Abstract
Background:
A number of hepatocellular carcinoma (HCC) patients have developed resistance against transcatheter arterial chemoembolization (TACE) treatment. In this study, we aimed to develop a panel of microRNAs (miRs) biomarkers to predict clinical outcomes in HCC patients after TACE treatment.
Methods:
The expression level of twenty miRs was evaluated in FFPE tissues collected from 33 HCC patients. We selected four differentially expressed miRs in TACE-responders versus non-responders and re-assessed their expression in 51 serum samples. The expressions of miRs associated with overall survival (OS), progression-free survival (PFS), and treatment outcomes were investigated. The diagnostic accuracy of these miRs in predicting patients’ response to TACE was also evaluated.
Results:
The baseline of miR-106b, miR-107 and miR-133b was significantly elevated (p<0.001) in sera of TACE-responders while miR-26a was elevated (p<0.001) in non-responders. miR-26a and miR-133b recorded the highest diagnostic performance as individual classifiers in response to TACE (AUC=1.0 and 100% sensitivity and specificity). Intriguingly, miR-133b distinguished complete responders from partial responders and non-responders (AUC ≥ 0.90). The PFS was improved (p<0.05) in the high expression group of miR-31, miR-200b, miR-133b and miR-181a over their low expression group.
Conclusion:
Circulating miR-133b, miR-26a, miR-107 and miR-106 in serum are potential candidates to be utilized as prognostic biomarkers for predication of TACE treatment outcomes in HCC patients.
Keywords: Hepatocellular carcinoma, TACE response, serum microRNAs, prognostic markers
1. Introduction
Hepatocellular carcinoma (HCC) is one of the leading cause of cancer death worldwide [1]. In Egypt, HCC is the one of the main health problem where the country has the highest annual incidence rate [2]. It has been reported that Egypt has the highest prevalence rate (14.7%) of hepatitis C virus infection [3]. Most of HCC patients are usually diagnosed at intermediate to advanced stages of the disease where the surgical intervention is very limited. One of the main options offered to HCC patients with larger lesions is the Transcatheter Arterial Chemoembolization (TACE). Although it is controversial, TACE is considered as an option for treatment of unresectable tumors in HCC patients [4, 5]. This intervention includes intra-arterial infusion with anti-tumor drugs such as doxorubicin and/or cisplatin followed by embolization of the artery with embolic agents [6–8]. In some cases, they developed resistance against doxorubicin and cisplatin where the drug efficacy is highly reduced and toxicities to normal hepatocytes increased. Thus, molecular biomarkers that can predict the response to such treatment are urgently needed to improve chemotherapeutic efficacy.
A growing evidence elucidates the role of microRNAs (miRs) in development and progression of HCC. Since miRs are released to the circulating blood, their potential value as diagnostic and prognostic markers in HCC have gained more attention [9–14]. There is an increasing interest in understanding the association of miR expression in HCC cells with their chemo- and radiosensitivity to increase the efficacy of the treatment modality in HCC patients. Experimental evidence demonstrated that miR mimics can normalize the gene regulatory network and cellular signaling pathways, and reverse the phenotype of cancer cells, a case may open new venue for more selective therapies [15]. For instance, miR-1268a acted as a prognostic marker for HCC and its downregulation predicted post-operative adjuvant TACE treatment [16]. The upregulation of miR-122 was associated with the emergence of early TACE refractory [17], while downregulation of miR-335 correlated with inferior response to TACE treatment [18].
Emergence of TACE resistance in HCC patients has several clinical complications including drug adverse effects, organs overload, development of aggressive forms of tumors, and therefore reduce the overall survival. Moreover, the economy is negatively affected by excess medical costs, loss of income and increase of social burdens. Because of tumor heterogeneity, patients’s genetic background and different treatment regimens, there is a clinical need for identifying new non-invasive panel of miRs to stratify HCC patients based on their response to TACE. In our previous study, we identified the signature of twelve miRs differentially regulated in non-responder group of HCC patients treated with TACE using doxorubicin and cisplatin [19]. However, the study was conducted on a limited number of patients (15 HCC patients), and data was not validated in serum samples. In the current study, we aimed to evaluate the expression level of twenty miRs that have been selected based upon our previous study [19] using larger number of formalin-fixed paraffin-embedded (FFPE) tissue specimens. Those miRs showed differential expression between HCC patients with and without TACE response were then re-assessed in sera of 51 HCC patients to evaluate their prognostic value. Our results demonstrated that four miRs (miR-26a, miR-133b, miR-106b and miR-107) were able to re-stratify HCC patients according to TACE treatment outcomes.
2. Material and methods
2.1. Patients.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a prior approval by the Institutional Review Board (IRB) of the National Cancer Institute, Cairo, Egypt. All specimens obtained prior to TACE treatment were approved after patients’s consents. The present study was carried out to determine miRs expression on two main levels; FFPE tissues and serum level as described in Figure 1. In FFPE specimens, we aimed to identify the candidate miRs that significantly correlated with response to TACE treatment. The FFPE tissue specimens included 33 patients with advanced HCC (Table S1). Tissue specimens were collected prior to TACE treatment with Cisplatin (50 mg) and Doxorubicin (50 mg) in addition to lipiodol as an embolization agent. In addition, FFPE normal liver tissue specimens were collected from 15 adult living liver donors prior to transplantation and considered as a normal control for FFPE tissues. Twenty miRs were analyzed by qPCR in FFPE tissue specimens in addition to the control group. The miRs that are significantly associated with response to TACE treatment were then validated in serum samples collected from HCC patients. As shown in Table S2, the serum samples included 51 patients who had advanced HCC. Serum samples collected prior to TACE treatment with doxorubicin (100 mg) and lipiodol. In addition, serum samples were also collected from another 15 age-matched healthy subjects, independent from the first normal subjects from which liver FFPE tissue biopsies were collected, and considered as a control group for serum samples. All normal controls used in this study were free from HCV or HBV and had normal liver functions. Patients were eligible for TACE treatment according to the Institutional Standard of Care [20]. Patients’ response to the treatment was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST VI. 1) [21]. In brief, complete response (CR) defined by disappearance of all large lesions, partial response (PR) evaluated by at least 30% reduction in lesion diameter, and no response (NR) if there was no tumor shrinkage. The overall survival (OS) and progression-free survival (PFS) of these patients were calculated as previously reported [22].
Figure 1.
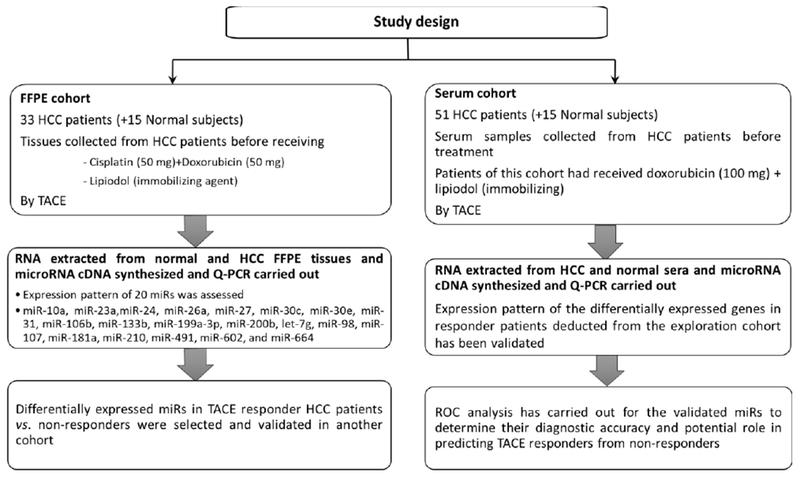
Flowchart diagram representing the experimental design of the current study.
2.2. Extraction of miRs from Formalin-Fixed Paraffin-Embedded (FFPE) tissues and serum samples.
Archived FFPE tissue specimens of HCC patients and normal controls were obtained. Total RNA from FFPE tissues (10-μm thickness per 5 cuts per sample) was extracted using miRNeasy FFPE kit according to the manufacturer’s instructions (Qiagen; Gaithersburg, MD). Total RNA was extracted from the blood sera obtained from HCC patients prior to TACE treatment and control group as described [9]. Briefly, total RNA from 200μL of serum was extracted using miRNeasy kit following the manufacture’s protocol (Qiagen; Gaithersburg, MD). RNA was purified and RNA concentrations were quantified using NanoDrop 2000 (Life Technologies Corp., Grand Island, NY, USA).
2.3. Quantitative Real-Time PCR (qPCR) analysis.
Total RNA was reverse transcribed using miScript RT kit (Qiagen; Gaithersburg, MD). For miR expression profiling, 1 μl of RT product was used as template in a 10 μL PCR reaction containing 1× SYBR Green master mix, 1× miR specific forward primer, and 1× universal primer (Qiagen; Gaithersburg, MD). Quantitative Real-Time PCR reactions were performed on ViiA 7 Real-Time PCR system (Applied Biosystems, Foster City, CA). Samples were analyzed in triplicates, and were repeated at least twice. RNU6-1 was used as an endogenous control for FFPE specimens and a combination of RNU6-1 and miR-16 was used as an internal control for serum samples. Data were analyzed using ΔΔCt comparative method regarding internal controls and the fold of change was calculated. Heatmap was performed with log2 fold change using GENE-E software (Broad Institute, Inc.).
2.4. Statistical analyses.
The obtained data were analyzed using SPSS, version 21 (SPSS, IBM, USA). Chi-square test was used to determine the association of miRs expression with each of the patients’ clinical pathological parameters. Statistical analysis comparisons were performed with Student’s t-test (two tailed) and Mann-Whitney U for miR expression analysis. Kaplan Mier survival curve followed by log-rank test was used to assess the association between survival and expression of miRs. All numerical data are expressed as mean and standard deviation or median and range. A p-value ≤0.05 was considered significant. Receiver operating characteristic (ROC) curves were constructed and AUC values were calculated to determine the accuracy of individual and combined miRs to segregate responders from non-responders to TACE treatment. To evaluate the diagnostic value of combined miRs, Logistic Regression was used to combine more than one miR and the predicted probability used to plot ROC (receiver operating characteristic) curves to evaluate the diagnostic accuracy of the combined miRs.
3. Results
3.1. Expression of miRs in FFPE tissue specimens collected from HCC patients compared to normal subjects.
We initiated our experiments by evaluating the level of the twenty miRs in 33 FFPE tissues of HCC patients compared to those collected from 15 healthy subjects. We found fourteen out of twenty miRs had a substantial difference in HCC patients compared to normal subjects (Table S3). The calculated ΔCT was used to generate an expression heat-map using online MORPHEUS software (Figure S1). Of these significantly altered miRs, miR-31 (p=0.015) and miR-98 (p=0.027) were downregulated while twelve miRs were significantly upregulated in HCC tissues over normal controls (Figure S2). The expression pattern of the studied 20 miRs was associated with clinicopathological features of the HCC patients. It was found that miR-27, miR-30c, and miR-199a had significant association with Child-Pugh score (p<0.05) whereas the levels of miR23a and miR-181a varied significantly with CLIP score (p<0.05) in HCC patients (Table S4).
3.2. Differentially expressed miRs discriminate HCC from normal subjects with high accuracy.
ROC analysis was performed to evaluate the diagnostic utilities of differentially expressed miRs. As shown in Table S5, the highest discriminatory power observed when miR-23a used as a predictor (AUC = 0.982, sensitivity = 96% & specificity = 93%). This diagnostic utility reached the maximum (AUC=1) when miR-23a combined with miR-106b or miR-644. The lowest diagnostic accuracy recorded when miR-98 used individually (AUC = 0.690, sensitivity = 93% & specificity = 60%).
3.3. Association of miRs expression level with survival data of HCC patients
The expression pattern of each miR in FFPE tissues was associated with OS and PFS of the HCC patients (Figure 2 and Table S6). We stratified the patients according to the median value of miR expression (cut-off value) into two groups; low and high expression groups. The median time of OS as well as PFS were established and compared to each group using Kaplan-Meier analysis. The p-value of the comparison was calculated by the two-sided log-rank test. The median OS and PFS were elevated in the high expression groups of each of miR-31, miR-200b and miR-181a relative to their low expression counterparts. For example, high expression level of miR-200b was associated with better OS (p=0.01) and PFS (p=0.04) (Figure 2 and Table S6). Likewise, high expression of miR-31 and miR-181a was accompanied with higher OS and PFS compared to lower expression group. In addition, the high expression group of miR-133b showed a better PFS rate compared to that of low expression level (p=0.038). Based on their clinico-pathological features, survival analyses demonstrated a statistical significance when OS or PFS of low AFP level (<200ng/ml) compared to that of high-level (≥200ng/ml) group. Indeed, the median OS was significantly increased (p=0.010) in patients whose AFP level was < 200 ng/ml versus > 200 ng/ml. Similarly, the PFS was also improved when AFP level < 200ng/ml compared to the higher level of AFP (p=0.001) as depicted in Table S6 and Figure 2.
Figure 2.
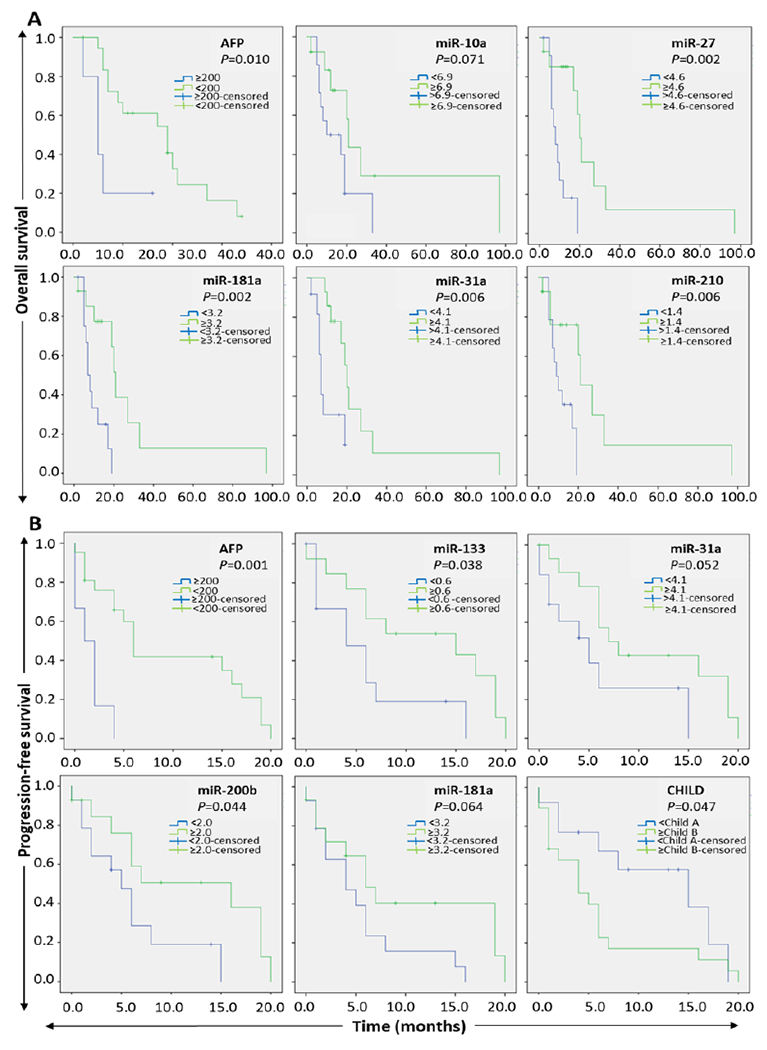
Kaplan–Meier survival analysis of HCC compared to normal controls. A: overall survival and B: progression-free survival according to the signature of five miRs and AFP. Patients were stratified into the low expression group and high expression group based on the median expression value of each miR. Significance was calculated using the log-rank test at p<0.05.
3.4. Association of miRs expression with response to TACE treatment in FFPE specimens.
Based on their response, the HCC patients categorized into two groups; responders (complete and partial responders together) and non-responders (Table S1). We compared the expression of miRs in both groups in order to select miRs associated with HCC patients’ response to TACE (Table S7 and Figure 3A). According to these data, responders had higher baseline of miR-26a (p=0.005), miR-106b (p=0.051) and miR-133 (p=0.017) than non-responders before receiving TACE. However, the baseline miR-107 was significantly lower in TACE responders compared to non-responders (p=0.03).
Figure 3.
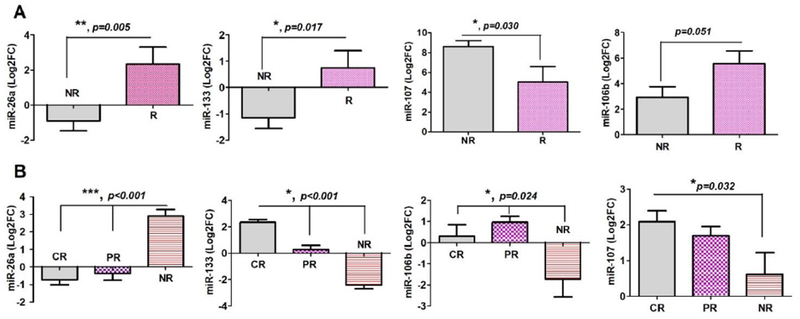
The differential expression of miRs in TACE-responder versus non-responders. The expression of miRs in FFPE tissues specimens (A) and serum samples (B) procured from HCC patients was evaluated by qPCR analysis. Bar charts of Log2FC values represented the differentially expression of four miRs in tissues and sera of TACE-responders (R) or partial responders (PR) compared to non-responders (NR) as described in the patients’ section. The Y-axis represents Log2FC normalized against internal control (RNU6-1) and the baseline expression in normal subjects. The mean Log2FC of the two groups compared by student t-test (two-tailed) and the significance level
3.5. Validation of miRs associated with TACE response in serum samples collected from HCC patients.
Patients categorized into three groups according to their TACE response; complete responders (CR), partial responders (PR) and non-responders (NR). As shown in Table 1, the baseline of miR-26a, miR-106b, miR-107 and miR-133b was significantly altered in sera of CR, PR and NR as depicted in Table 1 & Figure 3B. Interestingly, miR-26a and miR-133b recorded the highest significant differences (p<0.001) while miR-106b and miR-107 had a p-value of <0.05. In contrast to FFPE tissues, pairwise comparisons revealed that both CR and PR had significantly lower serum baseline of miR-26a than NR patients (p <0.001) for both comparisons using ANOVA followed by Gabriel Post-Hoc test. However, serum level of miR-26a in CR and PR was indistinguishable. This contrast between tissues and serum was also established by miR-107 but in the opposite direction. This evidenced by its significant elevation in CR compared to NR (p=0.032) but failed to achieve the significance in either CR-PR or PR-NR comparisons. Of note, miR-106b and miR-133b had a higher expression trend on tissues and serum levels in TACE responders compared to non-responders. The most striking observation in the serum samples was the highly elevated baseline of miR-133 in responders versus non-responders observed in all pairwise comparisons. This difference was not only demonstrated when miR-133b in CR compared to either NR or PR but also when we PR compared to NR (p<0.001).
Table 1: Validation of miRs expression pattern in the sera of TACE responders versus non-responders of HCC patients.
Data are represented as Log2FC normalized against internal control (U6) and the baseline expression in normal controls. *Pairwise comparisons of miR-26a and miR-133 carried out by Gabriel Post Hoc test. ** Pairwise comparison carried out according to Kruskal-Wallis Post Hoc. Data considered significant at n<0.05.
| 1. ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|
| miR-26a | miR-133 | |||||||
| Group | N | Mean | SEM | p† value | N | Mean | SEM | p† value |
| CR | 16 | −0.73 | 0.27 | <0.001 | 16.00 | 2.34 | 0.20 | <0.001 |
| PR | 17 | −0.37 | 0.39 | 17.00 | 0.27 | 0.32 | ||
| NR | 18 | 2.90 | 0.37 | 18.00 | −2.40 | 0.31 | ||
| 2. Kruskal-Wallis | ||||||
|---|---|---|---|---|---|---|
| miR-31 | miR-106b | miR-107 | ||||
| Group | Mean Rank | p‡ value | Mean Rank | p‡ value | Mean Rank | p‡ value |
| CR | 17.88 | 0.021 | 27.03 | 0.024 | 31.20 | 0.035 |
| PR | 27.71 | 32.38 | 26.24 | |||
| NR | 31.61 | 19.06 | 18.29 | |||
| 3. Pair-wise comparisons | ||||||
|---|---|---|---|---|---|---|
| p-value | ||||||
| Group | miR-26a* | miR-133* | miR-31** | miR-106b** | miR-107** | |
| CR-NR | <0.001 | <0.001 | 0.021 | 0.355 | 0.032 | |
| CR-PR | 0.849 | <0.001 | 0.173 | 0.904 | 0.315 | |
| PR-NR | <0.001 | <0.001 | 1.00 | 0.024 | 0.98 | |
CR: complete responders, PR: partial responders, NR: non-responders
3.6. Evaluation of prognostic utility of validated miRs as molecular classifiers to TACE response.
3.6.1. Using FFPE tissue specimens:
As shown in Figure S3 and Table S8, miR-26a recorded the highest AUC for individual miR used for the prediction of TACE response (AUC=0.818, sensitivity = 82% and specificity = 65%). This value jumped to 0.913 when miR-26a and miR-133 were combined. When miR-26a, miR-106b and miR-133b combined and used as a predictor, the diagnostic accuracy reached its maximum (AUC=1). Next miR in order recorded a good score of AUC is the miR-106b (AUC= 0.764, sensitivity = 80% and specificity = 71%) and miR-133b (AUC = 0.721, sensitivity = 60% and specificity = 78%). Basically, different combinations of miRs as predictors substantially improved the accuracy of discrimination. However, the combined miR-106b and miR-107 together established an AUC value of 0.767, which was less than the value recorded for miR-26a as an individual marker.
3.6.2. Using Serum samples:
Using the same approach applied in the FFPE specimens, miR-26a as a single predictor had the highest AUC value of 1.0 (the sensitivity and specificity were 100%, p<0.001) in discriminating CR form NR as illustrated in Table 2 and Figure 4A. This AUC value slightly dropped to 0.958 with 94% sensitivity and 83% specificity in discriminating PR from NR patients (p<0.001). In parallel, miR-133b differentiated CR from NR (AUC= 0.997, 100% sensitivity, 94% specificity; p<0.001), CR from PR (AUC=0.919, 93% sensitivity, 88% specificity; p<0.001) and PR from NR (AUC=0.935, 94% sensitivity, 83% specificity; p<0.001), and responders (CP&PR) from NR (AUC=0.965, 97% sensitivity, 83% specificity; p<0.001). A combination of miR-26a and miR-133 had excellent ability to differentiate responders from non-responders (Figure 4B) in high accuracy (AUC=1.0 when CR compared with NR, AUC=0.997 when PR compared with NR, AUC=0.919 when CR compared with PR, and AUC=0.998 when responders to TACE (CR&PR) compared with NR.
Table 2:
Prognostic ability of individual and combined miRs (Log2FC) to differentiate TACE responders from non-responders in serum samples collected from HCC patients
| Groups | Predictor miR(s) | Cut-off value | Sensitivity | Specificity | AUC | SEM | p-value |
|---|---|---|---|---|---|---|---|
| % | % | ||||||
| CR vs NR | miR-26a | 0.807 | 100.00 | 100.00 | 1.000 | 0.000 | <0.001 |
| miR-107 | 1.160 | 86.70 | 70.60 | 0.741 | 0.092 | 0.020 | |
| miR-133 | 0.037 | 100.00 | 94.40 | 0.997 | 0.006 | <0.001 | |
| miR-26a & miR-133 | 1.000 | 0.000 | <0.001 | ||||
| CR vs PR | miR-133 | 0.940 | 93.80 | 88.20 | 0.919 | 0.059 | <0.001 |
| miR-26a & miR-133 | 0.934 | 0.057 | <0.001 | ||||
| *(CR+PR) vs NR | miR-133 | −1.600 | 97.00 | 83.30 | 0.965 | 0.028 | <0.001 |
| miR-26a & miR-133 | 0.998 | 0.003 | <0.001 | ||||
| PR vs NR | miR-26a | 1.490 | 94.10 | 83.30 | 0.958 | 0.030 | <0.001 |
| miR-106b | 0.328 | 76.50 | 66.70 | 0.761 | 0.081 | <0.001 | |
| miR-133 | −1.530 | 94.10 | 83.30 | 0.935 | 0.050 | <0.001 | |
| miR-26a & miR-133 | 0.997 | 0.005 | <0.001 |
AUC=Area under ROC curve, CR=complete responders, PR=Partial responders, NR=non-responders to TACE.
Responders=CR+PR pooled together and compared to NR
Figure 4.
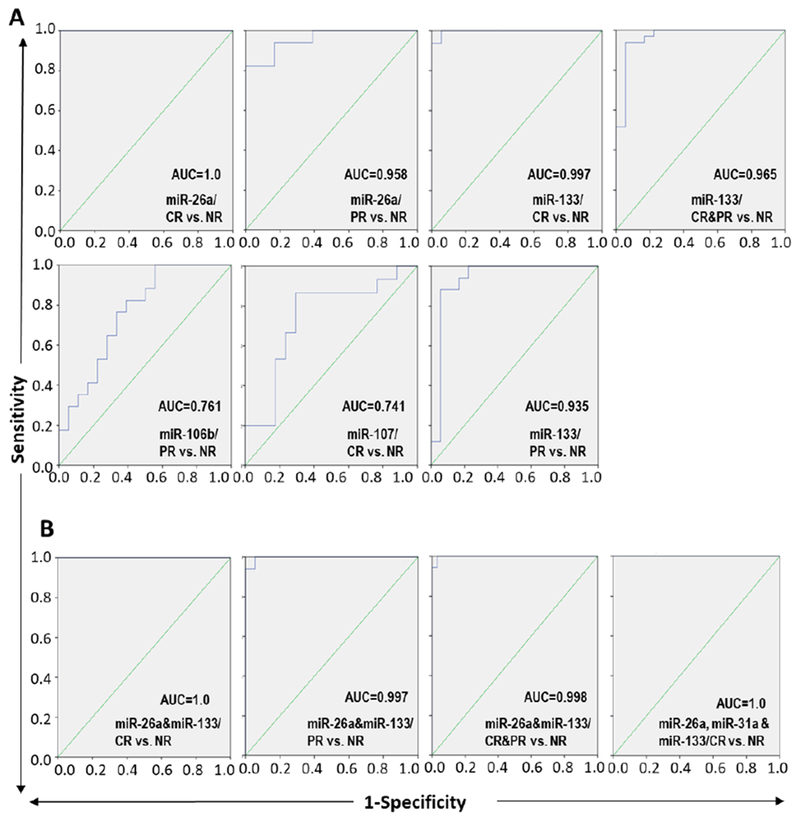
Prognostic accuracy of serum miRs in discriminating the response of HCC patients to TACE treatment. ROC curve of individual (A) and combined (B) miRs in differentiating TACE-responder HCC patients (complete responders “CR” or partial responders “PR”) from non-responder (NR) patients to the TACE treatment. The AUC was used to measure the prognostic accuracy of the altered miRs expression. CR&PR together represented the group of HCC patients responded to the TACE treatment.
4. Discussion
Although the prospective role of miRs in predicting drug response to TACE treatment has been investigated [17, 18, 23], the specificity and sensitivity of these miRs as prognostic markers are compromised. From our previous study [19], we selected twenty miRs and evaluated their expression in FFPE liver tissue specimens collected from 33 HCC patients. The top four miRs that showed differential expression in responders versus non-responders to TACE treatment were then re-assessed in serum samples collected from 51 HCC patients. The expression pattern of these miRs was established and associated with OS, PFS, and treatment outcomes. We evaluated the diagnostic accuracy of these miRs in predicting the response to TACE. Our results demonstrated that out of twenty miRs, fourteen miRs (12 up and 2 downregulated miRs) were able to differentiate HCC from normal subjects. Several previous studies have confirmed that some of these miRs were differentially expressed in HCC patients versus their normal counterparts. For instance, the serum level of miR-106b was higher in HCC patients at advanced versus early stages with ROC value of 0.885 [24]. Using miR-107 as a diagnostic marker, it separates HCC patients from normal subjects and the combination of miR-92 and miR-3126 improved their diagnostic accuracy [25]. Another marker, miR-31, was upregulated in liver tissues collected from HCC over normal controls and its upregulation was correlated with liver cirrhosis [26]. The median serum level of miR-30c in HCC was reduced compared to healthy control subjects [9]. We were able to show that levels of miR-31, miR-200b and miR-181a in the high expression group were positively correlated with better OS and PFS in HCC patients. In accordance with our findings, Nishida et al. reported that the higher level of serum miR-181a-5p predicts the early response to sorafenib treatment as well as the OS of HCC patients [27]. In addition, the higher expression of long non-coding RNA CARLo-5 inhibits miR-200b expression and predicts inferior OS in HCC patients [28].
Regarding TACE response in HCC patients, the baseline of miR-106b, miR-107 and miR-133b was elevated in the sera of TACE responders over non-responders while the level of miR-26a and miR-31 was elevated in the sera of non-responders. Of note, miR-26a and miR-133b recorded the highest diagnostic accuracy as classifiers for response to TACE (AUC=1.0). More specifically, miR-133b was able to discriminate complete from partial responders, complete from non-responders and partial responders from non-responders with AUC value of ≥ 0.90. That being said, miR-133b is among the potential markers for stratifying HCC patients in response to TACE treatment but multi-institutional studies are needed to validate this miR on a large scale. In the context of its biological significance, Chen et al. suggested that restoration of miR-133b suppresses ABCC1 and therefore increases the chemosensitivity of colorectal cancer cell lines to 5-FU and Vincristine [29]. In another study, overexpression of miR-133b enhances the sensitivity of resistant ovarian cancer cell lines to paclitaxel and cisplatin by silencing MDR1 and Glutathione-π [30]. We previously showed that miR-26a and miR-106b were upregulated in non-responders versus responders HCC patients to TACE treatment [19]. When Kim et al. combined three miRs including miR-26a; the panel of these miRs was able to predict TACE refractoriness in HCC patients within one year from receiving their TACE treatment [31].
Collectively, our results showed that the expression of miR-133b and miR-26a was significantly upregulated in responder compared to non-responder to TACE treatment. Although the expression pattern of miR-26a was not consistent in tissues and serum, its significant expression was recorded in TACE-responders. This change may originate from biological differences in miRs biogenesis and metabolism in liver tissues compared to circulating miRs in the blood. We showed that miR-26a had the highest AUC value as a single classifier in prediction of TACE response. However, the combination of more than two miRs, miR-26a, miR-106b and miR-133b, improved the AUC value to the unity. Instead of using individual miR, the combination of at least two miRs will substantially improve the diagnostic and prognostic abilities of miRs in HCC patients [25, 32]. We also demonstrated that miR-26a, as a single predictor, had absolute AUC value with 100% sensitivity and specificity for discriminating CR form NR. Next miR in rank was miR-133b, which can differentiate complete responders from non-responders with 100% sensitivity and 94% specificity and also was able to separate partial and complete responders from non-responders with 97% sensitivity and 83% specificity. The combination of miR-26a and miR-133 showed a remarkable capability of stratifying responders over non-responders after TACE treatment. Taken together, mir-133b, miR-26a and miR-106 are potential candidates to be utilized as non-invasive prognostic biomarkers for predicating TACE treatment outcomes in HCC patients. One of the mechanisms of developing TACE refractoriness is the ability of tumor cells to survive through dual blood supply carried by hepatic artery and portal vein [33, 34]. For instance, doxorubicin, cisplatin and sorafenib are used in TACE treatment to reduce tumor growth and induce tumor necrosis but they are acting by different mechanisms. Therefore, further studies are necessary to evaluate the current miRs panel as potential biomarker predicting TACE outcomes when another therapeutic agent replaces doxorubicin. Some of the limitations of this study are the sample size of the serum samples and unavailability of conducting multicentered studies. The strength of our study is the identification and validation of miRs that have not been fully described in response to TACE treatment. The second point of strength is the use of cross-validation approach to evaluate miRs signature in FFPE and serum samples. Moreover, we combined multiple circulating miRs to provide better accuracy and meet the diversity of HCC patients compared to utilizing individual miRs for predicting the response to TACE treatment. These findings warranted further studies to evaluate the clinical utilities of circulating miRs as prognostic markers for predicting TACE response in a large number of HCC patients’ samples.
Supplementary Material
Highlights.
Our objective was to develop a panel of microRNA biomarkers to predict the treatment outcomes of hepatocellular carcinoma (HCC) patients following transcatheter arterial chemoembolization (TACE).
The expression level of twenty miRs was evaluated in 33 HCC FFPE tissues, and four of them were re-assessed in 51 serum HCC samples in response to TACE.
The baseline of miR-106b, miR-107 and miR-133b was significantly elevated in sera of TACE responders while miR-26a was elevated in non-responders.
miR-26a and miR-133b recorded the highest diagnostic performance as individual classifiers in response to TACE (AUC=1.0 and 100% sensitivity and specificity).
Levels of miR-133b, miR-26a, miR-107 and miR-106 in serum are potential candidates to be utilized as prognostic biomarkers for predication of TACE treatment outcomes.
Acknowledgments
Funding
This study was partially supported by Texas A&M Health Science Center Rangel College of Pharmacy (ZYA), NIH/NCI R21CA194750 grant (ZYA) and the Science and Technology Development Fund (STDF), Ministry of Higher Education and Scientific Research, Egypt, Grant # 1729 (AHA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declared there is no conflict of interest related to this study.
References
- [1].C. Global Burden of Disease Liver Cancer, The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015, JAMA Oncol 3(12) (2017) 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Salim EI, Moore MA, Al-Lawati JA, Al-Sayyad J, Bazawir A, Bener A, Corbex M, El-Saghir N, Habib OS, Maziak W, Mokhtar HC, Seif-Eldrin IA, Sobue T, Cancer epidemiology and control in the arab world - past, present and future, Asian Pac J Cancer Prev 10(1) (2009) 3–16. [PubMed] [Google Scholar]
- [3].Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ, The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis, BMC Infect Dis 13 (2013) 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, Rodes J, Bruix J, G. Barcelona Liver Cancer, Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial, Lancet 359(9319) (2002) 1734–9. [DOI] [PubMed] [Google Scholar]
- [5].Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J, Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma, Hepatology 35(5) (2002) 1164–71. [DOI] [PubMed] [Google Scholar]
- [6].Gomes AS, Monteleone PA, Sayre JW, Finn RS, Sadeghi S, Tong MJ, Britten CD, Busuttil RW, Comparison of Triple-Drug Transcatheter Arterial Chemoembolization (TACE) With Single-Drug TACE Using Doxorubicin-Eluting Beads: Long-Term Survival in 313 Patients, AJR Am J Roentgenol 209(4) (2017) 722–732. [DOI] [PubMed] [Google Scholar]
- [7].Gomes AS, Rosove MH, Rosen PJ, Amado RG, Sayre JW, Monteleone PA, Busuttil RW, Triple-drug transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: assessment of survival in 124 consecutive patients, AJR Am J Roentgenol 193(6) (2009) 1665–71. [DOI] [PubMed] [Google Scholar]
- [8].Mabed M, Esmaeel M, El-Khodary T, Awad M, Amer T, A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol, doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma, Eur J Cancer Care (Engl) 18(5) (2009) 492–9. [DOI] [PubMed] [Google Scholar]
- [9].Ali HEA, Abdel Hameed R, Effat H, Ahmed EK, Atef AA, Sharawi SK, Ali M, Abd Elmageed ZY, Abdel Wahab AH, Circulating microRNAs panel as a diagnostic tool for discrimination of HCV-associated hepatocellular carcinoma, Clin Res Hepatol Gastroenterol 41(4) (2017) e51–e62. [DOI] [PubMed] [Google Scholar]
- [10].Keller T, Boeckel JN, Gross S, Klotsche J, Palapies L, Leistner D, Pieper L, Stalla GK, Lehnert H, Silber S, Pittrow D, Maerz W, Dorr M, Wittchen HU, Baumeister SE, Volker U, Felix SB, Dimmeler S, Zeiher AM, Improved risk stratification in prevention by use of a panel of selected circulating microRNAs, Sci Rep 7(1) (2017) 4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu HN, Wu H, Chen YJ, Tseng YJ, Bilegsaikhan E, Dong L, Shen XZ, Liu TT, Serum microRNA signatures and metabolomics have high diagnostic value in hepatocellular carcinoma, Oncotarget 8(65) (2017) 108810–108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Okajima W, Komatsu S, Ichikawa D, Miyamae M, Kawaguchi T, Hirajima S, Ohashi T, Imamura T, Kiuchi J, Arita T, Konishi H, Shiozaki A, Moriumura R, Ikoma H, Okamoto K, Taniguchi H, Itoh Y, Otsuji E, Circulating microRNA profiles in plasma: identification of miR-224 as a novel diagnostic biomarker in hepatocellular carcinoma independent of hepatic function, Oncotarget 7(33) (2016) 53820–53836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qu Z, Wu J, Wu J, Ji A, Qiang G, Jiang Y, Jiang C, Ding Y, Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis, Oncotarget 8(46) (2017) 80666–80678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sakabe T, Azumi J, Umekita Y, Toriguchi K, Hatano E, Hirooka Y, Shiota G, Prognostic relevance of miR-137 in patients with hepatocellular carcinoma, Liver Int 37(2) (2017) 271–279. [DOI] [PubMed] [Google Scholar]
- [15].Zhang W, Dolan ME, The emerging role of microRNAs in drug responses, Curr Opin Mol Ther 12(6) (2010) 695–702. [PMC free article] [PubMed] [Google Scholar]
- [16].Lu YL, Yao JG, Huang XY, Wang C, Wu XM, Xia Q, Long XD, Prognostic significance of miR-1268a expression and its beneficial effects for post-operative adjuvant transarterial chemoembolization in hepatocellular carcinoma, Sci Rep 6 (2016) 36104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim SS, Nam JS, Cho HJ, Won JH, Kim JW, Ji JH, Yang MJ, Park JH, Noh CK, Shin SJ, Lee KM, Cho SW, Cheong JY, Plasma micoRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma, J Gastroenterol Hepatol 32(1) (2017) 199–207. [DOI] [PubMed] [Google Scholar]
- [18].Cui L, Hu Y, Bai B, Zhang S, Serum miR-335 Level is Associated with the Treatment Response to Trans-Arterial Chemoembolization and Prognosis in Patients with Hepatocellular Carcinoma, Cell Physiol Biochem 37(1) (2015) 276–83. [DOI] [PubMed] [Google Scholar]
- [19].El-Halawany MS, Ismail HM, Zeeneldin AA, Elfiky A, Tantawy M, Kobaisi MH, Hamed I, Abdel Wahab AH, Investigating the pretreatment miRNA expression patterns of advanced hepatocellular carcinoma patients in association with response to TACE treatment, Biomed Res Int 2015 (2015) 649750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zeeneldin AA, Salem SE, Tabashy RH, Ibrahim AA, Alieldin NH, Transarterial chemoembolization for the treatment of hepatocellular carcinoma: a single center experience including 221 patients, J Egypt Natl Canc Inst 25(3) (2013) 143–50. [DOI] [PubMed] [Google Scholar]
- [21].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1), Eur J Cancer 45(2) (2009) 228–47. [DOI] [PubMed] [Google Scholar]
- [22].Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB, Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial, JAMA 304(19) (2010) 2154–60. [DOI] [PubMed] [Google Scholar]
- [23].Liu M, Liu J, Wang L, Wu H, Zhou C, Zhu H, Xu N, Xie Y, Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival, PLoS One 9(10) (2014) e109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shi BM, Lu W, Ji K, Wang YF, Xiao S, Wang XY, Study on the value of serum miR-106b for the early diagnosis of hepatocellular carcinoma, World J Gastroenterol 23(20) (2017) 3713–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, Li T, Qiu Y, Zhang T, Guo P, Ma X, Wei Q, Han L, Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma, Medicine (Baltimore) 96(2) (2017)e5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D, Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance, Mol Carcinog 52(4) (2013) 297–303. [DOI] [PubMed] [Google Scholar]
- [27].Nishida N, Arizumi T, Hagiwara S, Ida H, Sakurai T, Kudo M, MicroRNAs for the Prediction of Early Response to Sorafenib Treatment in Human Hepatocellular Carcinoma, Liver Cancer 6(2) (2017) 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dou C, Sun L, Jin X, Han M, Zhang B, Jiang X, Lv J, Li T, Long non-coding RNA CARLo-5 promotes tumor progression in hepatocellular carcinoma via suppressing miR-200b expression, Oncotarget 8(41) (2017) 70172–70182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen M, Li D, Gong N, Wu H, Su C, Xie C, Xiang H, Lin C, Li X, miR-133b downregulates ABCC1 and enhances the sensitivity of CRC to anti-tumor drugs, Oncotarget 8(32) (2017) 52983–52994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen S, Jiao JW, Sun KX, Zong ZH, Zhao Y, MicroRNA-133b targets glutathione S-transferase pi expression to increase ovarian cancer cell sensitivity to chemotherapy drugs, Drug Des Devel Ther 9 (2015) 5225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim SS, Cho HJ, Nam JS, Kim HJ, Kang DR, Won JH, Kim J, Kim JK, Lee JH, Kim BH, Lee MY, Cho SW, Cheong JY, Plasma MicroRNA-21, 26a, and 29a-3p as Predictive Markers for Treatment Response Following Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma, J Korean Med Sci 33(1) (2018) e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu M, Kong X, Wang H, Huang G, Ye C, He Z, A novel microRNAs expression signature for hepatocellular carcinoma diagnosis and prognosis, Oncotarget 8(5) (2017) 8775–8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kuroda C, Sakurai M, Monden M, Marukawa T, Hosoki T, Tokunaga K, Wakasa K, Okamura J, Kozuka T, Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases, Cancer 67(1) (1991) 81–6. [DOI] [PubMed] [Google Scholar]
- [34].L. European Association For The Study Of The, R. European Organisation For, C. Treatment Of, EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma, J Hepatol 56(4) (2012) 908–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


