Abstract
BACKGROUND
Drug toxicity is a common and even serious problem in the gastrointestinal tract that is thought to be caused by a broad spectrum of agents. Although withdrawal of the causative agent would cure the disease knowledge is scarce and mostly derives from case reports and series.
AIM
To investigate potential triggers of drug-induced colitis (DiC).
METHODS
We conducted a retrospective, observational case control study. Patients were assigned to DiC or one of two age- and gender-matched control groups (non-inflammatory controls and inflammatory colitis of another cause) based on histopathological findings. Histopathology was reassessed in a subset of patients (28 DiC with atherosclerosis, DiC without atherosclerosis and ischaemic colitis each) for validation purposes. Medical history was collected from the electronic database and patient records. Statistical analysis included chi-squared test, t-test, logistic and multivariate regression models.
RESULTS
Drug-induced colitis was detected in 211 endoscopically sampled biopsy specimens of the colon mucosa (7% of all screened colonoscopic biopsy samples); a total of 633 patients were included equally matched throughout the three groups (291 males, mean age: 62.1 ± 16.1 years). In the univariate analysis, DiC was associated with diuretics, dihydropyridines, glycosides, ASS, platelet aggregation inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), statins and fibrates, and with atherosclerosis, particularly coronary heart disease, and hyperlipoproteinaemia. Echocardiographic parameters did not show substantial differences. In the multivariate analysis only fibrates [odds ratio (OR) = 9.1], NSAIDs (OR = 6.7) and atherosclerosis (OR = 2.1) proved to be associated with DiC. Both DiC reassessment groups presented milder inflammation than ischaemic colitis. The DiC patients with atherosclerosis exhibited histological features from both DiC without atherosclerosis and ischaemic colitis.
CONCLUSION
Several drugs indicated for the treatment of cardiovascular and related diseases are associated with DiC. Atherosclerosis and microcirculatory disturbances seem to play an important pathogenetic role.
Keywords: Drug toxicity, Drug-induced colitis, Ischaemic colitis, Drug-associated gastrointestinal disease, Atherosclerosis, Colonic ischaemia, Nonsteroidal anti-inflammatory drugs, Fibrates
Core tip: Several drugs have been attributed to drug-induced colitis (DiC). In this systematical age- and gender-matched retrospective cohort study based on histopathological findings DiC was associated with drugs predominantly indicated for the treatment of cardiovascular and related diseases, nonsteroidal anti-inflammatory drugs, with atherosclerosis, particularly coronary heart disease, and hyperlipoproteinaemia. Histopathology was reassessed in three groups (DiC with atherosclerosis, DiC without atherosclerosis and ischaemic colitis each); both DiC groups presented milder inflammation than ischaemic colitis; DiC patients with atherosclerosis exhibited histological features from both other groups. In conclusion, atherosclerosis and microcirculatory disturbances seem to play an important pathogenetic role.
INTRODUCTION
Drug toxicity is a common and even serious problem in the gastrointestinal tract that is thought to be caused by a broad spectrum of drugs. Related symptoms are unspecific and assumed to cover the whole set of complaints known for colitis of any other cause, i.e., bloating, abdominal pain, cramping, diarrhoea, weight loss, mucosal bleeding or anaemia[1,2]. Furthermore, the continued intake of harmful medications can lead to structural bowel damage, i.e., development of strictures[3], perforation[4] or severe colitis with the need for emergency colectomy[5]. Although withdrawal of the respective trigger should cure the disease, data about drug-induced colitis (DiC) are scarce. Deeper insights into the phenotype of DiC, the underlying pathomechanisms and the identification of possible triggers are mandatory particularly since intake of multiple drugs hampers the determination of a single drug as the causative agent.
Various patterns of drug-induced damage at the colonic site have been described. These include nonsteroidal anti-inflammatory drug (NSAID) colonopathy, antraquinone-induced laxative-associated damage (melanosis coli), corticosteroid-associated damage (malakoplakia), gold compound-associated damage and other drug-induced conditions, such as microscopic colitis, antibiotics associated infectious and ischaemic colitis[6]. The main pathologies of drug-induced gastrointestinal disease are ulceration, stricture formation, variable inflammatory processes and ischaemia. Within these overall major patterns, microscopic clues, such as apoptosis, cytoplasmic vacuolation, increased intraepithelial lymphocytes, melanosis coli and eosinophils, are pointers to a drug-induced pathology, though all are far from specific[2].
In 2004, Cappell comprehensively collected and critically reviewed the knowledge about potential triggers and mechanisms of drug-induced colontoxicity and categorised them into well-established and probable associations[7]. Suspected agents having well-established associations with colonic ischaemia include cocaine, ergotamine and estrogens, and probable associations include alosetron, digitalis, dopamine, (nor)epinephrine, methysergide, NSAIDs, vasopressin, barbiturates, diuretics and tricyclic antidepressants. Gold compounds, NSAIDs and potassium chloride are thought to cause allergic, cytotoxic or inflammatory colitis; probable associations comprise alpha-methyldopa, salicylates, selective COX-2 inhibitors, carbamazepine, cimetidine, simvastatine, methodrexate, bisaodyl, penicillamine, isoretinoin[1,7]. Other drugs, such as immune checkpoint inhibitors (i.e., ipilimumab) and neuroleptics, have recently been identified as causing colitis[5,8]. Multiples of the associations mentioned above, such as statin use, rely on a single case report[9], a series of case reports and case series[7]. Up to now, no study has investigated potential triggers in a larger cohort of histologically suspected DiC. Hence, we aimed to analyse DiC in comparison to two different age- and gender-matched control groups.
MATERIALS AND METHODS
Study population
We conducted a single-centre retrospective cohort study of patients undergoing colonoscopy with biopsy between 2006 and 2016, referred by the Department of Gastroenterology and Hepatology of the University Hospital Bergmannsheil gGmbH in Bochum, Germany (Supplementary Table 1 and Table 1). All patients of whom a histopathological report was available were considered eligible for inclusion. Outpatients were excluded from analysis due to insufficient information about medical history.
Table 1.
Basic demographic characteristics n (%)
| Drug-induced colitis, n = 211 | Non-inflammatory controls, n = 211 | Inflammatory controls, n = 211 | |
| Basic characteristics | |||
| Age (yr) | 62.3 ± 16.4 | 62.2 ± 16.3 | 61.8 ±15.7 |
| Gender (male) | 97 (46.0) | 97 (46.0) | 97 (46.0) |
| Height (cm) | 168.9 ± 9.5 | 168.3 ± 12.4 | 170.0 ± 9.3 |
| Body weight (kg) | 76.9 ± 22.6 | 75.0 ± 20.3 | 72.9 ± 19.7 |
| BMI (kg/m2) | 26.9 ± 7.2a1b | 26.3 ± 6.8 | 25.0 ± 5.6a3 |
| ASAb2 | |||
| ASA 1 | 0 (0) | 3 (1.4) | 0 (0) |
| ASA 2 | 133 (63.0) | 169 (80.1) | 155 (73.5) |
| ASA 3 | 70 (33.2) | 37 (17.5) | 52 (24.6) |
| ASA 4 | 8 (3.8) | 2 (0.9) | 4 (1.9) |
| ECOGb2,b3 | |||
| ECOG 0 | 0 (0) | 1 (0.5) | 2 (0.9) |
| ECOG 1 | 8 (3.8) | 75 (35.5) | 13 (6.2) |
| ECOG 2 | 149 (70.6) | 104 (49.3) | 147 (69.7) |
| ECOG 3 | 41 (19.4) | 26 (12.3) | 43 (20.4) |
| ECOG 4 | 13 (6.2) | 5 (2.4) | 6 (2.8) |
| Indication of colonoscopyb1,a2 | |||
| Diarrhoea | 73 (34.6) | 95 (45.0) | 97 (46.0) |
| Constipation | 6 (2.8) | 5 (2.4) | 1 (0.5) |
| Gastrointestinal bleeding | 63 (29.9) | 31 (14.6) | 36 (17.1) |
| Abdominal pain | 47 (22.3) | 54 (25.6) | 46 (21.8) |
| Weight loss | 6 (2.8) | 5 (2.4) | 4 (1.9) |
| Scheduled survey | 2 (0.9) | 1 (0.5) | 2 (0.9) |
| Miscellaneous | 14 (6.6) | 20 (9.5) | 25 (11.8) |
Drug-induced colitis vs inflammatory controls.
Drug-induced colitis vs non-inflammatory controls.
Non-inflammatory controls vs inflammatory controls.
P < 0.05.
P < 0.01. Statistical analysis was carried out with χ2 test, ANOVA or t-test as appropriate. BMI: Body mass index.
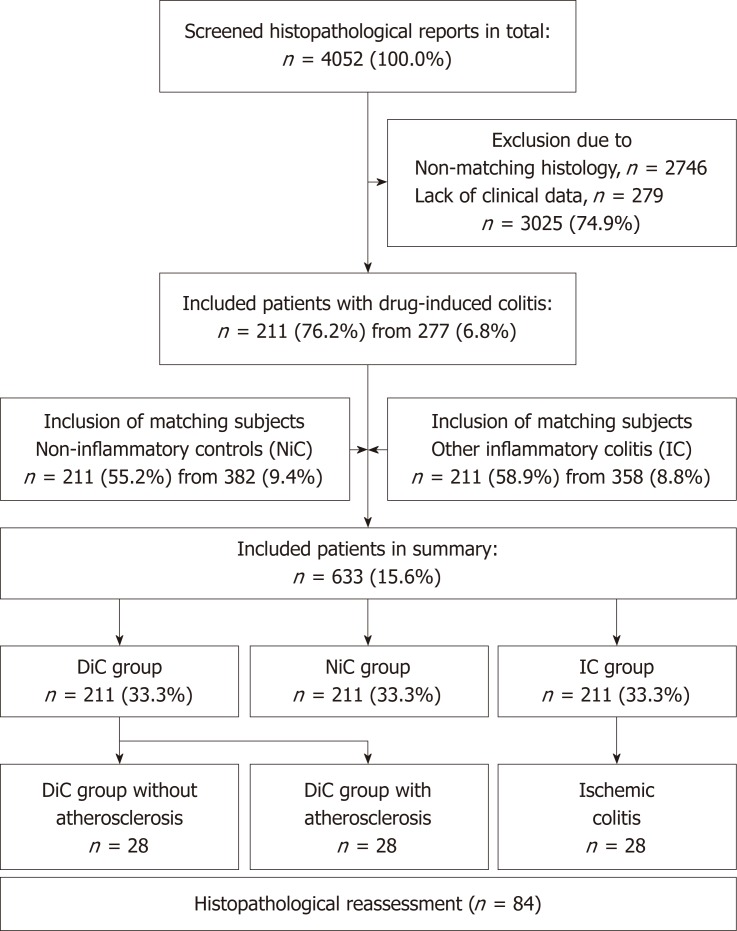
Patients were assigned to one of three groups based on histopathology: DiC, non-inflammatory controls (NiC) and inflammatory colitis of another cause (IC) (Figure 1). Patients of both control groups were age- and gender-matched; DiC patients without matching patients were excluded. Histology of microscopic colitis, pseudomembranous colitis and radiation-induced injury were reasons for exclusion for DiC patients. Non-inflammatory controls consisted of patients with irritable bowel syndrome, functional disorders or colorectal cancer screening. Inflammatory controls included diverticulitis, inflammatory bowel disease and ischaemic colitis.
Figure 1.
Flow-chart of inclusion. DiC: Drug-induced colitis; NiC: Non-inflammatory controls; IC: Inflammatory controls.
The primary histopathological assessment was validated in a second approach. From a clinical perspective, patients were divided into three groups: DiC without atherosclerosis, DiC with atherosclerosis, and ischaemic colitis. Patients from the first two groups derived from the DiC group while the latter group was gathered from the IC group. Hereby, 28 age- and gender-matched triplets were assembled.
Objectives
The aim of the study was to reveal associations between different agents and the presence of DiC. Secondary objectives were to describe the symptomatology, to identify cofactors that support the presence of DiC and to evaluate the reliability of the histopathological criteria.
Medical history
The electronic database and patient records have been reviewed to identify all drugs prescribed up to 21 d prior to colonoscopy. The specific agents were assigned to 36 different classes, as displayed in Supplementary Table 2.
Histopathological assessment
The histopathological assessment was performed by an expert pathologist based on international standards using haematoxylin and eosin stain including low- and high-power examination. Diagnosis of DiC was based on mixed, predominantly neutrophilic or lymphocytic inflammatory infiltrates, erosions, absence of granulomas, absence of basal plasmacellular infiltration and absence of crypt architectural distortion. Laxative-, corticosteroid- or gold compound-associated damage and well-defined drug-induced conditions, such as microscopic, infectious (including clostridium-associated colitis) and neutropenic colitis, were not regarded as suitable for inclusion.
Histopathological reassessment
Representative biopsy specimens were re-evaluated using haematoxylin and eosin stain with a magnification of 100-fold and assessed regarding oedema, haemorrhage, lymphocytic, granulocytic or eosinophilic infiltration, erosions, ulcerous lesions, necrosis, fibrin plaques on erosions, and fibrosis. The same team of pathologists evaluated the slides unaware of the former results and the respective group.
Statistical analysis
Statistical analysis was performed with SPSS Version 24 (IBM, Armonk, United States). Arithmetic mean and standard deviation were used for the evaluation of metric variables. Categorial data were stated as absolute and relative frequencies. Nominal variables, such as the dichotomous primary and secondary objectives, were compared using the chi-squared (χ2) test. Metric variables were analysed using the Analysis of Variance (ANOVA) and the two-tailed t-test. The binary logistic regression enter method was used for multivariate analysis. Analysis was considered statistically significant with a P-value ≤ 0.05.
Ethical concerns
The study protocol and amendment have been reviewed and approved by the institutional review board of the Ruhr-University Bochum (registration number 16-5963) based on the ethical guidelines of the Declaration of Helsinki and its later revisions. Written, informed consents were obtained from all patients before specific examinations and procedures such as colonoscopy and biopsy. For this retrospective study informed consent was neither practicable nor necessary and was exempted by the institutional review board of the Ruhr-university.
RESULTS
Basic characteristics
A total of 633 patients (291 male patients, mean age 62.1 ± 16.1 years) were included (Figure 1). Matching referring to gender and age resulted in homogenous groups with 211 subjects in either one (Supplementary Table 3). Medium body mass index (BMI) was 25.9 ± 6.1 kg/m2. Referring to American Society of Anaesthesiologist (ASA) scoring, DiC patients were characterised by higher grades, referring to the Eastern Cooperative Oncology Group (ECOG) scale, both DiC and IC patients were equally distributed, but revealed significantly higher scores than NiC patients. Indications for colonoscopy differed significantly; while diarrhoea predominated among both control groups, the major indication among DiC patients was gastrointestinal bleeding. Other indications, such as abdominal pain and scheduled survey, led to colonoscopy equally often.
Comorbidities
Most comorbidities were equally distributed, but some remarkable differences were found, as displayed in Table 2. Cardiac diseases were most common among the DiC group (52.1% vs 44.1% in NiC, P = 0.098 and 41.2% in IC, P = 0.025, χ2 test). Heart failure was less frequent in the NiC group (7.6% vs 16.6% and 14.7%, P = 0.014, χ2 test); atrial fibrillation did not differ. Coronary heart disease was most common among DiC patients (22.3% vs 12.3% and 16.6%, P < 0.001, χ2 test). While peripheral arterial occlusive disease did not differ, the overall manifestations of atherosclerosis appeared nearly twofold more often among DiC patients (35.1% vs 18.5% and 19.0%, P < 0.001, χ2 test). While most cardiovascular risk factors were equally distributed, hyperlipoprotenaemia was more common among DiC patients (10.4% vs 5.2% and 4.3%). Surgery during the same hospital stay was a relatively rare event but was overrepresented among the DiC patients (4.3% vs 0.5% and 1.4%, P = 0.024, χ2 test).
Table 2.
Comorbidities n (%)
| Comorbidity | Drug-induced colitis, n = 211 | Non-inflammatory controls, n = 211 | Inflammatory controls, n = 211 |
| Pulmonary | 39 (18.5) | 31 (14.7) | 37 (17.5) |
| Cardiac | 110 (52.1) | 93 (44.1) | 87 (41.2) |
| Neurological | 27 (12.8) | 23 (10.9) | 19 (9.0) |
| Psychiatric | 10 (4.7) | 9 (4.3) | 10 (4.7) |
| Endocrine | 47 (22.3) | 44 (20.9) | 30 (14.2) |
| Renal | 28 (13.2) | 18 (8.5) | 21 (10.0) |
| Hepatic | 11 (5.2) | 13 (6.2) | 11 (5.2) |
| Oncological | 14 (6.6) | 25 (11.8) | 17 (8.1) |
| Other | 19 (9.0) | 15 (7.1) | 16 (7.6) |
| Heart failure | 35 (16.6) | 16 (7.6)b2 | 31 (14.7)a3 |
| Renal insufficiency | 24 (11.4) | 14 (6.6) | 18 (8.5) |
| Atrial fibrillation | 26 (12.3) | 18 (8.5) | 22 (10.4) |
| Coronary heart disease | 47 (22.3) | 28 (13.3)a2 | 35 (16.6) |
| Peripheral arterial occlusive disease | 7 (3.3) | 10 (4.7) | 11 (5.2) |
| Atherosclerosis | 74 (35.1)b1 | 39 (18.5)b2 | 40 (19.0) |
| Arterial hypertension | 98 (46.4) | 97 (46.0) | 87 (41.2) |
| Diabetes mellitus | 46 (21.8) | 45 (21.3) | 32 (15.2) |
| Hypercholesterinaemia | 20 (9.5) | 16 (7.6) | 18 (8.5) |
| Hyperlipoproteinaemia | 22 (10.4)a1 | 11 (5.2)a2 | 9 (4.3) |
| Chronic obstructive lung disease | 29 (13.7) | 24 (11.4) | 27 (12.8) |
| Stroke | 14 (6.6) | 9 (4.3) | 11 (5.2) |
| Smoking | 77 (36.7) | 66 (31.3) | 78 (37.0) |
| Surgery | 9 (4.3) | 1 (0.5)a2 | 3 (1.4) |
| Intensive care therapy | 9 (4.3) | 2 (0.9)a2 | 4 (3.1) |
Drug-induced colitis vs inflammatory controls.
Drug-induced colitis vs non-inflammatory controls.
Non-inflammatory controls vs inflammatory controls.
P < 0.05.
P < 0.01.
Statistical analysis was carried out with χ2 test.
Histological assessment
Histopathological patterns are displayed in Supplementary Table 4. Inflammatory features were rarely seen among the NiC group (between 0% and 14.7%), except for lymphoplasmacellular and granulocytic infiltration (40.3%). Oedema, erosions, regeneratory hyperplasia of the crypts and subepithelial haemorrhage occurred equally often among DiC and IC patients (24.2%, 35.5%, 12.3% and 16.4%, respectively). Ischaemia and ulcers were most common among the IC group (5.2% vs 0.5% and 14.7% vs 7.6%, P < 0.001, χ2 test), while eosinophilia and mucosal fibrosis were reported most often in the DiC group (24.6% vs 21.8% vs 8.2%, P < 0.001 and 28.0% vs 21.8% vs 14.7%, P = 0.004, respectively).
Histological reassessment
A total of 28 age- and gender-matched triplets (84 patients altogether) were assembled for the reassessment. Among them, two-thirds were female patients (n = 57, 67.9%, mean age 75.6 ± 7.6 years). The inflammatory activity in both DiC groups was predominantly mild (89.3% and 82.1%, respectively), while patients with ischaemic colitis were uniformly distributed between mild, moderate and severe inflammation (Supplementary Table 5). The inflammation in both DiC groups occurred significantly more often in the ascending colon compared to ischaemic colitis (Supplementary Table 6). The same tendency, but less pronounced, was present regarding the coecum.
The histopathological reassessment revealed differences between both DiC groups and ischaemic colitis (Table 3 and Figure 2); for instance, eosinophilic infiltration was more common (42.9% and 25.0% vs 3.6%), while ulcers and necrosis occurred less frequently (14.3% and 10.7% vs 57.1% and 3.6% vs 57.1%, respectively). On the other hand, haemorrhage and erosions were equally present between DiC with atherosclerosis and ischaemic colitis but were more common than in the DiC group without atherosclerosis (28.6% and 21.4% vs 3.6% and 32.1% and 35.1% vs 7.1%, respectively). Oedema, lymphocytic and granulocytic infiltration, fibrin plaques and fibrosis were similar in both groups.
Table 3.
Histopathological reassessment n (%)
| Parameter (n = 28) | Drug-induced colitis without atherosclerosis | Drug-induced colitis with atherosclerosis | Ischaemic colitis |
| Oedema | 2 (7.1) | 2 (7.1) | 4 (14.3) |
| Haemorrhage | 1 (3.6)a2 | 8 (28.6)a1 | 6 (21.4) |
| Lymphocytic infiltration | 27 (96.4) | 27 (96.4) | 27 (96.4) |
| Granulocytic infiltration | 27 (96.4) | 26 (92.9) | 28 (100.0) |
| Eosinophilic infiltration | 12 (42.9)b2 | 7 (25.0) | 1 (3.6)a3 |
| Erosions | 2 (7.1)b2 | 9 (32.1)a1 | 10 (35.7) |
| Ulcerous lesions | 4 (14.3)b2 | 3 (10.7) | 16 (57.1)b3 |
| Necrosis | 1 (3.6)b2 | 1 (3.6) | 16 (57.1)b3 |
| Fibrin plaques on erosions | 2 (7.1) | 2 (7.1) | 3 (10.7) |
| Fibrosis | 3 (10.7) | 3 (10.7) | 7 (25.0) |
Drug-induced colitis without atherosclerosis vs drug-induced colitis with atherosclerosis.
Drug-induced colitis without atherosclerosis vs ischaemic colitis.
Drug-induced colitis with atherosclerosis vs ischaemic colitis.
P < 0.05.
P < 0.01.
Statistical analysis was carried out with χ2 test.
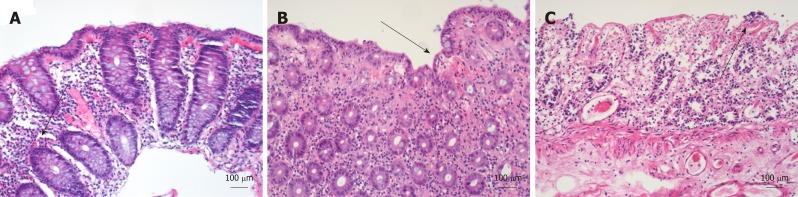
Figure 2.
Histological appearance of drug-induced colitis with atherosclerosis, drug-induced colitis without atherosclerosis, and ischaemic colitis in hematoxylin and eosin stain. A: Drug-induced colitis (DiC) with atherosclerosis; B: DiC without atherosclerosis; C: Ischaemic colitis. Three different groups of 28 patients each were collected for histological reassessment. DiC with atherosclerosis (A) is characterised by lymphocytic, granulocytic and eosinophilic infiltration (marked with an arrow) while haemorrhage, necrosis is rarely present. In ischaemic colitis, (C) ulcers, necrosis (marked with an arrow), and erosions predominate, and haemorrhage and fibrosis also occur. Eosinophilic infiltrations are rarely seen. DiC without atherosclerosis (B) shows features of both DiC without atherosclerosis and ischaemic colitis. These include haemorrhage, eosinophilic infiltration and erosions (marked with an arrow).
Drug assessment
Patients with DiC took significantly more drugs (mean 4.5 ± 2.8) than NiC (mean 3.9 ± 3.0, P = 0.042) and tendentially more than IC patients (3.9 ± 3.2, P = 0.071). The frequencies of specific drug classes are detailed in Table 4. Betablockers, angiotensin-converting enzyme (ACE) inhibitors, benzothiazines, aldosterone antagonists, nitrates, antiarrhythmic drugs, glycosides, metamizole, potassium, vitamin K antagonists, direct thrombin inhibitors, insulin, proton pump inhibitors, thyreostatics, antibiotics, tricyclic antidepressants, neuroleptics and sedatives were equally distributed between all three study groups.
Table 4.
Drug assessment n (%)
| Group | Drug-induced colitis, n = 211 | Non-inflammatory controls, n = 211 | Inflammatory controls, n = 211 |
| Betablocker | 97 (46.0) | 78 (37.0) | 83 (39.3) |
| ACE inhibitors | 70 (33.2) | 55 (26.1) | 62 (29.4) |
| Angiotensin II inhibitors | 20 (9.5)b1 | 24 (11.4) | 6 (2.8)b3 |
| Non-Dihydropyridines | 1 (0.5)a1 | 6 (2.8) | 7 (3.3) |
| Dihydropyridines | 34 (16.1)a1 | 27 (12.8) | 20 (9.5) |
| Diuretics | 55 (26.1)b1 | 29 (13.7)b2 | 33 (15.7) |
| Benzothiazines | 28 (13.3) | 33 (15.6) | 24 (11.4) |
| Aldosterone antagonists | 13 (6.2) | 13 (6.2) | 12 (5.7) |
| Nitrates | 7 (3.3) | 13 (6.2) | 5 (2.4) |
| Antiarrhythmic drugs | 7 (3.3) | 2 (0.9) | 2 (0.9) |
| Glycosides | 10 (4.7) | 2 (0.9)a2 | 8 (3.8) |
| ASS (100 mg to 300 mg) | 67 (31.8)b1 | 47 (22.3)a2 | 40 (19.0) |
| Platelet aggregation inhibitors | 20 (9.5)b1 | 10 (4.7) | 6 (2.8) |
| NSAIDs | 35 (16.6)b1 | 21 (10.0)a2 | 8 (3.8)a3 |
| Metamizole | 21 (10.0) | 16 (7.6) | 21 (10.0) |
| Potassium | 4 (1.9) | 3 (1.4) | 6 (2.8) |
| Vitman K antagonists/coumarin derivates | 16 (7.6) | 9 (4.3) | 11 (5.2) |
| Direct thrombin inhibitors | 6 (2.8) | 2 (0.9) | 4 (1.9) |
| Glucocorticosteroids | 13 (6.2)b1 | 14 (6.6) | 41 (19.4)b3 |
| Opioids | 20 (9.5) | 22 (10.4) | 23 (10.9) |
| Metformin | 8 (3.8) | 16 (7.6) | 4 (1.9)b3 |
| Insulin | 17 (8.1) | 15 (7.1) | 17 (8.1) |
| Statins | 56 (26.5)a1 | 42 (19.9) | 38 (18.0) |
| Fibrates | 7 (3.3)a1 | 1 (0.5)a2 | 1 (0.5) |
| Levothyroxine | 25 (11.8) | 39 (18.5) | 22 (10.4)a3 |
| Thyreostatics | 3 (1.4) | 3 (1.4) | 1 (0.5) |
| Proton pump inhibitors | 100 (47.4) | 85 (40.3) | 81 (38.4) |
| Penicillin derivates | 0 (0.0) | 2 (0.9) | 2 (0.9) |
| Macrolides | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Gyrase inhibitor | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Carbapenems | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Imidazoles | 3 (1.4) | 1 (0.5) | 1 (0.5) |
| Cephalosporins | 2 (0.9) | 2 (0.9) | 2 (0.9) |
| Antbiotics | 4 (1.9) | 5 (2.4) | 3 (1.4) |
| SSRIs | 14 (6.6) | 5 (2.4)a2 | 16 (7.6)a3 |
| Tricyclic antidepressants | 7 (3.3) | 10 (4.7) | 7 (3.3) |
| Neuroleptics | 5 (2.4) | 8 (3.8) | 6 (2.8) |
| Sedatives | 16 (7.6) | 16 (7.6) | 16 (7.6) |
| Others | 127 (60.2)a1 | 125 (59.2) | 147 (69.7)a3 |
| Number of drugsb | 4.5 ± 2.8 | 3.9 ± 3.0a2 | 3.9 ± 3.2 |
Drug-induced colitis vs inflammatory controls.
Drug-induced colitis vs non-inflammatory controls.
Non-inflammatory controls vs inflammatory controls.
P < 0.05.
P < 0.01.
Statistical analysis was carried out with χ2 test (a) or t-test (b). NSAIDs : Nonsteroidal anti-inflammatory drugs; SSRIs: Selective serotonin reuptake inhibitors; ACE: Angiotensin-converting enzyme.
Significant differences with the highest frequencies among DiC vs NiC vs IC patients were found regarding diuretics, dihydropyridines, glycosides, ASS (31.8% vs 22.3% vs 19.0%, P = 0.006), platelet aggregation inhibitors, NSAIDs, statins and fibrates.
The lowest frequencies in DiC vs NiC vs IC patients were found regarding non-dihydropyridines. The lowest frequencies in IC vs DiC vs NiC patients were found regarding angiotensin II inhibitors and metformin. The highest frequencies in IC vs DiC vs NiC group were found regarding glucocorticosteroids and other unspecified drugs. The highest frequency among the NiC vs DiC vs IC group was found for levothyroxine and lowest for selective serotonin reuptake inhibitors (SSRIs).
Among the reassessment subgroups (Supplementary Table 7), DiC patients without atherosclerosis took significantly fewer drugs than both other subgroups (4.8 ± 2.5 vs 6.6 ± 2.3, P < 0.01). The use of beta blockers, dihydropyridines, ASS and aldosterone antagonists was lower, though not significantly; statin use was significantly lowest (10.7% vs 60.7% vs 46.4%, P < 0.01). All other drug classes, including fibrates and NSAIDs, were distributed equally; particularly, there were no remarkable differences between DiC patients with atherosclerosis and ischaemic colitis patients.
Echocardiographic parameters
Heart ultrasound was available from 111 patients (17.5%), among them were 54 DiC (25.6%), 34 NiC (16.1%) and 23 IC (11.4%) patients (Table 5). Global left ventricular function was significantly better in NiC patients than in both other groups (P < 0.05) in which normal function was present in 72.2% (DiC) and 73.9% (IC), respectively. There was no moderate or severe decrease among NiC, while DiC patients presented in 9.3% and 11.1% and IC patients in 8.7% and 13.0%, respectively. Additionally, dilation of the left ventricular was reported in none of the NiC patients, but in 14.8% of DiC and even 26.1% of the IC patients (P = 0.036, χ2 test). Dilation of the right atrium was reported in 7.4% of DiC patients, 11.4% of NiC and 23.8% of IC patients (P = 0.142, χ2 test). No differences occurred regarding hypokinesia or diastolic dysfunction. Limited sample size among the reassessment groups inhibited the subgroup analysis.
Table 5.
Echocardiographic parameters n (%)
| Parameter | Drug-induced colitis, n = 54 of 211 (25.6) | Non-inflammatory controls, n = 34 of 211 (16.1) | Inflammatory controls, n = 24 of 211 (11.4) |
| Dilated right atrium | 4 (7.4)a1 | 4 (11.4) | 5 (23.8) |
| Dilated left ventricle | 8 (14.8) | 0 (0.0) | 6 (26.1)b3 |
| Hypokinesia | 10 (18.5) | 5 (14.3) | 3 (13.0) |
| Right heart failure | 5 (9.3) | 4 (11.4) | 2 (8.7) |
| Diastolic dysfunction | 22 (43.1) | 16 (48.5) | 7 (30.4) |
| LV Functionb2,b3 | |||
| Normal (> 50%) | 39 (72.2) | 29 (85.3) | 17 (73.9) |
| Slightly decreased (40%-50%) | 4 (7.4) | 5 (14.7) | 1 (4.3) |
| Moderately decreased (30%-40%) | 5 (9.3) | 0 (0.0) | 2 (8.7) |
| Severely decreased (< 30%) | 6 (11.1) | 0 (0.0) | 3 (13.0) |
Drug-induced colitis vs inflammatory controls.
Drug-induced colitis vs non-inflammatory controls.
Non-inflammatory controls vs inflammatory controls.
P < 0.05.
P < 0.01.
Statistical analysis was carried out with χ2 test. LV : Left ventricular.
Binary logistic regression models
Different models of binary logistic regression analysis were calculated (Table 6). The model comparing DiC patients and NiC that contained the variables chronic heart failure, any manifestation of atherosclerosis, on the one hand, and dihydropyridines, diuretics, digitalis glycosides, low-dose aspirin, any other platelet aggregation inhibitor, NSAIDs, statins and fibrates, on the other hand, revealed a statistically significant model (Omnibus test P < 0.001; Hosmer-Lemeshow test P = 0.598) with low predictive power (Nagelkerke’s R2 = 0.109). In this model, only NSAIDs (P = 0.010) and fibrates remained statistically significant, while atherosclerosis (P = 0.087) and diuretics (P = 0.070) showed a persisting tendency. The related odds ratios were 2.2 (CI: 1.2-4.0) and 8.9 (CI: 1.1-74.2), respectively. The resembling models without dihydropyridines or additional ASA or ECOG score showed comparable results (data not shown).
Table 6.
Binary logistic regression analysis
| Parameter | Drug-induced colitis vs inflammatory controls OR (95%CI) | Drug-induced colitis vs non-inflammatory controls OR (95%CI) |
| Heart failure | 0.6 (0.3-1.1) | 1.3 (0.6-2.8) |
| Atherosclerosis | 2.1 (1.2-3.7)b | 1.7 (0.9-3.1) |
| Dihydropyridines | 1.3 (0.7-2.5) | 1.0 (0.5-1.8) |
| Diuretics | 1.5 (0.8-2.6) | 1.7 (1.0-3.0) |
| Digitalis glycosides | 1.1 (0.4-3.1) | 3.7 (0.8-17.9) |
| Low-dose ASS | 1.7 (1.0-2.8) | 1.1 (0.7-1.9) |
| Platelet aggregation inhibitors | 2.0 (0.7-5.7) | 1.4 (0.6-3.3) |
| NSAIDs | 6.7 (3.0-15.1)b | 2.2 (1.2-4.0)a |
| Statins | 1.1 (0.6-1.9) | 0.9 (0.6-1.6) |
| Fibrates | 9.1 (1.1-74.3)a | 8.9 (1.1-74.2)a |
P < 0.05.
P < 0.01.
NSAIDs: Nonsteroidal anti-inflammatory drugs.
The same model comparing DiC and IC patients revealed a higher but still low predictive power (Omnibus test P < 0.001; Hosmer-Lemeshow test P = 0.432, Nagelkerke’s R2 = 0.170). Significant variables comprised atherosclerosis (P = 0.009), NSAIDs (P < 0.001) and fibrates (P = 0.043) while aspirin showed only a tendency (P = 0.059). The related ORs were 2.1 (CI: 1.2-3.7), 6.7 (CI: 3.0-15.1) and 9.1 (CI: 1.1-76.4). Skipping dihydropyridines or adding the ASA or ECOG score did not change the results substantially (data not shown).
DISCUSSION
In this age- and gender-matched retrospective cohort study, DiC was detected in about 7% of endoscopically sampled biopsy specimens of the colon mucosa. This prevalence coincides well with estimated numbers from a recently published overview[6,10]. Nevertheless, DiC (despite clostridium-associated[11,12] and microscopic colitis[13]) is a rarely reported entity in the literature and most knowledge derives from case reports or case series[7]. Therefore, to the best of our knowledge, this is the first study that systematically investigates a large set of potential triggers of DiC.
Potential triggers of DiC
In univariate analysis, DiC was associated with diuretics, dihydropyridines, glycosides, ASS, platelet aggregation inhibitors, NSAIDs, statins and fibrates. In addition to NSAIDs, that are well established to induce DiC[10,14,15], several drugs that are indicated for the treatment of heart failure, atherosclerosis and related conditions (such as fat-lowering medication) were associated with DiC. Cardiac and vascular comorbidity might, therefore, be a substantial confounding factor or the cause of the disease itself.
Several aspects were undertaken to control confounding factors. Two age- and gender-matched control groups were gathered, one of which consisted of patients with NiC while the other group was assembled from patients with IC. This matching resulted in rather equally distributed comorbidities and only a very few entities differed significantly. While overall cardiac comorbidity and atrial fibrillation were equally distributed between all three groups, atherosclerosis, particularly coronary heart disease, and hyperlipoproteinaemia were more common among DiC patients. Heart failure occurred similarly in the DiC and IC group, but more often than in NiC. Echocardiography was available for only a minority of patients (between 11% and 26%, respectively), but did not show restricted function among DiC patients. In detail, the left ventricular function did not differ between DiC and IC patients, while NiC patients preferentially showed a preserved ejection fraction. Dilation of the left ventricle and right atrium were most common among IC patients, while hypokinesia or diastolic dysfunction were the same among all three groups.
Only fibrates (OR = 9.1), NSAIDs (OR = 6.7), and atherosclerosis (OR = 2.1) proved to be associated with DiC in the multivariate analysis, while heart failure, dihydropyridines, glycosides, diuretics, low-dose aspirin, platelet aggregation inhibitors and statins lost their relevance. The effects of NSAIDs on the gastrointestinal tract have been extensively investigated, however, the association of fibrates and the role of atherosclerosis are novel findings.
Pathogenetic role of ischaemia
It has been proposed that medications can cause colonic ischaemia due to neuronal stimulation (e.g., cocaine), by promoting thrombosis due to hormonal effects (e.g., estrogen) or by extrinsic compression due to fibrosis (e.g., methysergide). Other drugs may promote colonic ischaemia by shunting blood away from the mesenteric vasculature (e.g., digitalis[16]) by decreasing fluid volume (e.g., diuretics[17]), inducing vasculitis or vascular spasms, or by other, not yet understood mechanisms[2,7,18,19]. It was hypothesised that drugs, such as digitalis and diuretics, may predispose to ischaemic colitis in elderly patients in a state of low blood flow, often due to heart failure[6], but up to now, there has been no proof of this concept. The overall results support the idea of disturbances in microperfusion at least in a subset of patients, maybe those patients with underlying atherosclerosis.
The subgroup analysis of either 28 matched DiC patients without atherosclerosis, with atherosclerosis and patients with ischaemic colitis further strengthens this concept. The DiC patients without atherosclerosis took significantly fewer remedies than both other subgroups and used beta blockers, dihydropyridines, ASS and aldosterone antagonists less often, though not significantly. While statin intake was significantly highest among the DiC subgroup with atherosclerotic manifestations, all other drug classes, including fibrates and NSAIDs, diversified similarly; there were no specific remarkable differences between DiC patients with atherosclerosis and ischaemic colitis patients. Indeed, this distribution does not prove but at least gives a distinct clue that atherosclerosis and focal hypoxia play a substantial role in the aetiopathogenesis of DiC in a subset of patients.
Reliability of histopathological assessment
The presence of DiC in the routine histological assessment was the major inclusion criterion. The pathological changes of neither drug-induced nor ischaemic colitis are specific and significant histological overlap exists between both entities[2,18,20]. Nevertheless, acute ischaemic colitis is observed predominantly in elderly (older than 65 years) subjects[21], while the patients of our cohort were distinctly younger (62 years). None of the three different histologic variants of colitis with ischaemic features that Jessurun recently distinguished has been included in the DiC group[20]. Given the common absence of strictly specific histopathological features, the diagnosis of DiC often relies upon thorough clinicopathological correlation, but in clinical practice it is difficult to establish the correlation between a certain medication and a particular pattern of injury. The optimal establishment of a causal relationship that results from improvement with withdrawal and recurrence with rechallenge of the suspected agent is rarely available and was not a common practice in our cohort. This approach is hardly realisable when dealing with a single patient who most commonly takes a multitude of drugs; the mean number in our cohort summed up to 4.5 different agents. Thus, it is the pathologist’s task to offer a strong suspicion concerning possible drug-related pathology[19].
The biopsy specimens were reassessed in a subset of patients and compared in three different groups: DiC patients with atherosclerosis, DiC patients without atherosclerosis and patients with ischaemic colitis, to gain deeper insights into the reliability of the histopathological assessment. Patients of both DiC groups presented milder inflammatory activity, suggesting common pathophysiological pathways that can be distinguished from purely ischaemic colitis. Indeed, DiC patients with atherosclerosis exhibited features from both DiC without atherosclerosis and ischaemic colitis: Haemorrhage and erosions were the same among atherosclerotic DiC and ischaemic colitis patients, while the presence of ulcers and necrosis were equal in both DiC groups. Interestingly, infiltration with eosinophils that is characteristic for DiC2 were found most often in the DiC group without atherosclerosis, while it rarely appeared among the ischaemic colitis group (42.9% vs 3.6%); atherosclerotic DiC patients ranked in between (25.0%). We, therefore, conclude that focal ischaemia due to disturbances of the microcirculation, probably supported by large vessel atherosclerosis, plays a substantial role particularly in a subset of patients with DiC.
Strengths and limitations
Most patients with acute colitis are diagnosed and treated based on a combination of clinical and laboratory findings without colonoscopy and mucosal biopsy analysis. Endoscopic evaluation is reserved for patients who present with severe or atypical clinical course, do not improve within an expected timeframe or fail to respond to standard treatment[20]. Therefore, the inclusion of histologically suspected DiC bears a potential selection bias. The histopathological approach was limited to colonic manifestations with morphological changes, while functional diarrhoea, alterations on a (sub)cellular level or manifestations of the small intestine were beyond the scope of the study. Despite all potential limitations, this is the largest study that investigates potential triggers of DiC and potential confounders systematically. The fact that NSAID use has clearly been shown to induce DiC[10,14] could have been reproduced in our cohort argues seriously for the reliability of the approach chosen.
CONCLUSION
In conclusion, histopathologically suspected DiC is associated with not only NSAIDs, angiotensin II inhibitors, dihydropyridines, diuretics, ASS and other platelet aggregation inhibitors, as well as statins and fibrates, but also coronary heart disease, hyperlipoproteinaemia and, partially, heart failure. Atherosclerosis (OR = 2.1) and the intake of NSAIDs (OR = 6.7) and fibrates (OR = 9.1) were associated most strongly with DiC in the multivariate analysis. Since a subset of DiC patients with atherosclerosis exhibited histological features of both DiC without atherosclerosis and ischaemia, we propose that focal disturbances of the microcirculation play a substantial role in the pathogenesis of a subgroup of DiC patients. The distribution of drug intake further supports this hypothesis, but the study design is not suitable to prove it. Prospective studies including larger cohorts with clearly defined cardiac function, pattern and severity of atherosclerosis and related comorbidities, such as hyperlipoprotenaemia, are warranted to unravel the underlying aetiology and pathophysiology of this under-recognised entity. Meanwhile, the histological suspicion of DiC might not necessarily reveal a drug-related mechanism but could also reflect focal ischaemia that is supported by macroangiopathy at least in a subset of patients.
ARTICLE HIGHLIGHTS
Research background
Drug-induced colitis is a common and even serious problem, but the knowledge about associated triggers is scarce.
Research motivation
Withdrawal of the respective trigger should cure the disease, so that its identification is crucial. Therefore, deeper insights into the aetiopathogenesis and knowledge about potential triggers is mandatory.
Research objectives
Consequently, the study aimed to identify potential triggers of histologically suspected drug-induced colitis.
Research methods
A retrospective case control study of 211 patients with histologically suspected drug-induced colitis and two age- and gender matched control groups was performed. The drug-induced colitis (DiC) patients showed histological changes attributable to drug-induced pathology, i.e., mixed, predominantly neutrophilic or lymphocytic inflammatory infiltrates, erosions, absence of granulomas, absence of basal plasmacellular infiltration and absence of crypt architectural distortion. The control groups consisted of patients with inflammatory colitis other than DiC and inflammatory bowel disease (i.e., diverticulitis, ischaemic colitis) and of patients without substantial histological changes (i.e., irritable bowel syndrome, cancer screening). Clinical data including drug history was obtained from the electronic data base.
In a second approach, patients were divided into three groups from a clinical perspective: DiC without atherosclerotic comorbidity, DiC with atherosclerotic comorbidity, and ischaemic colitis. Patients from the first two groups derived from the DiC group while the latter group was gathered from the inflammatory controls.
Research results
A total of 633 patients (291 male patients, mean age 62.1 ± 16.1 years) were included. Patients with DiC took more drugs (mean 4.5 ± 2.8) than patients from both other groups (mean 3.9 ± 3.0 and 3.9 ± 3.2, respectively). In univariate analysis, DiC was associated with diuretics, dihydropyridines, glycosides, ASS, platelet aggregation inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), statins and fibrates. In addition to NSAIDs, that are well established to induce DiC, several drugs that are indicated for the treatment of heart failure, atherosclerosis and related conditions were associated with DiC. Cardiac and vascular comorbidity might, therefore, be a substantial confounding factor or the cause of the disease itself. In fact, atherosclerosis was more common among patients with DiC. In multivariate analysis, atherosclerosis (OR = 2.1) and the intake of NSAIDs (OR = 6.7) and fibrates (OR = 9.1) were associated most strongly with DiC. Since a subset of DiC patients with atherosclerosis exhibited histological features of both DiC without atherosclerosis and ischaemia, we propose that focal disturbances of the microcirculation play a substantial role in the pathogenesis of a subgroup of DiC patients. A total of 28 age- and gender-matched triplets were assembled for the histological reassessment. Some DiC patients with atherosclerosis exhibited histological features of both control groups, DiC without atherosclerosis and ischaemia.
Research conclusions
While most knowledge of drug-induced colitis relies on case reports and case series this is the first study that systematically investigates potential triggers of DiC. This large case control study reveals that patients with the histopathological suspicion of drug-induced colitis take more different drugs than age- and gender-matched control patients. Associated remedies include drugs that are indicated for the treatment of heart failure, atherosclerosis and related conditions. Furthermore, atherosclerosis was more common among DiC patients. We therefore hypothesise that focal disturbances of the microcirculation play a substantial role in the pathogenesis of a subgroup of DiC patients, but the study design is not suitable to prove this hypothesis.
Research perspectives
Prospective studies including larger cohorts with clearly defined cardiac function, pattern and severity of atherosclerosis and related comorbidities, such as hyperlipoprotenaemia, are warranted to unravel the underlying aetiology and pathophysiology of this under-recognised entity. Meanwhile, the histological suspicion of DiC might not necessarily reveal a drug-related mechanism but could also reflect focal ischaemia that is supported by macroangiopathy at least in a subset of patients.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was approved by the institutional review board of the Ruhr-university Bochum [registration number 16-5963] on the basis of the ethical guidelines of the Declaration of Helsinki and its later revisions.
Informed consent statement: Written, informed consents were obtained from all patients before specific examinations and procedures such as colonoscopy and biopsy. For this retrospective study informed consent was neither practicable nor necessary.
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: All authors had unlimited access to the data.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Peer-review started: November 27, 2018
First decision: December 20, 2018
Article in press: January 26, 2019
P- Reviewer: Chiba T, Eleftheriadis NP, Tarnawski AS S- Editor: Ma RY L- Editor: A E- Editor: Yin SY
Contributor Information
Thorsten Brechmann, Department of Gastroenterology and Hepatology, Ruhr-University Bochum, Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil gGmbH, Bochum 44789, Germany. thorsten.brechmann@rub.de.
Katharina Günther, Department of Gastroenterology and Hepatology, Ruhr-University Bochum, Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil gGmbH, Bochum 44789, Germany.
Matthias Neid, Institute of Pathology, Ruhr-University Bochum, Bochum 44789, Germany.
Wolff Schmiegel, Department of Gastroenterology and Hepatology, Ruhr-University Bochum, Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil gGmbH, Bochum 44789, Germany; Department of Internal Medicine, University Hospital Knappschaftskrankenhaus, Ruhr-University Bochum, Bochum 44892, Germany.
Andrea Tannapfel, Institute of Pathology, Ruhr-University Bochum, Bochum 44789, Germany.
References
- 1.Sherid M, Ehrenpreis ED. Types of colitis based on histology. Dis Mon. 2011;57:457–489. doi: 10.1016/j.disamonth.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Price AB. Pathology of drug-associated gastrointestinal disease. Br J Clin Pharmacol. 2003;56:477–482. doi: 10.1046/j.1365-2125.2003.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Püspök A, Kiener HP, Oberhuber G. Clinical, endoscopic, and histologic spectrum of nonsteroidal anti-inflammatory drug-induced lesions in the colon. Dis Colon Rectum. 2000;43:685–691. doi: 10.1007/BF02235589. [DOI] [PubMed] [Google Scholar]
- 4.Hsu SC, Chang SS, Lee MG, Lee SH, Tsai YW, Lin SC, Chen ST, Weng YC, Porta L, Wu JY, Lee CC. Risk of gastrointestinal perforation in patients taking oral fluoroquinolone therapy: An analysis of nationally representative cohort. PLoS One. 2017;12:e0183813. doi: 10.1371/journal.pone.0183813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdalla S, Brouquet A, Lazure T, Costaglioli B, Penna C, Benoist S. Outcome of emergency surgery for severe neuroleptic-induced colitis: results of a prospective cohort. Colorectal Dis. 2016;18:1179–1185. doi: 10.1111/codi.13376. [DOI] [PubMed] [Google Scholar]
- 6.Pusztaszeri MP, Genta RM, Cryer BL. Drug-induced injury in the gastrointestinal tract: clinical and pathologic considerations. Nat Clin Pract Gastroenterol Hepatol. 2007;4:442–453. doi: 10.1038/ncpgasthep0896. [DOI] [PubMed] [Google Scholar]
- 7.Cappell MS. Colonic toxicity of administered drugs and chemicals. Am J Gastroenterol. 2004;99:1175–1190. doi: 10.1111/j.1572-0241.2004.30192.x. [DOI] [PubMed] [Google Scholar]
- 8.Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28:264–268. doi: 10.1097/CCO.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 9.Rea WE, Durrant DC, Boldy DA. Ulcerative colitis after statin treatment. Postgrad Med J. 2002;78:286–287. doi: 10.1136/pmj.78.919.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grattagliano I, Ubaldi E, Portincasa P. Drug-induced enterocolitis: Prevention and management in primary care. J Dig Dis. 2018;19:127–135. doi: 10.1111/1751-2980.12585. [DOI] [PubMed] [Google Scholar]
- 11.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016;22 Suppl 4:S63–S81. doi: 10.1016/j.cmi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Cotter TG, Pardi DS. Current Approach to the Evaluation and Management of Microscopic Colitis. Curr Gastroenterol Rep. 2017;19:8. doi: 10.1007/s11894-017-0551-3. [DOI] [PubMed] [Google Scholar]
- 14.Kwak HA, Hart J. The Many Faces of Medication-Related Injury in the Gastrointestinal Tract. Surg Pathol Clin. 2017;10:887–908. doi: 10.1016/j.path.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154:500–514. doi: 10.1053/j.gastro.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Keller HW, Lorenz R, Müller JM, Pichlmaier H. [Ischemic colitis following digitalis poisoning] Chirurg. 1984;55:830–831. [PubMed] [Google Scholar]
- 17.Sharefkin JB, Silen W. Diuretic agents: inciting factor in nonocclusive mesenteric infarction? JAMA. 1974;229:1451–1453. doi: 10.1001/jama.229.11.1451. [DOI] [PubMed] [Google Scholar]
- 18.Yang RD, Han MW, McCarthy JH. Ischemic colitis in a crack abuser. Dig Dis Sci. 1991;36:238–240. doi: 10.1007/BF01300764. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy AJ, Lauwers GY, Sheahan K. Iatrogenic pathology of the intestines. Histopathology. 2015;66:15–28. doi: 10.1111/his.12598. [DOI] [PubMed] [Google Scholar]
- 20.Jessurun J. The Differential Diagnosis of Acute Colitis: Clues to a Specific Diagnosis. Surg Pathol Clin. 2017;10:863–885. doi: 10.1016/j.path.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther. 2004;19:729–738. doi: 10.1111/j.1365-2036.2004.01903.x. [DOI] [PubMed] [Google Scholar]