Abstract
BACKGROUND
Procyanidins have beneficial effects on metabolic syndrome and antimicrobial activity, but the mechanisms underlying these effects are unclear.
AIM
To investigate the effects of procyanidin B2 (PB2) on non-alcoholic fatty liver disease and to explore the possible mechanism.
METHODS
Thirty male New Zealand white rabbits were randomized into three groups. All of them were fed either a high-fat-cholesterol diet (HCD) or chow diet. HCD-fed rabbits were treated with vehicle or PB2 daily for 12 wk. Body weight and food intake were evaluated once a week. Serum biomarkers, such as total cholesterols, triglycerides, and aspartate transaminase, were detected. All rabbits were sacrificed and histological parameters of liver were assessed by hematoxylin and eosin-stained sections. Moreover, several lipogenic genes and gut microbiota (by 16S rRNA sequencing) were investigated to explore the possible mechanism.
RESULTS
The HCD group had higher body weight, liver index, serum lipid profile, insulin resistance, serum glucose, and hepatic steatosis compared to the CHOW group. PB2 treatment prevented HCD-induced increases in body weight and hypertriglyceridemia in association with triglyceride accumulation in the liver. PB2 also ameliorated low-grade inflammation, which was reflected by serum lipopolysaccharides and improved insulin resistance. In rabbit liver, PB2 prevented the upregulation of steroid response element binding protein 1c and fatty acid synthase and the downregulation of carnitine palmitoyltransferase, compared to the HCD group. Moreover, HCD led to a decrease of Bacteroidetes in gut microbiota. PB2 significantly improved the proportions of Bacteroidetes at the phylum level and Akkermansia at the genus level.
CONCLUSION
Our results indicate the possible mechanism of PB2 to improve HCD-induced features of metabolic syndrome and provide a new dietary supplement.
Keywords: Procyanidin, Rabbit, Non-alcoholic fatty liver disease, Gut microbiota, 16S rRNA
Core tip: Procyanidins are widely recognized for their excellent antioxidant properties and fewer side effects compared to other drugs. In the past, the mechanism of procyanidin to improve insulin resistance mainly focused on the antioxidant effect. The effect of procyanidin on non-alcoholic fatty liver disease is not clear. We found that procyanidin can reduce fatty liver by remodeling intestinal flora, decreasing endotoxemia, and down-regulating fatty acid synthesis genes. Our results open a new chapter in the mechanism of action of plant compounds and suggest a safer method for the treatment of non-alcoholic fatty liver disease.
INTRODUCTION
Advances in industrialization have changed people's lifestyles and eating habits. The incidence of obesity has rapidly increased, along with an increase in obesity-related metabolic syndrome, such as non-alcoholic fatty liver disease (NAFLD). NAFLD refers to the pathological state in which lipid, carbohydrates, and other substances are metabolically disordered. It is complex and is a risk factor for cardiovascular diseases and diabetes[1]. NAFLD is characterized by insulin resistance (IR) caused by a combination of genetic and environmental factors. Common manifestations include endotoxemia, low-grade inflammation, and tissue damage[2-4]. The intestinal barrier serves as the first site of interaction between the diet and the host immune system. This interaction can affect the composition of the gut microbiota, which affects intestinal immune homeostasis and intestinal permeability[5,6]. Thus, the gut and its microflora are potential sources of pro-inflammatory molecules that can affect systemic metabolism and may be involved in early events related to IR and NAFLD.
Evidence indicates that changes in intestinal mucosal permeability caused by a dysfunctional gut microbiota and metabolites produced by bacteria initiate the development of obesity-related metabolic diseases[7,8]. Lipopolysaccharides (LPS) derived from the gut microbiota are effective inflammation-inducing agents and play important roles in the occurrence and development of inflammation and related NAFLD[7,9]. A high-fat diet is associated with elevated systemic circulating levels of LPS (endotoxemia). Moreover, it causes an imbalance in the gut microbiota, especially by changing the proportion of Firmicutes and Bacteroides, and this is thought to play a key role in the pathogenesis of NAFLD[10]. The treatment of mice with broad-spectrum antibiotics can reshape the gut microbiota and improve insulin sensitivity[11]. Therefore, the gut microbiota is a potential source of pro-inflammatory molecules that can affect systemic metabolism and appear before the development of obesity and metabolic syndrome.
Proanthocyanidins are polyphenolic plant compounds that are naturally present in the diet[12]. Owing to their anti-oxidative, anti-inflammatory, and anti-atherogenic properties, they have been studied extensively. Procyanidin B2 (PB2), an oligomeric anthocyanin precursor, is widely distributed in grapes, cranberries, and other berries. PB2 has been reported to improve dyslipidemia, hyperglycemia, and oxidative stress in individuals with metabolic syndrome through its anti-oxidative properties[13]. However, PB2 is a natural plant compound, and its bioavailability in humans is low[12,14]. The low levels of PB2 in the body make their superior effects difficult to explain. The mechanism underlying the beneficial effects of PB2 remains largely unknown. Recent growing evidence shows that procyanidin has strong antimicrobial effects against bacteria, fungi, and viruses and can rejuvenate the gut microbiota for health benefits[15]. A diet supplemented with proanthocyanidins can regulate the microbial composition in pig intestines and reduce the infection rate of swine mites. Given the close association between metabolic syndrome, the gut microbiota, and PB2, the purpose of this study was to determine the effects of PB2 on the metabolism of a high-fat-fed New Zealand white rabbits and to determine whether these effects are related to modulation of the gut microbiota.
MATIERIALS AND METHODS
Animal model, diet, and experimental procedures
Thirty New Zealand white rabbits (male, 2-mo-old, mean weight 2.0 ± 0.2 kg) were purchased from Shanghai Silaike Experimental Animal Co. LTD (Shanghai, China). Rabbits were housed in individual cages at a controlled temperature (22 ± 2 °C), with a 12-h light/dark period. Animals were fed either a chow (standard diet: 20% corn, 30% grass powder, 20% cardamom, 25% wheat bran, 5% multivitamins, FBSH Biotechnology Inc.) or a high-fat-cholesterol diet (HCD) (10% lard, 0.5% cholesterol, 5% sucrose, 1% maltodextrin, FBSH Biotechnology Inc.). After 2 wk of a high-fat, cholesterol diet, some rabbits were excluded because of very high (> 2 mmol/L) or low (< 0.5 mmol/L) cholesterol levels[16]. Group PB2 (fed a HCD, n = 8) received daily doses (150 mg/kg, dissolved in normal saline) of PB2 by gavage[17], whereas the other two groups [groups CHOW and HCD, fed a chow or HCD, n = 8] received the vehicle (normal saline) by gavage. Body weight gain and other parameters were evaluated once a week. Feces were collected at the end of 12 wk for 16S rRNA sequencing. After 12 wk, the rabbits were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for blood and tissue collection. The livers were weighed and the liver index (the ratio of liver to body weight) was calculated. The protocol was approved by the Animal Research Committee of the Second Xiangya Hospital, Central South University, Hunan, China (permit number: 20170722). The chemicals used in this study were of analytical grade. PB2 (≥ 95% purity) was purchased from Yuanye Biological Technology Co. Ltd. (Shanghai, China).
Measurement of serum biochemical markers
Blood samples were collected from the middle artery of the ear after overnight fasting at the end of 10 wk. The concentrations of serum total cholesterols (CH), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), serum alanine transaminase (ALT), aspartic acid transferase (AST), and glucose (GLU) were detected by enzymatic methods (BioMerieux, Lyon, France). At the end of the experiment, the intravenous glucose tolerance test (IVGTT) was performed. After fasting overnight, a bolus of glucose (0.6 g/kg body weight) was injected into the ear vein, and blood samples were collected from the middle artery at 0, 5, 10, 30, 60, and 120 min. The serum insulin concentrations were analyzed according to the protocols of the Rabbit Insulin ELISA Kit (Crystal Chem Co., Elk Grove Village, IL, United States). The serum LPS concentration was determined using a kit based on a Limulus amebocyte extract (LAL Kit; Lonza, Basel, Switzerland).
Histological analysis
After the rabbits were sacrificed, a morphological analysis of liver tissues was performed on hematoxylin and eosin-stained sections. Liver tissue samples were fixed with 10% neutral buffered formalin for 24 h, dehydrated with an ethanol solution, and embedded in paraffin. Tissue sections of 5 μm-6 μm in thickness were excised, deparaffinized, rehydrated, and stained with hematoxylin and eosin.
Quantitative real-time polymerase chain reaction (PCR)
Liver was homogenized and total RNA was isolated using the RNeasy kit (Qiagen, Hilden, Germany). A reverse transcription reaction was performed by Taqman Reverse Transcription reagent kit (Takara, Kusatsu, Japan) according to the manufacturer's instructions. The real-time PCR reaction was carried out on Roche real-time PCR system with cDNA sample containing SYBR Green PCR master mix (Takara) and primers. Primer sequences can be made available upon request. Relative expression of mRNA was calculated by the comparative cycle threshold method.
Bacterial DNA extraction and PCR amplification
Microbial genomic DNA was extracted from fecal samples using the QIAamp DNA Stool Mini Kit (Qiagen). The V4 hypervariable region of 16S rRNA was amplified by PCR using specific barcoded primers (515F: 5′-GTGCCAGCMGCCGCGGTAA-3’, 806R: 5’-GGACTACHVGGGTWTCTA AT-3’). All PCR procedures were performed using Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, United States). A mixture of PCR products was purified using the Qiagen Gel Extraction Kit. The PCR products were used to generate a sequencing library using the TruSeq DNA PCRFREE Sample Preparation Kit (Illumina, San Diego, CA, United States). Finally, the library was sequenced using the Illumina HiSeq 2500 platform.
Bioinformatics analysis of sequencing data
The 16S raw data for all samples were processed and analyzed using the QIIME pipeline (1.9.1). Operational taxonomic units (OTUs) were clustered with a 97% similarity. A representative sequence for each OTU was selected and was classified using the Greengene database gg_13_8. Based on the analysis of OTUs, the species with the highest abundance at each taxonomic level for each sample was selected to generate the relative abundances of species. An unweighted UniFrac principal coordinate analysis (PCoA) was used to analyze the similarity in the composition of fecal flora in rabbits.
Statistical analysis
The measurement data obtained in the study were expressed as means ± standard deviation, and the differences were analyzed by one-way analysis of variance. Statistical analyses were performed using SPSS 22.0 (Armonk, NY, United States). Differences were statistically significant when P < 0.05.
RESULTS
Effects of PB2 on body weight gain and the serum lipid profile
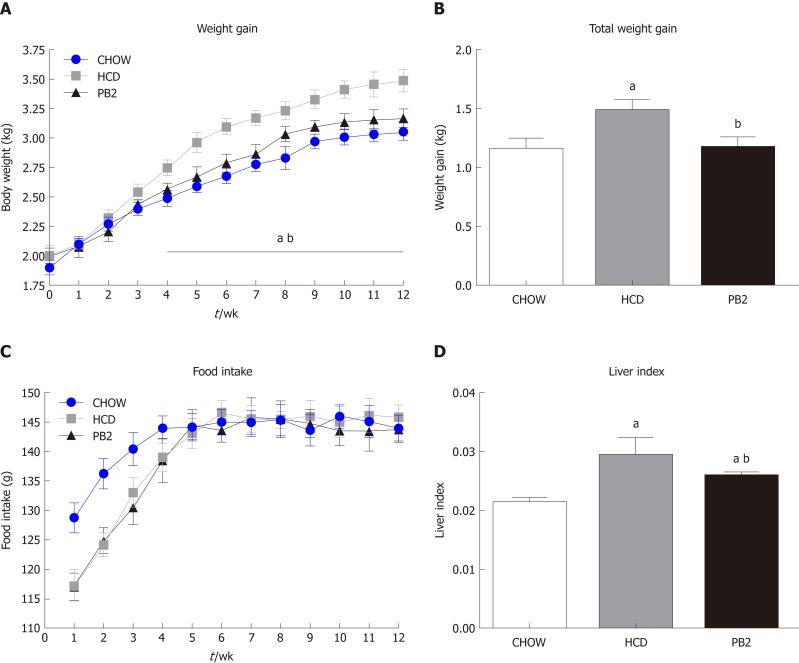
To investigate the metabolic effects of PB2 on HCD, body weight and serum biomarkers were detected. The treatment of HCD-fed rabbits with PB2 prevented an increase in body weight, and this effect was observed after just 3 wk (Figure 1A and B). By the end of 12-wk period, the Chow group gained the least body weight. The body weight of the PB2 group was slightly higher than that of the Chow group but was much lower than that of the HCD group (P < 0.05). The HCD and PB2 groups required a period of adaptation to the HCD and exhibited reduced food intake. Throughout the experiment, the PB2 group consumed the least amount of food. There was no significant difference in food intake between the three groups of rabbits after the acclimation period (Figure 1C). At the end of the experiment, the liver index of the PB2 group was significantly lower than that of the HCD group (Figure 1D).
Figure 1.
Effects of procyanidin B2 on body composition. A: Weight gain curves; B: Total weight gain; C: Food intake curves; D: Liver index. The data are expressed as means ± SD. aP < 0.05 vs CHOW group; bP < 0.05 vs high-fat-cholesterol diet group. HCD: High-fat-cholesterol diet; PB2: Procyanidin B2; SD: Standard deviation.
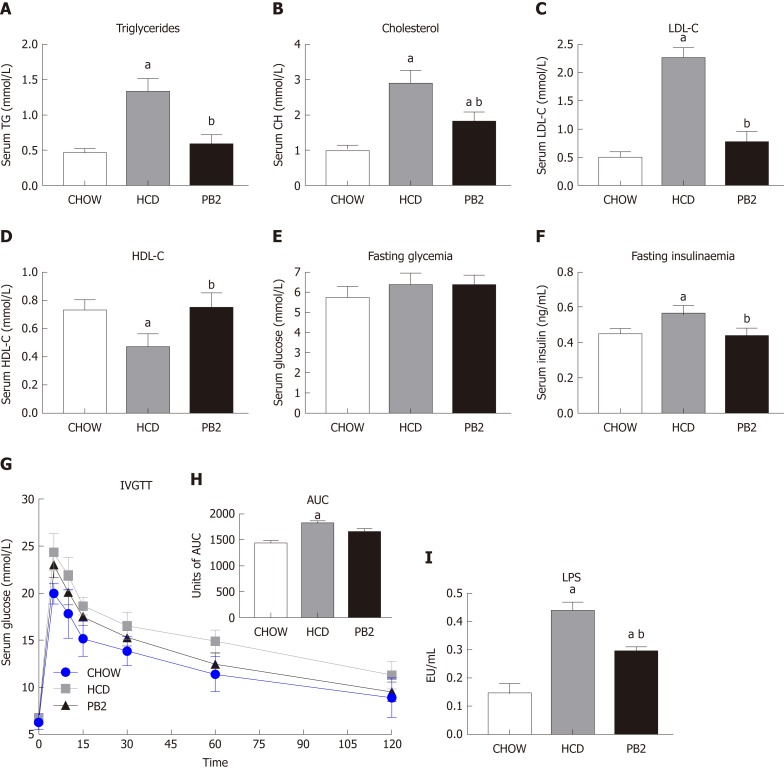
Several serum biochemical parameters, including TG, CH, LDL-C, HDL-C, GLU, and insulin, were determined. The values of TG, CH, and LDL-C were lowest in the Chow group. PB2 administration reduced serum TG, CH, and LDL-C levels compared with levels in the HCD group, ameliorating HCD-induced hypertriglyceridemia and hypercholesterolemia in these rabbits (P < 0.05) (Figure 2A-C). PB2 also significantly improved HDL-C levels compared with those in the HCD group (P < 0.05) (Figure 2D). Although PB2-treated HCD-fed rabbits did not show improved fasting glucose compared to that of the HCD group, these animals displayed lower concentrations of fasting insulin than those in untreated HCD-fed animals (P < 0.05) (Figure 2E and F). The IVGTT was performed, and changes in serum glucose are presented in Figure 2G. The incremental area under the curve (AUC) for glucose in the PB2 group was lower than that in the HCD group (Figure 2H), but the difference was not statistically significant. Twelve weeks of HCD feeding produced a significant increase in circulating LPS, which was partially prevented by PB2 administration (P < 0.05) (Figure 2I). Thus, PB2 alleviated the endotoxemia induced by HCD.
Figure 2.
Effects of procyanidin B2 on serum lipid profile, insulin resistance, and endotoxemia. Serum triglycerides (A), total cholesterol (B), low-density lipoprotein cholesterol (C), high-density lipoprotein cholesterol (D) and fasting glucose (E), fasting insulin (F) and the intravenous glucose tolerance test (G), units of area under the curve (H) and lipopolysaccharides (I). The data are expressed as means ± SD. aP < 0.05 vs CHOW group; bP < 0.05 vs high-fat-cholesterol diet group. HCD: High-fat-cholesterol diet; PB2: Procyanidin B2; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; IVGTT: Intravenous glucose tolerance test; AUC: Area under the curve; LPS: Lipopolysaccharides; SD: Standard deviation.
Effects of PB2 on diet-induced hepatic steatosis in rabbits
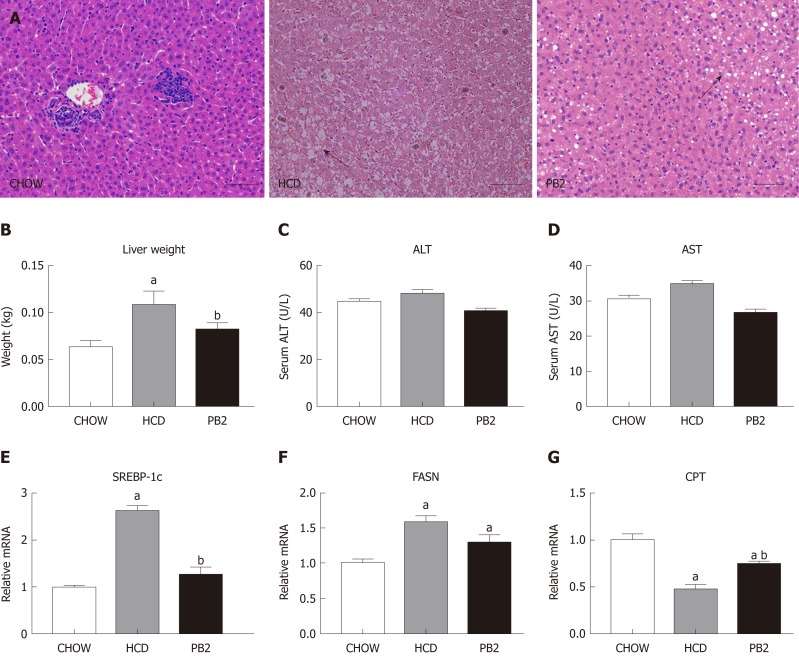
Since PB2 could inhibit hypertriglyceridemia and hypercholesterolemia in rabbits, we next examined the effects of PB2 on HCD-induced hepatic steatosis. We performed a histological analysis of the liver by staining with hematoxylin and eosin to investigate the effect of PB2 on hepatic triglyceride accumulation. The HCD group showed obvious triglyceride accumulation in the liver. The administration of PB2 resulted in significantly less triglyceride accumulation compared with that in the HCD group. The Chow group had undetectable triglycerides in the liver by hematoxylin and eosin staining (Figure 3A). At the end of the experiment, the liver weight in the HCD group was significantly higher than that of the Chow group (P < 0.05). PB2 treatment significantly reduced liver weight compared to the HCD group (P < 0.05) (Figure 3B). There was no significant difference in liver enzymes among the three groups (Figure 3C and D). Moreover, compared to the HCD group, PB2 treatment partially prevented the upregulation of the steroid response element binding protein 1c (SREBP‐1c) and the downregulation of carnitine palmitoyltransferase (P < 0.05) (Figure 3E-G). The expression of fatty acid synthase (FASN) was also decreased compared with the HCD group, but the difference was not significant.
Figure 3.
Effects of procyanidin B2 on hepatic steatosis. A: Hematoxylin and eosin staining of liver in CHOW group, HCD group and procyanidin B2 group; B: Liver weight; C: Alanine transaminase; D: Aspartate transaminase; E: Relative expression levels of steroid response element binding protein 1c; F: Relative expression levels of fatty acid synthase; G: Relative expression levels of carnitine palmitoyltransferase. The data are expressed as means ± SD. aP < 0.05 vs CHOW group; bP < 0.05 vs HCD group. HCD: High-fat-cholesterol diet; PB2: Procyanidin B2; ALT: Alanine transaminase; AST: Aspartate transaminase; SREBP-1c: Steroid response element binding protein 1c; FASN: Fatty acid synthase; CPT: Carnitine palmitoyltransferase; SD: Standard deviation.
Effects of procyanidin on the gut microbiota
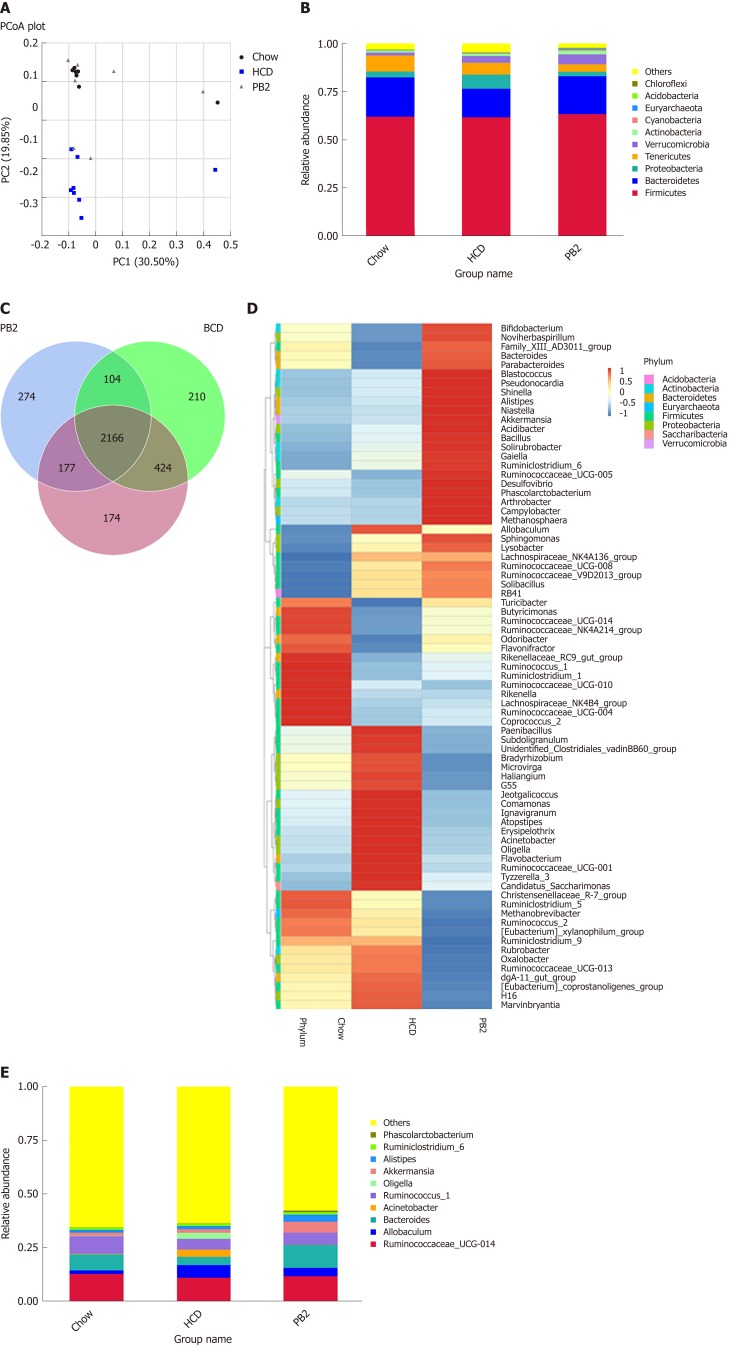
The overall composition of the gut microbiota in each group was investigated. For each sample, the V4 hypervariable region of the bacterial 16S rRNA gene sequence was amplified by PCR. After filtering out sequences with a low mass and short length, 1800802 high-quality reads were obtained (average, 78295 sequences per sample). We identified 3612 OTUs based on the conventional criterion of a 97% sequence identity (equal to the species level). The coverage index (sequencing depth index) for all samples ranged from 0.984 to 0.996.
Unweighted UniFrac PCoA differentiated microbial communities based on diet and treatment were generated. As shown in Figure 4A, PCoA revealed that the HCD diet promoted major alterations in the gut microbiota. Distinct clusters were observed for PB2-treated rabbits, Chow-fed rabbits, and HCD-fed rabbits. These results demonstrated that PB2 administration has a significant effect on gut microbial composition in HCD-fed rabbits. A total of 10 phyla were detected, and Firmicutes, Bacteroidetes, Proteobacteria, and Tenericutes were the most abundant phyla in these samples (Figure 4B). At the phylum level, the proportion of Bacteroidetes was significantly reduced in the HCD-fed rabbits (P < 0.05), whereas the relative abundance of Proteobacteria was significantly increased compared to that of the Chow-fed rabbits (P < 0.05). Similar trends for Bacteroidetes and Proteobacteria were observed in Chow-fed animals and PB2-treated animals. PB2 administration led to a low ratio of Firmicutes to Bacteroidetes (P < 0.05).
Figure 4.
Effects of procyanidin B2 on gut microbiota. A: Principal coordinate analysis of the gut microbiota based on unweighted UniFrac distances between groups; B: Relative abundance of gut microbiota in phylum; C: Venn diagrams described the number of operational taxonomic units that are distinct and shared across the groups; D: Heatmap: Community hierarchical clustering analysis of major taxonomic groups at genus level, the red represents high relative abundance and the blue represent low relative abundance; E: Relative abundance of gut microbiota in genus. HCD: High-fat-cholesterol diet; PB2: Procyanidin B2; PCoA: Principal coordinate analysis; OTUs: Operational taxonomic units.
At the genus level, there were significant alterations in Allobaculum, Ruminococcus, Bacteroidetes, and Akkermansia (Figure 4D and E). Compared with the HCD group, the relative abundance of Allobaculum was significantly reduced in the PB2 group and the relative abundances of Ruminococcus and Bacteroidetes were significantly increased. Of note, PB2 treatment was associated with a striking 3-fold increase in the relative abundance of Akkermansia compared with that of the HCD group.
Different numbers of OTUs were detected in each group, i.e. 2941 in the Chow group, 2940 in the HCD group, and 2712 in the PB2 group (Figure 4C). Among all OTUs, 2166 were shared by all groups. Additionally, each group had unique OTUs, including 174 in the Chow group, 210 in the HCD group, and 274 in the PB2 group.
DISCUSSION
In the current study, we used an HCD-induced model to investigate the effects of PB2 on several components of metabolic syndrome. Our results suggested that PB2 prevents HCD-induced weight gain and the development of NAFLD and improves insulin sensitivity. PB2 also prevented the upregulation of several lipogenic genes, including SREBP‐1c and FASN. Moreover, PB2 administration led to a dramatic alteration in the gut microbiota by increasing the proportion of Bacteroidetes at the phylum level and Akkermansia at the genus level.
Extensive evidence indicates that high polyphenol-rich fruit consumption is negatively correlated with several features of metabolic syndrome[18-20]. Although the positive health effects of fruit may be attributed to vitamins, minerals, and dietary fiber, a growing number of studies support the role of polyphenols in protection against obesity-related diseases[21]. Accordingly, we explored the impacts of PB2, a major component of berries, on several features of NAFLD[22,23]. Our results showed that PB2 reduces weight gain as well as the liver index, and this effect on weight was observed before significant change of food intake was detected. IVGTT revealed an improvement in IR by fasting and postprandial glucose in the PB2 group compared with the HCD group. PB2 treatment had an obvious lipid-lowing effect; it significantly reduced the serum levels of TG, TC, and LDL-C compared to the HCD group at the end of the experiment. Additionally, PB2 prevented the development of NAFLD to some extent by reducing liver TG deposition and weakened inflammation induced by the HCD.
SREBP-1c is a major regulator of fatty acids (FA) synthesis, which may be involved in IR and NAFLD development, with the goal of reducing lipogenesis and TG levels. Overexpressing SREBP1c increased FA synthase, leading to a higher level of liver FA and TG; whereas SREBP-1c knockout mice showed decreased FASN expression, liver FA, and serum TG levels[24]. Our results showed that PB2 significantly prevented the upregulation of SREBP-1c compared to the HCD group. The inhibition of SREBP1 upregulation may be included in the mechanisms of PB2 to alleviate the steatosis in hepatic cells.
A high ratio of Firmicutes to Bacteroidetes is often thought to be a key characteristic of obesity and NAFLD[25]. Ciubotaru et al[26] have reported that a high proportion of Bacteroidetes is associated with better glycemic control in humans. Rabot et al[27] found that the transplantation of a Bacteroides-rich microbiota improves glucose intolerance caused by an HCD in mice. Fernando reported that a lower Firmicutes to Bacteroidetes ratio is associated with improved glucose and insulin tolerance after cranberry extract administration[28]. Similar to previous results, we observed the partial recovery of IR and reshaping of gut microbiota induced by HCD after PB2 treatment. Future studies are warranted to determine whether the ratio of Firmicutes to Bacteroidetes influences host glucose homeostasis prior to fat mass accumulation.
The gut microbiota has a casual role in the pathogenesis of NAFLD[5]. Previous reports have suggested that polyphenolic plant compounds have poor absorption and are mainly absorbed in the intestines[13]. This prompted us to assess the impact of PB2 on gut microbiota. We demonstrated that an HCD leads to a dramatic shift in the gut microbiota of rabbits by decreasing the proportion of Bacteroidetes and increasing the ratio of Firmicutes to Bacteroidetes, whereas PB2 treatment changed this trend. This diet-induced remodeling of gut microbial communities in HCD-fed rabbits is a typical feature of obesity-driven dysbiosis and is in agreement with previous results[10].
According to our results, it is possible that PB2 influences metabolic phenotypes by regulating the relative abundance of Akkermansia. This is consistent with several previous studies that plant compounds can have a significant impact on the proportion of Akkermansia in the gut microbiota of an animal model[25-30]. The administration of cranberry extract to high fat-fed mice is associated with the relative proportion of Akkermansia, whereas a study revealed that treatment of HCD-fed mice with green tea polyphenols can modulate the gut microbial ecosystem (in vitro) by increasing the proportion of Akkermansia. In addition, the metabolic benefit of resveratrol is also associated with an increased intestinal abundance of the bacteria. Similarly, our results suggest that treatment with PB2, a plant compound, leads to an increase in the Akkermansia population and might be sufficient to prevent the negative metabolic phenotype caused by an HCD.
Hepatic steatosis involves the deposition of TG, which together with oxidative stress, constitutes the basis for the pathophysiology of NAFLD. In obese patients, LPS from the intestinal microbiota enters the liver through the portal vein and affects physiological metabolic processes in the host[31]. In our experiments, LPS levels in the HCD-fed rabbits were higher than those in the Chow group, and the change was partially reversed by treatment with PB2. Interestingly, the use of Akkermansia as a probiotic could reduce serum LPS levels in mice fed an HCD. This may be explained by the ability of Akkermansia to retain the thickness of the intestinal mucus layer, thus reducing intestinal permeability and LPS leakage. Therefore, the ability of PB2 to reduce serum LPS levels in our experiments could explain the reduction in liver TG accumulation and the protection against hepatic oxidative stress and inflammation in HCD-fed rabbits.
While our study provides evidence for the beneficial effects of PB2 for the treatment of NAFLD, some limitations must be acknowledged. Further studies should evaluate whether its effects on metabolic phenotype are mediated by the metabolic products of the gut microbiota, such as trimethylamine-N-oxide or bile acids.
In summary, we found that PB2 treatment protects against HCD-induced obesity, IR, and liver steatosis in rabbits. These effects were associated with the alleviation of intestinal inflammation and metabolic endotoxemia. These results led us to propose that PB2 may prevent obesity and NAFLD by a prebiotic effect on the gut microbiota.
ARTICLE HIGHLIGHTS
Research background
The mechanism of procyanidin to improve metabolic syndrome mainly focuses on its antioxidant effect. The latest studies show that procyanidin have commendable antibacterial properties. The evaluation of remodeling gut microbiota in non-alcoholic fatty liver disease (NAFLD) by procyanidin may provide a new therapeutic trend.
Research motivation
Procyanidin has been reported to improve dyslipidemia, hyperglycemia, and oxidative stress through its anti-oxidative properties. However, procyanidin is a natural plant compound, and its bioavailability in humans is low. The low levels of procyanidin B2 (PB2) in the body make their superior effects difficult to explain. The mechanism underlying the beneficial effects of procyanidin remains largely unknown.
Research objectives
To validate the effect of procyanidin on NAFLD and to clarify the possible mechanism of action.
Research methods
New Zealand white rabbits were fed chow or high-fat-cholesterol diet (HCD) for 12 wk. The body weight was investigated every week. The serum samples were analyzed after a 12-wk time. Hematoxylin and eosin staining of liver samples were performed, and fatty acid synthesis genes of liver were evaluated. The gut microbiota was sequenced by 16S rRNA analysis.
Research results
Our results show that procyanidin is associated with alleviated hepatic steatosis, decrease serum lipid, suppressed gut inflammation, and remolded gut microbiota.
Research conclusions
Procyanidin treatment protects against HCD-induced obesity, insulin resistance, and liver steatosis in rabbits. These effects were associated with the alleviation of intestinal inflammation and endotoxemia.
Research perspectives
Plant compounds, such as procyanidin, should be further explored for their potential therapeutic activity in NAFLD.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: This study was approved by the Animal Research Committee, Central South University, Hunan, China.
Conflict-of-interest statement: The authors declare no conflict of interests.
Data sharing statement: Raw sequencing data are available from the corresponding author at lgtmhu@163.com. Participants gave informed consent for data sharing.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guideline.
Peer-review started: December 17, 2018
First decision: January 18, 2019
Article in press: January 29, 2019
P- Reviewer: Choi MR S- Editor: Ma RY L- Editor: Filipodia E- Editor: Yin SY
Contributor Information
Ya-Wei Xing, Department of Gastroenterology, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Guang-Tao Lei, Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China. lgtmhu@163.com.
Qing-Hua Wu, Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Yu Jiang, Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Man-Xiang Huang, Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 3.Marette A. Mediators of cytokine-induced insulin resistance in obesity and other inflammatory settings. Curr Opin Clin Nutr Metab Care. 2002;5:377–383. doi: 10.1097/00075197-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 8.Geurts L, Lazarevic V, Derrien M, Everard A, Van Roye M, Knauf C, Valet P, Girard M, Muccioli GG, François P, de Vos WM, Schrenzel J, Delzenne NM, Cani PD. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:149. doi: 10.3389/fmicb.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24.e1-2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rune I, Rolin B, Larsen C, Nielsen DS, Kanter JE, Bornfeldt KE, Lykkesfeldt J, Buschard K, Kirk RK, Christoffersen B, Fels JJ, Josefsen K, Kihl P, Hansen AK. Modulating the Gut Microbiota Improves Glucose Tolerance, Lipoprotein Profile and Atherosclerotic Plaque Development in ApoE-Deficient Mice. PLoS One. 2016;11:e0146439. doi: 10.1371/journal.pone.0146439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bladé C, Arola L, Salvadó MJ. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res. 2010;54:37–59. doi: 10.1002/mnfr.200900476. [DOI] [PubMed] [Google Scholar]
- 13.Baba S, Osakabe N, Natsume M, Terao J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats. Free Radic Biol Med. 2002;33:142–148. doi: 10.1016/s0891-5849(02)00871-7. [DOI] [PubMed] [Google Scholar]
- 14.Sano A, Yamakoshi J, Tokutake S, Tobe K, Kubota Y, Kikuchi M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 15.Williams AR, Krych L, Fauzan Ahmad H, Nejsum P, Skovgaard K, Nielsen DS, Thamsborg SM. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS One. 2017;12:e0186546. doi: 10.1371/journal.pone.0186546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo F, Guo Y, Ruan GY, Long JK, Zheng XL, Xia Q, Zhao SP, Peng DQ, Fang ZF, Li XP. Combined use of metformin and atorvastatin attenuates atherosclerosis in rabbits fed a high-cholesterol diet. Sci Rep. 2017;7:2169. doi: 10.1038/s41598-017-02080-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Yamashita Y, Komeda S, Saito A, Ashida H. Absorption, metabolism, distribution and faecal excretion of B-type procyanidin oligomers in mice after a single oral administration of black soybean seed coat extract. Food Funct. 2018;9:5362–5370. doi: 10.1039/c8fo00852c. [DOI] [PubMed] [Google Scholar]
- 18.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Eshak ES, Iso H, Mizoue T, Inoue M, Noda M, Tsugane S. Soft drink, 100% fruit juice, and vegetable juice intakes and risk of diabetes mellitus. Clin Nutr. 2013;32:300–308. doi: 10.1016/j.clnu.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto A, Ohno Y, Tatsumi Y, Mizuno S, Watanabe S. Effects of healthy dietary pattern and other lifestyle factors on incidence of diabetes in a rural Japanese population. Asia Pac J Clin Nutr. 2012;21:601–608. [PubMed] [Google Scholar]
- 21.Corder R, Mullen W, Khan NQ, Marks SC, Wood EG, Carrier MJ, Crozier A. Oenology: red wine procyanidins and vascular health. Nature. 2006;444:566. doi: 10.1038/444566a. [DOI] [PubMed] [Google Scholar]
- 22.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575–594, ix. doi: 10.1016/j.cld.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Del Bas JM, Ricketts ML, Baiges I, Quesada H, Ardevol A, Salvadó MJ, Pujadas G, Blay M, Arola L, Bladé C, Moore DD, Fernandez-Larrea J. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol Nutr Food Res. 2008;52:1172–1181. doi: 10.1002/mnfr.200800054. [DOI] [PubMed] [Google Scholar]
- 25.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciubotaru I, Green SJ, Kukreja S, Barengolts E. Significant differences in fecal microbiota are associated with various stages of glucose tolerance in African American male veterans. Transl Res. 2015;166:401–411. doi: 10.1016/j.trsl.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabot S, Membrez M, Blancher F, Berger B, Moine D, Krause L, Bibiloni R, Bruneau A, Gérard P, Siddharth J, Lauber CL, Chou CJ. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci Rep. 2016;6:32484. doi: 10.1038/srep32484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, Fournier M, Lecours MA, Desjardins Y, Roy D, Levy E, Marette A. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017;6:1563–1573. doi: 10.1016/j.molmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu JD, Zhang QY, Mi MT. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio. 2016;7:e02210–e02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic fatty liver disease and insulin resistance: from bench to bedside. Diabetes Metab. 2013;39:16–26. doi: 10.1016/j.diabet.2012.11.002. [DOI] [PubMed] [Google Scholar]