Abstract
BACKGROUND
An in vitro injury model mimicking a corneal surface injury was optimised using human corneal epithelial cells (hCEC).
AIM
To investigate whether corneal-stroma derived stem cells (CSSC) seeded on an amniotic membrane (AM) construct manifests an anti-inflammatory, healing response.
METHODS
Treatment of hCEC with ethanol and pro-inflammatory cytokines were compared in terms of viability loss, cytotoxicity, and pro-inflammatory cytokine release, in order to generate the in vitro injury. This resulted in an optimal injury of 20% (v/v) ethanol for 30 s with 1 ng/mL interleukin-1 (IL-1) beta. Co-culture experiments were performed with CSSC alone and with CSSC-AM constructs. The effect of injury and co-culture on viability, cytotoxicity, IL-6 and IL-8 production, and IL1B, TNF, IL6, and CXCL8 mRNA expression were assessed.
RESULTS
Co-culture with CSSC inhibited loss of hCEC viability caused by injury. Enzyme linked immunosorbent assay and polymerase chain reaction showed a significant reduction in the production of IL-6 and IL-8 pro-inflammatory cytokines, and reduction in pro-inflammatory cytokine mRNA expression during co-culture with CSSC alone and with the AM construct. These results confirmed the therapeutic potential of the CSSC and the possible use of AM as a cell carrier for application to the ocular surface.
CONCLUSION
CSSC were shown to have a potentially therapeutic anti-inflammatory effect when treating injured hCEC, demonstrating an important role in corneal regeneration and wound healing, leading to an improved knowledge of their potential use for research and therapeutic purposes.
Keywords: Cornea, Corneal injuries, Injury model, Corneal epithelium, Corneal stroma-derived stem cells, Amnion, Anti-inflammatory, Cell therapy
Core tip: We designed a novel in vitro inflammation model of the human corneal surface using human corneal epithelial cells treated with 20% (v/v) ethanol, followed by stimulation with 1 ng/mL interleukin-1β. We then used this model to demonstrate the anti-inflammatory and regenerative healing properties of human cornea stroma-derived stem cells seeded on an amniotic membrane substrate in a co-culture model. This study is the first step in building a topical regenerative therapy for the treatment of inflammatory disorders of the front of the eye.
INTRODUCTION
The cornea is the transparent window of the eye. It functions to provide two thirds of the eye’s refractive power, as well as being the major barrier to the inner content of the eye. At present, when the cornea is damaged or diseased, transplantation of a donor cornea, known as keratoplasty, is the most effective technique to restore vision[1]. However, worldwide 8-10 million individuals have no access to a corneal transplant. Furthermore, patients may suffer from rejection of allogeneic corneal tissue or have to wait for long periods before finding a viable donor graft. For these reasons, corneal research has turned to the use of stem cell-based regenerative therapies for corneal tissue regeneration[2].
Since their discovery, mesenchymal stromal cells (MSCs) have been recognised by different characteristics: differentiation capacity into the adipogenic, chondrogenic, and osteogenic lineages; possible isolation from several tissues; and regeneration of myocardial tissues, tendon, and bone, amongst others in animal models[3]. The interest in MSCs has been enhanced for therapeutic applications due to their non-immunogenic potential[4]. MSCs can be obtained from autologous tissue and expanded in culture, producing anti-inflammatory factors which participate in normal wound repair[5]. Several studies have shown that MSCs have the ability to migrate to sites of tissue injury and stop an on going immune response by inhibiting T-cell proliferation[6]. Additionally, MSCs secrete growth factors and cytokines with autocrine and paracrine activities such as fibrosis inhibition and apoptosis, mitosis stimulation, suppression of the local immune system, angiogenesis enhancement, and stem cell differentiation. These effects can be either direct, causing intracellular signalling, or indirect (referred to as trophic effects), causing other cells to secrete functionally active factors which facilitate tissue regeneration[7].
In 2008, Polisetty et al[8] demonstrated the presence of MSCs in the human corneal limbus, which were shown to be similar to bone marrow-MSCs, indicating that these cells are unique in the adult stem cell niche. In 2012, Branch et al[9] characterised and analysed the peripheral and limbal corneal stromal cells, later referred to as corneal-stroma derived stem cells (CSSC), against the criteria of the International Society of Cellular Therapy for identification of MSCs. Finding evidence of plastic adhesion, trilineage potential differentiation, correct profile, and expression of the cell-surface markers, revealing that ≥ 95% of the cells expressed CD105, CD90, and CD73, but were negative for CD11b, CD19, CD34, and HLA-DR (≤ 2%). Further characterisation of these cells was performed to demonstrate their MSC-like phenotype in different media and the ability to differentiate back to a keratocyte-like state[10-12].
Recent in vitro studies have shown that CSSC contribute to corneal tissue homeostasis, presenting an immunomodulatory response, a non-immunogenic profile, and a regenerative role[13-15]. From this, we can infer that these cells have potential to control the microenvironment during local inflammation, and are candidates for allogeneic cell-based therapies. There have been several studies investigating the use of MSCs from other tissue (bone marrow or adipose tissue) in treating corneal disease to differing success[16-19]. The use of MSCs from tissues other than the cornea has shown limitations for corneal disease models. In 2015, Fuentes-Julián et al[20] aimed to prevent transplant rejection with an adipose-derived MSC treatment while increasing the length of graft survival in a rabbit corneal inflammation model. However, the treatment had the opposite effect and increased the inflammation. Additionally, it is well known that even if MSCs share biological functions and molecular expression profiles across different tissues, they retain a differentiation preference due to their tissue origins[21]. Thus, corneal-derived MSCs, such as CSSCs, may be considered a more appropriate cell source for corneal regeneration.
The amniotic membrane (AM) is the inner most membrane encapsulating the foetus in the amniotic cavity, and consists of a simple epithelium, avascular stroma, and basement membrane[22]. The first therapeutic application of AM was reported in 1913 as a surgical procedure for skin[23]. In the 1940s, AM was first used in the ophthalmology field as a patch to cover defects in the conjunctival epithelium[24]. Subsequently, several studies have demonstrated that AM maintains an anti-scarring and anti-inflammatory action during pregnancy[25], providing evidence of these properties for ocular disorders[26,27]. Alongside its therapeutic properties, AM has been widely used as a cell carrier for different conditions such as chemical burns, ocular cicatricial pemphigoid, severe pterygium, and Stevens-Johnson syndrome[28,29], providing an effective cell-delivery method and a more effective therapeutic effect[30].
In this study, the optimization of an in vitro injury model based on 20% (v/v) ethanol (EtOH) and pro-inflammatory cytokine stimulation has been performed using an immortalised human corneal epithelial cell (hCEC) line with the aim of assessing the therapeutic potential of both the CSSC and the AM in a co-culture system by performing the following analyses: cell viability, cytotoxicity, interleukin (IL)-6, IL-8 production, and quantitative reverse transcription polymerase chain reaction (RT-qPCR) for the genes IL1B, TNF, IL6, and CXCL8.
MATERIALS AND METHODS
Human tissue
Human corneoscleral rims and human AM were used with approval by the Nottingham Research Ethics Committee (07/H0403/140 and OY110101, respectively) and in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the donors and/or their relatives.
Culture of immortalised human corneal epithelial cells
SV40-immortalised human corneal epithelial cells (hCEC)[31] were cultured in supplemented basal epithelial cell medium EpiLife® (Gibco, ThermoFisher, United Kingdom) containing 5 mL human keratinocyte growth supplement (Gibco, ThermoFisher) and 1% (v/v) antibiotic-antimycotic (AbAm, Sigma-Aldrich, United Kingdom). Cells were incubated at 37 °C, 5% CO2 (standard conditions), and the medium changed every 2-3 d. hCEC were passaged at approximately 80% confluency using TrypLE Express dissociation reagent (ThermoFisher). hCEC were used between passages 24-31 and seeded at 5 × 104 cell/cm2 density.
Isolation and culture of CSSC
CSSC were isolated as previously described[11]. Briefly, corneoscleral rims were washed with PBS containing 1% (v/v) AbAm. Residual sclera was removed with a scalpel and the rim was cut in small pieces, placed into 1 mg/mL collagenase type IA (Sigma-Aldrich) solution in basal medium 199 (M199) with 1% (v/v) AbAm, before incubation at 37 °C under slow agitation for 7 h. Digests were filtered through a 40 µm cell-strainer to remove debris and the cells seeded in appropriate culture medium. Culture medium either consisted of M199 (Sigma-Aldrich) supplemented with 20% (v/v) foetal bovine serum (Sigma-Aldrich), 1% (v/v) L-Glutamine (Sigma-Aldrich), and 1% (v/v) AbAm or stem cell medium (SCM) consisting of Dulbecco’s modified Eagle’s medium: nutrient mixture F-12 (Gibco, ThermoFisher) supplemented with 20% (v/v) knockout serum replacement (Gibco, ThermoFisher), 1% (v/v) MEM non-essential amino acids (Gibco, ThermoFisher), 4 ng/mL basic-fibroblast growth factor (Gibco), 5 ng/mL human leukaemia inhibitory factor (Cell Signalling Technologies, United Kingdom), and 1% (v/v) AbAm. Finally, the cell suspension was transferred to a 0.1% (v/v) bovine gelatine (Sigma-Aldrich) coated T25 cm2 flask and incubated at standard conditions. Medium was changed every 3-4 d. CSSC passaging was performed using TrypLE Express dissociation reagent and cells were used experimentally between passages 4-6 and seeded at 2 × 104 cell/cm2 density, unless otherwise stated.
Experimental culture medium
To assess optimal growth conditions for co-culture of hCEC with CSSC, four media were tested: M199, SCM, EpiLife supplemented as previously mentioned, and keratinocyte-serum free medium (K-SFM, Gibco, ThermoFisher) supplemented with keratinocyte supplement (bovine pituitary extract, epidermal growth factor) and 1% (v/v) AbAm.
Cell viability and proliferation assay
PrestoBlue Cell Viability Reagent (Invitrogen, ThermoFisher) was used to assess cell viability and proliferation. At each time point, culture medium was removed from the cells and a 10% (v/v) PrestoBlue solution in Hank’s balanced salt solution was added to each well before incubation for 30 min at 37 °C. Aliquots of 100 µL from each well were transferred to a black 96-well plate and fluorescence readings were taken at 560 nm excitation/590 nm emission with a CLARIOstar microplate reader (BMG LabTech, Buckinghamshire, United Kingdom). Results were corrected for background fluorescence from blank readings.
Injury model optimisation
The effect of combinations of the following conditions were assessed on the hCEC: treatment with 20% (v/v) absolute ethanol (EtOH) in PBS for 30 seconds; application of 1 ng/mL human IL-1β (R and D Systems, United Kingdom) in the media and/or application of 10 ng/mL human tumour necrosis factor (TNF)-α (R and D Systems, United Kingdom). The effect of culturing with IL-1β and TNF-α was assessed on the CSSC. The final injury model used in further co-culture studies consisted of treatment of the hCEC with 20% EtOH for 30 s followed by incubation with 1 ng/mL IL-1β. CSSC were not treated with EtOH during any experiments.
Cytotoxicity
The Pierce lactate dehydrogenase (LDH) Cytotoxicity Assay Kit (ThermoScientific, United Kingdom) was used to quantify cytotoxicity caused by the injury model by measuring LDH release into the culture medium. The assay was performed according to the manufacturer’s protocol. Briefly, 50 µL of cell supernatant and 50 µL of reaction mixture were transferred into a 96-well plate, and incubated at room temperature for 30 min. The optical absorbance was read on the plate reader at 490 nm with background correction at 690 nm. Data was converted to a percentage using a maximum LDH release control reading to create a percentage cytotoxicity and corrected for cell viability.
Cytokine production
Human IL-6 and CXCL8/IL-8 DuoSet enzyme linked immunosorbent assay (R and D Systems, Abingdon, United Kingdom) were used in combination with the appropriate DuoSet Ancillary Reagent Kit (R and D Systems), according to manufacturer’s instructions, to detect cytokine protein levels in a sample. Briefly, the supernatant samples were diluted 1:20. Optical density at 450 nm with background correction at 540 nm was determined immediately after the addition of the stop solution. Cytokine concentration was determined using 4-parameter fit standard. Data was corrected for the number of cells using viability data.
LIVE/DEAD™ viability assay
The LIVE/DEAD viability kit for mammalian cells (Invitrogen, ThermoFisher) was used to simultaneously stain live (green) and dead (red) cells after injury was performed. hCEC were seeded in 8-well Permanox Lab-Tek chamber slides (ThermoFisher Scientific). Cell monolayers were incubated with a solution containing 2 µmol/L calcein AM and 4 µmol/L ethidium homodimer-1 in PBS for 30 min. After incubation, the staining solution was discarded, chambers removed, and slides mounted under coverslips in fluorescent mounting media (Dako, United Kingdom). Imaging was performed using an upright fluorescence microscope (BX51, Olympus, Southend-on-Sea, United Kingdom) with images captured with a black and white camera (XM-10, Olympus) and Cell^F software (Olympus).
Collection of conditioned medium
Conditioned medium was collected from CSSC at P4 cultured in SCM and SCM supplemented with 1 ng/mL IL-1β. CSSC were seeded at 10000 cells/cm2 in T75 cm2 flasks. Conditioned medium was produced by adding 12 mL appropriate media and culturing for 72 h, before collection and filtering through a 0.22 µm filter.
Co-culture
hCEC were seeded in 12-well plates at a density of 5 × 104 cell/cm2 and CSSC were seeded separately at 2 × 104 cell/cm2 into 12 mm transwells with 0.4 µm polyester membrane insert (Corning). On the day of the injury model, hCEC were treated with 20% (v/v) EtOH for 30 s, prior to the CSSC-seeded transwells being added to the co-culture. SCM containing 1 ng/mL IL-1β was then added to cover both cell types. Controls for co-culture experiments included no injury, hCEC only, and CSSC only to correct for background production.
RT-qPCR
RT-qPCR was performed as previously described[10]. Briefly, RNA was extracted from cells using an RNeasy mini kit (Qiagen, Manchester, United Kingdom) according to manufacturer’s instructions and quantified using an LVis-plate in a CLARIOstar plate reader. RNA transcription to single-stranded cDNA synthesis was performed with 1 µg of the total RNA using the SuperScript III First Strand Synthesis Kit (Invitrogen, ThermoFisher) using random hexamers. For PCR, 1 µL cDNA was used with inventoried TaqMan assays (Applied Biosystems, ThermoFisher) to detect GAPDH (Hs99999905_m1), IL1B (Hs01555410_m1), TNF (Hs00174128_m1), IL6 (Hs00985639_m1), and CXCL8 (Hs00174103_m1). Gene amplification was performed on an Mx3005P multi-colour 96-well PCR-system (Stratagene, Agilent Technologies). Results were analysed with the RT-qPCR Miner algorithm[32]. All readings were normalised using the endogenous reference gene GAPDH.
CSSC-AM construct preparation
Preparation of the dried, vacuum-packed AM was performed as previously described[33]. Briefly, AM was separated from chorion and triple washed in sodium chloride, before the spongy layer was removed. After incubation in 100 mmol/L raffinose pentahydrate (Acros Organics, United Kingdom) in PBS for 2 h, the AM was dried with main drying at a shelf temperature of 15 °C, vacuum 1.03 mbar for 30 min, followed by a final drying phase, shelf temperature 15 °C, vacuum pressure 0.001 mbar for 15 min in a cooled freeze dryer (Alpha 1-4 LSC, Advanced Freeze Dryer, Christ Osterode, Germany).
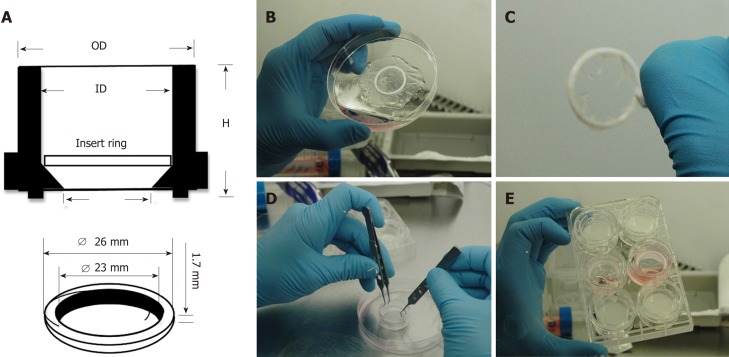
To culture CSSC on AM, a novel method designed in our laboratory was employed (Figure 1). Single use 30 mm diameter Millicell tissue culture inserts (Millipore, United Kingdom) were used without filtration membranes. A sterilisable polytetrafluoroethylene O-ring was placed in the centre of a 5 cm diameter rehydrated AM, epithelial side up. The AM was then trimmed to create a 1 cm frill around the inside of the O-ring, which was subsequently firmly wrapped over the O-ring edges creating a taut wrinkle free-surface. Forceps were used to position the O-ring-AM construct into the Millicell insert and the O-ring-AM construct was then gently pushed by the edges to the bottom. The AM-insert was placed in a 6-well tissue culture plate and the AM soaked in culture medium overnight. CSSC were seeded on the AM construct 7 d before inducing the injury model. On a separate 6-well plate, hCEC were seeded at 5 × 104 cell/cm2 density 72 h before injury. On the injury day, the hCEC were treated with 20% (v/v) EtOH for 30 s, the CSSC-AM constructs were moved into the wells for co-culture studies and SCM containing 1 ng/mL IL-1β added. Controls for CSSC-AM co-culture experiments included no-injury, hCEC only, CSSC only, and AM only.
Figure 1.
Culture system enabling seeding of corneal-stroma derived stem cells on amniotic membrane. A: Schematic showing dimensions and arrangement of polytetrafluoroethylene (PTFE) O-ring inside 30 mm tissue culture insert; B: The PTFE ring is placed on the epithelial side of hydrated amniotic membrane (AM); C: The AM is firmly wrapped over the O-ring edges leaving a taut membrane surface for cell seeding; D: The O-ring containing the AM is placed within the tissue culture insert; E: The tissue culture insert is placed within a 6-well plate and culture medium applied.
Statistical analysis
Statistical significances were calculated using GraphPad Prism v.7.00.
RESULTS
M199 and SCM support both hCEC and CSSC viability and proliferation
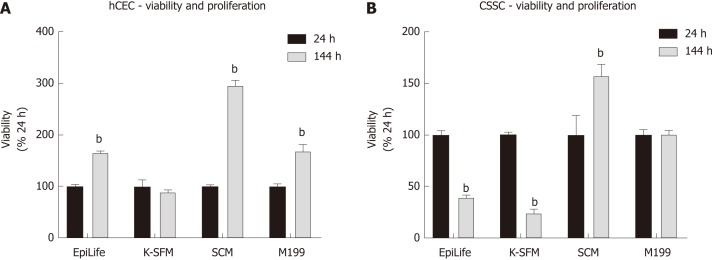
To determine an optimal medium for co-culturing hCEC and CSSC, both cell types were cultured in EpiLife, K-SFM, M199, and SCM with viability assays performed at 24 h and 144 h (Figure 2). hCEC showed significant proliferation in EpiLife, SCM, and M199 (Figure 2A). Contrary to expectations, hCEC in K-SFM did not proliferate. CSSC only demonstrated significant proliferation in SCM, but did not lose viability in M199. Culture in EpiLife and K-SFM significantly reduced CSSC viability (Figure 2B). As M199 and SCM supported both cell types, both media were selected for further experiments.
Figure 2.
Effect of culture medium on human corneal epithelial cells and corneal-stroma derived stem cells viability and proliferation. A, B: Human corneal epithelial cells (A) and corneal-stroma derived stem cells (B) were seeded in EpiLife, keratinocyte-serum free medium, stem cell medium, and M199. Presto Blue viability assay was performed at 24 h and 144 h. Each time point is represented relative to the viability in that media type at 24 h. Data shown as mean ± SEM of six replicates (n = 6) each with 3 samples. Statistical significance compared to 24 h, analysed by two-way ANOVA, represented by bP ≤ 0.0001. CSSC: Corneal-stroma derived stem cells; hCEC: Human corneal epithelial cells; K-SFM: Keratinocyte-serum free medium; SCM: Stem cell medium.
An injury model of 20% (v/v) EtOH and 1 ng/mL IL-1β creates a balance between cell viability reduction and pro-inflammatory cytokine production
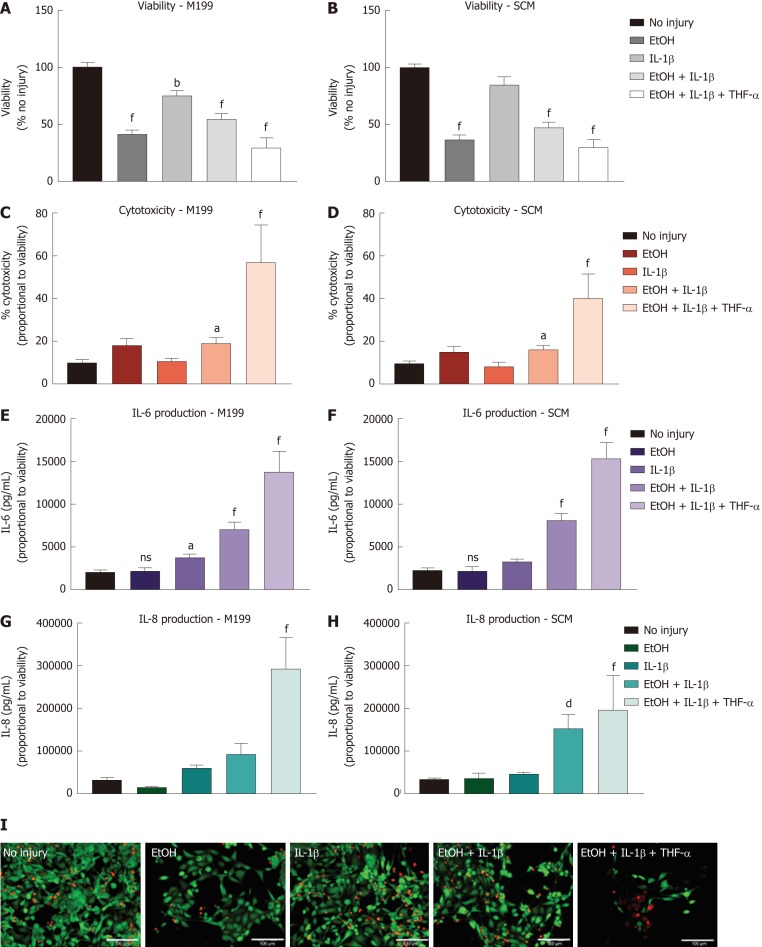
Different combinations of EtOH injury with and without inflammatory stimulus were tested on hCEC cultured in SCM and M199 to determine an optimised injury model (Figure 3). Viability assays were performed 72 h after injury with/without IL-1β and/or TNF-α supplementing the culture medium for the entire time (Figure 3A and 3B). For cells cultured in M199, all injury treatments caused significant reductions in cell viability, with EtOH with IL-1β and TNF-α treatment causing the largest reduction (Figure 3A). For cells cultured in SCM, there was no significant drop in viability when cells were treated with only IL-1β; the remaining treatments all caused over 50% reduction in viability (Figure 3B) with EtOH with IL-1β and TNF-α treatment causing the largest reduction.
Figure 3.
Injury model optimisation for human corneal epithelial cells. Injury model optimisation was performed with human corneal epithelial cells (hCEC) cultured in both M199 (A, C, E, G) and stem cell medium (B, D, F, H). Injuries consisted of the following treatments: 20% (v/v) ethanol (EtOH); 1 ng/mL interleukin (IL)-1β in the medium; 20% (v/v) EtOH with 1 ng/mL IL-1β in the medium; and 20% (v/v) EtOH with 1 ng/mL IL-1β and 10 ng/mL TNF-α in the medium. A, B: PrestoBlue viability assay performed 72 h after the different treatments. Data represented relative to reading for no injury control; C, D: LDH assay performed on supernatant 72 h after injury. Data displayed as percentage cytotoxicity and relative to cell viability; E, F: Concentration of IL-6 in the supernatant 72 h after injury. Data displayed relative to cell viability; G, H: Concentration of IL-8 in the supernatant 72 h after injury. Data displayed relative to cell viability. Data for all graphs shown as mean ± SEM of five independent experiments, with three to six replicates each. Statistical significance compared to no injury controls analysed by one-way ANOVA represented by aP ≤ 0.05, bP ≤ 0.01, dP ≤ 0.001, fP ≤ 0.0001; I: LIVE/DEAD staining performed on hCEC cultured in stem cell medium for hCEC untreated control (A), 20% (v/v) EtOH (B), 1 ng/mL IL-1β (C), EtOH + 1 ng/mL IL-1β (D), EtOH + 10 ng/mL IL-1β (E), and EtOH+ 1 ng/mL IL-1β + 10 ng/mL TNF-α (F). Live staining (green) is shown with FITC, and dead staining (red) is shown with TRITC. Scale bar = 100 µm. hCEC: Human corneal epithelial cells; IL: Interleukin; SCM: Stem cell medium; TNF: Tumour necrosis factor.
Cytotoxicity caused by the injury was measured by levels of the LDH enzyme within the supernatant 72 h after injury began (Figure 3C and 3D). In both M199 and SCM, only treatment with EtOH with IL-1β, or EtOH with IL-1β and TNF-α caused significant levels of cytotoxicity compared with the no injury controls. Levels of inflammation within the injury models were assessed by measuring IL-6 and IL-8 concentration within the hCEC supernatant 72 h after initial injury (Figure 3E, 3F, 3G, and 3H). Significant concentrations of IL-6 were found with treatment of EtOH with IL-1β, or EtOH with IL-1β and TNF-α in both M199 (Figure 3E) and SCM (Figure 3F). IL-8 production was only significant in M199 when treated with EtOH with IL-1β and TNF-α (Figure 3G), but in SCM was significant when treated with EtOH with IL-1β, or EtOH with IL-1β and TNF-α (Figure 3H). Representative LIVE/DEAD staining images of hCEC cultured in SCM 72 h after initial injury can be seen in Figure 3I. When EtOH treatment was not used, 1 ng/mL IL-1β treatment showed an almost comparable cell confluence to the control. All other treatments evidenced a decrease in cell number, with EtOH with IL-1β and TNF-α being the most toxic.
These results taken as a whole suggest that combining EtOH treatment with both IL-1β and TNF-α generates high, but unnecessary, inflammatory and toxic damage to the cells, leaving few cells alive for further analysis. The most balanced treatment was 20% (v/v) EtOH with 1 ng/mL IL-1β, which led to significant production of pro-inflammatory cytokines, significant cytotoxicity, and a significant but not excessive reduction in viability. Thus, this injury model was chosen for further experiments. It was also decided to continue co-culture experiments in SCM only.
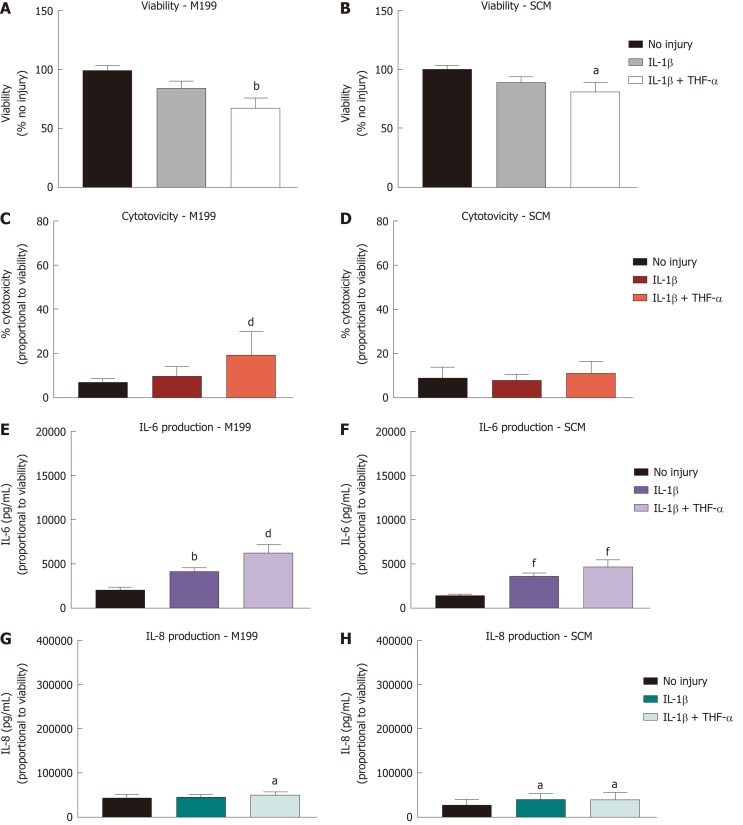
CSSC viability remains stable after treatment with IL-1β but production of IL-6 and IL-8 is increased
CSSC cultured in both M199 and SCM were stimulated with IL-1β and TNF-α and assessed with identical assays to those described above, in preparation for co-culture with hCEC (Figure 4). Viability assays were performed at 72 h of cytokine stimulation (Figure 4A and 4B). A significant reduction in viability compared to non-injured controls was seen when IL-1β and TNF-α together were present in the medium. LDH concentration in the media, indicating cytotoxicity, was assessed after 72 h stimulation (Figure 4C and 4D). Significant cytotoxicity was only seen in M199 when treated with both IL-1β and TNF-α. Assessment of IL-6 (Figure 4E and 4F) and IL-8 (Figure 4G and 4H) production showed significant production of IL-6 in both media due to IL-1β treatment alone and with TNF-α. IL-8 was produced in significant levels in SCM due to IL-1β treatment alone and with TNF-α but only in M199 when both cytokines were present. Overall production of IL-6 and IL-8 by CSSC was far lower than that of hCEC.
Figure 4.
Effect of treatment with pro-inflammatory cytokines on corneal-stroma derived stem cells. Treatment of corneal-stroma derived stem cells cultured in stem cell medium or M199 with 1 ng/mL IL-1β with/without 10 ng/mL tumour necrosis factor-α was performed for 72 h. A: PrestoBlue viability assay after 72 h stimulation. Data represented relative to reading for no injury control; B: Lactate dehydrogenase cytotoxicity assay performed on cell supernatants after 72 h treatment. Data displayed as percentage cytotoxicity and relative to cell viability; C: Concentration of IL-6 in the supernatant 72 h after injury. Data displayed relative to cell viability; D: Concentration of IL-8 in the supernatant 72 h after injury. Data displayed relative to cell viability. Data for all graphs shown as mean ± SEM of five independent experiments with three to six replicates each. Statistical significance compared to no injury controls analysed by one-way ANOVA represented by aP ≤ 0.05, bP ≤ 0.01, dP ≤ 0.001, fP ≤ 0.0001. IL: Interleukin; TNF: Tumour necrosis factor; SCM: Stem cell medium.
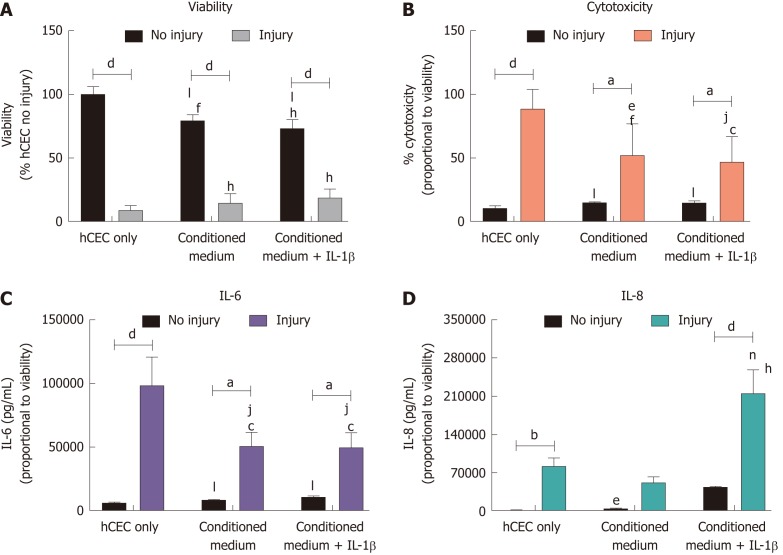
CSSC conditioned medium does not inhibit hCEC viability loss but shows some inhibition of LDH, IL-6, and IL-8 production after injury.
CSSC were used to obtain conditioned SCM medium in order to determine if secreted factors alone were adequate for producing an anti-inflammatory response. hCEC were injured with EtOH for 30 s. Subsequently, SCM control, CSSC-conditioned medium, or CSSC-conditioned medium pre-treated with IL-1β were applied along with the extra IL-1β inflammatory stimulus for 72 h before analysis (Figure 5). No inhibition in the reduction of viability due to the injury was seen in any group, although there was significant reduction in viability of non-injured hCEC due to the presence of conditioned medium (Figure 5A). There was significant reduction in the levels of cytotoxicity caused by the injury with treatment with both types of conditioned medium, however there was still significant levels of cytotoxicity compared to non-injured controls (Figure 5B). There was significant reduction in the production of IL-6 by injured hCEC when treated with CSSC-conditioned medium with and without IL-1β pre-treatment; however, levels were still significantly higher than non-injured controls (Figure 5C). IL-8 production by hCEC in response to injury was not significant compared to the non-injured control when treated with CSSC-conditioned medium. However, when treated with the conditioned medium from the CSSC that had IL-1β pre-treatment, there was a significant increase in the levels of IL-8, potentially due to the high levels of IL-1β (Figure 5D).
Figure 5.
Effect of corneal-stroma derived stem cells-conditioned medium on human corneal epithelial cells treated with the injury model. Human corneal epithelial cells (hCEC) were treated with an injury model consisting of 30 s ethanol treatment followed by stimulation with 1 ng/mL IL-1β for 72 h. Medium conditioned by corneal-stroma derived stem cells with and without pre-treatment with IL-1β was applied to the hCEC after ethanol treatment during stimulation with IL-1β. A: PrestoBlue viability assay after 72 h. Data represented relative to reading for hCEC no injury control; B: Lactate dehydrogenase cytotoxicity assay performed on cell supernatants after 72 h treatment. Data displayed as percentage cytotoxicity and relative to cell viability; C: Concentration of IL-6 in the supernatant 72 h after injury. Data displayed relative to cell viability; D: Concentration of IL-8 in the supernatant 72 h after injury. Data displayed relative to cell viability. Data shown as mean ± SEM of three independent experiments with four to six replicates each. Statistical significance analysed by two-way ANOVA. Significance compared to non-injured, same treatment represented by aP ≤ 0.05, bP ≤ 0.01, dP ≤ 0.0001. Significance compared to hCEC, no injury represented by cP ≤ 0.05, fP ≤ 0.01, hP ≤ 0.0001. Significance compared to hCEC, injury represented by eP ≤ 0.05, jP ≤ 0.01, nP ≤ 0.001, lP ≤ 0.0001. hCEC: Human corneal epithelial cells; IL: Interleukin.
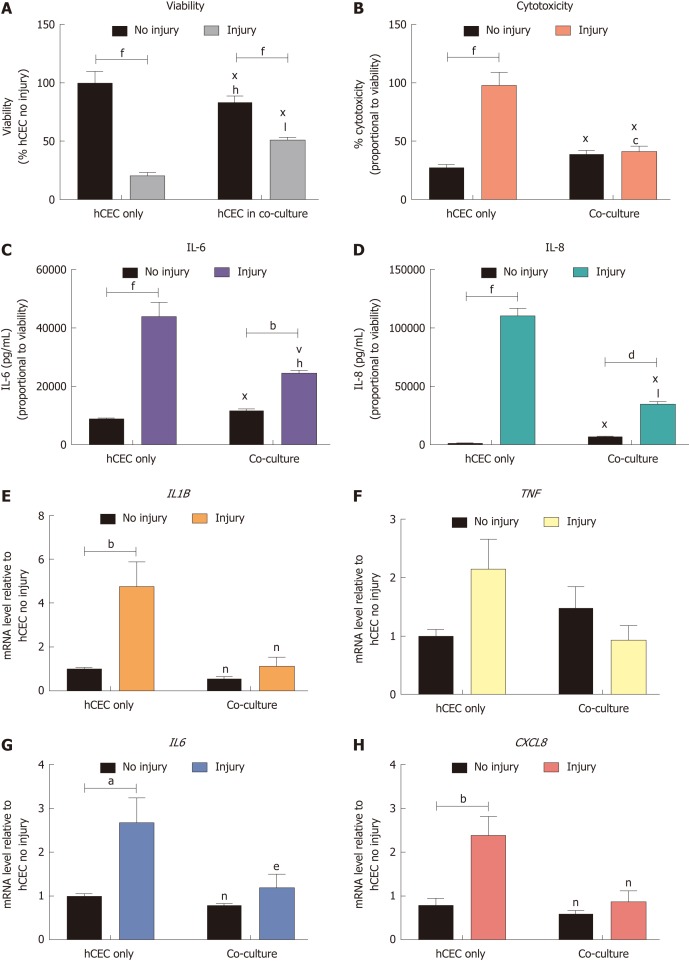
Co-culture of CSSC with injured hCEC improves hCEC viability and reduces post-injury inflammation
hCEC were treated with the standard injury of EtOH for 30 s followed by IL-1β stimulation. During the IL-1β treatment, co-cultures of CSSC were added and analysis of viability, cytotoxicity, cytokine production, and gene expression were performed after 72 h (Figure 6). There was a significant reduction in viability of the injured hCEC compared to non-injured cells, as previously shown. However, the addition of the CSSC in co-culture to the injured hCEC showed a significant inhibition of this viability decrease, with a significantly higher viability shown in the co-culture injured group than in non-co-culture (Figure 6A). Levels of cytotoxicity were significantly reduced in the co-culture systems when compared to hCEC only, with no significant difference shown between no injury and injury (Figure 6B). Both IL-6 (Figure 6C) and IL-8 (Figure 6D) production was significantly reduced when in co-culture compared to the hCEC only injured group. Gene expression of IL1B (Figure 6E), IL6 (Figure 6G) and CXCL8 (IL-8, Figure 6H) by injured hCEC was also significantly reduced by co-culture with CSSC. TNF expression was not significantly reduced (Figure 6F).
Figure 6.
Effect of co-culture with corneal-stroma derived stem cells on human corneal epithelial cells response after injury. Human corneal epithelial cells (hCEC) were treated with an injury model consisting of 30 s ethanol treatment followed by stimulation with 1 ng/mL IL-1β for 72 h. During IL-1β stimulation corneal-stroma derived stem cells (CSSC) were co-cultured with CSSC in a transwell system. A: PrestoBlue viability assay after 72 h. Data represented relative to reading for hCEC only, no injury control; B: Lactate dehydrogenase cytotoxicity assay performed on cell supernatants after 72 h treatment. Data displayed as percentage cytotoxicity and relative to cell viability; C: Concentration of IL-6 in the supernatant 72 h after injury. Data displayed relative to cell viability; D: Concentration of IL-8 in the supernatant 72 h after injury. Data displayed relative to cell viability; E-H: RT-qPCR performed to show mRNA expression of IL1B (E), TNF (F), IL6 (G), and CXCL8 (H). Expression of each target gene normalised to GAPDH and represented relative to hCEC only, no injury. Data for all graphs shown as mean ± SEM of three independent experiments with five replicates each. Statistical significance analysed by two-way ANOVA. Significance compared to non-injured, same group, represented by aP ≤ 0.05, bP ≤ 0.01, dP ≤ 0.001, fP ≤ 0.0001. Significance compared to hCEC only, no injury represented by cP ≤ 0.05, hP ≤ 0.01, jP ≤ 0.001, lP ≤ 0.0001. Significance compared to hCEC, injury represented by eP ≤ 0.05, nP ≤ 0.01, vP ≤ 0.001, xP ≤ 0.0001. CSSC: Corneal-stroma derived stem cells; hCEC: Human corneal epithelial cells; IL: Interleukin.
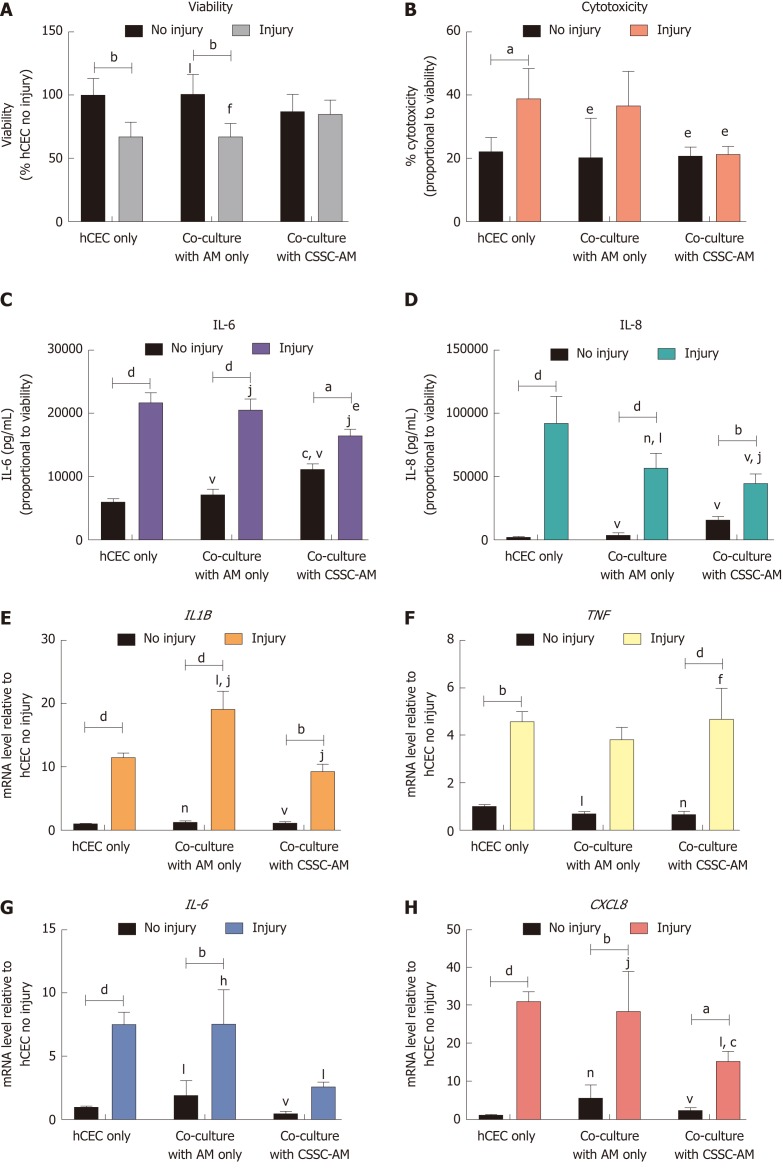
Co-culture of injured hCEC with the CSSC-AM construct improves hCEC viability while reducing pro-inflammatory cytokines and pro-inflammatory cytokine mRNA expression after injury
hCEC were treated with the standard injury of EtOH followed by IL-1β stimulation in the culture medium. During the IL-1β stimulation, constructs of AM only and CSSC-AM were added in co-culture. After 72 h, analysis of viability, cytotoxicity, cytokine production, and mRNA expression of the hCEC were performed (Figure 7). As seen previously, there was a loss in hCEC viability when the injury was performed without co-culture. This loss in viability was not inhibited by the presence of AM alone, but significant inhibition of viability loss was demonstrated when injured hCEC were co-cultured with the CSSC-AM construct (Figure 7A). Increased cytotoxicity was seen when hCEC were injured without co-culture; this was not inhibited by the AM construct, but was significantly inhibited by co-culture with the CSSC-AM construct (Figure 7B). IL-6 and IL-8 release into the media due to injury was significantly inhibited during co-culture with the CSSC-AM construct (Figure 7C and 7D). IL-8 production was also significantly inhibited by the presence of AM only. Unlike, the previous co-culture experiment, expression of IL1B was not significantly reduced by either co-culture during injury (Figure 7E). Expression of TNF was also unaffected by co-culture (Figure 7F). Expression of IL6 (Figure 7G) and CXCL8 (IL-8, Figure 7H) mRNA were significantly reduced when injured hCEC were co-cultured with CSSC-AM. This reduction did not occur with AM only.
Figure 7.
Effect of co-culture with corneal-stroma derived stem cells-amniotic membrane constructs on human corneal epithelial cells response after injury. Human corneal epithelial cells (hCEC) were treated with an injury model consisting of 30 s ethanol treatment followed by stimulation with 1 ng/mL IL-1β for 72 h. During IL-1β stimulation hCEC were co-cultured with either amniotic membrane (AM) only or a corneal-stroma derived stem cells-AM construct. A: PrestoBlue viability assay after 72 h. Data represented relative to reading for hCEC only, no injury control; B: Lactate dehydrogenase cytotoxicity assay performed on cell supernatants after 72 h treatment. Data displayed as percentage cytotoxicity and relative to cell viability; C: Concentration of IL-6 in the supernatant 72 h after injury. Data displayed relative to cell viability; D: Concentration of IL-8 in the supernatant 72 h after injury. Data displayed relative to cell viability; E-H: RT-qPCR performed to show mRNA expression of IL1B (E), TNF (F), IL6 (G), and CXCL8 (H). Expression of each target gene normalised to GAPDH and represented relative to hCEC only, no injury. Data for all graphs shown as mean ± SEM of three independent experiments with five replicates each. Statistical significance analysed by two-way ANOVA. Significance compared to non-injured, same group, represented by aP ≤ 0.05, bP ≤ 0.01, dP ≤ 0.0001. Significance compared to hCEC only, no injury represented by cP ≤ 0.05, fP ≤ 0.01, hP ≤ 0.001, jP ≤ 0.0001. Significance compared to hCEC, injury represented by eP ≤ 0.05, lP ≤ 0.01, nP ≤ 0.001, vP ≤ 0.0001. AM: Amniotic membrane; CSSC: Corneal-stroma derived stem cells; hCEC: Human corneal epithelial cells; IL: Interleukin.
DISCUSSION
In the last few decades, MSCs have been widely studied for viable medical applications due to their therapeutic properties[34,35]. Moreover, CSSC have a huge potential in the ophthalmology field as these stem cells may play an important role in corneal regeneration and wound healing[36]. Some of their important functions are the transdifferentiation capacity from the mesenchymal to the epithelial phenotype[15,37] and the generation of progenitors with MSC potential[38].
Although CSSC provide positive results as therapy for sight-threatening corneal diseases[14], an optimal cell-delivery format is essential for a cell-based therapy to succeed. The choice of the delivery method depends on both the tissue and the disease to be treated, looking for a high cell retention and integration capacity for the cells to repopulate, release healing factors, or help the surrounding tissues and cells to recover their normal functions[39]. For corneal disease, AM shows high promise as a biocompatible scaffold, demonstrating properties which enhance the delivery of a cell-based therapy to the eye’s surface through a topical application[26,30].
Herein, a comparison between four injury models was undertaken on hCEC to generate an in vitro model to mimic a keratitis condition in the cornea with the aim of demonstrating the therapeutic potential of CSSC. The second stage was to build a construct of CSSC on AM to investigate if the CSSC maintained their anti-inflammatory properties when using the membrane as a carrier and to discern if the AM added extra anti-inflammatory value.
Several factors had to be considered to develop a functional inflammatory model. Firstly, the comparison between K-SFM, EpiLife, SCM, and M199 took place in order to provide an optimal growth environment for both hCEC and CSSC when co-cultured. EpiLife is serum-free and commercially available for human epidermal keratinocytes, and it has been previously used and approved for hCEC culture[40]. K-SFM is another serum-free medium for epithelial cells, supplemented with epidermal growth factor and basic-fibroblast growth factor, which has been shown to be efficient for keratocyte[41] and hCEC[42] maintenance and proliferation. M199 was the only serum-containing medium tested in this study and it has previously demonstrated to maintain an MSC-like cell-surface marker profile when culturing CSSC[9,12]. Finally, SCM is mostly related with induced pluripotent stem cells and human embryonic stem cells. It contains basic-fibroblast growth factor, human leukaemia inhibitory factor, and knockout serum replacement and has been associated with the maintenance of pluripotency markers in CSSC cultures[10]. In this investigation, SCM and M199 maintained the proliferation and viability for both hCEC and CSSC, confirming previous studies performed on CSSC by our research group. However, as the ultimate outcome for this research is to translate the investigation into a cell-therapy based on maintenance and viability of the CSSC to heal the cornea and recover vision, an FBS-containing medium is not appropriate due to its variability in composition from batch-to-batch and its animal origin[43]. Moreover, despite the fact that M199 accomplished the minimal criteria for maintaining an MSC phenotype it does not share the advantages of SCM for the promotion of SC/progenitor markers while maintaining a MSC phenotype. Therefore, SCM is the best prospect for therapeutic applications[10].
Four models were initially tested against the hCEC to generate an injury. The 1 ng/mL IL-1β only treatment was not potent enough to significantly reduce the cell proliferation and it did not develop effective levels of inflammation. This response is supported by studies showing that IL-1β promotes differentiation of progenitor cells as well as maturation and survival of differentiated cells[44,45]. Treatment of 20% (v/v) EtOH only significantly reduced the cell viability/proliferation and caused an initial injury as supported by previous studies[46]. However, it did not activate the cells to generate inflammation through production of pro-inflammatory cytokines. In contrast, the IL-1β and TNF-α treatments both generated an inflammatory response. Combining both cytokines was too aggressive, allowing only a few cells to recover for further analysis. On the other hand, the 20% (v/v) EtOH with 1 ng/mL IL-1β treatment provided a balance between injury and inflammatory response, proving to be the optimal injury model to mimic an in vitro keratitis model. This outcome is consistent with the reviewed literature, as the inflammatory properties of IL-1β and TNF-α had only been tested individually[47,48]. However, to the best of the author’s knowledge this was the first time that these combinations were used for the generation of an in vitro injury model.
CSSC-conditioned medium was used on the hCEC injury model to assess potential anti-inflammatory action. The conditioned medium showed no significant effect improving hCEC viability, but did demonstrate some positive effects by reducing cytotoxicity and IL-6/IL-8 production, demonstrating that CSSC secrete factors into the media that have a positive anti-inflammatory effect.
Both the CSSC alone and the CSSC-AM constructs inhibited the hCEC viability loss seen when the injury was performed. Furthermore, both systems showed beneficial anti-inflammatory effects as production of pro-inflammatory cytokines, pro-inflammatory mRNA expression, and cytotoxicity levels were significantly reduced. The beneficial therapeutic effect of the CSSC was further evidenced by the fact that the AM construct without CSSC had few inhibitory effects on the injury. Investigations for similar constructs have been performed before with positive results; Li et al[49] developed a construct where corneal stroma and limbal epithelium with SCs promoted cell proliferation. Additionally, a model using real architecture for 3D-tissue tissue equivalents as a substrate for co-culturing limbal epithelial cells and CSSC showed a positive impact on the expansion of the epithelial cells while maintaining the MSC-markers CD73 and CD90, confirming the CSSC phenotype[50]. Contrary to our findings, previous work on burned rat corneas showed that MSCs increased IL6 expression but still reduced corneal inflammation[51].
In conclusion, an in vitro injury model was developed using hCEC culture, allowing the initial testing into the anti-inflammatory potential of CSSC. However, it is well known that in vitro observations are not always able to reflect an in vivo phenomenon; it would therefore be necessary to validate these findings in preclinical animal models in order to validate efficacy to proceed to clinical trials. Nevertheless, this investigation demonstrated that CSSC have an important role in corneal regeneration and wound healing and is a stepping stone towards further preclinical investigation.
ARTICLE HIGHLIGHTS
Research background
The cornea provides two thirds of the eye’s refractive power as well as being the major barrier to the inner content of the eye. At present, the most effective treatment of a diseased or damaged cornea is transplantation of a donor cornea (keratoplasty). However, this is not feasible for 8-10 million individuals. Corneal research has turned to the use of stem cell-based regenerative therapies for corneal tissue regeneration. Recent in vitro studies have shown that mesenchymal stem cell-like cells from the corneal stroma (corneal-derived stromal stem cells, CSSC) contribute to corneal tissue homeostasis, presenting an immunomodulatory response, a non-immunogenic profile, and a regenerative role. Thus, CSSC may be considered an appropriate cell source for the treatment of inflammatory disorders of the cornea.
Research motivation
To investigate whether CSSC seeded on an amniotic membrane (AM) substrate have the ability to modulate an inflamed environment, and therefore whether they were suitable candidates for developing a cell therapy for the front of the eye.
Research objectives
The first objective was to optimise an in vitro injury model mimicking a corneal surface using human corneal epithelial cells (hCEC) stimulated with various injurious agents. Once optimised the second objective was to investigate whether CSSC conditioned media and then CSSC in co-culture could modulate the injury environment. The final objective was to seed CSSC on AM and investigate whether the CSSC-AM construct provided an anti-inflammatory, healing response to the injury.
Research methods
Treatment of hCEC with ethanol and pro-inflammatory cytokines were compared in terms of viability loss, cytotoxicity, and pro-inflammatory cytokine release in order to generate the novel in vitro injury. Co-culture experiments were performed with CSSC alone and with CSSC-AM constructs. The effect of injury and co-culture on viability, cytotoxicity, interleukin (IL)-6 and IL-8 production, and IL1B, TNF, IL6, and CXCL8 mRNA expression were assessed.
Research results
An optimal injury of 20% (v/v) ethanol for 30 s with 1 ng/mL IL-1 beta was developed. Co-culture of the injury model with CSSC inhibited loss of hCEC viability caused by injury. Enzyme linked immunosorbent assay and PCR showed a significant reduction in the production of IL-6 and IL-8 pro-inflammatory cytokines and reduction in pro-inflammatory cytokine mRNA expression during co-culture with CSSC alone and with the AM construct.
Research conclusions
The novel findings of this study confirm the therapeutic potential of the CSSC and the possible use of AM as a cell carrier for application to the ocular surface.
Research perspectives
The novel injury model developed in this study can be adapted for studying the therapeutic effects of many different agents. The findings of this study may lead to the development of practise changing cell therapies for treatment of inflammatory disorders of the cornea in clinic.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: Human corneoscleral rims and human AM were used with approval by the Nottingham Research Ethics Committee (Ethics approval numbers: 07/H0403/140 and OY110101, respectively).
Informed consent statement: All corneal tissue was supplied anonymously through Manchester Eye Bank, and all informed consent forms are held at this institution. For amniotic membrane, all donors provided written informed consent prior to tissue collection. During research studies all tissue was anonymised to the researchers.
Conflict-of-interest statement: There are no potential conflicts of interest relevant to this study reported by the authors.
Peer-review started: July 24, 2018
First decision: October 5, 2018
Article in press: January 1, 2019
P- Reviewer: Liu L, Micheu MM, Vladimir H S- Editor: Ji FF L- Editor: Filipodia E- Editor: Bian YN
Contributor Information
Mariana Lizeth Orozco Morales, Academic Ophthalmology, Division of Clinical Neuroscience, University of Nottingham, Nottingham, NG7 2UH, United Kingdom.
Nagi M Marsit, Academic Ophthalmology, Division of Clinical Neuroscience, University of Nottingham, Nottingham, NG7 2UH, United Kingdom.
Owen D McIntosh, Academic Ophthalmology, Division of Clinical Neuroscience, University of Nottingham, Nottingham, NG7 2UH, United Kingdom.
Andrew Hopkinson, Academic Ophthalmology, Division of Clinical Neuroscience, University of Nottingham, Nottingham, NG7 2UH, United Kingdom.
Laura E Sidney, Academic Ophthalmology, Division of Clinical Neuroscience, University of Nottingham, Nottingham, NG7 2UH, United Kingdom. laura.sidney@nottingham.ac.uk.
References
- 1.Thompson RW, Jr, Price MO, Bowers PJ, Price FW., Jr Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 2.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 4.Rohaina CM, Then KY, Ng AM, Wan Abdul Halim WH, Zahidin AZ, Saim A, Idrus RB. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl Res. 2014;163:200–210. doi: 10.1016/j.trsl.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 8.Polisetty N, Fatima A, Madhira SL, Sangwan VS, Vemuganti GK. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 9.Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012;53:5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 10.Sidney LE, Branch MJ, Dua HS, Hopkinson A. Effect of culture medium on propagation and phenotype of corneal stroma-derived stem cells. Cytotherapy. 2015;17:1706–1722. doi: 10.1016/j.jcyt.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Sidney LE, McIntosh OD, Hopkinson A. Phenotypic Change and Induction of Cytokeratin Expression During In Vitro Culture of Corneal Stromal Cells. Invest Ophthalmol Vis Sci. 2015;56:7225–7235. doi: 10.1167/iovs.15-17810. [DOI] [PubMed] [Google Scholar]
- 12.Sidney LE, Hopkinson A. Corneal keratocyte transition to mesenchymal stem cell phenotype and reversal using serum-free medium supplemented with fibroblast growth factor-2, transforming growth factor-β3 and retinoic acid. J Tissue Eng Regen Med. 2018;12:e203–e215. doi: 10.1002/term.2316. [DOI] [PubMed] [Google Scholar]
- 13.Bray LJ, Heazlewood CF, Munster DJ, Hutmacher DW, Atkinson K, Harkin DG. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy. 2014;16:64–73. doi: 10.1016/j.jcyt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Veréb Z, Póliska S, Albert R, Olstad OK, Boratkó A, Csortos C, Moe MC, Facskó A, Petrovski G. Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing. Sci Rep. 2016;6:26227. doi: 10.1038/srep26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashmani K, Branch MJ, Sidney LE, Dhillon PS, Verma M, McIntosh OD, Hopkinson A, Dua HS. Characterization of corneal stromal stem cells with the potential for epithelial transdifferentiation. Stem Cell Res Ther. 2013;4:75. doi: 10.1186/scrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Z, Li F, Zeng X, Lv Y, Zhao S. The effects of local administration of mesenchymal stem cells on rat corneal allograft rejection. BMC Ophthalmol. 2018;18:139. doi: 10.1186/s12886-018-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song HB, Park SY, Ko JH, Park JW, Yoon CH, Kim DH, Kim JH, Kim MK, Lee RH, Prockop DJ, Oh JY. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol Ther. 2018;26:162–172. doi: 10.1016/j.ymthe.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, Shah DI, Sahu SK, Chauhan SK. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports. 2016;7:583–590. doi: 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X, Li J, Yin L, Pan J, Zhang Y, Jiang Z. Role of microRNA 146a on the healing of cornea alkali burn treated with mesenchymal stem cells. Mol Med Rep. 2018;18:3203–3210. doi: 10.3892/mmr.2018.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuentes-Julián S, Arnalich-Montiel F, Jaumandreu L, Leal M, Casado A, García-Tuñon I, Hernández-Jiménez E, López-Collazo E, De Miguel MP. Adipose-derived mesenchymal stem cell administration does not improve corneal graft survival outcome. PLoS One. 2015;10:e0117945. doi: 10.1371/journal.pone.0117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong HS, Kim YH, Son Y. Perspectives on mesenchymal stem cells: tissue repair, immune modulation, and tumor homing. Arch Pharm Res. 2012;35:201–211. doi: 10.1007/s12272-012-0201-0. [DOI] [PubMed] [Google Scholar]
- 22.Tseng SC, He H, Zhang S, Chen SY. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul Surf. 2016;14:100–112. doi: 10.1016/j.jtos.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituting skin grafts: A preliminary report. JAMA. 1913;60:973–974. [Google Scholar]
- 24.SORSBY A, SYMONS HM. Amniotic membrane grafts in caustic burns of the eye (burns of the second degree) Br J Ophthalmol. 1946;30:337–345. [PubMed] [Google Scholar]
- 25.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408–413. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Bauer D, Wasmuth S, Hennig M, Baehler H, Steuhl KP, Heiligenhaus A. Amniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2009;50:3188–3198. doi: 10.1167/iovs.08-3041. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303–312. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- 29.Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi N, Inatomi T, Quantock AJ, Fullwood NJ, Dota A, Kinoshita S. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000;19:65–71. doi: 10.1097/00003226-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 32.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen CL, Clare G, Stewart EA, Branch MJ, McIntosh OD, Dadhwal M, Dua HS, Hopkinson A. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One. 2013;8:e78441. doi: 10.1371/journal.pone.0078441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 36.Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye (Lond) 2006;20:482–490. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 37.Perrella G, Scott CA, Spelat R, Brusini P, D'Aurizio F, De Pol I, Dua HS. Cultured hyman keratocytes from the limbus and cornea both express epithelial cytokeratin 3: Possible mesenchymal-epithelial transition. Int J Ophthalmic Pathol. 2012;1:1–7. [Google Scholar]
- 38.Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelm JM, Fussenegger M. Scaffold-free cell delivery for use in regenerative medicine. Adv Drug Deliv Rev. 2010;62:753–764. doi: 10.1016/j.addr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Lekhanont K, Choubtum L, Chuck RS, Sa-ngiampornpanit T, Chuckpaiwong V, Vongthongsri A. A serum- and feeder-free technique of culturing human corneal epithelial stem cells on amniotic membrane. Mol Vis. 2009;15:1294–1302. [PMC free article] [PubMed] [Google Scholar]
- 41.Kawakita T, Espana EM, He H, Smiddy R, Parel JM, Liu CY, Tseng SC. Preservation and expansion of the primate keratocyte phenotype by downregulating TGF-beta signaling in a low-calcium, serum-free medium. Invest Ophthalmol Vis Sci. 2006;47:1918–1927. doi: 10.1167/iovs.05-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postnikoff CK, Pintwala R, Williams S, Wright AM, Hileeto D, Gorbet MB. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS One. 2014;9:e96448. doi: 10.1371/journal.pone.0096448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wappler J, Rath B, Läufer T, Heidenreich A, Montzka K. Eliminating the need of serum testing using low serum culture conditions for human bone marrow-derived mesenchymal stromal cell expansion. Biomed Eng Online. 2013;12:15. doi: 10.1186/1475-925X-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills KH, Dunne A. Immune modulation: IL-1, master mediator or initiator of inflammation. Nat Med. 2009;15:1363–1364. doi: 10.1038/nm1209-1363. [DOI] [PubMed] [Google Scholar]
- 45.Vela JM, Molina-Holgado E, Arévalo-Martín A, Almazán G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20:489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- 46.Oh JY, Yu JM, Ko JH. Analysis of ethanol effects on corneal epithelium. Invest Ophthalmol Vis Sci. 2013;54:3852–3856. doi: 10.1167/iovs.13-11717. [DOI] [PubMed] [Google Scholar]
- 47.Mohan RR, Mohan RR, Kim WJ, Wilson SE. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest Ophthalmol Vis Sci. 2000;41:1327–1336. [PubMed] [Google Scholar]
- 48.Kimura K, Teranishi S, Nishida T. Interleukin-1beta-induced disruption of barrier function in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:597–603. doi: 10.1167/iovs.08-2606. [DOI] [PubMed] [Google Scholar]
- 49.Li DQ, Wang Z, Yoon KC, Bian F. Characterization, isolation, expansion and clinical therapy of human corneal epithelial stem/progenitor cells. J Stem Cells. 2014;9:79–91. [PubMed] [Google Scholar]
- 50.Kureshi AK, Dziasko M, Funderburgh JL, Daniels JT. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Sci Rep. 2015;5:16186. doi: 10.1038/srep16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]