Abstract
BACKGROUND
Novel strategies are needed for improving guided bone regeneration (GBR) in oral surgery prior to implant placement, particularly in maxillary sinus augmentation (GBR-MSA) and in lateral alveolar ridge augmentation (LRA). This study tested the hypothesis that the combination of freshly isolated, unmodified autologous adipose-derived regenerative cells (UA-ADRCs), fraction 2 of plasma rich in growth factors (PRGF-2) and an osteoinductive scaffold (OIS) (UA-ADRC/PRGF-2/OIS) is superior to the combination of PRGF-2 and the same OIS alone (PRGF-2/OIS) in GBR-MSA/LRA.
CASE SUMMARY
A 79-year-old patient was treated with a bilateral external sinus lift procedure as well as a bilateral lateral alveolar ridge augmentation. GBR-MSA/LRA was performed with UA-ADRC/PRGF-2/OIS on the right side, and with PRGF-2/OIS on the left side. Biopsies were collected at 6 wk and 34 wk after GBR-MSA/LRA. At the latter time point implants were placed. Radiographs (32 mo follow-up time) demonstrated excellent bone healing. No radiological or histological signs of inflammation were observed. Detailed histologic, histomorphometric, and immunohistochemical analysis of the biopsies evidenced that UA-ADRC/PRGF-2/OIS resulted in better and faster bone regeneration than PRGF-2/OIS.
CONCLUSION
GBR-MSA with UA-ADRCs, PRGF-2, and an OIS shows effectiveness without adverse effects.
Keywords: Case report, Cell-based therapy, Guided bone regeneration, Maxillary sinus augmentation, Lateral alveolar ridge augmentation, Unmodified autologous adipose-derived regenerative cells, Stem cells
Core tip: Novel strategies are needed in oral surgery for improving guided bone regeneration in maxillary sinus augmentation prior to implant placement. We demonstrate that the combination of freshly isolated, unmodified autologous adipose-derived regenerative cells, fraction 2 of plasma rich in growth factors and an osteoinductive scaffold is superior to the combination of fraction 2 of plasma rich in growth factors and the same osteoinductive scaffold alone. This novel procedure may contribute to a decreased healing period and increased bone quality in rehabilitation of the edentulous posterior maxilla as well as in other regenerative techniques in pre-implant bone augmentation procedures.
INTRODUCTION
The main causes of tooth loss are periodontal disease and dental caries[1]. Replacing missing or lost teeth with osseointegrated dental implants has been highly successful in the treatment of single, partial, or complete edentulism[2]. However, insufficient bone volume is a common problem occurring in the rehabilitation of the edentulous posterior maxilla with implant-supported prostheses[3-5]. Both the presence of the maxillary sinus and atrophy of the alveolar process after tooth extraction or loss contribute to this problem. Therefore, it is often necessary to perform vertical alveolar ridge augmentation to enable implant placement and integration. A well-studied technique in this context is maxillary sinus membrane elevation with autologous bone or a variety of biomaterials (i.e., a form of guided bone regeneration in maxillary sinus augmentation; thereafter, “GBR-MSA”)[3-6]. However, for the following reasons there is a need for developing novel strategies for improving GBR-MSA.
First, autologous bone is considered to be the gold standard in GBR-MSA due to its osteogenic, osteoinductive, and osteoconductive properties including lack of immunogenicity[7,8]. However, autologous bone grafts may show a number of disadvantages, such as increased operation time, donor site morbidity, post-operative discomfort, limitations in bone quantity and volume, unpredictable bone quality, reduced volume stability, and fast resorption rate[9-13]. It may also be only effective under good recipient conditions. Furthermore, the intraoral amount of autologous bone is limited, and therefore extraoral donor sites are needed for larger defects. Extraoral donor sites like the iliac crest may lead to further discomfort for the patient and an even higher morbidity rate compared to intraoral donor sites.
Second, while allografts have osteogenic properties, their probable osteoinductive and osteoconductive functions are still discussed contradictorily[7,11,14-16]. Especially the osteoconductive property of bone allografts leads to a significant higher volume stability compared to autologous bone, although it is still resorbable and will be degraded into autologous bone. Demineralized freeze-dried bone allografts were shown to be osteoinductive and osteoconductive due to the release of bone morphogenetic proteins[17], although clinical outcomes comparing mineralized and demineralized freeze-dried bone allografts were reported to be similar[18]. Studies in vitro and animal investigations revealed osteoinductive functions of demineralized freeze-dried bone allografts by recruiting cells and ectopic bone formation[19]. Disadvantages of allogeneic materials may be a protracted vascularization, slow remodeling and resorption or longer time for osseointegration, and the risk of immunogenic reactions[15-18].
Third, several experimental studies on animal models[20,21], clinical studies[22-24], and an earlier meta-analysis[25] indicated that platelet-rich plasma (PRP) can increase new bone formation in maxillary sinus augmentation when used in combination with autologous or allogeneic graft material. The use of PRP is based on the premise that it contains large quantities of growth factors, including platelet derived growth factor, insulin-like growth factor-1, and transforming growth factor-β that may enhance osteogenesis[26-28]. However, a number of recent systematic reviews and meta-analyses came to the conclusion that PRP has no significant impact on bone formation as well as on implant survival in maxillary sinus augmentation[29-31].
Based on multidisciplinary expert consultation the aim of the present study was to test (using a first-in-human, split-mouth single case study design) the hypothesis that in GBR-MSA the combination of freshly isolated, unmodified autologous adipose derived regenerative cells (UA-ADRCs), fraction 2 of plasma rich in growth factors (PRGF-2), and an osteoinductive scaffold (OIS) is superior to the combination of PRGF-2 and the same OIS alone. Due to the fact that preliminary data were not available, the present study tested the null hypothesis that the combination of UA-ADRCs, PRGF-2, and an OIS in GBR-MSA is not more effective than the combination of PRGF-2 and the same OIS alone.
CASE PRESENTATION
Chief complaints
A 79-year-old male patient presented with a partly failing maxillary dentition to the clinic of the principal investigator who specialized in periodontology and implant dentistry. The patient reported that his major concern was a functional occlusion resulting in restoration of an aesthetic smile.
History of present illness
The patient reported extensive restorative treatment in the past as well as the loss of several premolar and molar teeth.
History of past illness
No specific past illness was reported that was directly related to the present illness. However, the patient reported reduced oral hygiene in the past, including lack of supragingival plaque control and limited motivation for oral hygiene.
Personal and family history
No specific personal and family history was reported that was directly related to the present illness.
Physical examination upon admission
The clinical examination upon admission revealed a reduced vertical dimension of occlusion and loss of several premolar and molar teeth. Furthermore, advanced periodontal defects were present around several teeth in the anterior maxilla as well as around maxillary and mandibular molar teeth. Most of the remaining maxillary teeth had a guarded to hopeless prognosis.
Laboratory examinations
No laboratory examinations were performed upon admission.
Imaging examinations
A panoramic radiograph was performed upon admission (i.e., at time point T0; the time course of the present study is summarized in Table 1) showed the loss of several premolar and molar teeth and revealed that the residual bone height of the edentulous posterior maxilla below the antrum and the ridge crest was less than 3 mm on both sides (Class D according to[32]) (Figure 1).
Table 1.
Overview on the treatments performed in the present study.
| Treatments | Time point | Procedures |
| Pre-phase | T0 | Evaluation of the patient |
| Preparatory steps | T1 (4 mo after T0) | Preparation of plasma rich in growth factors (PRGF-2); isolation of unmodified, autologous adipose derived regenerative cells (UA-ADRCs) |
| 1 | T1 (GBR-MSA) | Extraction of tooth # 14; ridge preservation and external sinus lift procedures |
| 2 | T2 (6 wk after T1) | Extraction of teeth # 11, #12 and #22; collection of the first biopsies |
| 3 | T3 (34 wk after T1) | Placement of implants; collection of the second biopsies |
| 4 | T4 (1 yr after T3) | Extraction of tooth #18; placement of healing abutments |
| 5 | T5 (6 wk after T4) | Placement of the definitive prosthetic telescopic bridge |
| T6 (32 mo after T1) | Last radiologic follow-up |
In order to prevent potential identification of the patient the timeline was coded.
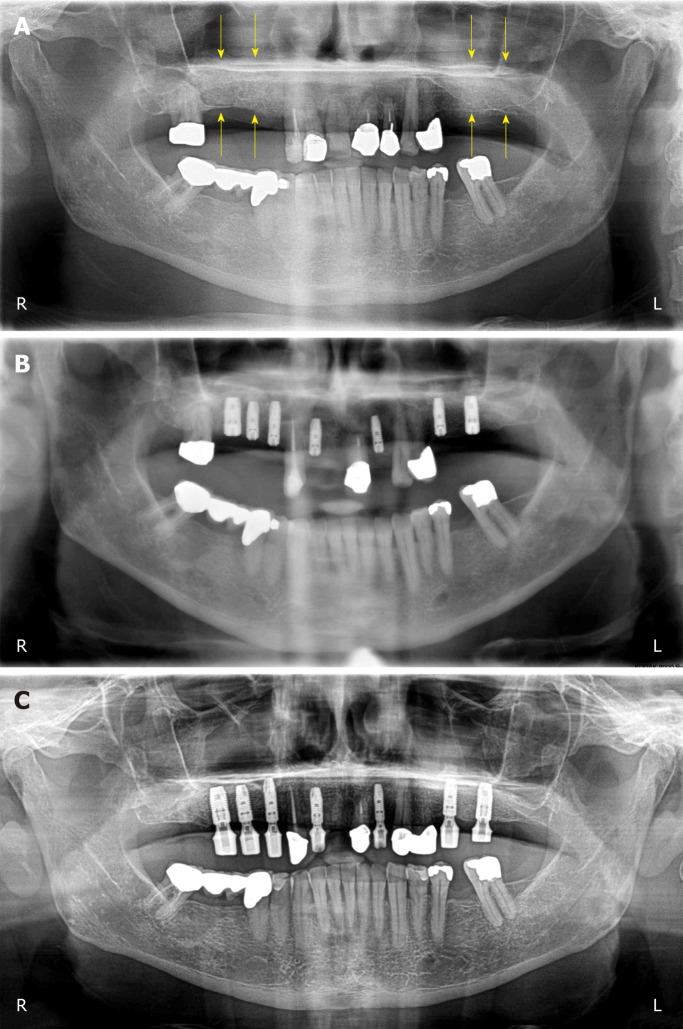
Figure 1.
Panoramic radiograph at T0. The yellow arrows indicate the reduced bone height of the edentulous posterior maxilla below the antrum and the ridge crest of less than 3 mm on both sides. R: Right; L: Left.
MULTIDISCIPLINARY EXPERT CONSULTATION
Önder Solakoglu, DDS, Head of the Clinic for Periodontology and Implantology (Hamburg, Germany)
The residual bone height of the edentulous posterior maxilla as well as of the alveolar bone crest height around the remaining maxillary teeth did not justify immediate implant placement. Therefore, extensive bone augmentation procedures (GBR-MSA) were necessary prior to implant placement. According to the literature, residual bone height of the edentulous posterior maxilla below the antrum and the ridge crest of less than 3 mm shall be treated with a lateral approach involving a bone grafting material and delayed implant placement[33].
To date, the combination of PRGF-2 instead of PRP and an OIS appears to be the most advanced procedure for GBR-MSA. This is due to the fact that a recent experimental study on rats showed that PRGF-2 has more availability for bone regeneration than PRP[34]. The use of a combination of PRGF-2 (prepared using the PRGF-Endoret technology; BTI, Miñano, Spain) and a mineralized cancellous bone particulate allograft (MCBPA) (Puros Cancellous Particulate Allograft; Zimmer Biomet Dental, Palm Beach Gardens, FL, United States) is the current standard procedure for GBR-MSA at the Clinic for Periodontology and Implantology (Hamburg, Germany).
Eckhard U. Alt, MD, PhD, Professor, Chairman of the Board of InGeneron (Houston, TX, United States) and of Isar Klinikum (Munich, Germany)
It appears feasible to further improve GBR-MSA by the application of stem cells (for recent reviews on the use of stem cells in regenerative dentistry[35-37]). Among the different types of mesenchymal stem cells, cells derived from adipose tissue (either freshly isolated or culture-expanded) have emerged as a promising tool for GBR[4,38,39]. The freshly isolated cells are named stromal vascular fraction or ADRCs, and the cultured cells are named adipose-derived stem cells (ASCs). It should be mentioned that some studies on animal models suggested that bone marrow stem cells (BMSCs) may demonstrate greater differentiation into osteoblasts than ADRCs and ASCs[40,41] and might be more useful than ADRCs or ASCs in GBR-MSA[42]. However, bone marrow has a significantly lower stem cell density than adipose tissue (0.01% vs 5%), and harvesting adipose tissue is much less painful than harvesting bone marrow because the former is less invasive than the latter[43-45]. Furthermore, focusing exclusively on the potential to differentiate into osteoblasts would fail to take into account the known effect of indirect stimulation of bone regeneration by ADRCs and ASCs, which led to the same amount of measured regenerated bone volume after 6 wk in a study that compared the bone regeneration effect of BMSCs and ASCs in a rabbit craniectomy model[40].
Furthermore, several studies demonstrated that the combination of ADRCs or ASCs with an OIS is a more effective strategy for GBR than the use of an OIS alone (reviewed in[46-48]). Moreover, it was hypothesized that the application of ADRCs or ASCs in combination with an OIS and osteogenic/angiogenic growth factors may help to optimize clinical procedures[46]. In this regard the following must be kept in mind: (1) Compared to cultured and potentially modified ASCs, freshly isolated, unmodified ADRCs have the advantage of lower safety requirements because it is not necessary to culture and/or modify the cells; (2) Several non-enzymatic and enzymatic systems for isolating ADRCs were developed (reviewed in[49]). It was reported that cell yield may vary considerably[50]. Moreover, it was shown that in general, non-enzymatic isolation of ADRCs yielded fewer cells than enzymatic (mechanical) isolation[51,52]. To our knowledge, the greatest difference in cell yield between enzymatic and non-enzymatic isolation of ADRCs was reported for the Transpose RT system and the proprietary Matrase Reagent (both from InGeneron, Inc., Houston, TX, United States)[53] (this study is discussed in detail below); and (3) Some clinical studies on cell-based therapies reported the production of donor-specific antibodies after application of allogeneic cells[54,55]. This is not the case when using autologous cells.
In summary, the combination of enzymatically isolated, UA-ADRCs with PRGF-2 and an OIS may be optimal for GBR-MSA.
Werner Götz, MD, PhD, Director of the Laboratory for Orthodontic Basic Research, University of Bonn (Bonn, Germany)
The use of a combination of UA-ADRCs, PRGF-2, and an OIS appears promising but has not yet been reported in GBR-MSA or in guided bone regeneration in general. Thus, it is justified to test this combination in a first-in-human, split-mouth single case study. Furthermore, it is recommended to perform comprehensive histologic, histomorphometric, and immunohistochemical analysis of biopsies of the newly formed bone collected at different time points after GBR-MSA.
Christoph Schmitz, MD, PhD, Head of the Department of Neuroanatomy at LMU Munich (Munich, Germany)
Contemporary histomorphometric analysis of bone biopsies may be insufficient to assess the significance of a combination of UA-ADRCs, PRGF-2, and an OIS in GBR-MSA because it does not provide a detailed analysis of the relative amount (area/area) of bone, allograft, fibrin and connective tissue, adipocytes, arteries, and veins in biopsies of the newly formed bone. However, the latter can be achieved with design-based stereology[56].
FINAL DIAGNOSIS
Loss of several premolar and molar teeth, with residual bone height of the edentulous posterior maxilla as well as of the alveolar bone crest height around the remaining maxillary teeth considered insufficient for implant placement. Justification for treatment with a novel combination of UA-ADRCs, PRGF-2, and an OIS in a first-in-human, split-mouth single case study, carefully controlled by comprehensive histologic, design-based stereologic, and immunohistochemical analysis of biopsies of the newly formed bone collected at different time points after GBR-MSA.
TREATMENT
The present single-case study was approved by the ethics committee of the Federal Dental Association Hamburg (Hamburg, Germany) (no. PV5211). Written informed consent was obtained from the patient to participate in this study after verbal and written information provided by the principal investigator.
During a 4 mo pre-phase the patient’s reduced oral hygiene was significantly improved by oral hygiene advice and supragingival plaque control.
During the first treatment, venous blood (8 × 9 mL = 72 mL) was withdrawn from the patient’s arm and was processed into PRGF using the PRGF-Endoret technology (BTI, Miñano, Spain) according to the manufacturer’s instructions for use. After centrifugation, PRGF-2 was collected and activated with PRGF Activator (BTI).
Furthermore, human subcutaneous adipose tissue was obtained using liposuction. To this end, the periumbilical abdominal area was surgically disinfected. Then, local anesthesia was achieved by infiltrating the periumbilical subcutaneous adipose tissue with 150 mL of modified Klein tumescent solution[57], comprising lactated Ringer solution, adrenaline (1:1000; 1 mg/mL) and 2% lidocaine (20 mg/mL). Fifteen minutes later a stab incision was made 15 cm lateral of the umbilicus, bilaterally. Lipoaspiration was performed using the Coleman method[58] using a 4-hole blunt tipped cannula (3 mm × 150 mm) (part of the LCK-15 Lipoaspiration Collection Kit; InGeneron) and a 60 cm3 Luer-Lock Toomey-Syringe (also part of the LCK-15 Kit; InGeneron). After liposuction, pressure was applied to the wounds. Then the wounds were closed using adhesive bandage strips (Leukosilk; BSN Medical GmbH, Hamburg, Germany). The lipoaspirate (100 mL) was divided into four aliquots of 25 mL each. Then, all aliquots were processed using the Transpose RT system (InGeneron) for isolating UA-ADRCs from the adipose tissue. Specifically, each aliquot was incubated together with Matrase Reagent (InGeneron) for 1 h. The latter was performed in the processing unit under agitation at 39 °C according to the manufacturer’s instructions for use. The total procedure time was 70 min.
Approximately 7 g (2 × 2 cubic centimeters of 0.25-1.0 mm particle size and 1 x 3 cubic centimeters of 1-2 mm particle size) of Puros Cancellous Particulate Allograft (Zimmer Biomet Dental, Palm Beach Gardens, FL, United States) were rehydrated with 1.5 mL of PRGF-2 and a suspension of UA-ADRCs (approximately 50 × 106 cells in 3 mL saline) (hereafter referred to as MCBPA/PRGF-2/UA-ADRCs). This was done within 30 min after completion of the PRGF processing and immediately after completion of isolating the UA-ADRCs (Figure 2A). Another 7 g of the same MCBPA were rehydrated with 1.5 ml of PRGF-2 and 3 mL of saline (hereafter referred to as MCBPA/PRGF-2/saline).
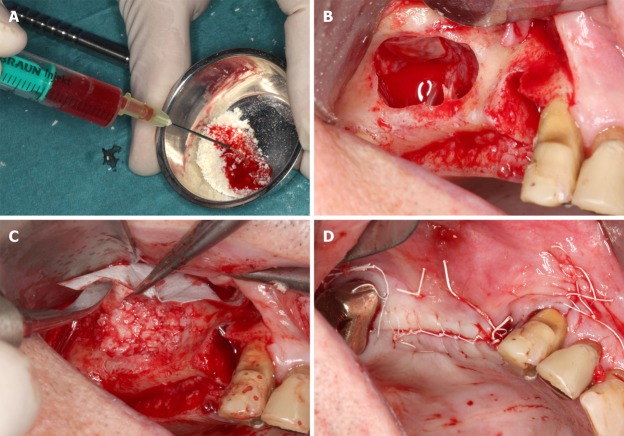
Figure 2.
Details of the procedures performed immediately before and during the first surgery (guided bone regeneration-maxillary sinus augmentation). A: Rehydration of the MCBPA with PRGF-2 and UA-ADRCs; B: Preparation of the right maxillary sinus with a lateral external window after atraumatic extraction of tooth # 14 (the Schneiderian membrane was elevated); C: Filling of the right sinus cavity with loosely packed MCBPA/PRGF-2/UA-ADRCs; D: Achievement of primary closure without tension on the right side using horizontal mattress as well as a continuous half-hitch sutures. MCBPA: Mineralized cancellous bone particulate allograft; PRGF-2: Fraction 2 of plasma rich in growth factors; UA-ADRCs: Unmodified autologous adipose-derived regenerative cells.
After the patient was prepared for surgery and anesthetized via local infiltration with Ultracain-DS Forte (Sanofi-Aventis, Frankfurt/Main, Germany), the maxillary tooth 14 was extracted using a minimal traumatic approach with periotomes. Following careful extraction with special emphasis on preservation of the buccal plate of bone, the extraction sockets were examined for potential perforation and fenestration. Curettage of the extraction site was performed to remove all soft tissue debris as well as granulation tissue and to promote healing by stimulating bleeding from the osseous base. Additionally, a bilateral external sinus lift procedure using the Tatum technique[59] was performed. The lateral window access was prepared in the areas of the first molars on both sides using a very atraumatic piezosurgery approach (Mectron, Cologne, Germany) to a size of approximately 10 mm × 7 mm (length × height) in order to allow for sufficient overview into the sinus cavity and to minimize the reduction of cortical bone for overall stability (Figure 2B). Then, the MCBPA/PRGF-2/UA-ADRCs was loosely packed into the prepared right maxillary sinus (Figure 2C), and the MCBPA/PRGF-2/saline into the prepared left maxillary sinus. Afterwards, all sites were covered with a resorbable native pericardium membrane (CopiOs Extend Membrane; Zimmer Biomet Dental) in order to promote the ingrowth of new bone by excluding epithelial migration into the grafted sites. Finally, a soft tissue flap extension[60,61] was surgically performed and primary closure without tension was achieved using vertical and horizontal cross mattress sutures using Gore-Tex Suture (W.L. Gore and Associates, Inc., Flagstaff, AZ, United States) (Figure 2D). The anterior maxillary teeth 11, 12 and 22 were initially preserved for aesthetic reasons and scheduled for extraction 6.5 wk later (Figure 3A).
Figure 3.
Clinical findings. A: Panoramic radiograph taken immediately after the first surgery (GBR-MSA). The yellow arrows indicate the restored bone height of the edentulous posterior maxilla on both sides; B: Panoramic radiograph taken after the third surgery (placement of implants at 34 wk after GBR-MSA); C: Panoramic radiograph taken at 32 mo after the first treatment. R: Right; L: Left.
Analgesics (Paracetamol 500 mg, t.i.d.) and prophylactic antibiotics (Amoxicillin 500 mg, t.i.d.) were prescribed for 7 d postoperatively. Tooth brushing in the surgical area was restricted for the first 2 wk. In addition, chlorhexidine mouthwash was prescribed to maintain the oral flora and prevent infection. Sutures were removed on the 10th postoperative day, and routine monitoring appointments were held at monthly intervals to evaluate healing.
Six weeks after the first treatment, the patient was reappointed and the maxillary anterior teeth # 11, 12, and 22 were extracted (second treatment) using the same approach as applied to the maxillary posterior tooth # 14 during the first treatment. During this procedure, biopsies of the areas grafted during the first treatment with the MCBPA/PRGF-2/UA-ADRCs (right) and the MCBPA/PRGF-2/saline (left) were collected using a trephine bur (length: 18 mm; inner diameter: 2.6 mm; outer diameter: 3.2 mm; Trepan Bur 227A.204.032; Komet Dental, Lemgo, Germany). Biopsies were fixed by immersion in 4% buffered formaldehyde at room temperature. After at least 1 d of fixation they were prepared for histologic and immunohistological analysis (described in detail below).
The postoperative management (prescription of analgesics, antibiotics, and chlorhexidine mouthwash, restriction of tooth brushing in the surgical area, removal of sutures, and routine monitoring) was the same as performed after the first treatment.
The patient was reappointed again at 34 wk after the second treatment and prepared for dental implant placement surgery (third treatment). Anesthesia was induced via local infiltration of Ultracain-DS Forte (Sanofi-Aventis). Then, an osteotomy for implant placement was initially prepared in the alveolar bone using a trephine bur that was identical to the one used during the second treatment (Trepan Bur 227A.204.032; Komet Dental), and biopsies of the areas grafted during the first treatment with the MCBPA/PRGF-2/UA-ADRCs (right side) and the MCBPA/PRGF-2/saline (left side) were collected (these biopsies were handled and processed in the same way as the biopsies that were collected during the second treatment). The osteotomies for implant placement were prepared by sequential cutting to the radiographically determined length with surgical drills in graduated diameters according to the dental implant manufacturer’s instructions of use. Implants were then placed according to the manufacturer’s recommendations (ASTRA TECH implants with cover screw, Dentsply, Mannheim, Germany). Implants of the following dimensions were inserted into the finally prepared osteotomies: regions 16 and 26: 5.0 S × 11 mm; region 14: 4.0 S × 13 mm; regions 12, 15, and 25: 4.0 S × 11 mm; and region 22: 3.5 S × 11 mm. All implants achieved a high primary stability of 25-30 Ncm insertion torque. The implant access holes were closed with cover screws prior to primary soft tissue closure as performed during the first treatment (Figure 3B). The postoperative management was the same as performed after the first and second treatments.
Twelve months after the third treatment, radiographs were taken and demonstrated excellent bone healing around the dental implants, within the augmented sinus, and the extraction sockets (Figure 4) (fourth treatment) (note that the long time between the third and the fourth treatment was due to constraints of time on the side of the patient; from a medical point of view the fourth treatment could have been performed already at 4 mo after the third treatment). No radiological signs of inflammation were observed. The patient was reappointed again and prepared for uncovering of the implants and implant abutment placement surgery as well as extraction of tooth #18. Anesthesia was induced via local infiltration of Ultracain-DS Forte (Sanofi-Aventis). Localized mucoperiosteal flaps were raised and the cover screws were removed from the implant access holes. The implant access holes were rinsed using 2% chlorhexidine solution and were then covered with ASTRA Tech Implant healing abutments (‘Gingivaformer’) of the following dimensions: regions 16 and 26: 5.5 mm × 4.0 mm; regions 12, 14, 15, and 25: 4.5 mm × 4.0 mm; and region 22: 3.5 mm × 4.0 mm.
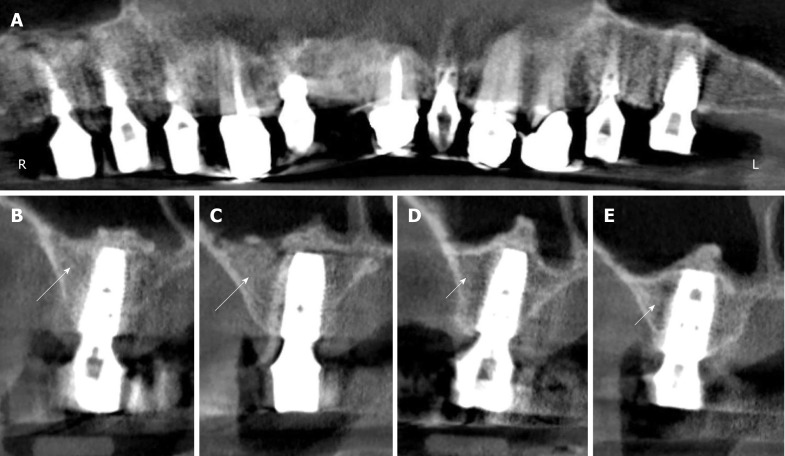
Figure 4.
Digital volume tomography radiographs taken after the fourth surgery (1 year after the placement of implants and 20 mo after guided bone regeneration-maxillary sinus augmentation). A: Panoramic view; B-E: Detailed view on selected regions 15 (B), 16 (C), 25 (D), and 26 (E). With the application of unmodified autologous adipose-derived regenerative cells (UA-ADRCs) the bone around the implants in regions 15 and 16 appeared larger in area and denser (arrows in B, C) than without the application of UA-ADRCs in regions 25 and 26 (arrowheads in D, E).
Prior to closure of the implant access hole, the healing abutment screws were covered with 2% chlorhexidine gel in order to minimize bacterial contamination. The healing abutments were placed using a torque of 20 Ncm according to the manufacturer’s recommendations. All implants were surrounded with very sufficient three-dimensional bone volume and demonstrated excellent stability (Figure 4). No clinical signs of inflammation were observed. Tooth # 18 was carefully extracted using a piezosurgery device as described above. Following achievement of primary closure around the healing abutments as described for the other treatments, the same postoperative care regimen was administered as described above and the sutures were removed approximately 10 d postoperatively.
Six weeks after placement of the healing abutments, the definitive prosthetic telescopic bridge (prepared by Dr. Johanna Hevelke, Winsen/Luhe, Germany) was placed.
Histologic processing of the biopsies was performed at the Department of Orthodontics, Center of Dento-Maxillo-Facial Medicine, University of Bonn, Bonn, Germany. To this end, the fixed biopsies were decalcified in 4.1% EDTA solution at room temperature for about 7 d, changing the EDTA solution every 24 h. Then, the biopsies were hydrated, followed by rehydration in an ascending series of ethanol. Afterwards they were embedded in paraffin and cut into 3 µm thick serial longitudinal sections. The sections were mounted on Superfrost Plus slides (Gerhard Menzel, Braunschweig, Germany). Sections representing positions within the biopsies near the longitudinal axis were stained with hematoxylin and eosin stain, or were processed by immunohistochemistry. Finally, all sections were coverslipped with DePeX (Serva, Heidelberg, Germany).
Relative amounts (area/area) of bone, allograft, connective tissue and fibrin, adipocytes, arteries, and veins were determined on the sections that were stained with hematoxylin and eosin stain using point counting as described in detail in[56] (Figure 1B). The distance between the points was 110 µm in XY directions, resulting in a mean total number of 943 (range, 662-1172) points per section. Analyses were performed with a computerized stereology workstation consisting of a modified light microscope (Axioskop; Carl Zeiss Microscopy, Jena, Germany) with Plan-Neofluar objectives 2.5 × (numerical aperture [NA] = 0.075) and 10 × (NA = 0.3) (Carl Zeiss Microscopy), motorized specimen stage (BioPrecision2; Ludl Electronics, Hawthorne, NY, United States), stage controller (MAC 6000 XY; Ludl Electronics), focus encoder (MT 1271; Heidenhain, Traunreut, Germany), CCD color video camera (1,600 x 1,200 pixels; MBF Bioscience, Williston, VT, United States), and stereology software (Stereo Investigator version 10; MBF Bioscience).
After deparaffinizing and rehydrating, sections were rinsed for 10 min in TBS. Histochemical detection of tartrate-resistant acid phosphatase was performed with a specific Acid Phosphatase staining kit (Sigma-Aldrich, Steinheim, Germany) according to the staining protocol of the manufacturer. For immunohistochemistry, endogenous peroxidase was blocked in a methanol/H2O2 (Merck, Darmstadt, Germany) solution for 45 min in the dark. Then, sections were pretreated with TBS containing 1% bovine serum albumin at room temperature for 20 min. Afterwards, sections were digested with 0.4% pepsin at 37 °C for 10 min, followed by incubation with the primary antibodies in a humid chamber. Table 2 summarizes details of the antibodies and the incubation protocols.
Table 2.
Details of antibodies and incubation protocols used in the present study
| Antibody | Isotype | Manufacturer | Dilution/incubation |
| runX2 | Goat polyclonal | Santa Cruz Biotechnology (Dallas, TX, United States) | 1:30, 4 °C, ON |
| Collagen type I | Rabbit monoclonal | Abcam (Cambridge, United Kingdom) | 1:400, RT, 1 h |
| Alkaline phosphatase | Rabbit polyclonal | Quartett (Berlin, Germany) | Ready to use, 4 °C, ON |
| Von Willebrand factor | Rabbit polyclonal | Linaris (Dossenheim, Germany) | 1:200, RT, 1h |
| CD146 | Rabbit monoclonal | Abcam | 1:50, RT, 1h |
| CD73 | Mouse monoclonal | Antibodies-online (Atlanta, GA, United States) | 1:100, 4 °C, ON |
| PPARγ | Mouse monoclonal | Santa Cruz Biotechnology | 1:25, 4 °C, ON |
ON: Overnight; RT: Room temperature.
Antibody binding was detected with the peroxidase-conjugated EnVision anti-mouse system (Dako, Glostrup, Denmark) or the horseradish peroxidase-conjugated EnVision anti-rabbit/anti-goat secondary antibodies (Dako) that were diluted 1:50 and incubated at room temperature for 30 min. Visualization of peroxidase activity was performed using diaminobenzidine resulting in a brown staining product. Mayer’s hematoxylin was used for counterstaining the sections. In order to perform specificity controls, primary antibodies were omitted and TBS or normal horse serum was applied. In other control experiments, both primary and secondary antibodies were omitted. Fetal human bone or mandibular bone (i.e., tissues carrying known antigens) were used as positive controls. Qualitative histological evaluations were performed blinded by one of the authors.
Digital photography was used to produce the photomicrographs shown in Figure 5A and 5B. To this end, on average 41 (range, 30-45) images were captured for each composite using a computerized stereology workstation. The latter consisted of a modified light microscope (Axio Imager 2; Carl Zeiss Microscopy) with an EC Plan Neofluar 10 × objective (NA = 0.3) (Carl Zeiss Microscopy), motorized specimen stage for automatic sampling (H101A; Prior Scientific Instruments, Cambridge, United Kingdom), focus encoder (MT 1271; Heidenhain), CCD color video camera (1388 x 1040 pixels; AxioCam MRc; Carl Zeiss Microscopy), and stereology software (Stereo Investigator version 10; MBF Bioscience). The Virtual Slice module of the Stereo Investigator software (MBF Bioscience) was used to create the montages. The photomicrographs shown in Figure 5C and 5D, Figure 6 and Figure 7 were produced by digital photography (all components from Carl Zeiss Microscopy) using an AxioCam MRc camera attached to an AxioScope 2 microscope and AxioVision 4.7 software using the following objectives: Epiplan 20 × (NA = 0.40) and Plan-Neofluar 40 × (NA = 0.75). Corel Photo-Paint X7 and Corel Draw X7 (both versions 17.5.0.907; Corel, Ottawa, Canada) were used to construct the final figures. Contrast and brightness were only marginally adjusted, which did not alter the appearance of the original materials.
Figure 5.
Histological findings. Representative photomicrographs of 3 µm thick paraffin sections stained with hematoxylin and eosin of biopsies that were collected at 6 wk (A, B, E, F) or 34 wk (C, D, G, H) after guided bone regeneration maxillary sinus augmentation with the application of UA-ADRCs (A, C, E, G) or without UA-ADRCs (B, D, F, H). In A-D the asterisks indicate newly formed bone and the arrows indicate empty osteocyte lacunae in allogeneic fragments. Furthermore, in A and B the arrowheads point to osteoblasts with underlying osteoid, while in C and D the arrowheads indicate adipocytes. In E-H the arrows indicate cells on the surface of newly formed bone. The scale bar in H represents 150 µm in A-D and 75 µm in E-H. UA-ADRCs: Unmodified autologous adipose-derived regenerative cells.
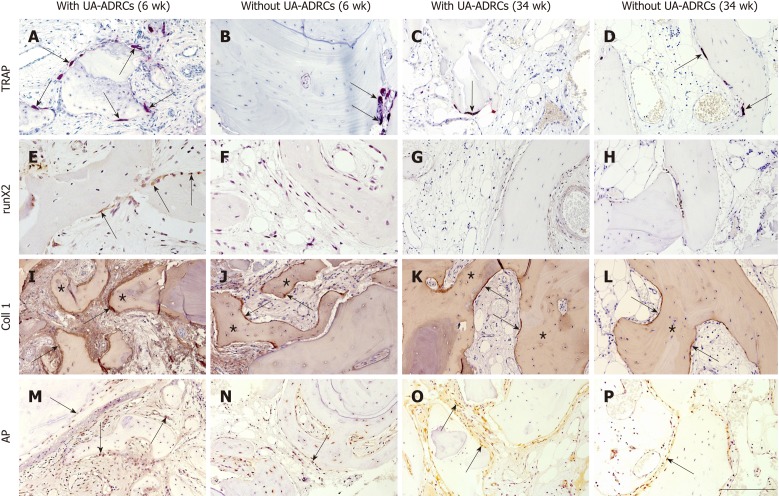
Figure 6.
Histological findings. Representative photomicrographs of histochemical detection of tartrate-resistant acid phosphatase (TRAP) (A-D) as well as of immunohistochemical detection of Runt-related transcription factor 2 (runX2) (E-H), collagen 1 (Coll 1) (I-L), and alkaline phosphatase (AP) (M-P) in 3 µm thick paraffin sections of biopsies that were collected at 6 wk (A, B, E, F, I, J, M, N) or 34 wk (C, D, G, H, K, L, O, P) after GBR-MSA with the application of UA-ADRCs (A, C, E, G, I, K, M, O) or without UA-ADRCs (B, D, F, H, J, L, N, P). In A-D the arrows point to osteoclasts, in E to osteoblasts, in I-L to type I collagen in osteoid seams, and in M-P to AP immunostaining found in osteoblasts, osteoblast-like cells, and fibroblasts in the intertrabecular connective tissue. Furthermore, in I-L the asterisks indicate type I collagen in the matrix of newly formed bone trabeculae. The scale bar in P represents 200 µm in A-D and I-P, and 100 µm in E-H. UA-ADRCs: Unmodified autologous adipose-derived regenerative cells.
Figure 7.
Histological findings. Representative photomicrographs of immunohistochemical detection of factor VIII/von Willebrand factor (vWF) (A-D), CD146 (E-H), CD73 (I-L), and peroxisome proliferator-activated receptor gamma (PPARγ) (M-P) in 3 µm thick paraffin sections of biopsies that were collected at 6 wk (A, B, E, F, I, J, M, N) or 34 wk (C, D, G, H, K, L, O, P) after GBR-MSA with the application of UA-ADRCs (A, C, E, G, I, K, M, O) or without UA-ADRCs (B, D, F, H, J, L, N, P). In all panels the arrows point to vessels. Furthermore, the arrowheads indicate sinusoidal vessels in D, and fibroblasts and osteoblasts in I-L, M, and O. The scale bar in P represents 200 µm in A-D and I-P, and 100 µm in E-H. GBR-MSA: Guided bone regeneration maxillary sinus augmentation; UA-ADRCs: Unmodified autologous adipose-derived regenerative cells.
OUTCOME AND FOLLOW-UP
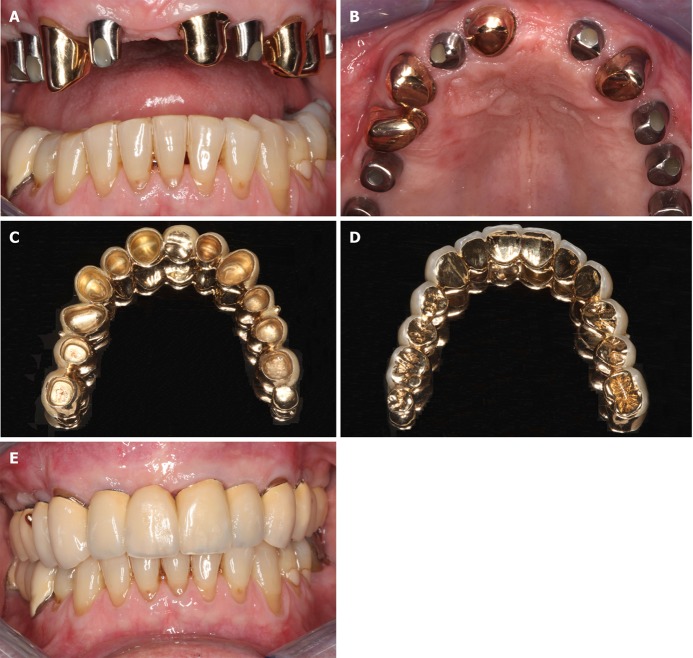
The patient was very satisfied with the maxillary restoration regarding aesthetics, function, phonetics, and cleansibility (Figure 8). The last radiologic follow-up examination took place at 32 mo after the GBR-MSA surgery (Figure 3C).
Figure 8.
Documentation of clinical outcome. A, B: Intraoral ventral (A) and occlusal (B) views on the healing abutments in regions 12, 14, 15, 16, 22, 25, and 26 after the fourth treatment (1 year after the placement of implants and 20 mo after guided bone regeneration maxillary sinus augmentation). Note that the teeth # 13, 21, 23, and 24 are crowned; C, D: External (C) and internal (D) view of the prosthetic telescopic bridge; E: Intraoral view of the final prosthetic reconstruction.
The biopsies that were collected at 6 wk after GBR-MSA showed the formation of a network of cancellous bony trabeculae by appositional membranaceous osteogenesis in different developmental stages around allogeneic fragments. The newly formed bone (asterisks in Figure 5A and 5B) consisted of fibrous bone. Typically, newly formed bone spicules contained nuclei of allogeneic remnants that showed basophilic staining and contained empty osteocyte lacunae (arrows in Figure 5A and 5B). Most surfaces of the newly formed trabeculae were covered by osteoblasts with underlying osteoid (arrowheads in Figure 5A and 5B). With the application of UA-ADRCs a higher bone lining cell density was achieved than without UA-ADRCs. No signs of active inflammation or necrosis could be recognized in either biopsy.
The biopsies that were collected at 34 wk after GBR-MSA showed a similar morphology as the biopsies that were collected at 6 wk after GBR-MSA. However, bone formation seemed to be increased with a decreased percentage of allogeneic remnants (arrows in Figure 5C and 5D) and fibrous bone. Newly formed cancellous bone was in an advanced stage of remodeling, appearing as lamellar bone (asterisks in Figure 5C and 5D) with fibrous bone remnants incorporated. Fewer adipocytes developed after the application of UA-ADRCs than without UA-ADRCs (arrowheads in Figure 5C and 5D).
At higher magnification, the biopsy that was collected at 6 wk after GBR-MSA with the application of UA-ADRCs showed regions with early osteogenic condensations within a highly cellular surrounding. Specifically, osteoclasts appeared on the surface of natural and allogeneic bone (arrowheads in Figure 5E). Such regions with early osteogenic condensations were not observed at 6 wk after GBR-MSA without application of UA-ADRCs. Rather, osteogenesis appeared more discrete in this case (Figure 5F). Likewise, at 34 wk after GBR-MSA a higher cell density around newly formed cancellous bone was found after the application of UA-ADRCs (arrowheads in Figure 5G) than without UA-ADRCs (arrowheads in Figure 5H).
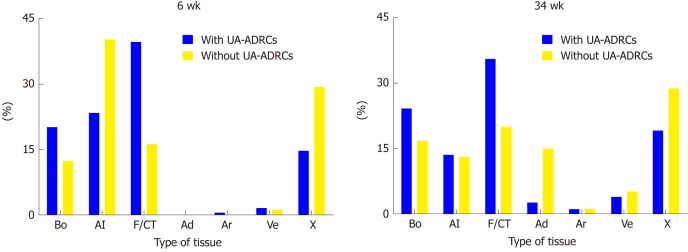
The results of the histomorphometric analysis are summarized in Figure 9. The application of UA-ADRCs resulted in a higher relative amount (area/area) of newly formed bone (+ 63% at 6 wk; + 44% at 34 wk), a higher relative amount of fibrin and collagen (+ 144% at 6 wk; + 78% at 34 wk) and a lower relative amount of adipocytes (- 83% at 34 wk) compared to the situation without application of UA-ADRCs. Besides this, the ratio of the relative amounts (area/area) of veins to arteries was 3.5 after the application of ADRCs and 4.7 without ADRCs at 34 wk after GBR-MSA.
Figure 9.
Results of histomorphometric analyses. Relative amounts (area/area) of bone (Bo), allograft (Al), fibrin and connective tissue (F/CT), adipocytes (Ad), arteries (Ar), veins (Ve) and artifacts (X) in 3 µm thick paraffin sections stained with hematoxylin and eosin stain of biopsies that were collected at 6 wk (on the left) or 34 wk (on the right) after guided bone regeneration maxillary sinus augmentation with the application of unmodified autologous adipose-derived regenerative cells (UA-ADRCs) or without UA-ADRCs. UA-ADRCs: Unmodified autologous adipose-derived regenerative cells.
Tartrate-resistant acid phosphatase immunostaining revealed middle-sized osteoclasts on the surface of newly formed and allogeneic bone and on debris accumulations at 6 wk after GBR-MSA, with a higher osteoclast density after the application of UA-ADRCs than without UA-ADRCs (arrows in Figure 6A and 6B). Only a few osteoclasts were found at 34 wk after GBR-MSA (arrows in Figure 6C and 6D).
Anti-runt-related transcription factor 2 immunoreactivity was found at 6 wk after GBR-MSA with the application of UA-ADRCs, but not in GBR-MSA without UA-ADRCs, in most osteoblasts and osteoblast-like cells (arrows in Figure 6E). Only very weak anti-runt-related transcription factor 2 immunoreactivity was found at 34 wk after GBR-MSA (Figure 6G and 6H).
Type I collagen appeared in osteoid seams (arrows in Figure 6I and 6J) and in a weak manner in the matrix of newly formed bone trabeculae (asterisks in Figure 6I and 6J) at 6 wk after GBR-MSA. At 34 wk after GBR-MSA the pattern of immunoreactivity for type I collagen was similar to the pattern observed at 6 wk after GBR-MSA but weaker (arrows and asterisks in Figure 6K and 6L).
The application of UA-ADRCs resulted in strong alkaline phosphatase (AP) immunoreactivity in osteoblasts, osteoblast-like cells, and fibroblasts in the intertrabecular connective tissue at 6 wk after GBR-MSA, while in GBR-MSA without UA-ADRCs only a few osteoblasts were immunoreactive for AP (arrows in Figure 6M and 6N). At 34 wk after GBR-MSA AP immunoreactivity appeared in some osteoblasts and osteoblast-like cells in a weak to moderate manner (arrows in Figure 6O and 6P).
Immunohistochemical detection of factor VIII/von Willebrand factor showed dense vascularization in all biopsies (arrows in Figure 7A-D), with the highest density at 6 wk after GBR-MSA with the application of UA-ADRCs. In GBR-MSA without UA-ADRCs, larger sinusoidal vessels were found at 34 wk after GBR-MSA and were located within the intertrabecular connective tissue (arrowheads in Figure 7D).
Vessel walls were strongly immunoreactive for CD146 in all biopsies (arrows in Figure 7E-H).
A strong intracellular, granular anti-CD 73 immunoreactivity was seen in all biopsies in subsets of fibroblasts and osteoblasts (arrows in Figure 7I-L), as well as in vessel walls (arrowheads in Figure 7I-L).
Nearly all osteoblasts and fibroblasts as well as a subset of osteocytes showed immunoreactivity for PPARγ at 6 wk after GBR-MSA (arrows in Figure 7M and 7N). Furthermore, PPARγ immunoreactivity was also found in vessel walls in areas with osteogenesis (arrowheads in Figure 7M and 7N). In general, a very similar pattern was found at 34 wk after the application of UA-ADRCs, whereas without UA-ADRCs immunoreactivity for PPARγ was restricted to vessel walls (arrows and arrowheads in Figure 7O and 7P).
The immunohistochemically negative control specimens displayed no immunoreactivity.
DISCUSSION
The present study is the first one in which a combination of freshly isolated UA-ADRCs, PRGF-2, and an OIS was used for GBR-MSA. Furthermore, our study is the first one in which cellular and histological effects of mesenchymal stem cells in human GBR-MSA were investigated with design-based stereology, histochemistry, and immunohistochemistry. The analysis of the biopsies that were collected at 6 wk and again at 34 wk after GBR-MSA showed that the combination of UA-ADRCs, PRGF-2, and the OIS resulted in better and faster bone regeneration than the combination of PRGF-2 and the same OIS alone. It is of note that our design-based stereologic finding of a higher relative amount of newly formed bone after GBR-MSA with the application of UA-ADRCs than without UA-ADRCs (+ 63% at 6 wk; + 44% at 34 wk) (Figure 9) was in line with our radiologic finding of more dense bone after the application of UA-ADRCs in regions 15 and 16 than without UA-ADRCs in regions 25 and 26 at 20 mo after GBR-MSA (Figure 4).
The results of the present study may open new horizons for the rehabilitation of the edentulous posterior maxilla and potentially for other regenerative techniques in pre-implant bone augmentation procedures like lateral and horizontal alveolar ridge augmentation, socket preservation, and bone grafting following large cystectomies. Specifically, the novel GBR-MSA approach presented here could result in a superior bone-implant-contact due to advanced new bone formation (Figure 9) and could also serve as the basis for reducing the time between GBR-MSA and the placement of implants in patients with residual bone height below the antrum and the ridge crest of less than 3 mm (Class D[32]). Besides this, our novel approach may allow for immediate implant placement in combination with bone augmentation procedures when the primary stability of the implant is provided. Moreover, the harvesting of ADRCs may result in less morbidity of the patient compared to bone marrow aspiration from the iliac crest area (addressed in the next paragraph). However, it will be the task of future studies to test these hypotheses.
GBR-MSA using mesenchymal stem cells (except of the use of UA-ADRCs) has been investigated in many preclinical and clinical studies, with and without OIS, and with and without use of PRP (reviewed in[62-64]). In most of these studies BMSCs, autologous bone marrow aspirate concentrate (BMAC), or periosteal derived stem cells were used. Histomorphometric analysis showed considerable differences in the relative amount (area/area) of newly formed bone at 24 wk after surgery, ranging between 13.5% using BMSCs and bovine bone material (BBM)[65] and 55.2% using BMAC and BBM[66]. This considerable difference and the fact that the relative amount (area/area) of cancellous bone in normal human bone is only approximately 25%[67] should give reason to accept the results of related studies only after having critically scrutinized the corresponding methodological details. For example, in a study that reported a relative amount of newly bone of 55.2% using BMAC and BBM[67] only small portions of the investigated specimens were shown, and histomorphometry was performed by inspecting the specimens with a 1.25× objective, which precludes to distinguish between empty osteocyte lacunae (representing scaffold) and cell bodies within osteocyte lacunae (representing newly formed bone). In any case, a meta-analysis of nine studies (seven animal and two human studies) in which the combination of mesenchymal stem cells and OIS versus OIS alone were compared, found a statistically significant positive effect of stem cells on the bone re-growth in GBR-MSA[63].
At first glance our design-based results (relative amount of newly formed bone of 24.2% at 34 wk after GBR-MSA) do not speak in favor of our approach compared to the use of BMSCs (among the six studies reviewed in[63] in which BMSCs were applied and histomorphometric analysis was performed at 24 wk after surgery, three studies reported a relative amount of newly formed bone of more than 24%). However, our decision to use UA-ADRCs rather than other types of cells (including ASCs, BMSCs, periosteal derived stem cells, allogeneic and/or modified ASCs/BMSCs, dental-derived mesenchymal stem cell-like cells (reviewed in[68]) or induced pluripotent stem cells) was based on the fact that UA-ADRCs are the only type of cell that allows immediate usage at point of care, with the lowest safety concerns in cell-based therapy as no culturing or modification is required. This is fundamentally different from all other types of cells that have been considered for cell-based therapies in dentistry (reviewed in[69]). Regarding safety concerns one must keep in mind that, e.g., potential oncogenesis currently limits the clinical translation of induced pluripotent stem cells[70,71], and applying allogeneic cells may cause the production of donor-specific antibodies and cell induced immune response[54,55]. The only other cell-based therapy that allows immediate usage at point of care is autologous BMAC, which was investigated in two studies on GBR-MSA (BBM as OIS). One of these studies was a case report on a 46-year-old partially edentulous man, showing a relative amount of newly formed bone of 27% at 12 wk after surgery without control treatment[72]. The other study[73] was a randomized controlled trial (from the same lab as[72], published in the same year, and using exactly the same procedure for harvesting bone marrow aspirate as used in[72]), reporting a mean relative amount of newly formed bone of only 12.6% at 12 wk after surgery (n = 34 patients), with a mean relative amount of newly formed bone of only 14.3% at 12 wk after grafting BBM and autologous bone (n = 11 patients)[73]. It is of note that only in the latter study[73] whole specimens were shown and methodological details of the histomorphometric analysis were provided. The data of the latter study[73] are in line with the data obtained with our control treatment (Figure 7) and indicates that the use of UA-ADRCs may be more effective than the use of BMAC in GBR-MSA.
Detection of factors involved in osteogenesis and bone remodeling using histochemistry and immunohistochemistry is not frequently applied in studies on GBR-MSA in dentistry. Mostly, only selected factors were investigated. In line with former studies[74,75] we applied a broader panel of antibodies including vessel markers like factor VIII/von Willebrand factor. The immunostaining pattern obtained revealed similar findings as for remodeling of allografts[76]. However, the direct comparison of the immunolabeling of osteogenic factors like runt-related transcription factor 2 and AP between both sides showed stronger immunopositivity after the application of UA-ADRCs, which underlines better and faster bone regeneration. The finding of middle-sized osteoclasts on the surface of newly formed and allogeneic bone and on debris accumulations at 6 wk after GBR-MSA, with a higher osteoclast density after the application of UA-ADRCs than without UA-ADRCs (Figure 5E, 5F, and Figure 6A-D), was in line with earlier reports in the literature[77,78] about osteoclasts involved in bone remodeling (which was increased after application of UA-ADRCs compared to the situation without UA-ADRCs). This phenomenon must not be confused with foreign material resorption by multinucleated giant cells[77]. That was not observed in the present study. The denser vascularization on the cell treated side (detected with both histomorphometry and anti-factor VIII/von Willebrand factor immunohistochemistry) that was treated with UA-ARDCs underlines the importance of the coupling of angiogenesis and osteogenesis in bone regeneration[79].
We did not characterize the UA-ADRCs isolated from lipoaspirate with the Transpose RT system and Matrase Reagent (both from InGeneron) in the present study. However, a very recent study compared cell suspensions that were obtained by isolating cells from lipoaspirate from 12 healthy donors using the Transpose RT system and Matrase Reagent (thereafter: "TRT-MR cell suspensions") with cell suspensions that were obtained by isolating cells from lipoaspirate from the same donors just mechanically (i.e., using the Transpose RT system but without Matrase Reagent) (thereafter: "TRT cell suspensions")[53]. It was found that the mean cell yield (numbers of cells/g of processed lipoaspirate) was approximately twelve times higher in TRT-MR cell suspensions than in TRT cell suspensions (P < 0.001), and cells in TRT-MR cell suspensions formed on average 16 times more colony forming units (considered to be an indicator of stemness) per g lipoaspirate than cells in TRT cell suspensions (P < 0.001)[53]. Of note, the mean relative number of viable cells in TRT-MR cell suspensions (85.9% ± 1.1%; mean ± SE) exceeded the proposed minimum threshold for the viability of cells in the stromal vascular fraction of 70% established by the International Federation for Adipose Therapeutics and Science[80], whereas the mean relative number of viable cells in TRT suspensions (61.7% ± 2.6%) did not (P < 0.001)[53]. On the other hand, cells in TRT-MR cell suspensions exhibited no statistically significant differences in the expression of regenerative cell-associated genes such as Oct4, Hes1, and Klf4 compared to cells in TRT cell suspensions[53].
The same study demonstrated that upon stimulation with specific differentiation media cells in TRT-MR and TRT cell suspensions were independently able to differentiate into cells from all three germ layers (i.e., into the adipogenic, osteogenic, hepatogenic, and neurogenic lineages)[53]. The latter is in line with earlier findings that adult stem cells may obtain any of the lineages but depend on constant induction of differentiation and re-confirmation by signals released and communicated from the local microenvironment[81-83]. If this information and confirmation is missing or ceases, adult stem cells stop differentiating[84,85]. In fact, only true stem cells are able to continue their expected differentiation pathway as supported by the local microenvironment[86-88]. This is one of several reasons why adult stem cell therapy with UA-ARDCs is very safe[89-91]. In contrast, safety concerns have been raised regarding stem cell therapy with cultured adult stem cells because with higher passages an increased rate of potential malignant transformation may occur[92-94].
A study on fresh, uncultured cells that were isolated from adipose tissue of pigs using the Transpose RT system and Matrase Reagent showed that approximately 40% of cells in the stromal vascular fraction were immunopositive for CD29 (thereafter: CD29+) and CD44+[95], which are markers of adipose tissue-derived stem cells[50,96]. Furthermore, on average only 9% of the cells were CD45+ (a marker of blood derived cells[50]), and on average only 11% of the cells were CD31+ (a marker of endothelial cells[50]). Another study on fresh, uncultured cells that were isolated from adipose tissue of horses using a predecessor of the Transpose RT system (ARC system; InGeneron) and Matrase Reagent found the highest relative gene expression of osteocalcin (a gene associated with the osteogenic lineage[97]) when investigating these cells for the relative gene expression of CD44, CD73, CD90, CD105, CD146, and CD166 (mesenchymal stem cell surface markers), CD34 and CD45 (hematopoietic markers), CD31 (endothelial cell marker), type-1 collagen, PPARγ2 (a gene associated with the adipogenic lineage), and osteocalcin[98]. Collectively, these data underline the osteogenic potential of the UA-ADRCs used in the present study.
We used PRGF-2 rather than PRP because our clinical experience is in line with data from a recent experimental study on rats that showed that PRGF-2 has more availability for bone regeneration than PRP[34]. The content of PRGF-2 prepared using the PRGF-Endoret technology (BTI) was investigated in several studies in the literature. Most relevant to the results of the present study was the demonstration of high amounts of growth factors in PRGF-2[98], i.e. on average approximately 14000 pg/mL of platelet derived growth factor-AB, 46000 pg/mL of transforming growth factor-β 1, 220 pg/mL of vascular endothelial growth factor, 400 pg/mL hepatocyte growth factor, 83000 pg/mL insulin-like growth factor-1, and 600 pg/mL endothelial growth factor (note that what was named "PRGF F3" in[98] is now named "Fraction 2 of PRGF" according to BTI, and the latter terminology was used in the present study). Several pilot studies described the use of PRGF in GBR-MSA[99-101]. Furthermore, it was shown that PRGF can stimulate the proliferation, migration, and chemotaxis of osteoblasts in vitro and enhance the osteblasts’ autocrine expression of vascular endothelial growth factor and hepatocyte growth factor that are proangiogenic factors[98]. Both vascular endothelial growth factor and hepatocyte growth factor are contained in PRGF-2[98] and were also identified within the ASCs’ secretome (reviewed in[102]). A recent study showed that PRGF can induce proliferation and migration of human-derived ASCs and reduce senescence and autophagocytosis of these cells in vitro[103]. The same study[103] confirmed our own finding that human-derived ASCs can efficiently differentiate into osteocytes or adipocytes when cultured in osteogenic or adipogenic induction medium, respectively[53,104], but also demonstrated that this process is enhanced in the presence of PRGF[103]. Accordingly, our finding that the treatment with MCBPA/PRGF-2/UA-ADRCs resulted in the formation of 44% more bone and 83% fewer adipocytes in the biopsies collected at 34 wk after GBR-MSA (Figure 9) cannot be directly attributed to the action of PRGF-2 on UA-ADRCs. Rather, it is reasonable to hypothesize that cues from the local extracellular environment affected the properties of the UA-ADRCs in terms of proliferation and specific osteogenic differentiation (c.f.[105]), and this process was enhanced by PRGF-2.
GBR-MSA has been performed using a number of grafting materials, including autologous bone grafts, allografts, and xenografts[106]. An earlier meta-analysis published in 1998 found that the survival rates of implants placed in grafted maxillary sinuses did not depend on whether autologous, allogeneic, or alloplastic grafts were used[107]. However, when focusing on the total bone volume, another meta-analysis published in 2010 concluded that autologous bone should still be considered the gold standard in MSA surgery[108]. On the other hand, the latter study stated that the consequence of the total bone volume for implant survival is still unknown[108]. A recent meta-analysis based on 16 original studies found that the implant survival rate was 99.6% when a biomaterial was used during surgery compared to 96.0% when no graft material was used (the follow-up period was 48 to 60 mo in this study)[109]. However, these data should be handled cautiously because only two studies in this meta-analysis were performed with autologous bone grafts, and in 10 out of the 16 included studies (six out of seven studies without interpositional graft material) the average preoperative bone height was more than 5 mm, which represents Class C according to[32] and does not resemble the situation addressed in the present study. We used the Puros Cancellous Particulate Allograft (Zimmer Biomet Dental) because it fulfills all of the following criteria that are considered essential for an OIS in the literature (reviewed in[110]: volume stability of at least 4 mo after implantation, full biocompatibility and resorbability, and possibility for loading with stem cells and growth factors.
The patient who was investigated in our study was 79-years-old. The statistical life expectancy of 80-year-old men and women has increased 7.7 years and 9.2 years, respectively in Germany[111]. Thus, one can expect an increasing demand for the restoration of an aesthetic smile and a functional occlusion by patients aged 79 and older (and of course by younger patients) in the future. On the other hand, some authors have argued that age-related progressive decline in mechanical strength of tissue could be due to loss of resident stem cell number and function and have raised concerns regarding the use of autologous adult stem cell therapy in older patients[112]. Indeed, an earlier study[113] found that the number and multilineage differentiation potential of ASCs declined with the age of healthy volunteers, combined with increased expression of cyclin-dependent kinase inhibitor p16ink4a and CHEK1 (i.e., genes associated with senescence)[112]. These and other data reported in the literature have motivated patients to start cryopreserving ADRCs from lipoaspirate[112]. However, a recent pilot study showed that two samples of ADRCs collected from a healthy person at age 72 years and again at age 84 years showed the same cell yield and ADRC subpopulation composition without change in the proliferation rates of ASCs (obtained by culturing the ADRCs), as well as the capability of tri-lineage differentiation of both cell cultures[112]. Another recent pilot study showed that protein expression profiles of human umbilical vein endothelial cells that were co-cultured under oxidative stress conditions with ADRCs from three healthy persons aged 42, 45, and 47 years did not differ from protein expression profiles of human umbilical vein endothelial cells that were co-cultured under identical conditions with ADRCs from three healthy persons aged 61 and 62 (two persons) years[114]. Collectively, these data with the results of the present study, indicate (albeit preliminary) that using freshly isolated UA-ADRCs from patients aged 79 years and older is a valid approach for GBR-MSA. Effects of aging on PRGF-2 have, to our knowledge, not been reported.
There are limitations to the present study. First, only a single patient was investigated. However, from an ethical point of view, this appears justified in a first-in-human pilot study. It goes without saying that the efficacy and safety of our novel approach must be confirmed in future studies (including well-designed randomized controlled trials) on a larger number of patients. Second, only a single combination of a certain dose of UA-ADRCs, PRGF-2, and a certain OIS was tested. However, the same was done in most other feasibility studies on cell-based therapies for GBR-MSA. Third, we did not investigate the combination of UA-ADRCs and MCBPA. Rather, we used MCBPA/PRGF-2/saline as a control treatment because we wanted to compare the effects of UA-ADRCs in GBR-MSA with an established procedure (a similar decision was taken in an earlier study[79] that compared a combination of BMAC and BBM with a combination of autologous bone and BBM in GBR-MSA rather than BBM alone). Fourth, we were unable to determine whether (and, if so, how many) UA-ADRCs differentiated into osteoblasts. This is due to the fact that UA-ADRCs can in principle not be labeled and, thus not be quantified.
CONCLUSION
The present study suggests that GBR-MSA with a combination of UA-ADRCs, PRGF-2, and an OIS is effective, leading to a significant increase in the relative amount of newly formed bone and of dense fibrous tissue as well as less unwanted new adipose tissue formation without adverse effects. The results of our study support further evaluation of UA-ADRCs (including the isolation procedure used), dose, and combination with PRFG-2 and an OIS in future clinical trials under strict criteria. Besides this, the results presented here may also be of relevance to other fields of regenerative dentistry using stem cells (reviewed in[115-117]), as well as for GBR in general. The results depicted in Figure 9 indicate that the addition of stem cells induces more bone formation already after 6 wk than achieved without stem cells after 6 mo. This clinically relevant shortening of time to implant should be evaluated by future studies.
ACKNOWLEDGEMENTS
We thank Aschauer B, Müller-Bay I, and van Dyck S for technical support.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Informed consent statement: Consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: All authors have no conflicts of interest to report.
Peer-review started: October 9, 2018
First decision: November 14, 2018
Article in press: January 11, 2019
P- Reviewer: Fatkhudinov T, He SQ, Zheng YW S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Bian YN
Contributor Information
Önder Solakoglu, External Visiting Lecturer, Dental Department of the University Medical Center Hamburg-Eppendorf, Hamburg 20246, Germany; Clinic for Periodontology and Implantology, Hamburg 22453, Germany. solakoglu@fpi-hamburg.de.
Werner Götz, Department of Orthodontics, Center of Dento-Maxillo-Facial Medicine, University of Bonn, Bonn 53111, Germany.
Maren C Kiessling, Institute of Anatomy, Faculty of Medicine, LMU Munich, Munich 80336, Germany.
Christopher Alt, InGeneron GmbH, Munich 80331, Germany.
Christoph Schmitz, Institute of Anatomy, Faculty of Medicine, LMU Munich, Munich 80336, Germany.
Eckhard U Alt, InGeneron GmbH, Munich 80331, Germany; InGeneron, Inc., Houston, TX 77054, United States; Isar Klinikum Munich, 80331 Munich, Germany.
References
- 1.Laudenbach JM, Simon Z. Common dental and periodontal diseases: evaluation and management. Med Clin North Am. 2014;98:1239–1260. doi: 10.1016/j.mcna.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Moraschini V, Poubel LA, Ferreira VF, Barboza Edos S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44:377–388. doi: 10.1016/j.ijom.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Lundgren S, Cricchio G, Hallman M, Jungner M, Rasmusson L, Sennerby L. Sinus floor elevation procedures to enable implant placement and integration: techniques, biological aspects and clinical outcomes. Periodontol 2000. 2017;73:103–120. doi: 10.1111/prd.12165. [DOI] [PubMed] [Google Scholar]
- 4.Esposito M, Felice P, Worthington HV. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database Syst Rev. 2014;(5):CD008397. doi: 10.1002/14651858.CD008397.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starch-Jensen T, Jensen JD. Maxillary Sinus Floor Augmentation: a Review of Selected Treatment Modalities. J Oral Maxillofac Res. 2017;8:e3. doi: 10.5037/jomr.2017.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Dajani M. Recent Trends in Sinus Lift Surgery and Their Clinical Implications. Clin Implant Dent Relat Res. 2016;18:204–212. doi: 10.1111/cid.12275. [DOI] [PubMed] [Google Scholar]
- 7.Jamjoom A, Cohen RE. Grafts for Ridge Preservation. J Funct Biomater. 2015;6:833–848. doi: 10.3390/jfb6030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg. 2012;23:323–327. doi: 10.1097/SCS.0b013e318241dcba. [DOI] [PubMed] [Google Scholar]
- 9.Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology-is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017;3:23. doi: 10.1186/s40729-017-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanz M, Vignoletti F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent Mater. 2015;31:640–647. doi: 10.1016/j.dental.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2:224–247. doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M, Egusa H. Current bone substitutes for implant dentistry. J Prosthodont Res. 2018;62:152–161. doi: 10.1016/j.jpor.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol. 2014;7 Suppl 2:S203–S217. [PubMed] [Google Scholar]
- 14.García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–121. doi: 10.1016/j.bone.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Troeltzsch M, Troeltzsch M, Kauffmann P, Gruber R, Brockmeyer P, Moser N, Rau A, Schliephake H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J Craniomaxillofac Surg. 2016;44:1618–1629. doi: 10.1016/j.jcms.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Danesh-Sani SA, Engebretson SP, Janal MN. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: a systematic review and meta-analysis. J Periodontal Res. 2017;52:301–312. doi: 10.1111/jre.12402. [DOI] [PubMed] [Google Scholar]
- 17.Monje A, O'Valle F, Monje-Gil F, Ortega-Oller I, Mesa F, Wang HL, Galindo-Moreno P. Cellular, Vascular, and Histomorphometric Outcomes of Solvent-Dehydrated vs Freeze-Dried Allogeneic Graft for Maxillary Sinus Augmentation: A Randomized Case Series. Int J Oral Maxillofac Implants. 2017;32:121–127. doi: 10.11607/jomi.4801. [DOI] [PubMed] [Google Scholar]
- 18.Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol. 2012;83:329–336. doi: 10.1902/jop.2011.110270. [DOI] [PubMed] [Google Scholar]
- 19.Miron RJ, Sculean A, Shuang Y, Bosshardt DD, Gruber R, Buser D, Chandad F, Zhang Y. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin Oral Implants Res. 2016;27:668–675. doi: 10.1111/clr.12647. [DOI] [PubMed] [Google Scholar]
- 20.Roldán JC, Knueppel H, Schmidt C, Jepsen S, Zimmermann C, Terheyden H. Single-stage sinus augmentation with cancellous iliac bone and anorganic bovine bone in the presence of platelet-rich plasma in the miniature pig. Clin Oral Implants Res. 2008;19:373–378. doi: 10.1111/j.1600-0501.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Choi BH, Jung JH, Zhu SJ, Lee SH, Huh JY, You TM, Li J. Maxillary sinus floor augmentation using autogenous bone grafts and platelet-enriched fibrin glue with simultaneous implant placement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:329–333. doi: 10.1016/j.tripleo.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71:1654–1661. doi: 10.1902/jop.2000.71.10.1654. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg. 2003;61:157–163. doi: 10.1053/joms.2003.50041. [DOI] [PubMed] [Google Scholar]
- 24.Wiltfang J, Schlegel KA, Schultze-Mosgau S, Nkenke E, Zimmermann R, Kessler P. Sinus floor augmentation with beta-tricalciumphosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin Oral Implants Res. 2003;14:213–218. doi: 10.1034/j.1600-0501.2003.140212.x. [DOI] [PubMed] [Google Scholar]
- 25.Bae JH, Kim YK, Myung SK. Effects of platelet-rich plasma on sinus bone graft: meta-analysis. J Periodontol. 2011;82:660–667. doi: 10.1902/jop.2010.100529. [DOI] [PubMed] [Google Scholar]
- 26.Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008;14:249–258. doi: 10.1089/ten.teb.2008.0062. [DOI] [PubMed] [Google Scholar]
- 27.Intini G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials. 2009;30:4956–4966. doi: 10.1016/j.biomaterials.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 28.Roffi A, Di Matteo B, Krishnakumar GS, Kon E, Filardo G. Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review. Int Orthop. 2017;41:221–237. doi: 10.1007/s00264-016-3342-9. [DOI] [PubMed] [Google Scholar]
- 29.Lemos CA, Mello CC, dos Santos DM, Verri FR, Goiato MC, Pellizzer EP. Effects of platelet-rich plasma in association with bone grafts in maxillary sinus augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:517–525. doi: 10.1016/j.ijom.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Pocaterra A, Caruso S, Bernardi S, Scagnoli L, Continenza MA, Gatto R. Effectiveness of platelet-rich plasma as an adjunctive material to bone graft: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Oral Maxillofac Surg. 2016;45:1027–1034. doi: 10.1016/j.ijom.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Abdalla RIB, Alqutaibi AY, Kaddah A. Does the adjunctive use of platelet-rich plasma to bone graft during sinus augmentation reduce implant failure and complication? Systematic review and meta-analysis. Quintessence Int. 2018;49:139–146. doi: 10.3290/j.qi.a39616. [DOI] [PubMed] [Google Scholar]
- 32.Misch CE. Louis: Mosby; 2008. Available bone and dental implant treatment plans. In: Misch CE, editor. Contemporary Implant Dentistry. 3rd ed. St; pp. 178–199. [Google Scholar]
- 33.Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998;13 Suppl:11–45. [PubMed] [Google Scholar]
- 34.Eda T, Takahashi K, Kanao S, Aoki A, Ogura N, Ito K, Tsukahara H, Suemitsu M, Kuyama K, Kondoh T. Comparison study between plasma rich in growth factors and platelet-rich plasma for osteoconduction in rat calvaria. J Oral Maxillofac Surg Med Pathol. 2017;29:563–569. [Google Scholar]
- 35.Parnia F, Yazdani J, Maleki Dizaj S. Applications of Mesenchymal Stem Cells in Sinus Lift Augmentation as a Dental Implant Technology. Stem Cells Int. 2018;2018:3080139. doi: 10.1155/2018/3080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niño-Sandoval TC, Vasconcelos BC, D Moraes SL, A Lemos CA, Pellizzer EP. Efficacy of stem cells in maxillary sinus floor augmentation: systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2018 doi: 10.1016/j.ijom.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Miguita L, Mantesso A, Pannuti CM, Deboni MCZ. Can stem cells enhance bone formation in the human edentulous alveolar ridge? A systematic review and meta-analysis. Cell Tissue Bank. 2017;18:217–228. doi: 10.1007/s10561-017-9612-y. [DOI] [PubMed] [Google Scholar]
- 38.Barba M, Di Taranto G, Lattanzi W. Adipose-derived stem cell therapies for bone regeneration. Expert Opin Biol Ther. 2017;17:677–689. doi: 10.1080/14712598.2017.1315403. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya I, Ghayor C, Weber FE. The Use of Adipose Tissue-Derived Progenitors in Bone Tissue Engineering - a Review. Transfus Med Hemother. 2016;43:336–343. doi: 10.1159/000447494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han DS, Chang HK, Kim KR, Woo SM. Consideration of bone regeneration effect of stem cells: comparison of bone regeneration between bone marrow stem cells and adipose-derived stem cells. J Craniofac Surg. 2014;25:196–201. doi: 10.1097/SCS.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82:238–247. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Zhang X, Wang S, Xu L, Zhang M, Wang G, Jin Y, Zhang X, Jiang X. Comparison of the use of adipose tissue-derived and bone marrow-derived stem cells for rapid bone regeneration. J Dent Res. 2013;92:1136–1141. doi: 10.1177/0022034513507581. [DOI] [PubMed] [Google Scholar]
- 43.Yu H, Lu K, Zhu J, Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. 2017;121:135–154. doi: 10.1093/bmb/ldw059. [DOI] [PubMed] [Google Scholar]
- 44.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 46.Tajima S, Tobita M, Mizuno H. Current status of bone regeneration using adipose-derived stem cells. Histol Histopathol. 2018;33:619–627. doi: 10.14670/HH-11-942. [DOI] [PubMed] [Google Scholar]
- 47.Romagnoli C, Brandi ML. Adipose mesenchymal stem cells in the field of bone tissue engineering. World J Stem Cells. 2014;6:144–152. doi: 10.4252/wjsc.v6.i2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanetti AS, Sabliov C, Gimble JM, Hayes DJ. Human adipose-derived stem cells and three-dimensional scaffold constructs: a review of the biomaterials and models currently used for bone regeneration. J Biomed Mater Res B Appl Biomater. 2013;101:187–199. doi: 10.1002/jbm.b.32817. [DOI] [PubMed] [Google Scholar]
- 49.Oberbauer E, Steffenhagen C, Wurzer C, Gabriel C, Redl H, Wolbank S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen (Lond) 2015;4:7. doi: 10.1186/s13619-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dongen JA, Tuin AJ, Spiekman M, Jansma J, van der Lei B, Harmsen MC. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med. 2018;12:e261–e274. doi: 10.1002/term.2407. [DOI] [PubMed] [Google Scholar]
- 51.Condé-Green A, Kotamarti VS, Sherman LS, Keith JD, Lee ES, Granick MS, Rameshwar P. Shift toward Mechanical Isolation of Adipose-derived Stromal Vascular Fraction: Review of Upcoming Techniques. Plast Reconstr Surg Glob Open. 2016;4:e1017. doi: 10.1097/GOX.0000000000001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. doi: 10.1186/s40064-015-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela N, Alt C, Winnier GE, Alt EU. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of enzymatic reagent. 2018 Preprint. bioRxiv:485318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 55.Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, Blanco F, Martínez-Taboada VM, Taylor P, Martín-Martín C, DelaRosa O, Tagarro I, Díaz-González F. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76:196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 56.Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 57.Klein JA. Tumescent technique for local anesthesia improves safety in large-volume liposuction. Plast Reconstr Surg. 1993;92:1085–98; discussion 1099-100. [PubMed] [Google Scholar]
- 58.Coleman WP 4th, Hendry SL 2nd. Principles of liposuction. Semin Cutan Med Surg. 2006;25:138–144. doi: 10.1016/j.sder.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Tatum H., Jr Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986;30:207–229. [PubMed] [Google Scholar]
- 60.El Chaar ES. Soft tissue closure of grafted extraction sockets in the posterior maxilla: the rotated pedicle palatal connective tissue flap technique. Implant Dent. 2010;19:370–377. doi: 10.1097/ID.0b013e3181ed06cd. [DOI] [PubMed] [Google Scholar]
- 61.Ronda M, Stacchi C. A Novel Approach for the Coronal Advancement of the Buccal Flap. Int J Periodontics Restorative Dent. 2015;35:795–801. doi: 10.11607/prd.2232. [DOI] [PubMed] [Google Scholar]
- 62.Mangano FG, Tettamanti L, Sammons RL, Azzi L, Caprioglio A, Macchi A, Mangano C. Maxillary sinus augmentation with adult mesenchymal stem cells: a review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:717–723. doi: 10.1016/j.oooo.2012.09.087. [DOI] [PubMed] [Google Scholar]
- 63.Mangano FG, Colombo M, Veronesi G, Caprioglio A, Mangano C. Mesenchymal stem cells in maxillary sinus augmentation: A systematic review with meta-analysis. World J Stem Cells. 2015;7:976–991. doi: 10.4252/wjsc.v7.i6.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Correia F, Pozza DH, Gouveia S, Felino A, Faria E Almeida R. The applications of regenerative medicine in sinus lift procedures: A systematic review. Clin Implant Dent Relat Res. 2018;20:229–242. doi: 10.1111/cid.12561. [DOI] [PubMed] [Google Scholar]
- 65.Wildburger A, Payer M, Jakse N, Strunk D, Etchard-Liechtenstein N, Sauerbier S. Impact of autogenous concentrated bone marrow aspirate on bone regeneration after sinus floor augmentation with a bovine bone substitute--a split-mouth pilot study. Clin Oral Implants Res. 2014;25:1175–1181. doi: 10.1111/clr.12228. [DOI] [PubMed] [Google Scholar]
- 66.Pasquali PJ, Teixeira ML, de Oliveira TA, de Macedo LG, Aloise AC, Pelegrine AA. Maxillary Sinus Augmentation Combining Bio-Oss with the Bone Marrow Aspirate Concentrate: A Histomorphometric Study in Humans. Int J Biomater. 2015;2015:121286. doi: 10.1155/2015/121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parisien M, Cosman F, Morgan D, Schnitzer M, Liang X, Nieves J, Forese L, Luckey M, Meier D, Shen V, Lindsay R, Dempster DW. Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. J Bone Miner Res. 1997;12:948–957. doi: 10.1359/jbmr.1997.12.6.948. [DOI] [PubMed] [Google Scholar]
- 68.Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 2012;59:203–227. doi: 10.1111/j.1600-0757.2012.00443.x. [DOI] [PubMed] [Google Scholar]
- 69.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--part I: stem cell sources. J Prosthodont Res. 2012;56:151–165. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed RP, Ashraf M, Buccini S, Shujia J, Haider HKh. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen Med. 2011;6:171–178. doi: 10.2217/rme.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS One. 2011;6:e19012. doi: 10.1371/journal.pone.0019012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmelzeisen R, Gutwald R, Oshima T, Nagursky H, Vogeler M, Sauerbier S. Making bone II: maxillary sinus augmentation with mononuclear cells--case report with a new clinical method. Br J Oral Maxillofac Surg. 2011;49:480–482. doi: 10.1016/j.bjoms.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 73.Sauerbier S, Rickert D, Gutwald R, Nagursky H, Oshima T, Xavier SP, Christmann J, Kurz P, Menne D, Vissink A, Raghoebar G, Schmelzeisen R, Wagner W, Koch FP. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial--per-protocol analysis. Tissue Eng Part A. 2011;17:2187–2197. doi: 10.1089/ten.TEA.2010.0516. [DOI] [PubMed] [Google Scholar]
- 74.Götz W, Gerber T, Michel B, Lossdörfer S, Henkel KO, Heinemann F. Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone(r)) osteogenesis: a study on biopsies from human jaws. Clin Oral Implants Res. 2008;19:1016–1026. doi: 10.1111/j.1600-0501.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- 75.Friedmann A, Gissel K, Konermann A, Götz W. Tissue reactions after simultaneous alveolar ridge augmentation with biphasic calcium phosphate and implant insertion--histological and immunohistochemical evaluation in humans. Clin Oral Investig. 2015;19:1595–1603. doi: 10.1007/s00784-014-1385-0. [DOI] [PubMed] [Google Scholar]
- 76.Hawthorne AC, Xavier SP, Okamoto R, Salvador SL, Antunes AA, Salata LA. Immunohistochemical, tomographic, and histological study on onlay bone graft remodeling. Part III: allografts. Clin Oral Implants Res. 2013;24:1164–1172. doi: 10.1111/j.1600-0501.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- 77.Brunel G, Brocard D, Duffort JF, Jacquet E, Justumus P, Simonet T, Benqué E. Bioabsorbable materials for guided bone regeneration prior to implant placement and 7-year follow-up: report of 14 cases. J Periodontol. 2001;72:257–264. doi: 10.1902/jop.2001.72.2.257. [DOI] [PubMed] [Google Scholar]
- 78.Mordenfeld A, Hallman M, Johansson CB, Albrektsson T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin Oral Implants Res. 2010;21:961–970. doi: 10.1111/j.1600-0501.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 79.Tian T, Zhang T, Lin Y, Cai X. Vascularization in Craniofacial Bone Tissue Engineering. J Dent Res. 2018;97:969–976. doi: 10.1177/0022034518767120. [DOI] [PubMed] [Google Scholar]
- 80.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rezza A, Sennett R, Rendl M. Adult stem cell niches: cellular and molecular components. Curr Top Dev Biol. 2014;107:333–372. doi: 10.1016/B978-0-12-416022-4.00012-3. [DOI] [PubMed] [Google Scholar]
- 82.Dong L, Hao H, Han W, Fu X. The role of the microenvironment on the fate of adult stem cells. Sci China Life Sci. 2015;58:639–648. doi: 10.1007/s11427-015-4865-9. [DOI] [PubMed] [Google Scholar]
- 83.Wabik A, Jones PH. Switching roles: the functional plasticity of adult tissue stem cells. EMBO J. 2015;34:1164–1179. doi: 10.15252/embj.201490386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trosko JE. Induction of iPS cells and of cancer stem cells: the stem cell or reprogramming hypothesis of cancer? Anat Rec (Hoboken) 2014;297:161–173. doi: 10.1002/ar.22793. [DOI] [PubMed] [Google Scholar]
- 85.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 86.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 87.Kara RJ, Bolli P, Karakikes I, Matsunaga I, Tripodi J, Tanweer O, Altman P, Shachter NS, Nakano A, Najfeld V, Chaudhry HW. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbán E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 90.Hoffman AM, Dow SW. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells. 2016;34:1709–1729. doi: 10.1002/stem.2377. [DOI] [PubMed] [Google Scholar]
- 91.Toyserkani NM, Jørgensen MG, Tabatabaeifar S, Jensen CH, Sheikh SP, Sørensen JA. Concise Review: A Safety Assessment of Adipose-Derived Cell Therapy in Clinical Trials: A Systematic Review of Reported Adverse Events. Stem Cells Transl Med. 2017;6:1786–1794. doi: 10.1002/sctm.17-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 93.Xu S, De Becker A, De Raeve H, Van Camp B, Vanderkerken K, Van Riet I. In vitro expanded bone marrow-derived murine (C57Bl/KaLwRij) mesenchymal stem cells can acquire CD34 expression and induce sarcoma formation in vivo. Biochem Biophys Res Commun. 2012;424:391–397. doi: 10.1016/j.bbrc.2012.06.118. [DOI] [PubMed] [Google Scholar]
- 94.Pan Q, Fouraschen SM, de Ruiter PE, Dinjens WN, Kwekkeboom J, Tilanus HW, van der Laan LJ. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med (Maywood) 2014;239:105–115. doi: 10.1177/1535370213506802. [DOI] [PubMed] [Google Scholar]
- 95.Haenel A, Ghosn M, Karimi T, Vykoukal J, Kettlun C, Shah D, Dave A, Valderrrabano M, Schulz D, Azares A, Raizner A, Alt E. Unmodified, autologous adipose-derived regenerative cells improve cardiac function, structure and revascularization in a porcine model of chronic myocardial infarction. 2018 Preprint. BioRxiv:286468 [Google Scholar]
- 96.Badimon L, Oñate B, Vilahur G. Adipose-derived Mesenchymal Stem Cells and Their Reparative Potential in Ischemic Heart Disease. Rev Esp Cardiol (Engl Ed) 2015;68:599–611. doi: 10.1016/j.rec.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 97.Lombardi G, Perego S, Luzi L, Banfi G. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine. 2015;48:394–404. doi: 10.1007/s12020-014-0401-0. [DOI] [PubMed] [Google Scholar]
- 98.Anitua E, Tejero R, Zalduendo MM, Orive G. Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J Periodontol. 2013;84:1180–1190. doi: 10.1902/jop.2012.120292. [DOI] [PubMed] [Google Scholar]
- 99.Anitua E, Prado R, Orive G. A lateral approach for sinus elevation using PRGF technology. Clin Implant Dent Relat Res. 2009;11 Suppl 1:e23–e31. doi: 10.1111/j.1708-8208.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- 100.Taschieri S, Corbella S, Del Fabbro M. Use of plasma rich in growth factor for schneiderian membrane management during maxillary sinus augmentation procedure. J Oral Implantol. 2012;38:621–627. doi: 10.1563/AAID-JOI-D-12-00009. [DOI] [PubMed] [Google Scholar]
- 101.Khouly I, Pardiñas López S, Aliaga I, Froum SJ. Long-Term Implant Survival After 100 Maxillary Sinus Augmentations Using Plasma Rich in Growth Factors. Implant Dent. 2017;26:199–208. doi: 10.1097/ID.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 102.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 103.Mellado-López M, Griffeth RJ, Meseguer-Ripolles J, Cugat R, García M, Moreno-Manzano V. Plasma Rich in Growth Factors Induces Cell Proliferation, Migration, Differentiation, and Cell Survival of Adipose-Derived Stem Cells. Stem Cells Int. 2017;2017:5946527. doi: 10.1155/2017/5946527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bai X, Sadat S, Gehmert S, Alt E, Song YH. VEGF receptor Flk-1 plays an important role in c-kit expression in adipose tissue derived stem cells. FEBS Lett. 2007;581:4681–4684. doi: 10.1016/j.febslet.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 105.Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017;26:617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 106.Hegde R, Prasad K, Shroff KK. Maxillary sinus augmentation using sinus membrane elevation without grafts - A Systematic Review. J Indian Prosthodont Soc. 2016;16:317–322. doi: 10.4103/0972-4052.191289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tong DC, Rioux K, Drangsholt M, Beirne OR. A review of survival rates for implants placed in grafted maxillary sinuses using meta-analysis. Int J Oral Maxillofac Implants. 1998;13:175–182. [PubMed] [Google Scholar]
- 108.Klijn RJ, Meijer GJ, Bronkhorst EM, Jansen JA. A meta-analysis of histomorphometric results and graft healing time of various biomaterials compared to autologous bone used as sinus floor augmentation material in humans. Tissue Eng Part B Rev. 2010;16:493–507. doi: 10.1089/ten.TEB.2010.0035. [DOI] [PubMed] [Google Scholar]
- 109.Silva LD, de Lima VN, Faverani LP, de Mendonça MR, Okamoto R, Pellizzer EP. Maxillary sinus lift surgery-with or without graft material? A systematic review. Int J Oral Maxillofac Surg. 2016;45:1570–1576. doi: 10.1016/j.ijom.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 110.Hosseinpour S, Ghazizadeh Ahsaie M, Rezai Rad M, Baghani MT, Motamedian SR, Khojasteh A. Application of selected scaffolds for bone tissue engineering: a systematic review. Oral Maxillofac Surg. 2017;21:109–129. doi: 10.1007/s10006-017-0608-3. [DOI] [PubMed] [Google Scholar]
- 111.Destatis Statistisches Bundesamt. 2018. Die Angeforderte Seite IST Nicht Verfügbar. Available from: URL: https://www.destatis.de/DE/ZahlenFakten/GesellschaftStaat/Bevoelkerung/Sterbefaelle/Tabellen/Lebenserwartung.pdf. [Google Scholar]
- 112.Kokai LE, Traktuev DO, Zhang L, Merfeld-Clauss S, DiBernardo G, Lu H, Marra KG, Donnenberg A, Donnenberg V, Meyer EM, Fodor PB, March KL, Rubin JP. Adipose Stem Cell Function Maintained with Age: An Intra-Subject Study of Long-Term Cryopreserved Cells. Aesthet Surg J. 2017;37:454–463. doi: 10.1093/asj/sjw197. [DOI] [PubMed] [Google Scholar]
- 113.Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Kim SW, Choi JW, Lee CY, Lee J, Shin S, Lim S, Lee S, Kim IK, Lee HB, Hwang KC. Effects of donor age on human adipose-derived adherent stromal cells under oxidative stress conditions. J Int Med Res. 2018;46:951–964. doi: 10.1177/0300060517731684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--Part II: Clinical applications. J Prosthodont Res. 2012;56:229–248. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Miran S, Mitsiadis TA, Pagella P. Innovative Dental Stem Cell-Based Research Approaches: The Future of Dentistry. Stem Cells Int. 2016;2016:7231038. doi: 10.1155/2016/7231038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amrollahi P, Shah B, Seifi A, Tayebi L. Recent advancements in regenerative dentistry: A review. Mater Sci Eng C Mater Biol Appl. 2016;69:1383–1390. doi: 10.1016/j.msec.2016.08.045. [DOI] [PubMed] [Google Scholar]