elective hospitalisation and increased rates of hepatorenal syndrome (HRS) in patients with SBP. Pathophysiologically, circulating blood volume depletion during large volume paracentesis for refractory ascites and the systemic inflammatory response during SBP may threaten the already limited cardiac reserve of decompensated patients, while beta-blockade further impairs restoration of renal and systemic perfusion pressures[7]. Furthermore, patients with advanced stages of decompensation and a more amplified hyperdynamic circulation are likely to receive higher doses of beta-blockers, thus exaggerating detrimental effects on systemic haemodynamics. Accordingly, a retrospective nationwide study of 3719 Danish patients with cirrhosis found a reduction in mortality for propranolol doses < 160 mg/d but an increase in mortality for doses > 160 mg/d[15].
The ‘window hypothesis’: Consequently, the ‘window hypothesis’ by Krag et al[16] proposes the existence of a specific time frame of opportunity during the natural course of cirrhosis, only within the bounds of which NSBB therapy exerts beneficial effects on survival. It is propositioned that the window opens with the development of moderate/large varices and closes during advanced cirrhosis with the advent of refractory ascites, SBP, HRS or with the occurrence of alcoholic hepatitis[6,16]. An elegantly-designed randomized controlled trial by Groszmann et al[17] demonstrated that NSBBs are ineffective in the pre-primary prophylaxis of variceal development and even result in adverse effects in patients without varices, since the absence of a hyperdynamic circulation in patients with subclinical portal hypertension (Hepato-Venous Pressure Gradient (HVPG) < 10 mmHg) attenuates the portal pressure lowering effects of beta-blockers[18,19]. A more recent meta-analysis by Kumar et al[20] also found no difference in incidence of first variceal haemorrhage and development of large varices comparing patients receiving beta-blockers and patients not receiving beta-blockers, yet a significantly higher rate of adverse events [relative risk (RR) 4.66, 95% confidence interval (CI) 1.36–15.91] was observed in patients receiving beta-blockers. With cirrhosis progression, portal pressure heightens while effective circulating volume decreases as a result of splanchnic vasodilatation and raised portal inflow. At this level of disease progression, the drop in effective circulating blood volume is compensated for by enhanced sympathetic stimulation of the cardiocirculatory system in the context of preserved cardiac reserves[4,21]. It is precisely at this stage that NSBBs are proposed to effectively counteract portal hypertension and abrogate the hyperdynamic state, thus improving patient survival[4]. With further advancement of cirrhosis and portal hypertension, however, the cardiac response to stimuli such as volume depletion by variceal haemorrhage becomes limited and beta-blockade impedes restoration of systemic and renal perfusion pressures, thus negatively impacting patient survival[7,21]. In line with the ‘window hypothesis’, Kim et al[22] conducted a nested case-control study in patients awaiting liver transplantation, matching 205 patients with AKI to 205 patients without AKI. On multivariate analysis, patients with ascites receiving beta-blockers had a 3-fold increased [Hazard ratio (HR) 3.31] risk of developing AKI, while patients without ascites receiving beta-blockers had a 5-fold reduced (HR 0.19) risk of developing AKI[21,22].
As a consequence of these concerns, widespread withholding of NSBB therapy in patients with advanced cirrhosis ensued. Yet, data from additional studies, performed after this initial apprehension had surfaced, provide counteracting evidence. A recent post-hoc analysis of 1188 patients with cirrhotic ascites from three satavaptan RCTs showed no association between NSBB therapy and increased mortality[23]. Similarly, a retrospective study by Leithead et al[24] (105 beta-blocker users propensity risk score matched to 105 beta-blocker non-users) correlated NSBB use with improved survival in patients with ascites awaiting orthotopic liver transplantation (HR 0.55, 95% CI 0.32-0.95), and even on subgroup analysis of patients with refractory ascites, beta-blockers remained independently associated with improved survival (HR 0.35, 95% CI 0.14-0.86). Furthermore, Onali et al[25] evaluated 316 consecutive cirrhotic patients with ascites undergoing assessment for liver transplantation. Beta-blockers were associated with reduced overall mortality (HR 0.55, 95% CI 0.33-0.94) and on sub-group analysis of patients with refractory ascites no difference in mortality was observed (HR 0.43, 95% CI 0.20-1.11). The numerous observational studies investigating the risks and benefits of NSBBs in advanced cirrhosis are summarised in Table 1. A recent meta-analysis of three RCTs and 13 observational studies summarising the effect of NSBBs on mortality in cirrhotic patients with ascites found that survival was comparable between NSBB and control groups for both the overall population (HR 0.86, 95% CI 0.71-1.03, P = 0.11) and the refractory ascites subgroup (HR 0.90, 95% CI 0.45-1.79, P = 0.76) with significant heterogeneity between included studies[26].
Table 1.
Observational studies investigating the effects of non-selective beta-blockers in advanced cirrhosis
| Study | Study type | Study population | Type of NSBB | Outcomes |
| Sersté et al[12] | Single-centre, prospective cohort study | Cirrhotic inpatients with refractory ascites (n = 151; mean MELD 18.8 ± 4.1; 69% diuretic-intractable, 31% diuretic-resistant), all treated with large-volume paracentesis + IV albumin Median follow-up: 8 mo (range 1-47) | Propranolol (40-160 mg per day) | Median survival was 5.0 mo (95% CI 3.5-6.5 mo) for patients on propranolol compared to 20.0 mo (95% CI 4.8-35.2 mo) for patients not on propranolol (P < 0.0001) |
| Sersté et al[13] | Single centre, retrospective cohort study | Cirrhotic inpatients with alcoholic hepatitis (n = 139; mean MELD score 27.3 ± 7.6; mean Maddrey score 71.0 ± 34.4), all treated with methylprednisolone | Propranolol (40-160 mg per day) | At 168-d follow-up: AKI incidence was 89.6% (95% CI 74.9%-95.9%) for patients on propranolol compared to 50.4% (95% CI 39.0%-60.7%) for patients not on propranolol (P < 0.0001) Transplant-free mortality was 56.8% (95% CI 41.3%-69.7%) in NSBB users compared to 46.7% (95% CI 35.0%-57.6%) in non-users (P = 0.25) |
| Mandorfer et al[14] | Single-centre retrospective observational study | Cirrhotic outpatients with ascites (n = 607; mean MELD 17.5 ± 10.6), all treated with large-volume paracentesis + IV albumin Follow-up: 660 person-years | Propranolol (20-120 mg per day); Carvedilol (6.25-25 mg per day) | In patients without SBP: NSBB use was associated with higher transplant-free survival (HR 0.75, 95% CI 0.581-0.968) and with reduced length of hospitalisation In patients with SBP: NSBB use was associated with reduced transplant free survival (HR 1.58, 95% CI 1.098-2.274), development of HRS (24% vs 11%, P = 0.03), and increased length of hospitalisation |
| Bang et al[15] | Multicentre, retrospective, propensity-adjusted, longitudinal study of Danish databases | Decompensated cirrhotic in- and outpatients (n = 702 propranolol-users matched to n = 702 non-users). Stratified into mild decompensation (1-4 previous paracenteses) and severe decompensation (> 4 paracenteses) | Propranolol (< 80 mg, 80-160 or > 160 mg per day) | At 2-year follow-up: Propranolol use was associated with lower mortality in patients with mildly decompensated cirrhosis (HR 0.7, 95% CI 0.6-0.9) and severely decompensated cirrhosis (HR 0.6, 95% CI 0.4-0.9). Survival benefit was only found for propranolol doses < 160 mg/d. |
| Kim et al[22] | Single-centre, retrospective, nested case-control study | Cirrhotic patients listed for liver transplantation who developed AKI (n = 205 patients with AKI matched to n = 205 patients without AKI) Median follow-up: 18.2 mo (range 1-198 mo) | Propranolol and nadolol (propranolol equivalent 40 mg per day, IQR 30.0–60.0 mg) | In patients with ascites: NSBB use was associated with an increased risk of AKI (HR 3.31, 95% CI 1.57-6.95) In patients without ascites: NSBB use was associated with a reduced risk of AKI (HR 0.19, 95% CI 0.06-0.60) |
| Bossen et al[23] | Post-hoc observational analysis of three multicentre RCTs (satavaptan vs placebo) | Cirrhotic patients with diuretic-responsive (n = 600) and refractory (n = 588) ascites (n = 559 NSBB users, n = 629 non-users) | Propranolol and carvedilol (doses not specified) | At 52-wk follow-up: In patients with refractory ascites, the cumulative mortality in NSBB users was 30.5% compared to 30.9% in non-users (HR 1.02, 95% CI 0.74-1.39). In patients with diuretic-responsive ascites, the cumulative mortality in NSBB users was 17.0% compared to 19.5% in non-users (HR 0.78, 95% CI 0.53-1.16) |
| Leithead et al[24] | Single-centre, retrospective, propensity-adjusted, observational study | Consecutive cirrhotic patients with ascites listed for liver transplantation (n = 105 NSBB users matched to n = 105 non-users) Median follow-up: 72 d (IQR 27-162 d) | Propranolol (median dose 80mg per day, range 10-240 mg); Carvedilol (median dose 6.25 mg per day, range 3.125-12.5) | In patients with diuretic-responsive ascites: NSBB users showed lower waitlist mortality compared to non-users (HR 0.55, 95% CI 0.32-0.95) In patients with refractory ascites: NSBB users showed lower waitlist mortality compared to non-users (HR 0.35, 95% CI 0.14-0.85) |
| Onali et al[25] | Single-centre, retrospective audit | Consecutive cirrhotic patients with ascites assessed for liver transplant suitability (n = 316, median MELD score 15, range 6-40) Median follow-up: 7 mo (± 12) | Propranolol (median dose 80 mg per day, IQR 40); Carvedilol (median dose 6.25 mg per day, IQR not specified) | In the whole population, NSBB use was associated with lower mortality (HR 0.55, 95% CI 0.33-0.94). In patients with refractory ascites, there was no difference in survival in NSBB users compared to non-users (HR 0.43, 95% CI 0.20-1.11) |
AKI: Acute kidney injury; CI: Confidence interval; HR: Hazard ratio; HRS: Hepatorenal syndrome; IQR: Interquartile range; IV: Intravenous; MELD: Model for end stage liver disease; NSBB: Non-selective beta-blocker; RCT: Randomised controlled trial; SBP: Spontaneous bacterial peritonitis.
Carvedilol: With the hypothesis that these discrepancies regarding the effect of beta-blockers in patients with refractory and non-refractory ascites may be related to the type of beta-blocker administered, a meta-analysis of nine observational studies in cirrhotic patients with ascites by Njei et al[27] found that the traditional NSBBs propranolol and nadolol were not associated with increased mortality, but that carvedilol, a NSBB with additional anti-alpha-1-adrenergic receptor activity, demonstrated a statistically significant association with increased all-cause mortality (RR 1.75, 95% CI 1.06-2.90)[27]. Previous studies had shown that carvedilol is more potent in reducing HVPG than traditional NSBBs[28] and is able to achieve a haemodynamic response in a high percentage (56%) of non-responders to propranolol[29]. In rodents, carvedilol administration was shown to modulate inflammatory cytokine generation and augment antioxidant production, resulting in diminished liver fibrogenesis[30,31]. The anti-alpha-1 mediated reduction in intrahepatic resistance brought about by carvedilol may be of benefit to patients with less advanced portal hypertension, where intrahepatic resistance still constitutes a major factor in portal pressure[32]. While traditional beta-blockers were shown to be ineffective in the primary prevention of variceal development, carvedilol achieved a delay in progression of small to large varices from 18.7 to 20.8 mo in a RCT by Bhardwaj et al[33]. However, carvedilol also shows a trend towards a more potent reduction in systemic arterial pressure compared to traditional beta-blockers[19,28] and could thus further destabilise the delicately-balanced haemodynamic state in cirrhotic patients with ascites, as Njei et al[27] hypothesise in view of the detrimental effect of carvedilol on their cohort. In contrast, Sinha et al recently investigated the effects of long-term, low-dose (12.5 mg) carvedilol treatment in a retrospective, propensity score matched cohort of 264 patients with ascites. After a median follow-up of 2.3 years, carvedilol therapy was associated with a hazard ratio of 0.47 (95% CI 0.29-0.77) in patients with mild ascites and was not associated with increased mortality in patients with moderate to severe ascites[30]. Zacharias et al[34] recently conducted a Cochrane systematic review of 10 RCTs and 810 patients comparing the safety and efficacy of carvedilol versus traditional NSBBs in the primary and secondary prevention of variceal haemorrhage; they identified no differences in the incidence of mortality, variceal haemorrhage and serious adverse events between both groups despite greater reductions in HVPG for the carvedilol group. Due to the low quality of assessed evidence, these findings were associated with substantial uncertainty.
Haemodynamically-independent potential of beta-blockers: Additional observa-tional studies have proposed the existence of haemodynamically independent effects of beta-blockers. Senzolo et al[35] and Bang et al[15] observed protective effects of NSBB administration on SBP occurrence in patients with cirrhotic ascites. Similarly, Merli et al[36] prospectively followed 400 cirrhotic inpatients, finding that beta-blockade generally protected against infection and that infected patients showed lower morbidity and mortality when receiving beta-blocker therapy. Mookerjee et al[37] investigated the immunomodulatory effect of beta-blockers in 349 patients with acute-on-chronic liver failure (AoCLF) and observed that beta-blockade favourably affected 28-d mortality (24% vs 34%, P = 0.048). Patients on beta-blockers demonstrated reduced severity of AoCLF, slower progression of AoCLF and lower white blood cell counts on admission[37]. Facciorusso et al[38] analysed 107 cirrhotics admitted to a single centre with sepsis, noting that NSBB users had significantly reduced in-hospital mortality (18.7% vs 42.3%, P = 0.01), hospital stay duration (15.0 vs 18.5 d, P = 0.03), and white blood cell counts (9.2 vs 12.1, P = 0.004) compared to non-users.
A potential pathophysiological mechanism underlying these observations was provided by Reiberger et al[39] who measured reduced serum levels of interleukin 6 and lipopolysaccharide binding protein in cirrhotic patients receiving beta-blockers, indicative of decreased bacterial translocation. Bacterial translocation secondary to intestinal permeability and hypomotility is augmented in liver cirrhosis and appears to play a pivotal role in causing systemic immune dysfunction in these patients[21]. In animal models with ascites, cirrhotic rats were observed to have significantly reduced intestinal permeability as well as faster intestinal transit following propranolol administration[40]. Recently, Gimenez et al[41] observed that monocytes and granulocytes of cirrhotic patients on long-term NSBB therapy displayed significantly raised phagocytic capacity in the presence of bacterial DNA compared to NSBB-naïve patients.
Other potential effects of beta-blockers upon cirrhotic patients: To further investigate the haemodynamically independent effects of beta-blockade in patients with cirrhosis, Thiele et al[42] performed a meta-analysis of 23 RCTs on 2618 patients finding a reduced incidence of hepatocellular carcinoma (HCC) in patients receiving NSBBs [risk difference 0.026, 95% CI 0.052-0.001, number needed to treat (NNT) 38 patients]. Recently, Herrera et al[43] prospectively evaluated 173 patients included in the early HCC detection program for a median follow-up time of 11 years. The cumulative incidence of HCC was significantly lower in NSBB users compared to non-users (6% vs 19% at 10 years, 16% vs 24% at 15 years), with beta-blockade being the only parameter inversely correlated with HCC development on multivariate analysis. Regarding a pathophysiological mechanism underlying this finding, the authors propose that the reduction in bacterial translocation effected by beta-adrenergic blockade may diminish the portal load of pathogen-associated molecular patterns and thus hepatic inflammation[44]. Secondly, beta-adrenergic blockade may impede angiogenesis through inhibition of vascular endothelial growth factor production. Both hepatic inflammation and neo-angiogenesis are critical drivers in the pathogenesis of HCC[44].
Clinical guidelines and future directions: Presently, the only effective surrogate marker for assessing response to beta-blocker administration is HVPG. HVPG reduction by 20% or to < 12 mmHg was demonstrated to significantly decrease the risk of variceal bleeding and improve survival[9,45], yet the clinical utility of HVPG testing is limited by its invasiveness and availability[32]. Clinical practitioners predominantly titrate beta-blockers to reach a specific target heart rate (50-55 beats per minute) despite insufficient evidence that reductions in heart rate correlate with reductions in HVPG[32]. It is imperative that future research focuses on the identification of novel, non-invasive biomarkers for the assessment of beta-blocker response, since it is critical to identify the change from benefit to detriment of beta-blocker treatment during the natural progression of cirrhosis, so that beta-blocker treatment can be individualised and patient benefit maximised.
While beta-blockers continue to occupy a pivotal role in the treatment of portal hypertension, recent evidence has not only outlined additional, haemodynamically-independent beneficial effects of beta-blockers in cirrhosis, but also described potentially debilitating effects in advanced cirrhotics. Concerning clinical practice, D’Amico et al[46] recommend that beta-blocker therapy should: (1) Not be used in compensated cirrhotics with no evidence of varices; (2) be used in cirrhotic patients with varices at risk of bleeding or re-bleeding independent of the absence/presence of ascites; and (3) be used with caution in cirrhotic patients with refractory ascites and discontinued if haemodynamic or renal compromise arises. Currently, the Braveno VI consensus and 2017 AASLD guidelines recommend temporarily reducing or withholding beta-blockers in patients with refractory ascites and circulatory dysfunction (serum sodium < 130 mEq/L, systolic BP < 90 mmHg)[5,47,48]. Similarly, the 2018 EASL guidelines recommend discontinuation of beta-blockers in patients who develop hypotension (systolic BP < 90 mmHg), sepsis, bleeding, AKI or SBP, followed by an attempt at re-introduction of beta-blocker therapy after recovery. Presently, EASL does not recommend the use of carvedilol[49].
Overall, the evidence-base for the use of NSBBs in cirrhotic patients remains disputed with the requirement for further assessment of safety and efficacy. A large body of evidence regarding the use of beta-blockers in advanced cirrhosis comes from observational studies which are at risk of indication bias as patients receiving beta-blockers are likely to have relatively severe liver disease with clinically significant portal hypertension and large varices[4]. This disparity in liver disease severity between patient cohorts is difficult to account for without randomisation[4].
Lactulose and rifaximin
Lactulose: From the 1980s onwards, non-absorbable disaccharides (lactulose and lactitol) have been the mainstay of treatment for HE and have been recommended as first line therapy ever since lactulose was shown to be equally as effective but safer than neomycin[50-52]. The adverse effect profile of non-absorbable disaccharides is well-characterised, and is predominantly characterised by non-serious gastrointestinal side effects such as bloating, flatulence and diarrhoea[52]. Lactulose exerts its main effect through reducing production of ammonia. Ammonia has been implicated in the pathogenesis of HE by causing direct neurotoxicity and astrocytic swelling. Lactulose is metabolised to lactic acid by colonic bacteria, resulting in intestinal acidification. The acidic environment both promotes the transfer of ammonia from tissues into the intestinal lumen and impedes the growth of ammoniagenic coliforms. Additionally, the cathartic effects of lactulose aid in decreasing intestinal bacterial load[53].
In 2004 a systematic review of 22 RCTs demonstrated that non-absorbable disaccharides had no significant effect on mortality or HE incidence after exclusion of trials with high risk of bias, and concluded that evidence to support the use of non-absorbable disaccharides in the treatment of HE was insufficient[51]. As a consequence, further trials assessing the efficacy of lactulose in the management of HE were performed. A RCT of 140 patients by Sharma et al[54] investigating the efficacy of lactulose for secondary prophylaxis of HE found that, after 14-mo follow-up, a significantly lower proportion of patients receiving lactulose (19.6%) redeveloped HE compared to patients receiving placebo (46.8%, P = 0.001). Similarly, Agrawal et al[55] also investigated the efficacy of lactulose for secondary prevention of HE in a RCT (n = 235), finding that lactulose therapy was associated with significantly reduced HE recurrence compared to no intervention (26.2% vs 56.9%, P = 0.001). Another RCT by Sharma et al[56], this time investigating the efficacy of lactulose for primary prophylaxis of HE, found that, after 12-mo follow-up, a significantly lower proportion of patients receiving lactulose (11%) developed overt HE compared to patients receiving placebo (28%, P = 0.02). Furthermore, lactulose therapy was associated with improvement in both cognitive function and health-related quality of life in patients with minimal HE[57]. Mittal et al[58] then assessed the effectiveness of lactulose in the treatment of minimal HE in the RCT setting (n = 322), finding significant improvements in minimal HE in the lactulose group (47.5%) compared to the no intervention group (10%, P = 0.006) as well as significant reductions in arterial ammonia levels (- 8.57 vs - 0.52, P = 0.0001). Incorporating the new evidence generated after 2004, a recent Cochrane review including 38 RCTs and 1828 patients found moderate quality evidence for beneficial effects of non-absorbable disaccharides on mortality (RR 0.59, 95% CI 0.40-0.87), HE (RR 0.58, 95% CI 0.50-0.69), and other complications of cirrhosis such as liver failure, variceal haemorrhage and HRS (RR 0.47,95% CI 0.36-0.60), when compared to placebo or no intervention[52]. In view of the current evidence-base for non-absorbable disaccharides, EASL and AASLD recommend lactulose as the first-choice agent for the treatment of episodic overt HE and prevention of recurrent HE[59].
Rifaximin: As described above, the cathartic and ammonia-lowering effects of the non-absorbable disaccharide lactulose have been the cornerstone for treating acute, overt HE and maintaining remission in recurrent HE for decades[60,61]. However, its long-term therapeutic value has been limited as a consequence of treatment non-adherence due to its numerous, unpleasant side effects[62]. Lately, the semi-synthetic, non-absorbable antibiotic rifaximin surged in popularity as an alternative or additive to lactulose based on its gut-selective antimicrobial activity favouring non-pathogenic species and its favourable safety and tolerability profile[61]. A randomized, double-blinded trial in 103 patients found the impact of rifaximin on the portosystemic encephalopathy index to be superior to the effect of lactitol[60,63]. A further placebo-controlled RCT by Bass et al[64] established the safety and efficacy of rifaximin therapy in the maintenance of remission in 299 patients with recurrent HE over a follow-up period of 6 mo, finding that a lower proportion of patients receiving rifaximin experienced an overt episode of HE (22.1%) and were hospitalised with HE (13.6%) compared to patients receiving placebo (45.9% and 22.6%, respectively)[60,64]. As a sequel to this trial, Mullen et al[62] performed an open-label, 24-mo maintenance study in 326 patients to investigate the long-term safety profile and treatment effect durability of rifaximin, finding that long-term rifaximin administration achieves a persistent reduction in HE-related and all-cause hospitalisation without a corresponding increase in infection or antibiotic resistance rates. A meta-analysis of 21 RCTs and 2258 patients demonstrated that rifaximin reduced mortality in overt HE, but not minimal HE, when compared to placebo, and had no effect on mortality when compared to non-absorbable disaccharides. In this study, rifaximin also decreased the risk of serious adverse events and had a potential beneficial effect on quality of life[65]. However, the evidence analysed in this meta-analysis was of low-to-moderate quality[65]. Another meta-analysis by Wu et al[66] incorporating 8 RCTs outlined that there was no significant difference in the efficacy of preventing and treating HE between rifaximin and lactulose, but that rifaximin therapy was correlated with less adverse events. A recent retrospective analysis of 1042 patients with HE by Kang et al[67] correlated rifaximin-lactulose combination therapy with significantly improved mortality (HR 0.67, P = 0.024) and reduced risk of recurrent HE (HR 0.45, P < 0.001), variceal haemorrhage (HR 0.43, P = 0.011), and SBP (HR 0.21, P < 0.001), compared to lactulose alone. This was only evident in the non-HCC cohort (n = 421), however. Kabeshova et al[68] evaluated the cost-effectiveness of long-term rifaximin-lactulose combination therapy compared to lactulose monotherapy for recurrent HE in the French healthcare system. This study found that rifaximin-lactulose combination therapy incurs a cost of 18517 EUR (approximately 16000 GBP or 21000 USD) to gain one additional QALY compared to lactulose monotherapy, concluding that rifaximin is a cost-effective treatment in the context of the cost-effectiveness threshold range of 23000-34000 EUR (20000-30000 GBP) adopted by NICE. In view of the current evidence, EASL and AASLD state that rifaximin is effective as an add-on therapy to lactulose for secondary prevention of HE recurrence[59].
The potential uses of rifaximin beyond hepatic encephalopathy: Additionally, recent evidence has indicated that the therapeutic effects of rifaximin may extend beyond its original indication of treating HE. An initial, prospective, observational study conducted by Vlachogiannakos et al[69] observed reduced incidence of variceal haemorrhage, HE, SBP and HRS in 23 patients receiving rifaximin compared to controls. Similarly, Hanouneh et al[70] investigated 404 patients with cirrhotic ascites and demonstrated a 72% reduction in SBP incidence with rifaximin therapy compared to placebo. Norfloxacin is a systemic antibiotic, currently recommended as the first line agent in the prevention of SBP by both AASLD and EASL[71,72]. Recently, Praharaj et al[73] conducted a RCT (n = 117) comparing rifaximin and norfloxacin for both primary and secondary prevention of SBP. Praharaj et al[73] established that patients on norfloxacin had a significantly higher rate of SBP development compared to patients on rifaximin in secondary prophylaxis (39% vs 7%, P = 0.007), while no significant difference (20% vs 14%, P = 0.73) was manifested in primary prophylaxis. A meta-analysis performed by Sidhu et al[74]—incorporating three RCTs and one prospective observational study - compared norfloxacin and rifaximin with the primary outcome of SBP occurrence and secondary outcomes of mortality and adverse events. The included studies, of moderate quality evidence, either demonstrated superior efficacy of rifaximin or no significant difference between rifaximin and norfloxacin in SBP prevention, leading the authors to conclude that rifaximin could be a safe and efficacious alternative to norfloxacin in SBP prophylaxis for patients with hepatitis C virus (HCV) cirrhosis. Furthermore, a small prospective study in 13 patients with advanced cirrhosis suggests improvements in renal function after intestinal decontamination by rifaximin[75]. Baik et al[76] compared propranolol monotherapy to propranolol-rifaximin combination therapy in 65 patients with advanced cirrhosis, finding amplification of mean HVPG reduction with combination therapy. However, a recent RCT investigating the haemodynamic effect of rifaximin in 54 patients with cirrhotic ascites observed no difference in HVPG, cardiac output or glomerular filtration rate compared to placebo[77]. Due to its relative novelty, the evidence-base for the impact of rifaximin on outcomes in cirrhotic patients, particularly regarding its effects outside HE treatment, is lacking in robustness, with the majority of conducted studies featuring very limited sample sizes. A United Kingdom based, multicentre RCT evaluating the role of rifaximin in preventing secondary infections in cirrhotic patients, including both community and hospital acquired infections, is currently being undertaken and its results are awaited[78].
L-ornithine L-aspartate and acarbose: As outlined, ammonia has been identified as the pivotal neurotoxin implicated in the pathogenesis of HE and its reduction is a central objective in the therapeutic approach to HE management. LOLA has demonstrated ammonia-lowering properties by enhancing residual hepatic urea cycle activity and skeletal muscle glutamine synthesis[79,80]. Goh et al[79] performed a recent Cochrane systematic review of 36 RCTs and 2377 patients summarising the evidence of LOLA in the prevention and treatment of HE. The authors found very low quality evidence that LOLA had beneficial effects on mortality, HE and serious adverse events compared to placebo. However, these findings were not upheld when only trials with low risk of bias were considered. On subgroup analysis, there was no difference between intravenous and oral LOLA administration or between minimal and overt HE. In comparison to lactulose and rifaximin, LOLA demonstrated no effect on mortality, HE and serious adverse events. The uncertainty stemming from data quality concerns led the authors to conclude that new, high-quality RCTs are required for the definitive evaluation of evidence[79]. A randomised, placebo-controlled, quadruple blinded, phase IV trial investigating the efficacy of LOLA in treating overt HE is currently in progress and its results are awaited[81].
One randomised, double-blinded, placebo-controlled trial in 107 cirrhotic patients with HE and type 2 diabetes mellitus provided encouraging data for the safety and efficacy of acarbose in treating HE with the intervention group demonstrating lower blood ammonia levels, improved encephalopathy global score and reduced Child-Pugh score[82]. However, the generalisability of these findings is diminished by the highly selective study population of compensated Child-Pugh A cirrhotics with predominantly Grade 2 encephalopathy, as well as the scarcity of further studies investigating the efficacy of acarbose in treating HE[83]. Acarbose is not mentioned in current EASL and AASLD guidelines for HE management.
Diuretics
The most common complication of cirrhosis is ascites[84]. Diuretics are the mainstay of treatment for moderate ascites (Grade 2)[72,85]. Excessive aldosterone generation secondary to splanchnic vasodilatation and systemic hypotension is considered the primary causative factor for increased sodium reabsorption in the kidneys of patients with cirrhotic ascites[86]. Consequently, spironolactone monotherapy was shown superior to loop diuretics for initial ascites management, with furosemide frequently used as an addition to potentiate diuresis in recurrent ascites[72]. Side-effects may result from excessive diuresis causing hypovolaemia and renal dysfunction along with electrolyte disturbances such as hyponatraemia, hypokalaemia and hyperkalaemia[72,87,88]. Furthermore, diuretic therapy may precipitate HE; although the underlying mechanism remains unknown[72,89,90], this is likely related to a combination of electrolyte disturbance and hypovolaemia. To forestall these adverse effects, it is recommended to adjust diuretic dosage so that daily weight loss does not exceed 800 g[91]. In cirrhotic patients who develop ascites, one-year survival ranges from 60 to 85%[92]. Sodium restriction and diuretic therapy enable mobilisation of ascites in approximately 90% of those patients.
α-1 and α-2 adrenergic agonists: One-year survival substantially declines to 25% with the development of refractory ascites (diuretic-resistant or diuretic-intractable), when ascites mobilisation fails due to a lack of effect or advent of complications such as SBP, hyponatraemia and renal impairment[84,93]. First-line management for refractory ascites is serial large-volume paracentesis[49]. However, recent evidence has proposed a role for α1 and α2-adrenergic agonists in managing refractory ascites. A group of heterogenous studies with differing dosing and follow-up regimes demonstrated that midodrine, a peripheral α1-adrenergic agonist acting as a splanchnic vasoconstrictor, increases mean arterial pressure, urine output as well as urine sodium output, and decreases plasma renin and aldosterone activity in patients with refractory ascites[84]. Two small randomised pilot studies found that midodrine plus standard medical therapy transiently (3 mo) improves the mobilisation of refractory ascites compared to diuretic therapy alone[94,95]. Guo et al[96] performed a meta-analysis of 10 RCTs (n = 462) finding that midodrine enhances response rates to diuretics, but does not increase survival in patients with refractory ascites. A recent small-scale prospective observational study also suggests that oral midodrine and subcutaneous octreotide combination therapy could ameliorate cirrhosis-induced hyponatraemia (pre-treatment serum Na: 124 mmol/L vs post-treatment serum Na: 130 mmol/L, P = 0.00001)[97]. AASLD advise to consider the administration of midodrine in patients with refractory ascites, while EASL have not recommended its use in view of limited sample size of existing trials[49,71]. Clonidine, a central α2-adrenergic agonist with sympatholytic activity, is thought to ameliorate sympathetic nervous system overstimulation which leads to renal hypoperfusion and excessive renin-angiotensin system activation in refractory ascites[84,98]. Similar to midodrine, clonidine has been shown to improve urine output and urine sodium output, while lowering plasma aldosterone and renin activity in patients with refractory ascites. Multiple small-scale RCTs with limited follow-up investigated the clinical efficacy of clonidine in such patients, demonstrating that clonidine reduced diuretic requirements and induced earlier diuretic response[94,98]. In animal models of cirrhosis, clonidine improved renal function at low doses, but negatively influenced mean arterial pressure and urine sodium output at high doses[99]. Clonidine is currently not recommended by AASLD or EASL as an adjunct to diuretics in refractory ascites management due to the non-existence of sufficiently powered, long-term studies[49,71].
Vaptans: To avoid the increased incidence of adverse effects with high-dose conventional diuretic therapy, the so-called vaptans, selective antagonists to the vasopressin V2 receptors in principal cells of collecting ducts, have gained interest in recent decades as they are able to achieve a highly hypotonic diuresis without impacting on the electrolyte balance[100]. Thus vaptans might be valuable in the correction of hyponatraemia, an important predictor of mortality in cirrhotic patients with ascites. However, controversies exist with regards to their expense and their effect on clinically relevant outcomes and prognosis in patients with cirrhosis[100]. In their meta-analysis (16 RCTs, n = 2620) Yan et al[100] found that vaptans showed a significant aquaretic effect and increased serum sodium significantly both when used alone and when used in combination with traditional diuretics, and concluded that vaptans could play a major role in the pharmacological treatment of refractory ascites with insufficient response to traditional diuretics in order to alleviate ascites volume and the need for paracentesis without incurring a higher rate of adverse events. Yet, despite the substantial beneficial impact of vaptans on diuresis and hyponatraemia, vaptan therapy made no significant difference to short-term or long-term survival in these cirrhotic patients. An adverse effect of vaptan treatment observed in the included trials was excessive correction of serum sodium, which may lead to osmotic demyelination, thus necessitating monitoring when administering vaptans[100]. Furthermore, the Food and Drug Administration has recommended to avoid the use of vaptans in patients with chronic liver disease, as tolvaptan has the potential to induce serious liver injury[101]. In a phase 3, placebo-controlled, randomized trial (n = 1200), satavaptan therapy showed no difference to placebo regarding ascites control, but was superior to placebo in improving serum sodium of hyponatraemic patients. However, in one of the trial groups, satavaptan therapy was associated with increased mortality (HR 1.47; 95% CI 1.01-2.15). No specific causes for this mortality increase were identified, but the majority of deaths were attributed to known complications of cirrhosis. The authors concluded that vaptan therapy is ineffective in controlling ascites, but may have a role in managing hypervolaemic hyponatraemia in cirrhotic patients[102]. Current EASL and AASLD guidelines do not endorse the use of vaptans in cirrhotic patients in light of their costs, risks and lacking efficacy in clinical settings[49,71].
Statins
Although statins are the mainstay for preventing atherosclerosis and have well-defined beneficial effects on cardiovascular health, they are under-prescribed in cirrhotic patients due to concerns over hepatotoxicity[103]. However, recent evidence points towards an unexpected, potentially beneficial impact of statins on cirrhosis resulting from their pleiotropic properties which comprise antioxidant, anti-fibrotic, anti-infective and anti-inflammatory effects[103,104]. Endothelial dysfunction and diminished nitric oxide generation play essential roles in the establishment of the pro-inflammatory and pro-fibrotic microenvironment of cirrhosis[104]. Numerous pre-clinical studies in vitro and in vivo have demonstrated that statins upregulate Kruppel-like factor 2 and nitric oxide (NO) availability as well as down-regulate hepatic stellate cell activation, thus exerting favourable effects on fibrogenesis, endothelial function and portal hypertension[103]. In addition to findings from pre-clinical studies, simvastatin enhanced hepatosplanchnic NO output and reduced intrahepatic vascular resistance in a study involving 30 cirrhotic patients[105]. A multicentre RCT conducted by Abraldes et al[106] in 59 cirrhotic patients with portal hypertension described that simvastatin significantly decreased HVPG by 8.3% and improved hepatocyte perfusion without discernible harmful effects on the systemic circulation[103,104,106].
In a retrospective study of patients with predominantly Child-Pugh A cirrhosis (81 statin users and 162 controls), statin use was associated with lower mortality (HR 0.56) and decompensation (HR 0.55) risk on multivariate analysis after 36-mo follow-up[107]. Similarly, another recent retrospective analysis of patients with compensated HCV cirrhosis (685 statin users and 2062 controls) also described statin use to be associated with lower mortality (HR 0.56) and decompensation rates (HR 0.55) at 10-year follow-up after adjusting for age, serum albumin, model for end stage liver disease (MELD) and Child-Pugh scores[108]. Using the Taiwanese National Health Insurance database, Chang et al[109] conducted a nested case-control study with 13-year follow-up, matching 675 statin users with 675 statin non-users. Following a dose-response relationship, statin use correlated with a significantly decreased risk of decompensation (HR 0.39), HCC development (HR 0.52), and mortality (HR 0.46). The most robust evidence regarding the therapeutic benefit of statins in cirrhosis comes from a multicentre RCT of 158 cirrhotic patients who received either standard therapy (beta-blocker and band ligation) after variceal haemorrhage, or standard therapy plus simvastatin. After 24-mo follow-up, addition of simvastatin had no effect on the rate of re-bleeding but significantly reduced all-cause mortality compared to the control group (9% vs 22%, P = 0.03). However, this survival benefit was only observed in the Child-Pugh A/B cohort, not in Child-Pugh C patients, and no difference was found in the rate of cirrhosis-related complications between the intervention and control groups. Notably, two patients with decompensated cirrhosis developed rhabdomyolysis, thus again raising questions about the safety of statin use in advanced cirrhosis[103,104,110].
Recently, the evidence regarding the impact of statin use on cirrhosis and its complications was summarized by three meta-analyses. Kamal et al[111] performed one meta-analysis of 9 studies (2 RCTs, 7 observational studies) and 166000 patients (40950 statin users) finding moderate-quality evidence that statin use is associated with reduced fibrosis progression (HR 0.55, 95% CI 0.49-0.61) and rate of decompensation (HR 0.54, 95% CI 0.46-0.65) as well as low-quality evidence that statin use is associated with reduced mortality (0.62, 95% CI 0.43-0.91). The second meta-analysis by Kim et al[112] included 121000 patients (46% statin users) and found moderate-quality evidence of an association between statin use and decreased risk of decompensation (RR 0.54, 95% CI 0.47-0.61), mortality (RR 0.54, 95% CI 0.47-0.61) and variceal bleeding (RR 0.73, 95% CI 0.59-0.91). Thirdly, Singh et al[113] performed a large meta-analysis including 1.4 million patients with 4298 cases of HCC, associating statin use with a 37% risk reduction [odds ratio (OR) 0.63, 95% CI 0.52-0.76] of HCC development. This risk reduction was significantly more pronounced in the Asian population, in which chronic hepatitis B virus infection is the predominant risk factor for HCC development. In this population, the NNT to prevent one case of HCC per year was estimated at 5209. As a consequence of this high NNT in combination with the significant heterogeneity between included studies, the authors conclude that they cannot recommend statins for chemoprevention of HCC[103,113]. In the context of increasingly stretched healthcare budgets, the inexpensiveness of statins and the encouraging evidence presented above calls for the implementation of a phase 3 RCT in patients with Child-Pugh A/B cirrhosis with the primary endpoint of decompensation or death[114].
While the use of statins in cirrhotic patients remains disputed due to safety concerns, multiple studies have demonstrated that statins are safe to use in patients with compensated non-alcoholic fatty liver disease (NAFLD) irrespective of liver enzyme elevations[115,116]. Due to the strong association between NAFLD and cardiovascular morbidity and mortality, both EASL and AASLD guidelines recommend the initiation of cholesterol-lowering therapy with statins in patients with compensated NAFLD[117,118]. However, current AASLD guidelines advise against the use of statins in patients with decompensated cirrhosis in view of the potentially deleterious complications in this population of patients[117]. Since the majority of evidence regarding the pleiotropic effects of statins in chronic liver disease is derived from observational studies and small-scale trials, adequately powered RCTs are required to assess whether the pleiotropic properties of statins can modulate clinical endpoints in cirrhotic patients[119].
Proton pump inhibitors
Despite suboptimal evidence for their efficacy in cirrhosis and growing concerns over their promotion of bacterial overgrowth and translocation, proton pump inhibitors (PPI) are frequently prescribed to patients with cirrhosis receiving multidrug treatment for variceal haemorrhage or portal hypertensive gastropathy in order to forestall peptic complications[120]. In fact, 46%-78% of cirrhotic patients are reported to use PPIs[121]. Initially, concerns about the safety of PPI use in cirrhosis were raised by a variety of low-powered observational studies. A prospective study of 272 patients with overall survival as the primary endpoint demonstrated that PPI treatment was associated with higher MELD scores, ascites and mortality[122]. In addition, a meta-analysis of four studies and 772 patients identified a significant correlation between PPI use and SBP development (OR 2.77)[123]. A prospective cohort study of 400 patients hospitalized with cirrhosis established that PPI use was an independent risk factor for the development of infection (OR 2.0), while beta-blocker use was a protective factor (OR 0.46)[36].
Recently, two large, retrospective analyses fuelled the established fear over the impact of PPI use on SBP and HE development. Dam et al[121] retrospectively analysed data from three large, multicentre RCTs (n = 865) for the use of satavaptan in cirrhotic ascites, describing that current PPI use was associated with an increased risk of HE (HR 1.36, 95% CI 1.01-1.84) and SBP development (HR 1.72, 95% CI 1.10-2.69) compared to non-users. Additionally, the authors described that only 56% of PPI users had a valid indication for PPI prescription[121,124]. Using data from the Taiwan National Health Insurance database, Tsai et al[125] conducted a nested case-control study of 1166 cirrhotic patients with HE matched to 1166 cirrhotic patients without HE, observing a dose-response relationship between PPI use and risk of HE development. The pathophysiological mechanism hypothesized to underlie this observed association between PPI use and SBP/HE development is based on PPIs weakening the stomach acid barrier by increasing gastric pH, thus promoting bacterial dysbiosis, translocation and small intestinal bacterial overgrowth (SIBO)[121,124]. Since the majority of hepatic blood supply is derived from the gut, there is an intimate relationship between the liver and the intestinal microbiome. Gut dysbiosis with consequent translocation of pathogenic bacterial taxa and endotoxins into the portal and systemic circulation promotes immune system dysfunction as well as hepatic inflammation and injury[126]. In line with this hypothesis, Bajaj et al[127] recently conducted a cohort study, observing the readmission rates in 343 cirrhotic inpatients and conducting stool microbiota analyses in 137 cirrhotic outpatients. In the inpatient cohort, PPI use was independently associated with higher readmission rates, while in the outpatient cohort, the microbiota composition of PPI users showed increased oral-origin taxa and decreased beneficial autochthonous taxa. In contrast, a large (n = 1191), retrospective analysis by Ratuapli et al[128] described no significant difference in the frequency of SIBO diagnosis, as established by glucose hydrogen breath testing, between PPI users and non-users. Alternatively, Assaraf et al[129] hypothesise that PPI use and HE development may be linked to a cerebral build-up of toxic metabolites as PPIs act as agonists to ATP-binding cassette efflux transporters found on the blood-brain barrier.
However, this negative trend for PPI use in cirrhosis has recently come under question. Khan and colleagues published two articles highlighting the limitations of the retrospective analyses by Dam et al[121] and Tsai et al[125]. In both studies, the ORs achieved were below 3, an indication that the observed associations may be due to residual confounding rather than causality[124,130]. In the Tsai study, patients receiving PPIs had higher rates of comorbidities, thus representing a potential source of channelling bias[130]. The Dam study analysed data not collected specifically for the purpose of assessing the impact of PPIs on SBP and HE incidence and could thus not account for all potential confounders[121]. Therefore, Khan et al[124,130] concluded that the current evidence-base against PPIs is not of sufficient quality to withhold PPIs from cirrhotic patients with a reasonable indication for PPI treatment. A large (n = 519), prospective, multicentre study by Terg et al[131] observed no significant difference in PPI use between infected and non-infected patients (44.3% vs 42.8%) or between patients with SBP and patients with ascites and no SBP (46% vs 42%). Furthermore, no difference was observed in the causative species or infection origin between PPI users and non-users who developed SBP[131]. The largest meta-analysis (16 studies, 10 case-control/6 cohort, n = 8145) on this issue by Yu et al[132] found that the harmful association between PPI use and SBP incidence was present in case-control studies, but not cohort studies, and that there was no association between PPI use and mortality during hospitalisation in both case-control and cohort studies. The authors therefore concluded that causality between PPI administration and increased SBP incidence and mortality could not be established[132]. In view of the conflicting evidence and substantial limitations of the conducted studies outlined above, there is the need for a randomized, placebo-controlled, adequately powered trial investigating the effects of long-term PPI administration in cirrhotic patients with the primary outcomes of HE and SBP occurrence.
Anticoagulation
Historically, cirrhotic patients were viewed as ‘auto-anticoagulated’, since cirrhosis is associated with a global impairment in clotting factor synthesis excluding factor VII and von Willebrand factor. More recently, it has been recognized that the relationship between cirrhosis and the coagulation cascade is more complex and that routine haemostatic tests, such as the international normalized ratio or activated partial thromboplastin time, lack accuracy in predicting coagulation status in these patients. In light of a coexisting reduction in anticoagulant factors (namely antithrombin III, protein S and C), recent studies have highlighted the importance of procoagulant complications in cirrhotic patients, with higher rates of venous thromboembolism (VTE) and splanchnic vein thrombosis being observed. These findings have suggested the potential role for anticoagulation in cirrhosis. Furthermore, there is emerging evidence that coagulation proteins may activate hepatic myofibroblasts to accelerate fibrogenesis. As such, there has been the suggestion that anticoagulant medication may thus have a further role in impeding fibrosis progression[133-135].
Retrospective studies investigating the role of prophylactic anticoagulation in cirrhotic patients at risk of VTE have provided heterogenous results with some studies failing to demonstrate a reduction in VTE incidence with anticoagulative treatment[133,136]. In a single-centre, non-blinded RCT of 70 cirrhotic outpatients, Villa et al[137] investigated the safety and efficacy of low-molecular-weight heparin in preventing portal vein thrombosis (PVT). The major findings were that subcutaneous enoxaparin not only reduced PVT incidence (0% vs 27.7%, P = 0.001) but also protected against hepatic decompensation (11.7% vs 59.4%, P < 0.001) with no apparent increase in haemorrhagic complications. The authors attribute the observed reduction in decompensating events to improvements in hepatic microcirculation with consequent diminution of bacterial translocation and immune dysfunction[134,137]. Further prospective and randomised studies are required to delineate the role of prophylactic anticoagulation in cirrhosis.
Regarding patients with established non-malignant PVT, numerous studies have demonstrated a favourable effect of low-molecular weight heparin therapy on portal vein recanalization rates, achieving re-permeation in 40%-90% of treated patients compared to 0% of untreated controls. Early initiation of anticoagulative therapy was the most important factor associated with successful re-permeation. Furthermore, these studies indicate that anticoagulation therapy has an acceptable safety profile in cirrhotic patients with no significant increase in bleeding rates provided that oesophageal varices are adequately screened for and managed[134,138-141]. At present, however, there is a need for detailed guidelines regarding the use of anticoagulation therapy in patients with cirrhosis.
Further treatments
A number of different agents with potentially therapeutic effects upon patients with cirrhosis are currently at different stages of pre-clinical and clinical assessment. These are summarised in Table 2.
Table 2.
Summary of further pharmacological agents with potentially therapeutic effects
| Treatment | Potential mechanism of action | Indications and Evidence |
| Human Serum Albumin (HAS) | Oncotic properties: Acts as a plasma expander to counteract splanchnic arterial vasodilatation in cirrhosis; Non-oncotic properties: Modulation of the inflammatory response through binding of reactive oxygen species and NO. Also affects capillary integrity. Furthermore, cirrhosis affects the capacity of albumin to bind endogenous and exogenous substances, which may be compensated for by albumin infusion. | A recent meta-analysis found that HAS infusion in combination with antibiotics decreases the incidence of renal failure and mortality in patients with SBP; Several studies demonstrate that HAS infusion together with vasoconstrictors reduces mortality in patients with type 1 HRS; A meta-analysis by Bernardi et al showed that HAS infusion was effective in preventing paracentesis-induced circulatory dysfunction; A multicentre RCT by Caraceni et al[148] (n = 440) in cirrhotic patients with uncomplicated ascites showed that long-term HAS plus diuretics prolonged survival compared to diuretics alone[142-148]. |
| Faecal microbiota transplant (FMT) | As described previously, cirrhosis and its progression have been closely linked to gut dysbiosis with a predominance of pathogenic bacterial taxa and SIBO. Amelioration or even reversion of dysbiosis may be achieved through direct manipulation of the intestinal microbiome using FMT. | Although FMT has been extremely successful in repopulating the healthy intestinal microbiome of patients with C. difficile diarrhoea and has even been endorsed in guidelines for the treatment of recurrent C. difficile diarrhoea, studies of FMT in patients with liver disease are smaller and more limited. A pilot study suggested a reduced burden of HE in patients given a single FMT enema compared to standard-of-care therapy. Currently, Woodhouse et al[126] are in the process of conducting a randomised, single-blinded, placebo-controlled trial in 32 cirrhotic patients in order to assess the safety and tolerability of FMT delivered by upper GI endoscopy[126,149-151]. |
| Caffeine/ Coffee | Caffeine antagonizes the A2a adenosine receptor on hepatic stellate cells, the effector cells of fibrogenesis. Activation of the A2a adenosine receptor has been directly associated with matrix production in rodent models. However, decaffeinated coffee has also been shown to lower transaminases. Hence there may be additional anti-fibrotic constituents of coffee such as polyphenols which are potent anti-inflammatories and anti-oxidants. | Both retrospective and prospective observational studies have indicated that an inverse dose-response relationship exists between coffee consumption and cirrhosis risk as well as cirrhosis-related complications. Corrao et al[155] retrospectively analysed 274 decompensated cirrhotic patients and 458 matched controls with chronic liver disease, finding that daily consumption of one cup of coffee conferred an odds ratio of 0.47, while daily consumption of four cups of coffee even conferred an odds ratio of 0.16 for cirrhosis risk. Similarly, a prospective cohort study of patients with advanced hepatitis C induced liver disease found that liver-related mortality and complication rates declined with increasing coffee consumption (12.1/100 person years for > 1 cup/d; 8.2/100 for 1-3 cups/d; 6.3/100 for > 3 cups/d; p-trend = 0.001). A recent prospective cohort study analysed non-invasive liver stiffness measurements in the general Dutch population (n = 2424), observing that coffee consumption was inversely correlated with liver stiffness. Similarly, recent data from a meta-analysis showed a favourable effect of coffee consumption on risk of HCC development (RR 0.55, 95% CI 0.44-0.67). At present, no guidelines exist regarding the prescription of coffee to patients with cirrhosis or chronic liver disease[152-158]. |
CI: Confidence interval; FMT: Faecal microbiota transplant; GI: Gastrointestinal; HAS: Human Serum Albumin; HCC: Hepatocellular carcinoma; HE: Hepatic encephalopathy; HRS: Hepatorenal syndrome; NO: Nitric oxide; RCT: Randomised controlled trial; RR: Relative risk; SBP: Spontaneous bacterial peritonitis; SIBO: Small intestinal bacterial overgrowth.
CONCLUSION
The evidence presented above clearly demonstrates that, while pharmacotherapy plays an important role for the long-term supportive management of the cirrhotic outpatient, its application is also highly complex and controversial. Many questions regarding the effect of the individual agents in the different stages of cirrhosis remain unanswered and require further research, particularly in a randomized, controlled setting with well-defined cohorts of cirrhotic patients. In this article we have provided an update with regards to the latest studies and international consensus on specific treatments. To maximize benefits and minimize drawbacks of chronic pharmacotherapy in cirrhosis, it is essential that these drugs are prescribed and administered only in close accordance with up-to-date guidelines and that patients are reviewed frequently, so that adverse effects can be recognized early. To heighten the effectiveness of pharmacotherapy in cirrhosis, drug administration needs to be individualized with respect to the following criteria: current complications of cirrhosis, stage of cirrhosis, type of drug used (especially for beta-blockers) and dose of drug used. Pharmacotherapy is only one part of the holistic management of the cirrhotic outpatient and will only achieve its full effectiveness if used in conjunction with treatment of the underlying aetiology of liver disease, nutritional management and patient education in a specialist clinic setting.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None declared for all authors.
Peer-review started: December 9, 2018
First decision: January 11, 2019
Article in press: January 26, 2019
P- Reviewer: Ikura Y, Marcos M, Ozenirler S S- Editor: Yan JP L- Editor: A E- Editor: Yin SY
Contributor Information
David Kockerling, Liver Unit/Division of Integrative Systems Medicine and Digestive Disease, Imperial College London, London W2 1NY, United Kingdom.
Rooshi Nathwani, Liver Unit/Division of Integrative Systems Medicine and Digestive Disease, Imperial College London, London W2 1NY, United Kingdom.
Roberta Forlano, Liver Unit/Division of Integrative Systems Medicine and Digestive Disease, Imperial College London, London W2 1NY, United Kingdom.
Pinelopi Manousou, Liver Unit/Division of Integrative Systems Medicine and Digestive Disease, Imperial College London, London W2 1NY, United Kingdom.
Benjamin H Mullish, Liver Unit/Division of Integrative Systems Medicine and Digestive Disease, Imperial College London, London W2 1NY, United Kingdom.
Ameet Dhar, Liver Unit/Division of Integrative Systems Medicine and Digestive Disease, Imperial College London, London W2 1NY, United Kingdom. a.dhar@imperial.ac.uk.
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 2.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: A review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, Ferguson J, Forton D, Foster G, Gilmore I, Hickman M, Hudson M, Kelly D, Langford A, Lombard M, Longworth L, Martin N, Moriarty K, Newsome P, O'Grady J, Pryke R, Rutter H, Ryder S, Sheron N, Smith T. Addressing liver disease in the UK: A blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 4.Moctezuma-Velazquez C, Kalainy S, Abraldes JG. Beta-blockers in patients with advanced liver disease: Has the dust settled? Liver Transpl. 2017;23:1058–1069. doi: 10.1002/lt.24794. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 7.Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66:849–859. doi: 10.1016/j.jhep.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Gluud LL, Morgan MY. Endoscopic therapy and beta-blockers for secondary prevention in adults with cirrhosis and oesophageal varices Cochrane Database of systematic Reviews 2017; (6): Art. No.: CD012694 [Google Scholar]
- 9.Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401–1407. doi: 10.1016/0016-5085(90)91168-6. [DOI] [PubMed] [Google Scholar]
- 10.Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, Lebrec D. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532–1538. doi: 10.1056/NEJM199105303242202. [DOI] [PubMed] [Google Scholar]
- 11.Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: A controlled study. N Engl J Med. 1981;305:1371–1374. doi: 10.1056/NEJM198112033052302. [DOI] [PubMed] [Google Scholar]
- 12.Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 13.Sersté T, Njimi H, Degré D, Deltenre P, Schreiber J, Lepida A, Trépo E, Gustot T, Moreno C. The use of beta-blockers is associated with the occurrence of acute kidney injury in severe alcoholic hepatitis. Liver Int. 2015;35:1974–1982. doi: 10.1111/liv.12786. [DOI] [PubMed] [Google Scholar]
- 14.Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680–90.e1. doi: 10.1053/j.gastro.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Bang UC, Benfield T, Hyldstrup L, Jensen JE, Bendtsen F. Effect of propranolol on survival in patients with decompensated cirrhosis: A nationwide study based Danish patient registers. Liver Int. 2016;36:1304–1312. doi: 10.1111/liv.13119. [DOI] [PubMed] [Google Scholar]
- 16.Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of β-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967–969. doi: 10.1136/gutjnl-2011-301348. [DOI] [PubMed] [Google Scholar]
- 17.Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva C, Aracil C, Colomo A, Lopez-Balaguer JM, Piqueras M, Gonzalez B, Torras X, Guarner C, Balanzo J. Clinical trial: A randomized controlled study on prevention of variceal rebleeding comparing nadolol + ligation vs. hepatic venous pressure gradient-guided pharmacological therapy. Aliment Pharmacol Ther. 2009;29:397–408. doi: 10.1111/j.1365-2036.2008.03880.x. [DOI] [PubMed] [Google Scholar]
- 19.Mandorfer M, Reiberger T. Beta blockers and cirrhosis, 2016. Dig Liver Dis. 2017;49:3–10. doi: 10.1016/j.dld.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Sharma P, Anikhindi SA, Prajapati R, Agarwal R, Sharma B, Bansal N, Singla V, Arora A. Can Non-Selective Beta-Blockers (NSBBs) Prevent Enlargement of Small Esophageal Varices in Patients with Cirrhosis? A Meta-analysis. J Clin Exp Hepatol. 2017;7:275–283. doi: 10.1016/j.jceh.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelmann C, Jalan R. Non selective beta-blocker in cirrhosis: Not ‘whether’but ‘who and how’. AME Med J. 2017;2:90. [Google Scholar]
- 22.Kim SG, Larson JJ, Lee JS, Therneau TM, Kim WR. Beneficial and harmful effects of nonselective beta blockade on acute kidney injury in liver transplant candidates. Liver Transpl. 2017;23:733–740. doi: 10.1002/lt.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016;63:1968–1976. doi: 10.1002/hep.28352. [DOI] [PubMed] [Google Scholar]
- 24.Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, Ferguson JW. Non-selective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015;64:1111–1119. doi: 10.1136/gutjnl-2013-306502. [DOI] [PubMed] [Google Scholar]
- 25.Onali S, Kalafateli M, Majumdar A, Westbrook R, O'Beirne J, Leandro G, Patch D, Tsochatzis EA. Non-selective beta-blockers are not associated with increased mortality in cirrhotic patients with ascites. Liver Int. 2017;37:1334–1344. doi: 10.1111/liv.13409. [DOI] [PubMed] [Google Scholar]
- 26.Facciorusso A, Roy S, Livadas S, Fevrier-Paul A, Wekesa C, Kilic ID, Chaurasia AK, Sadeq M, Muscatiello N. Nonselective Beta-Blockers Do Not Affect Survival in Cirrhotic Patients with Ascites. Dig Dis Sci. 2018;63:1737–1746. doi: 10.1007/s10620-018-5092-6. [DOI] [PubMed] [Google Scholar]
- 27.Njei B, McCarty TR, Garcia-Tsao G. Beta-blockers in patients with cirrhosis and ascites: type of beta-blocker matters. Gut. 2016;65:1393–1394. doi: 10.1136/gutjnl-2016-312129. [DOI] [PubMed] [Google Scholar]
- 28.Sinagra E, Perricone G, D'Amico M, Tinè F, D'Amico G. Systematic review with meta-analysis: The haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557–568. doi: 10.1111/apt.12634. [DOI] [PubMed] [Google Scholar]
- 29.Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, Heinisch BB, Trauner M, Kramer L, Peck-Radosavljevic M Vienna Hepatic Hemodynamic Lab. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. 2013;62:1634–1641. doi: 10.1136/gutjnl-2012-304038. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R, Lockman KA, Mallawaarachchi N, Robertson M, Plevris JN, Hayes PC. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol. 2017;67:40–46. doi: 10.1016/j.jhep.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Araújo Júnior RF, Garcia VB, Leitão RF, Brito GA, Miguel Ede C, Guedes PM, de Araújo AA. Carvedilol Improves Inflammatory Response, Oxidative Stress and Fibrosis in the Alcohol-Induced Liver Injury in Rats by Regulating Kuppfer Cells and Hepatic Stellate Cells. PLoS One. 2016;11:e0148868. doi: 10.1371/journal.pone.0148868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandorfer M, Peck-Radosavljevic M, Reiberger T. Prevention of progression from small to large varices: are we there yet? An updated meta-analysis. Gut. 2017;66:1347–1349. doi: 10.1136/gutjnl-2016-312814. [DOI] [PubMed] [Google Scholar]
- 33.Bhardwaj A, Kedarisetty CK, Vashishtha C, Bhadoria AS, Jindal A, Kumar G, Choudhary A, Shasthry SM, Maiwall R, Kumar M, Bhatia V, Sarin SK. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: A randomised placebo-controlled trial. Gut. 2017;66:1838–1843. doi: 10.1136/gutjnl-2016-311735. [DOI] [PubMed] [Google Scholar]
- 34.Zacharias AP, Jeyaraj R, Hobolth L, Bendtsen F, Gluud LL, Morgan MY. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochrane Database Syst Rev. 2018;10:CD011510. doi: 10.1002/14651858.CD011510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, Burroughs AK. beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: A meta-analysis. Liver Int. 2009;29:1189–1193. doi: 10.1111/j.1478-3231.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 36.Merli M, Lucidi C, Di Gregorio V, Giannelli V, Giusto M, Ceccarelli G, Riggio O, Venditti M. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int. 2015;35:362–369. doi: 10.1111/liv.12593. [DOI] [PubMed] [Google Scholar]
- 37.Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, Macnaughtan J, Bendtsen F, Coenraad M, Sperl J, Gines P, Moreau R, Arroyo V, Jalan R CANONIC Study Investigators of the EASL-CLIF Consortium. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64:574–582. doi: 10.1016/j.jhep.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Facciorusso A, Villani R, Bellanti F, Bruno R, Fioravanti G, Vendemiale G, Serviddio G. In-hospital mortality and length of stay in cirrhotic patients with sepsis treated with non-selective beta-blockers. Digest Liver Dis. 2017;49:e15. [Google Scholar]
- 39.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H Vienna Hepatic Hemodynamic Lab. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43–48. doi: 10.1002/hep.510310109. [DOI] [PubMed] [Google Scholar]
- 41.Gimenez P, Garcia-Martinez I, Francés R, Gonzalez-Navajas JM, Mauri M, Alfayate R, Almenara S, Miralles C, Palazon JM, Carnicer F, Pascual S, Such J, Horga JF, Zapater P. Treatment with non-selective beta-blockers affects the systemic inflammatory response to bacterial DNA in patients with cirrhosis. Liver Int. 2018;38:2219–2227. doi: 10.1111/liv.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: A meta-analysis of randomized trials. Liver Int. 2015;35:2009–2016. doi: 10.1111/liv.12782. [DOI] [PubMed] [Google Scholar]
- 43.Herrera I, Pascual S, Zapater P, Carnicer F, Bellot P, María Palazón J. The use of β-blockers is associated with a lower risk of developing hepatocellular carcinoma in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:1194–1197. doi: 10.1097/MEG.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 44.Thiele M, Wiest R, Gluud LL, Albillos A, Krag A. Can non-selective beta-blockers prevent hepatocellular carcinoma in patients with cirrhosis? Med Hypotheses. 2013;81:871–874. doi: 10.1016/j.mehy.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 45.Feu F, García-Pagán JC, Bosch J, Luca A, Terés J, Escorsell A, Rodés J. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346:1056–1059. doi: 10.1016/s0140-6736(95)91740-3. [DOI] [PubMed] [Google Scholar]
- 46.D’Amico G, Malizia G, Bosch J. Beta-blockers in 2016: Still the safest and most useful drugs for portal hypertension? Hepatology. 2016;63:1771–1773. doi: 10.1002/hep.28502. [DOI] [PubMed] [Google Scholar]
- 47.Peeraphatdit TB, Kamath PS, Shah VH. Beta-blockers in patients with advanced cirrhosis: Red light, green light, yellow light…. Liver Transpl. 2017;23:725–726. doi: 10.1002/lt.24780. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 49.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, Levy LL. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573–583. [PubMed] [Google Scholar]
- 51.Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: Systematic review of randomised trials. BMJ. 2004;328:1046. doi: 10.1136/bmj.38048.506134.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;4:CD003044. doi: 10.1002/14651858.CD003044.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Elkington SG. Lactulose. Gut. 1970;11:1043–1048. doi: 10.1136/gut.11.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: An open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885–891, 891.e1. doi: 10.1053/j.gastro.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 55.Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: An open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107:1043–1050. doi: 10.1038/ajg.2012.113. [DOI] [PubMed] [Google Scholar]
- 56.Sharma P, Sharma BC, Agrawal A, Sarin SK. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: An open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol. 2012;27:1329–1335. doi: 10.1111/j.1440-1746.2012.07186.x. [DOI] [PubMed] [Google Scholar]
- 57.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 58.Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2011;23:725–732. doi: 10.1097/MEG.0b013e32834696f5. [DOI] [PubMed] [Google Scholar]
- 59.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 60.Iadevaia MD, Prete AD, Cesaro C, Gaeta L, Zulli C, Loguercio C. Rifaximin in the treatment of hepatic encephalopathy. Hepat Med. 2011;3:109–117. doi: 10.2147/HMER.S11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zullo A, Hassan C, Ridola L, Lorenzetti R, Campo SM, Riggio O. Rifaximin therapy and hepatic encephalopathy: Pros and cons. World J Gastrointest Pharmacol Ther. 2012;3:62–67. doi: 10.4292/wjgpt.v3.i4.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12:1390–7.e2. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodríguez-Martínez D, Fernández-Rodríguez C, Coll I, Pardo A Spanish Association for the Study of the Liver Hepatic Encephalopathy Cooperative Group. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: Results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38:51–58. doi: 10.1016/s0168-8278(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 64.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, Teperman L, Hillebrand D, Huang S, Merchant K, Shaw A, Bortey E, Forbes WP. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 65.Kimer N, Gluud LL, Morris RW, Morgan MY. Rifaximin for the prevention and treatment of hepatic encephalopathy: A systematic review with meta-analyses of randomised controlled trials. J Clin Exp Hepatol. 2017;7:S78–S79. [Google Scholar]
- 66.Wu D, Wu SM, Lu J, Zhou YQ, Xu L, Guo CY. Rifaximin versus Nonabsorbable Disaccharides for the Treatment of Hepatic Encephalopathy: A Meta-Analysis. Gastroenterol Res Pract. 2013;2013:236963. doi: 10.1155/2013/236963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, Yoo JJ, Cho YY, Cho EJ, Yu SJ, Kim MY, Kim YJ, Baik SK, Yoon JH. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46:845–855. doi: 10.1111/apt.14275. [DOI] [PubMed] [Google Scholar]
- 68.Kabeshova A, Ben Hariz S, Tsakeu E, Benamouzig R, Launois R. Cost-effectiveness analysis of rifaximin-α administration for the reduction of episodes of overt hepatic encephalopathy in recurrence compared with standard treatment in France. Therap Adv Gastroenterol. 2016;9:473–482. doi: 10.1177/1756283X16644249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 70.Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, Hazratjee N, Smith T, Zein NN. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46:709–715. doi: 10.1097/MCG.0b013e3182506dbb. [DOI] [PubMed] [Google Scholar]
- 71.Runyon BA AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: An update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 72.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Praharaj D, Taneja S, Duseja A, Chawla Y, Dhiman RK. Randomized Control Trial of Rifaximin and Norfloxacin in Primary and Secondary Prophylaxis of Spontaneous Bacterial Peritonitis (SBP) in Cirrhotic Patients. J Clin Exp Hepatol. 2017;7:S71. [Google Scholar]
- 74.Sidhu GS, Go A, Attar BM, Mutneja HR, Arora S, Patel SA. Rifaximin versus norfloxacin for prevention of spontaneous bacterial peritonitis: A systematic review. BMJ Open Gastroenterol. 2017;4:e000154. doi: 10.1136/bmjgast-2017-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–818. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 76.Baik S, Lim Y, Cho Y, Kim M, Jang Y, Suk K, Cheon G, Kim Y, Choi D. G03: Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy in the hepatic venous pressure gradient response and propranolol dose reduction–A pilot study. J Hepatol. 2015;62:S188. [Google Scholar]
- 77.Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, Møller S, Bendtsen F Copenhagen Rifaximin (CoRif) Study Group. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: A randomized, double-blind, placebo-controlled trial. Hepatology. 2017;65:592–603. doi: 10.1002/hep.28898. [DOI] [PubMed] [Google Scholar]
- 78.Nathwani R. Rifaximin to reduce infection in decompensated cirrhosis. [accessed 2017 Sep 11]. In: ISRCTN registry [Internet] Available from: https://doi.org/10.1186/ISRCTN10994757 ISRCTN number: ISRCTN10994757.
- 79.Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2018;5:CD012410. doi: 10.1002/14651858.CD012410.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bai M, Yang Z, Qi X, Fan D, Han G. l-ornithine-l-aspartate for hepatic encephalopathy in patients with cirrhosis: A meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2013;28:783–792. doi: 10.1111/jgh.12142. [DOI] [PubMed] [Google Scholar]
- 81.Sidhu SS. L-ornithine L-aspartate in Overt Hepatic Encephalopathy (HEAL). [accessed 2017 Aug 2]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01722578 ClinicalTrials.gov. Identifier: NCT01722578 [Google Scholar]
- 82.Gentile S, Guarino G, Romano M, Alagia IA, Fierro M, Annunziata S, Magliano PL, Gravina AG, Torella R. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:184–191. doi: 10.1016/s1542-3565(04)00667-6. [DOI] [PubMed] [Google Scholar]
- 83.Mullen KD, Howard R. Is acarbose an effective drug for treating patients with cirrhosis and hepatic encephalopathy? Nat Clin Pract Gastroenterol Hepatol. 2005;2:264–265. doi: 10.1038/ncpgasthep0194. [DOI] [PubMed] [Google Scholar]
- 84.Ventura-Cots M, Santos B, Genescà J. α1 and α2-adrenergic agonists on cirrhotic patients with refractory ascites. Liver Int. 2016;36:177–180. doi: 10.1111/liv.12941. [DOI] [PubMed] [Google Scholar]
- 85.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1–v12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122–134. doi: 10.1007/s12072-017-9815-0. [DOI] [PubMed] [Google Scholar]
- 87.Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 88.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: Report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 89.Sherlock S, Walker JG, Senewiratne B, Scott A. The complications of diuretic therapy in patients with cirrhosis. Ann N Y Acad Sci. 1966;139:497–505. doi: 10.1111/j.1749-6632.1966.tb41223.x. [DOI] [PubMed] [Google Scholar]
- 90.Laffi G, La Villa G, Carloni V, Foschi M, Bartoletti L, Quartini M, Gentilini P. Loop diuretic therapy in liver cirrhosis with ascites. J Cardiovasc Pharmacol. 1993;22 Suppl 3:S51–S58. doi: 10.1097/00005344-199322003-00007. [DOI] [PubMed] [Google Scholar]
- 91.Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. J Hepatol. 2017;67:184–185. doi: 10.1016/j.jhep.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Senousy BE, Draganov PV. Evaluation and management of patients with refractory ascites. World J Gastroenterol. 2009;15:67–80. doi: 10.3748/wjg.15.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukerji AN, Patel V, Jain A. Improving survival in decompensated cirrhosis. Int J Hepatol. 2012;2012:318627. doi: 10.1155/2012/318627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh V, Singh A, Singh B, Vijayvergiya R, Sharma N, Ghai A, Bhalla A. Midodrine and clonidine in patients with cirrhosis and refractory or recurrent ascites: A randomized pilot study. Am J Gastroenterol. 2013;108:560–567. doi: 10.1038/ajg.2013.9. [DOI] [PubMed] [Google Scholar]
- 95.Singh V, Dhungana SP, Singh B, Vijayverghia R, Nain CK, Sharma N, Bhalla A, Gupta PK. Midodrine in patients with cirrhosis and refractory or recurrent ascites: A randomized pilot study. J Hepatol. 2012;56:348–354. doi: 10.1016/j.jhep.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 96.Guo TT, Yang Y, Song Y, Ren Y, Liu ZX, Cheng G. Effects of midodrine in patients with ascites due to cirrhosis: Systematic review and meta-analysis. J Dig Dis. 2016;17:11–19. doi: 10.1111/1751-2980.12304. [DOI] [PubMed] [Google Scholar]
- 97.Patel S, Nguyen DS, Rastogi A, Nguyen MK, Nguyen MK. Treatment of Cirrhosis-Associated Hyponatremia with Midodrine and Octreotide. Front Med (Lausanne) 2017;4:17. doi: 10.3389/fmed.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lenaerts A, Codden T, Meunier JC, Henry JP, Ligny G. Effects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous system. Hepatology. 2006;44:844–849. doi: 10.1002/hep.21355. [DOI] [PubMed] [Google Scholar]
- 99.Sansoè G, Aragno M, Mastrocola R, Parola M. Dose-dependency of clonidine's effects in ascitic cirrhotic rats: Comparison with α1-adrenergic agonist midodrine. Liver Int. 2016;36:205–211. doi: 10.1111/liv.12905. [DOI] [PubMed] [Google Scholar]
- 100.Yan L, Xie F, Lu J, Ni Q, Shi C, Tang C, Yang J. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015;15:65. doi: 10.1186/s12876-015-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pose E, Cardenas A. Translating Our Current Understanding of Ascites Management into New Therapies for Patients with Cirrhosis and Fluid Retention. Dig Dis. 2017;35:402–410. doi: 10.1159/000456595. [DOI] [PubMed] [Google Scholar]
- 102.Wong F, Watson H, Gerbes A, Vilstrup H, Badalamenti S, Bernardi M, Ginès P Satavaptan Investigators Group. Satavaptan for the management of ascites in cirrhosis: Efficacy and safety across the spectrum of ascites severity. Gut. 2012;61:108–116. doi: 10.1136/gutjnl-2011-300157. [DOI] [PubMed] [Google Scholar]
- 103.Vargas JI, Arrese M, Shah VH, Arab JP. Use of Statins in Patients with Chronic Liver Disease and Cirrhosis: Current Views and Prospects. Curr Gastroenterol Rep. 2017;19:43. doi: 10.1007/s11894-017-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cabrera L, Abraldes JG. Statins: The Panacea of Cirrhosis? Curr Hepatol Rep. 2016;15:1–7. [Google Scholar]
- 105.Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 106.Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: A randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 107.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: A retrospective cohort study. Dig Dis Sci. 2014;59:1958–1965. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 108.Mohanty A, Tate JP, Garcia-Tsao G. Statins Are Associated With a Decreased Risk of Decompensation and Death in Veterans With Hepatitis C-Related Compensated Cirrhosis. Gastroenterology. 2016;150:430–40.e1. doi: 10.1053/j.gastro.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896–907. doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 110.Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F, Calleja JL, Albillos A, Bosch J BLEPS Study Group. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160–1170.e3. doi: 10.1053/j.gastro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 111.Kamal S, Khan MA, Seth A, Cholankeril G, Gupta D, Singh U, Kamal F, Howden CW, Stave C, Nair S, Satapathy SK, Ahmed A. Beneficial Effects of Statins on the Rates of Hepatic Fibrosis, Hepatic Decompensation, and Mortality in Chronic Liver Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:1495–1505. doi: 10.1038/ajg.2017.170. [DOI] [PubMed] [Google Scholar]
- 112.Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1521–1530.e8. doi: 10.1016/j.cgh.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Tsochatzis EA, Bosch J. Statins in cirrhosis-Ready for prime time. Hepatology. 2017;66:697–699. doi: 10.1002/hep.29277. [DOI] [PubMed] [Google Scholar]
- 115.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–1292. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 116.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 117.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 118.European Association for the Study of the Liver (EASL) ; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts. 2016;9:65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moctezuma-Velázquez C, Abraldes JG, Montano-Loza AJ. The Use of Statins in Patients With Chronic Liver Disease and Cirrhosis. Curr Treat Options Gastroenterol. 2018;16:226–240. doi: 10.1007/s11938-018-0180-4. [DOI] [PubMed] [Google Scholar]
- 120.Lodato F, Azzaroli F, Di Girolamo M, Feletti V, Cecinato P, Lisotti A, Festi D, Roda E, Mazzella G. Proton pump inhibitors in cirrhosis: Tradition or evidence based practice? World J Gastroenterol. 2008;14:2980–2985. doi: 10.3748/wjg.14.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 122.Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther. 2015;41:459–466. doi: 10.1111/apt.13061. [DOI] [PubMed] [Google Scholar]
- 123.Trikudanathan G, Israel J, Cappa J, O'Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract. 2011;65:674–678. doi: 10.1111/j.1742-1241.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 124.Khan MA, Cholankeril G, Howden CW. Are proton pump inhibitors a threat for spontaneous bacterial peritonitis and hepatic encephalopathy in cirrhosis? Not so fast. Hepatology. 2017;65:393. doi: 10.1002/hep.28858. [DOI] [PubMed] [Google Scholar]
- 125.Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Lee FY, Su TP, Lu CL. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152:134–141. doi: 10.1053/j.gastro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 126.Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. 2018;47:192–202. doi: 10.1111/apt.14397. [DOI] [PubMed] [Google Scholar]
- 127.Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, Hylemon PB, Fuchs M, Puri P, Schubert ML, Sanyal AJ, Sterling RK, Stravitz RT, Siddiqui MS, Luketic V, Lee H, Sikaroodi M, Gillevet PM. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol. 2018;113:1177–1186. doi: 10.1038/s41395-018-0085-9. [DOI] [PubMed] [Google Scholar]
- 128.Ratuapli SK, Ellington TG, O'Neill MT, Umar SB, Harris LA, Foxx-Orenstein AE, Burdick GE, Dibaise JK, Lacy BE, Crowell MD. Proton pump inhibitor therapy use does not predispose to small intestinal bacterial overgrowth. Am J Gastroenterol. 2012;107:730–735. doi: 10.1038/ajg.2012.4. [DOI] [PubMed] [Google Scholar]
- 129.Assaraf J, Weiss N, Thabut D. Proton Pump Inhibitor Administration Triggers Encephalopathy in Cirrhotic Patients by Modulating Blood-Brain Barrier Drug Transport. Gastroenterology. 2017;152:2077. doi: 10.1053/j.gastro.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 130.Khan MA, Cholankeril G, Howden CW. Proton Pump Inhibitors and the Possible Development of Hepatic Encephalopathy in Cirrhotic Patients: True Association or Residual Confounding? Gastroenterology. 2017;152:2076. doi: 10.1053/j.gastro.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 131.Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, Niveyro C, Benavides J, Marino M, Colombato L, Berbara D, Silva M, Salgado P, Barreyro F, Fassio E, Gadano A Study Group of Cirrhosis Complications of the Argentine Association for the Study of Liver Disease. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: A multicenter prospective study. J Hepatol. 2015;62:1056–1060. doi: 10.1016/j.jhep.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 132.Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L. Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: A meta-analysis. Dig Liver Dis. 2016;48:353–359. doi: 10.1016/j.dld.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 133.Dhar A, Mullish BH, Thursz MR. Anticoagulation in chronic liver disease. J Hepatol. 2017;66:1313–1326. doi: 10.1016/j.jhep.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 134.Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: A new paradigm? Clin Mol Hepatol. 2017;23:13–21. doi: 10.3350/cmh.2016.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bitto N, Liguori E, La Mura V. Coagulation, Microenvironment and Liver Fibrosis. Cells. 2018;7:pii: E85. doi: 10.3390/cells7080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137:1145–1149. doi: 10.1378/chest.09-2177. [DOI] [PubMed] [Google Scholar]
- 137.Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S, Lei B, Bernabucci V, Vukotic R, De Maria N, Schepis F, Karampatou A, Caporali C, Simoni L, Del Buono M, Zambotto B, Turola E, Fornaciari G, Schianchi S, Ferrari A, Valla D. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260.e4. doi: 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 138.Senzolo M, M Sartori T, Rossetto V, Burra P, Cillo U, Boccagni P, Gasparini D, Miotto D, Simioni P, Tsochatzis E, A Burroughs K. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919–927. doi: 10.1111/j.1478-3231.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 139.Chen H, Liu L, Qi X, He C, Wu F, Fan D, Han G. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:82–89. doi: 10.1097/MEG.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 140.Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García-Criado A, Abraldes JG, de la Peña J, Bañares R, Albillos A, Bosch J, García-Pagán JC. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–783. doi: 10.1016/j.cgh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 141.Amitrano L, Guardascione MA, Menchise A, Martino R, Scaglione M, Giovine S, Romano L, Balzano A. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol. 2010;44:448–451. doi: 10.1097/MCG.0b013e3181b3ab44. [DOI] [PubMed] [Google Scholar]
- 142.Bernardi M, Maggioli C, Zaccherini G. Human albumin in the management of complications of liver cirrhosis. Crit Care. 2012;16:211. doi: 10.1186/cc11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 144.Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 145.Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: A meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11:123–30.e1. doi: 10.1016/j.cgh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: A meta-analysis of randomized trials. Hepatology. 2012;55:1172–1181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 148.Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, Levantesi F, Airoldi A, Boccia S, Svegliati-Baroni G, Fagiuoli S, Romanelli RG, Cozzolongo R, Di Marco V, Sangiovanni V, Morisco F, Toniutto P, Tortora A, De Marco R, Angelico M, Cacciola I, Elia G, Federico A, Massironi S, Guarisco R, Galioto A, Ballardini G, Rendina M, Nardelli S, Piano S, Elia C, Prestianni L, Cappa FM, Cesarini L, Simone L, Pasquale C, Cavallin M, Andrealli A, Fidone F, Ruggeri M, Roncadori A, Baldassarre M, Tufoni M, Zaccherini G, Bernardi M ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): An open-label randomised trial. Lancet. 2018;391:2417–2429. doi: 10.1016/S0140-6736(18)30840-7. [DOI] [PubMed] [Google Scholar]
- 149.Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, Moore DJ, Colville A, Bhala N, Iqbal TH, Settle C, Kontkowski G, Hart AL, Hawkey PM, Goldenberg SD, Williams HRT. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67:1920–1941. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 150.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Woodhouse C. Trial of faecal microbiota transplantation in cirrhosis. ISRCTN registry. [accessed 2018 May 21]. In: ISRCTN registry [Internet] Available from: https://doi.org/10.1186/ISRCTN11814003 ISRCTN number: ISRCTN11814003.
- 152.Dranoff JA. Coffee Consumption and Prevention of Cirrhosis: In Support of the Caffeine Hypothesis. Gene Expr. 2018;18:1–3. doi: 10.3727/105221617X15046391179559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salomone F, Galvano F, Li Volti G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients. 2017;9:pii: E85. doi: 10.3390/nu9010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Petta S, Marchesini G. Coffee and tea breaks for liver health. J Hepatol. 2017;67:221–223. doi: 10.1016/j.jhep.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 155.Corrao G, Zambon A, Bagnardi V, D'Amicis A, Klatsky A Collaborative SIDECIR Group. Coffee, caffeine, and the risk of liver cirrhosis. Ann Epidemiol. 2001;11:458–465. doi: 10.1016/s1047-2797(01)00223-x. [DOI] [PubMed] [Google Scholar]
- 156.Alferink LJM, Fittipaldi J, Kiefte-de Jong JC, Taimr P, Hansen BE, Metselaar HJ, Schoufour JD, Ikram MA, Janssen HLA, Franco OH, Darwish Murad S. Coffee and herbal tea consumption is associated with lower liver stiffness in the general population: The Rotterdam study. J Hepatol. 2017;67:339–348. doi: 10.1016/j.jhep.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 157.Yu C, Cao Q, Chen P, Yang S, Deng M, Wang Y, Li L. An updated dose-response meta-analysis of coffee consumption and liver cancer risk. Sci Rep. 2016;6:37488. doi: 10.1038/srep37488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freedman ND, Everhart JE, Lindsay KL, Ghany MG, Curto TM, Shiffman ML, Lee WM, Lok AS, Di Bisceglie AM, Bonkovsky HL, Hoefs JC, Dienstag JL, Morishima C, Abnet CC, Sinha R HALT-C Trial Group. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50:1360–1369. doi: 10.1002/hep.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]