Abstract
Background
Cancer immunotherapy is under development as a promising alternative strategy for treating advanced non‐small cell lung cancer (NSCLC). However, the development of novel biomarkers to optimize the use of immune checkpoint inhibitors (ICIs) is still ongoing. Gut microbiota are known to regulate a host's immunity and are associated with the response to ICIs in melanoma. Therefore, we analyzed the association between ICI treatment efficacy and bowel movement condition in patients with NSCLC.
Methods
This retrospective study analyzed patients with advanced NSCLC who were treated with ICIs between December 2015 and March 2018 at University Hospital Kyoto Prefectural University of Medicine in Kyoto, Japan. The association between stool abnormalities and ICI efficacy was investigated. We defined patients with constipation or those who used a laxative as the stool abnormality group.
Results
We retrospectively enrolled 40 patients with advanced NSCLC who were treated with ICIs. The median age was 69.5 years; 20 patients had a stool abnormality and 20 patients did not. The disease control rates were lower in NSCLC patients with stool abnormalities than in those without stool abnormalities (20% vs. 77.8%, respectively; P = 0.0016). The time to treatment failure with ICI treatment was shorter in NSCLC patients with stool abnormalities compared with those without stool abnormalities (P = 0.003; odds ratio, 3.09; 95% confidence interval 1.41–6.78).
Conclusion
Stool abnormality might be a predictive biomarker for the clinical benefit of ICI treatment in patients with NSCLC. Further investigations are warranted to validate our findings.
Keywords: Biomarker, bowel movement condition, immunotherapy, non‐small cell lung cancer, retrospective study
Introduction
Lung cancer is the leading cause of cancer‐related deaths worldwide.1 Some molecularly targeted therapies and angiogenesis inhibitors have been successfully introduced for treating subpopulations of patients with advanced lung cancer. Cancer immunotherapy, such as programmed cell death protein 1 (PD‐1)/programmed death ligand 1 (PD‐L1) checkpoint inhibitors, has recently been developed as a promising alternative strategy for treating advanced non‐small cell lung cancer (NSCLC). Of them, nivolumab, pembrolizumab, and atezolizumab have received approval in the USA, Japan, and other countries for the treatment of patients with metastatic NSCLC based on some phase III clinical trials.2, 3, 4 Several mechanisms of a favorable response to immune checkpoints have been reported, such as a high level of PD‐L1 expression and high tumor mutation burden in tumors, and the accumulation of tumor‐infiltrating lymphocytes in the tumor microenvironment.5 However, the development of a potent biomarker to optimize the use of immune checkpoint inhibitors (ICIs) is still ongoing. Further studies are required to identify methods of detecting ICI responders among NSCLC patients.
A recent report showed that gut microbiota has received much attention as a critical factor of tumor development, as it modulates inflammation and antitumor immunity.6, 7, 8 Gopalakrishnan et al. showed that the abundance of gut bacteria belonging to the Ruminococcaceae family was associated with the clinical response to anti‐PD‐1 treatment in patients with melanoma.9 Therefore, the comprehensive analysis of commensal microbiota might lead to promising novel biomarkers in NSCLC patients treated with ICIs. However, the association between fecal character and the efficacy of ICIs in NSCLC remains unknown.
Here, we focused on baseline bowel abnormalities in NSCLC patients treated with ICIs, and analyzed the association between ICI treatment efficacy and patient characteristics, including bowel movement condition, to discover a novel biomarker of ICI responders.
Methods
Patients
We enrolled 40 patients diagnosed with advanced NSCLC who were treated with ICIs at University Hospital Kyoto Prefectural University of Medicine in Kyoto, Japan between December 2015 and March 2018 regardless of any previous treatment with cytotoxic chemotherapy. We obtained each patient's clinical data from a retrospective medical record review, and retrieved information on age, sex, histological subtype, PD‐L1 expression level in tumors, epidermal growth factor receptor mutation status, disease staging, Eastern Cooperative Oncology Group Performance Status (ECOG‐PS), smoking status, bowel movement condition, laboratory findings at baseline (including serum C‐reactive protein [CRP] level), overall survival (OS), time to treatment failure (TTF), response rate, and disease control rate of patients receiving ICI treatment based on the Response Evaluation Criteria in Solid Tumors version 1.1. The study protocol was approved by our hospital's ethics committees. Tumor–node–metastasis stage was classified using the tumor–node–metastasis stage classification system version 7.
Tumor PD‐L1 analysis
PD‐L1 expression was analyzed at SRL Inc. (Tokyo, Japan) using the PD‐L1 IHC 22C3 pharmDx assay or 28‐8 pharmDx assay (Agilent Technologies, Santa Clara, CA, USA). The PD‐L1 tumor proportion score was calculated as the percentage of at least 100 viable tumor cells for complete or partial membrane staining. The pathologists of the commercial vendor interpreted the tumor proportion score.
Immunotherapy
The anti‐PD‐1 antibodies administered included nivolumab and pembrolizumab, which were intravenously administered at the doses of 3 mg/kg every two weeks and 200 mg every three weeks, respectively. These treatments generally continued until disease progression, intolerable toxicity, or patient refusal occurred.
Definition of stool abnormality
We obtained each patient's data regarding constipation from the retrospective medical record review and defined the patients with stool abnormality as follows: (i) constipation condition according to Common Terminology Criteria for Adverse Events version 4.0 for more than three days in a week before and after ICI administration; or (ii) taking oral laxatives during ICI treatment. The term “laxative” refers to constipation medicine therapy based on the 2017 chronic constipation clinical practice guideline.
Statistical analysis
Cox proportional hazards models including age, sex, smoking history, performance status, histological type, epidermal growth factor receptor mutation status, bowel movement condition, serum CRP levels, metastatic lesions, staging, and tumor PD‐L1 expression levels were created. To analyze the TTF and OS, times to events were estimated using the Kaplan–Meier method and compared using the log–rank test. The TTF and OS were censored at the date of the last visit for patients who were still alive without any documented disease progression. The tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1. All statistical analyses were carried out using GraphPad Prism (version 7.02; GraphPad Software Inc., La Jolla, CA, USA). P‐values <0.05 were considered statistically significant.
Results
Patient characteristics
A total of 40 NSCLC patients treated with ICIs between December 2015 and March 2018 at our hospital in Japan were included. Of them, 27 (67.5%) were men and 29 (72.5%) were never‐smokers; the median age of all patients was 69.5 years (range 46–83 years). The histological subtypes were adenocarcinoma in 20 (50%) and squamous cell carcinoma in 12 (30%). Performance statuses were 0 and 1 in 30 (75%), and >2 in 10 (25%). Sites of metastatic disease were detected in the liver of five patients (12.5%), and in the brain of 14 patients (35%). Of the 40 patients, 33 (82.5%) had stage IV disease, and seven (17.5%) had postoperative recurrence. Treatment consisted of nivolumab in 26 (65%) and pembrolizumab in 14 (35%). Epidermal growth factor receptor mutations were detected in seven patients (17.5%). The baseline clinicopathological characteristics of the patients are summarized in Table 1.
Table 1.
Patient characteristics at baseline (n = 40)
| Items | n (%) |
|---|---|
| Age | |
| Median (range) | 69.5 (46–83) |
| Gender | |
| Male | 27 (67.5) |
| Female | 13 (32.5) |
| ECOG‐PS | |
| 0–1 | 30 (75) |
| 2–4 | 10 (25) |
| Histology | |
| Adenocarcinoma | 20 (50) |
| Squamous cell carcinoma | 12 (30) |
| Other | 8 (20) |
| Smoking status | |
| Never smoker | 29 (72.5) |
| Current or former smoker | 11 (27.5) |
| Staging | |
| Stage IV | 33(82.5) |
| Postoperative recurrence | 7 (17.5) |
| EGFR mutations | |
| Positive | 7 (17.5) |
| Negative | 33 (82.5) |
| PD‐L1 TPS | |
| ≥50% | 11 (27.5) |
| 1–49% | 5 (12.5) |
| <1% | 5 (12.5) |
| Not evaluated | 19 (47.5) |
| Metastasis | |
| Liver metastasis | 5 (12.5) |
| Brain metastasis | 14 (35) |
| Defecation status | |
| With constipation | 20 (50) |
| Without constipation | 20 (50) |
| Medication history | |
| Laxative | 15 (37.5) |
| Opioid | 8 (20) |
| Response to treatment | |
| CR/PR | 7 (17.5) |
| SD | 10 (25) |
| PD | 16 (40) |
| Not evaluated | 7 (17.5) |
| ICI | |
| Nivolumab | 26 (65) |
| Pembrolizumab | 14 (35) |
CR/PR, complete response/partial response; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; PD, progressive disease; SD, stable disease; TPS, tumor proportion score.
Association between clinical features and ICI outcomes in NSCLC patients
Of the 40 NSCLC patients, none experienced a complete response (0%), seven experienced a partial response (17.5%), 10 experienced stable disease (25%), and 16 experienced progressive disease (40%), whereas seven were non‐evaluable (17.5%) when treated with ICIs per Response Evaluation Criteria in Solid Tumors criteria, indicating a 21.2% response rate and 51.5% disease control rate (Table 1). Median TTF and median OS were 83 and 262 days in all NSCLC patients, respectively (Fig 1a,b).
Figure 1.
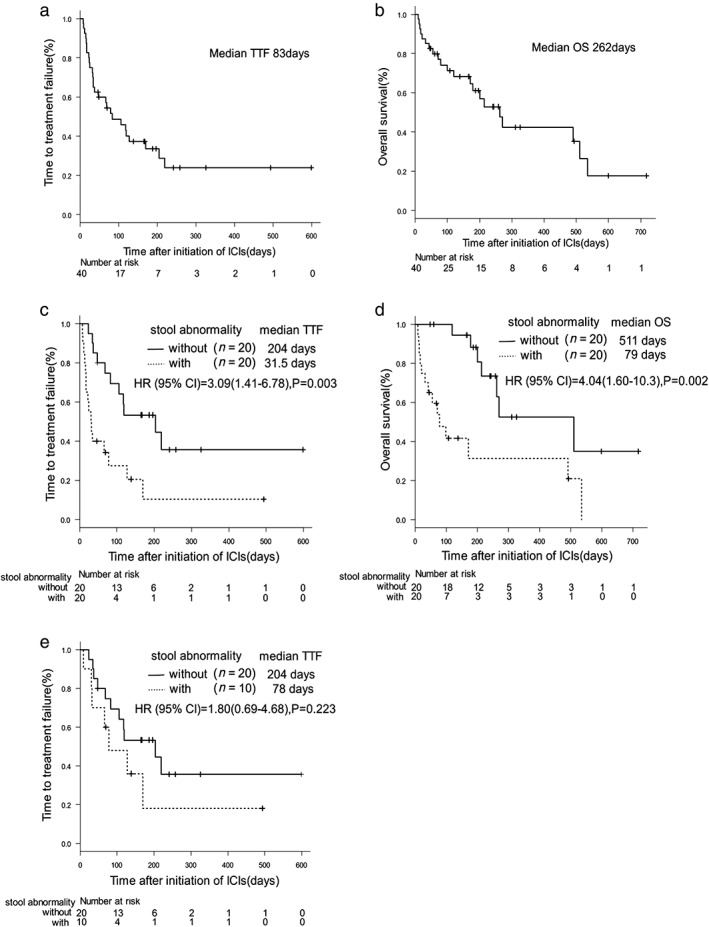
Kaplan–Meier survival curves of time to treatment failure (TTF) and overall survival (OS). (a) TTF of all patients (n = 40) on immune checkpoint inhibitor treatment. (b) OS of all patients (n = 40) on immune checkpoint inhibitor treatment. (c) TTF of patients with constipation (n = 20) or without constipation (n = 20) on immune checkpoint inhibitor treatment. (d) OS of patients with constipation (n = 20) or without constipation (n = 20) on immune checkpoint inhibitor treatment. (e) TTF of patients with constipation (n = 20) or without constipation (n = 20) with an Eastern Cooperative Oncology Group Performance Status of 0 or 1 on immune checkpoint inhibitor treatment.
TTF with ICI treatment and OS were significantly correlated with constipation, ECOG‐PS, serum CRP, and the presence of liver metastasis on univariate analysis among patient profiles (Table 2).
Table 2.
Cox proportional hazards models
| TTF (Univariate analysis) | OS (Univariate analysis) | |||
|---|---|---|---|---|
| Items | HR (95% CI) | P‐value | HR (95% CI) | P‐value |
| Age >75years | 1.38 (0.520–3.64) | 0.521 | 1.39 (0.497–3.90) | 0.53 |
| Male gender | 1.05 (0.470–2.36) | 0.902 | 1.39 (0.534–3.60) | 0.502 |
| Smoker | 1.30 (0.582–2.91) | 0.521 | 1.29 (0.526–3.16) | 0.578 |
| ECOG‐PS ≥2 | 11.1 (3.74–32.71) | <0.001 | 30.2 (6.13–149) | <0.001 |
| Squamous histology | 0.928 (0.405–2.12) | 0.86 | 0.833 (0.313–2.22) | 0.715 |
| EGFR mutation status | 1.33 (0.534–3.3) | 0.542 | 1.03 (0.343–3.10) | 0.956 |
| Constipation | 3.09 (1.41–6.78) | 0.005 | 4.04 (1.59–10.2) | 0.003 |
| CRP ≥1.0 | 2.86 (1.28–6.41) | 0.01 | 3.36 (1.32–8.51) | 0.01 |
| Liver metastasis | 5.77(1.96–17.0) | 0.001 | 5.11 (1.31–19.9) | 0.02 |
| Brain metastasis | 1.06 (0.497–2.33) | 0.891 | 1.05 (0.43–2.57) | 0.914 |
| PD‐L1 TPS >1% | 0.925 (0.253–3.39) | 0.906 | 0.726 (0.140–3.76) | 0.702 |
| PD‐L1 TPS >50% | 0.623 (0.201–1.93) | 0.412 | 1.83 (0.435–7.68) | 0.41 |
CI, confidence interval; CRP, C‐reactive protein; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; OR, odds ratio; PD‐L1, programmed death ligand 1; TPS, tumor proportion score; TTF, time to treatment failure; OS, overall survival.
To evaluate the impact of stool condition in ICI treatment, we next determined the association between stool abnormality and ICI efficacy. In our criteria, as shown in the Methods section, 20 patients with stool abnormality and 20 patients without stool abnormality were identified. Of them, 10 patients (50%) in the stool abnormality group and no patients (0%) in the normal stool group had an ECOG‐PS >2. In the stool abnormality group, 15 patients (75%) had a medication history of laxative use. TTF with ICI treatment and OS were more prolonged in NSCLC patients without stool abnormalities than in those with stool abnormalities (204 vs. 31.5 days, 511 days vs. 79 days, P = 0.003, P = 0.002, respectively; Fig 1c,d). Regarding cases with an ECOG‐PS of 0 or 1, patients without constipation had a longer TTF than those with constipation (204 days vs. 78 days, respectively, P = 0.223; Fig 1e). The disease control rates were lower in NSCLC patients with stool abnormalities than in those without stool abnormalities (20.0% vs. 77.8%, P = 0.0016; Fig 2).
Figure 2.
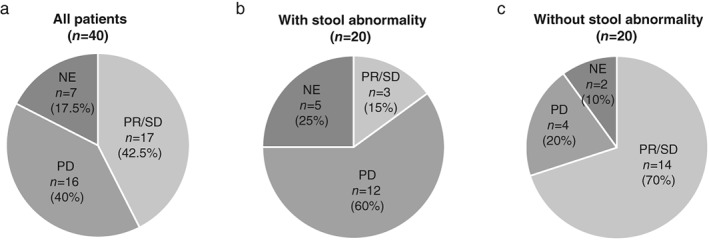
Frequency of best overall response to immune checkpoint inhibitors in patients with non‐small cell lung cancer. (a) Frequency of best overall response to immune checkpoint inhibitor treatment among all patients (n = 40). (b) Frequency of best overall response to immune checkpoint inhibitor treatment among all patients with constipation (n = 20). (c) Frequency of best overall response to immune checkpoint inhibitor treatment across all patients without constipation (n = 20). CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
To further assess whether the clinical features might determine constipation in NSCLC patients, we next sought to evaluate the differences in clinical profiles between patients with and those without stool abnormalities. A poor PS was significantly correlated with stool abnormality (P = 0.0004), whereas poor PS, but not constipation, was significantly associated with poor TTF and poor OS on multivariate analysis (Table 3, Table S1).
Table 3.
Patient characteristics at baseline classified by constipation
| Items | Without constipation (n = 20) | With constipation (n = 20) | P‐value |
|---|---|---|---|
| Gender | |||
| Male | 15 (75%) | 12 (60%) | 0.501 |
| Female | 5 (25%) | 8 (40%) | |
| ECOG‐PS | |||
| 0–1 | 20 (100%) | 10 (50%) | 0.0004 |
| 2–4 | 0 (0%) | 10 (50%) | |
| Histology | |||
| Adenocarcinoma | 13 (65%) | 7 (35%) | 0.301 |
| Squamous cell carcinoma | 4 (20%) | 8 (40%) | |
| Other | 3 (15%) | 5 (25%) | |
| Smoking status | |||
| Never smoker | 4 (20%) | 7 (35%) | 0.48 |
| Smoker | 16 (80%) | 13 (65%) | |
| Staging | |||
| Stage IV | 17 (85%) | 16 (80%) | 1 |
| Postoperative recurrence | 3 (15%) | 4 (20%) | |
| EGFR mutations | |||
| Positive | 5 (25%) | 2 (10%) | 0.407 |
| Negative | 15 (75%) | 18 (90%) | |
| PD‐L1 TPS | |||
| ≥50% | 6 (30%) | 6 (30%) | 0.297 |
| 1–49% | 4 (20%) | 2 (10%) | |
| <1% | 1 (5%) | 4 (20%) | |
| Not evaluated | 9 (45%) | 8 (40%) | |
| Metastasis | |||
| Liver metastasis | 1 (5%) | 4 (20%) | 0.342 |
| Brain metastasis | 8 (40%) | 6 (30%) | 0.741 |
| Medication history | |||
| Opioid | 2 (10%) | 6 (30%) | 0.235 |
ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
These observations showed that a baseline stool abnormality might be one of the potent predictors of ICI response among NSCLC patients.
Discussion
Gut microbiota plays critical roles in the development and progression of tumors through modulating inflammation and the host immune response. Several previous reports showed that the gut microbiome influences antitumor immune responses through innate and adaptive immunity,10, 11 thereby improving therapeutic responses.6, 12 Thus, gut microbiota is likely an important factor in the development and progression of tumors through inflammation and host immune response modulation.6, 7, 8 Although the fecal characteristics might be a useful factor in identifying ICI responders, this issue remains completely unknown in NSCLC patients.
The general microbiome contains more types of bacteria, such as Firmicutes, Actinobacteria, Proteobacteria, Bacteroidetes, and Verrucomicrobia, related to good responses to immunotherapy in metastatic melanoma.9, 13, 14 Although these observations encourage us to seek the optimal microbiome condition involved in ICI responses using a comprehensive analysis in each patient, it is difficult to establish a novel analysis method for clinical use considering a diversity of microbiota consisting of 500–1000 unique bacterial strains in the human colon.15 Previous studies reported that stool consistency and frequency are closely associated with fecal microbial richness.16, 17, 18 Therefore, instead of searching for individual microbiota, we focused on baseline bowel movement condition as a potential simple method for identifying ICI treatment responders, and sought to evaluate its role in ICI treatment efficacy in NSCLC patients. Our results showed that OS and TTF after ICI treatment were markedly shorter in NSCLC patients with baseline constipation than in those without constipation. To our knowledge, this is the first report to reveal the potent role of stool abnormality in the identification of ICI treatment non‐responders among NSCLC patients.
In our retrospective cohort, we additionally evaluated the correlation between patient profiles and clinical outcomes of ICI treatment that were reported as the predictive factors for the therapeutic effect of ICI treatment in patients with NSCLC. Of them, liver metastasis, ECOG‐PS, and serum CRP were significantly correlated with ICI response, a finding that is consistent with those of previous reports19, 20, 21. The multivariate analysis showed that a poor PS was the predictive factor of ICI treatment failure and a shortened OS, which was closely associated with persistent constipation in palliative care patients.22, 23 Stool abnormality was significantly related to poor PS in terms of ICI response in the present study. Further investigations are required to elucidate the impact of ECOG‐PS status on the roles of stool abnormality in ICI treatment response in NSCLC patients.
The present study had several limitations. First, it was retrospective in design, carried out in a single center, and included relatively small sample sizes. Second, the definition of constipation is ambiguous, because each patient's stool frequency history was based on the medical record, and the presence of constipation was unknown in patients taking oral laxatives. Finally, stool frequency was greatly affected by each patient's background, such as general health condition and history of opioid use. Therefore, a further prospective study is warranted to identify the role of stool abnormality as a predictive factor of ICI response in NSCLC patients.
In summary, we revealed that stool frequency can be a novel predictive factor of ICI treatment response, and a prognostic factor in patients with advanced NSCLC. As constipation assessments are simple and cost‐effective, this predictive factor could be a useful tool for identifying NSCLC patients who will respond poorly to ICI treatment, and assist clinical decision‐making processes regarding therapeutic intervention with ICIs. Further prospective studies are warranted to validate our findings.
Disclosure
No authors report any conflict of interest.
Supporting information
Appendix S1. Cox proportional models (Multivariate analysis).
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin 2012; 62 (5): 283–98. [DOI] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373 (17): 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373 (2): 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet (London, England) 2016; 387 (10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 5. Soo RA, Lim SM, Syn NL et al Immune checkpoint inhibitors in epidermal growth factor receptor mutant non‐small cell lung cancer: Current controversies and future directions. Lung Cancer (Amsterdam, Netherlands) 2018; 115: 12–20. [DOI] [PubMed] [Google Scholar]
- 6. Iida N, Dzutsev A, Stewart CA et al Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (New York, NY) 2013; 342 (6161): 967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell 2016; 165 (2): 276–87. [DOI] [PubMed] [Google Scholar]
- 8. Pushalkar S, Ji X, Li Y et al Comparison of oral microbiota in tumor and non‐tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol 2012; 12: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gopalakrishnan V, Spencer CN, Nezi L et al Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science (New York, NY) 2018; 359 (6371): 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paulos CM, Wrzesinski C, Kaiser A et al Microbial translocation augments the function of adoptively transferred self/tumor‐specific CD8+ T cells via TLR4 signaling. J Clin Invest 2007; 117 (8): 2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sivan A, Corrales L, Hubert N et al Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science (New York, NY) 2015; 350 (6264): 1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viaud S, Saccheri F, Mignot G et al The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (New York, NY). 2013; 342 (6161): 971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matson V, Fessler J, Bao R et al The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science (New York, NY) 2018; 359 (6371): 104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaput N, Lepage P, Coutzac C et al Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017; 28 (6): 1368–79. [DOI] [PubMed] [Google Scholar]
- 15. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375 (24): 2369–79. [DOI] [PubMed] [Google Scholar]
- 16. Vandeputte D, Falony G, Vieira‐Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016; 65 (1): 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tigchelaar EF, Bonder MJ, Jankipersadsing SA, Fu J, Wijmenga C, Zhernakova A. Gut microbiota composition associated with stool consistency. Gut 2016; 65 (3): 540–2. [DOI] [PubMed] [Google Scholar]
- 18. Hadizadeh F, Walter S, Belheouane M et al Stool frequency is associated with gut microbiota composition. Gut 2017; 66 (3): 559–60. [DOI] [PubMed] [Google Scholar]
- 19. Tumeh PC, Hellmann MD, Hamid O et al Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5 (5): 417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bagley SJ, Kothari S, Aggarwal C et al Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2017; 106: 1–7. [DOI] [PubMed] [Google Scholar]
- 21. Brustugun OT, Sprauten M, Helland A. C‐reactive protein (CRP) as a predictive marker for immunotherapy in lung cancer. J Clin Oncol 2016; 34: e20623. [Google Scholar]
- 22. Dzierzanowski T, Cialkowska‐Rysz A. Behavioral risk factors of constipation in palliative care patients. Support Care Cancer 2015; 23 (6): 1787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fallon MT, Hanks GW. Morphine, constipation and performance status in advanced cancer patients. Palliat Med 1999; 13 (2): 159–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Cox proportional models (Multivariate analysis).


