Abstract
Background
Pulmonary emphysema is a major component of chronic obstructive pulmonary disease and lung cancer. However the prognostic significance of quantitative emphysema severity in patients with lung cancer is unclear. We analyzed whether numerical emphysema value is a prognostic factor for recurrence in patients with surgically resected non‐small cell lung cancer.
Methods
We quantified emphysema severity of the whole lung and regional lobes in 45 patients (mean age 68.0 years) using an automated chest computed tomography‐based program. Predictive factors for recurrence were investigated using a Cox proportional hazards model. Recurrence‐free and overall survival was compared after dichotomization of patients according to whole lung emphysema severity.
Results
The mean percentage emphysema ratio of the whole lung was 1.21 ± 2.04. Regional lobar emphysema severity was highest in the right middle lobe (1.93 ± 0.36), followed by right upper (1.35 ± 2.50), left upper (1.34 ± 2.12), left lower (1.05 ± 2.52), and right lower (0.78 ± 2.28) lobes. The low severity group showed significantly longer overall survival compared to the high severity group (log‐rank test, P = 0.018). Quantitative emphysema severity of the whole lung (hazard ratio 1.36; 95% confidence interval 1.0–1.73) and stage III (hazard ratio 6.17; 95% confidence interval 1.52–25.0) were independent predictors of recurrence after adjusting for age, gender, smoking status, and forced expiratory volume in one second.
Conclusion
The severity of whole lung emphysema was independently associated with recurrence. Patients with non‐small cell lung cancer and marginal pulmonary emphysema at lower severity survive longer after curative‐intent surgery.
Keywords: Emphysema, non‐small cell lung cancer, recurrence, surgical resection, survival
Introduction
Pulmonary emphysema is defined as an abnormal and permanent enlargement of airspaces distal to the terminal bronchioles and the destruction of alveolar walls.1 Emphysema is one of the two major pathological changes associated with chronic obstructive pulmonary disease (COPD). Chronic airway inflammation caused by the inhalation of cigarette smoke and other noxious particles induces the narrowing of small airways and pulmonary emphysema, which lead to the development of COPD.2 Although a diagnosis of COPD is based on spirometry, pulmonary emphysema assessed by computed tomography (CT) can be used as an imaging biomarker of COPD. CT measurements of pulmonary emphysema are significantly associated with spirometric parameters, including forced expiratory volume in one second (FEV1) and the ratio of FEV1/forced vital capacity (FVC).3, 4 Assessments of emphysema aid the diagnosis of COPD and emphysema is a useful biomarker in the absence of spirometry.5 Furthermore, in the general population with a smoking history, the presence of emphysema on CT is an independent predictor for all‐cause mortality.6, 7
Pulmonary emphysema also implies diverse clinical implications in patients with lung cancer. The presence of radiographic emphysema is a significant factor related to lung cancer occurrence after adjustment for gender, age, pack‐years of smoking, and airflow obstruction.8 Increasing emphysema severity is associated with increasing tumor size.9 Higher regional emphysema severity is associated with the presence of lung cancer.10 Furthermore, using quantitative CT analysis, previous studies have shown an association between the presence of emphysema and prognosis in patients with lung cancer.11, 12, 13 The authors quantified the emphysema severity of the whole lung using CT‐based software. Patients were dichotomized according to the percentage ratio of emphysema volume/whole lung volume; cutoff values were diverse, ranging from 5% to 20%.
In the current study, we analyzed whether the numerical emphysema value as a continuous variable is a prognostic factor for recurrence and overall survival (OS) in patients with surgically resected non‐small cell lung cancer (NSCLC). An automated chest CT‐based program was used to measure the severity of emphysema in the whole lung and regional lobes, and the prognostic significance of each value was assessed. Furthermore, after dichotomizing the enrolled patients, we compared recurrence and OS between groups with emphysema of low and high severity.
Methods
Study patients
A total of 45 patients with newly diagnosed NSCLC treated with curative‐intent surgical resection from January 2013 to December 2014 at Gyeongsang National University Hospital, Jinju, South Korea, were recruited. The institutional review board of our institution approved this retrospective study. Medical records were reviewed and patients’ baseline characteristics, clinical information, and preoperative data, including age, gender, body mass index, pulmonary function, smoking status, pack‐years of smoking, and comorbid diseases, were collected. Spirometry was performed preoperatively according to the international guideline14 and spirometric values included FEV1, FVC, and the FEV1/FVC ratio. Tumor histology was divided into adenocarcinoma, squamous cell carcinoma, and NSCLC not otherwise specified. Pathologic staging was determined using the seventh edition American Joint Committee on Cancer Tumor Node Metastasis (TNM) Classification after surgery. The exclusion criteria were: (i) patients with small cell lung cancer; (ii) patients with metastatic lung cancer; (iii) inability to verify pulmonary function; and (iv) absence of emphysema on chest CT.
Chest computed tomography scan and emphysema quantification
All patients underwent contrast‐enhanced chest CT for diagnosis and clinical staging of lung cancer. Chest CT scans were obtained using 64‐channel multidetector CT scanners (Brilliance‐64; Philips Healthcare, Amsterdam, Netherlands) during a full‐inspiration breath‐hold using the following parameters: detector configuration of 64 × 0.625 mm, tube voltage of 120 kVp, tube current of 180–200 mAs, pitch of 0.923 or 0.987, and gantry rotation time of half a second. The data was reconstructed in 2 mm thick axial images with 1 mm overlap with a standard reconstruction kernel. An experienced chest radiologist who did not participate in the process of lobe segmentation or quantification of emphysema determined the location of the nodule or mass.
For quantification of emphysema, all chest CT scans were transferred to a workstation (IntelliSpace Portal 7.0), and pulmonary‐dedicated software (CT COPD) was used (Philips Healthcare, Cleveland, OH, USA). One radiologist blinded to the purpose of the study performed all image analyses. CT data were loaded first for the quantification procedure. Three‐dimensional (3D) results were displayed in each step. The borders of each lung, fissures, and central airways were detected automatically. First, the tracheobronchial tree up to the subsegmental level was identified. Both lungs were then differentiated from the surrounding chest wall and mediastinal structures. Lobe segmentation was performed to allow automated delineation for each of the five pulmonary lobes using an automatic lobe segmentation algorithm. When lobe segmentation was completed, axial, sagittal, coronal, and volume‐rendered images were displayed. A colored mask was superimposed onto the CT images using a different color for each lobe. The operator could evaluate if the automated lobar segmentation was adequate by scrolling through the multiplanar images. When lobar limits were not correct, they could be corrected with adjustment for interlobar boundaries in multiplanar images. Attenuation of each voxel within segmented lungs was computed automatically. Emphysema volume was calculated as the sum of voxels with attenuation below −950 Hounsfield units (HU). Lung volume, emphysema volume, and the percentage ratio between lung volume and emphysema volume in the lungs as a whole in each separate lung and in individual lobes were calculated. The percentage ratios between lung volume and emphysema volume in both lungs as a whole and in individual lobes are referred as “whole‐lung emphysema ratio” and “lobar emphysema ratio,” respectively.
Postoperative follow‐up
All patients were followed up at the outpatient department at three month intervals during the first two years after surgical resection, then at six month intervals by chest CT, and if necessary, 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET). Recurrence was determined by clinical assessment, chest CT, and other data, including tissue biopsy, bronchoscopy, and FDG‐PET. The date of death was identified by first medical record review, and if identification was not possible, data from the Korean Death Registry on 22 September 2017 were used.
Statistical analyses
A Pearson's chi‐squared test was used to compare categorical variables, and an unpaired t‐test was used to compare continuous variables. A Cox proportional hazard regression model was used to identify independent risk factors for recurrence and OS. Confounding variables including age, gender, smoking status, and FEV1 were selected on the basis of a previous study regarding the prognostic index of NSCLC and adjusted.15 Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated. The probability of recurrence and OS was calculated using the Kaplan–Meier method, and differences were assessed by the log‐rank test. The follow‐up interval for analysis of recurrence or OS was defined as date of diagnosis of lung cancer to the date of recurrence or death, or the date of the last outpatient visit. The optimal cutoff value of emphysema severity to predict lung cancer recurrence was determined by receiver operating curve curve analysis. A P value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
During the study period, 54 patients with lung cancer underwent surgical resection for curative intent in our hospital. After the exclusion of nine patients, 45 patients were included in the final analysis (Fig 1). The mean age of the patients was 68.0 ± 7.80 years and 36 (80%) patients were male (Table 1). The mean FEV1 was 2.26 ± 0.63 L, and the mean predicted value of FEV1 was 83.3 ± 17.1%. There were 12 (26.7%) current smokers, 20 (44.4%) former smokers, and 13 (28.9%) never‐smokers. The pathological stages included: 29 (64.4%) patients at stage I, 7 (15.6%) at stage II, and 9 (20.0%) at stage III. The histologic types were: 26 adenocarcinoma and 18 squamous cell carcinoma. COPD, followed by diabetes and hypertension were common comorbid diseases.
Figure 1.
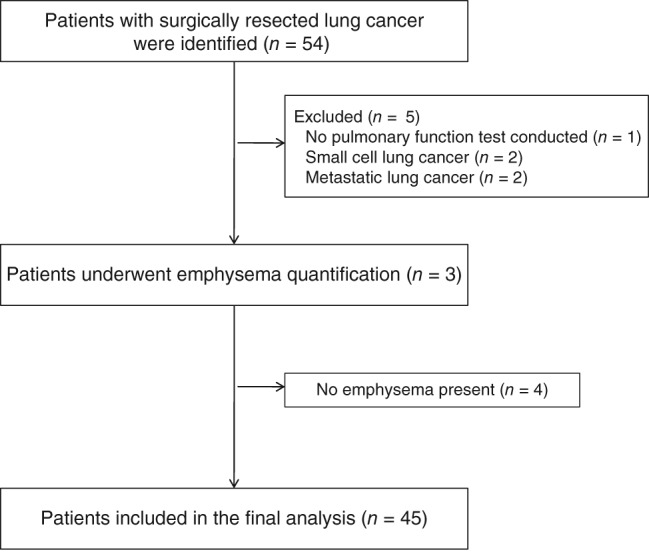
Flow chart of the study patients.
Table 1.
Baseline characteristics of the study sample
| Characteristic | All patients (n = 45) |
|---|---|
| Age, years | 68.0 ± 7.80 |
| Gender | |
| Male | 36 (80.0) |
| Female | 9 (20.0) |
| Body mass index, kg/m2 | 23.1 ± 2.83 |
| FEV1/FVC, % | 69.1 ± 10.7 |
| FEV1, L | 2.26 ± 0.63 |
| FEV1, % | 83.3 ± 17.1 |
| Smoking status | |
| Current smoker | 12 (26.7) |
| Former smoker | 20 (44.4) |
| Never‐smoker | 13 (28.9) |
| Pack‐years of smoking | 36.8 ± 18.4 |
| Tumor size (cm) | 3.12 ± 2.24 |
| SUVmax | 8.08 ± 4.28 |
| Status of regional lymph nodes | |
| 0 | 34 (75.6) |
| 1 | 5 (11.1) |
| 2 | 6 (13.3) |
| Lung cancer stage | |
| I | 29 (64.4) |
| II | 7 (15.6) |
| III | 9 (20.0) |
| Histology | |
| Adenocarcinoma | 26 (57.8) |
| Squamous cell | 18 (40.0) |
| NSCLC, NOS | 1 (2.2) |
| Total emphysema scores | 1.21 ± 2.04 |
| Comorbid disease | |
| COPD | 23 (51.1) |
| Hypertension | 20 (44.4) |
| Diabetes | 9 (20.0) |
| Cardiovascular disease | 2 (4.4) |
| ILD | 2 (4.4) |
Values are presented as mean ± standard deviation or N (%).
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ILD, interstitial lung disease; NOS, not otherwise specified; NSCLC, non‐small cell lung cancer; SUVmax, maximum standardized uptake value.
Severity of emphysema and location of lung cancer
The mean percentage ratio of emphysema volume/whole lung volume (mean whole‐lung emphysema ratio) was 1.21 ± 2.04. The mean emphysema ratio of the regional lobe was highest in the right middle lobe (1.93 ± 5.36), followed by the right upper (1.35 ± 2.50), left upper (1.34 ± 2.12), left lower (1.05 ± 2.52), and right lower (0.78 ± 2.28) lobes (Table 2). Lung cancer developed in the right lower (n = 12, 26.7%), left lower (n = 12, 26.7%), right upper (n = 10, 22.2%), left upper (n = 6, 13.3%), and right middle (n = 5, 11.1%) lobes (Table 2). The quantitative lobar emphysema ratio was not associated with the location of lung cancer development (data not shown).
Table 2.
Quantitative severity of lobar emphysema and lung cancer location
| Lobe | Emphysema ratio | Number of lung cancers in each lobe |
|---|---|---|
| Right upper | 1.35 ± 2.50 | 10 (22.2) |
| Right middle | 1.93 ± 5.36 | 5 (11.1) |
| Right lower | 0.78 ± 2.28 | 12 (26.7) |
| Left upper | 1.34 ± 2.12 | 6 (13.3) |
| Left lower | 1.05 ± 2.52 | 12 (26.7) |
Values are presented as mean ± standard deviation or N (%).
Comparison and predictive factors of recurrence‐free and overall survival
A whole‐lung emphysema ratio of 0.31 was considered as the cutoff value with maximal sensitivity and specificity for recurrence of lung cancer following receiver operating curve analysis. The sensitivity was 55.6% and the specificity was 63.0% (Fig 2). The median follow‐up interval from lung cancer diagnosis to the last outpatient visit or death was 45.8 months. The median recurrence‐free survival (RFS) was 30.1 months in the low severity emphysema group and 22.0 months in the high severity emphysema group (log‐rank test, P = 0.26) (Fig 3a). OS was significantly longer in the low severity emphysema group compared to the high severity emphysema group (log‐rank test, P = 0.018) (Fig 3b). Cox proportional hazards regression analysis revealed that the quantitative whole‐lung emphysema ratio (HR 1.36, 95% CI 1.06–1.73) and stage III (HR 6.17, 95% CI 1.52–25.0) were independently associated with recurrence after adjustment for age, gender, smoking status, and FEV1 (Table 3). However, Cox hazards regression analysis did not reveal any risk factors for OS (data not shown).
Figure 2.
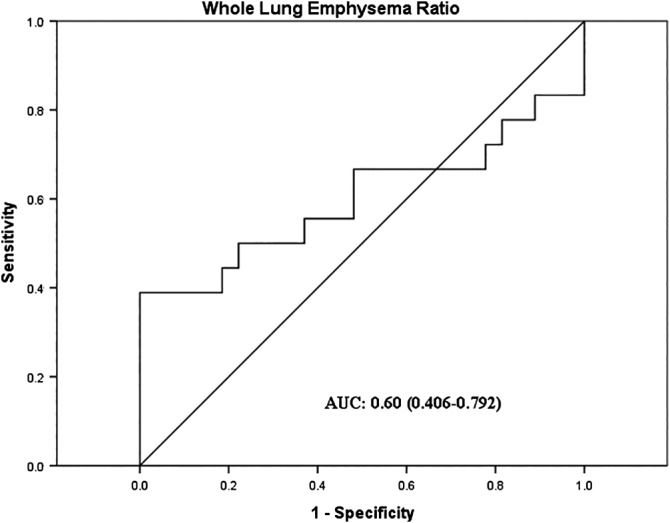
Receiver operator characteristic curve of whole‐lung emphysema ratio to predict lung cancer recurrence. AUC, area under the curve.
Figure 3.
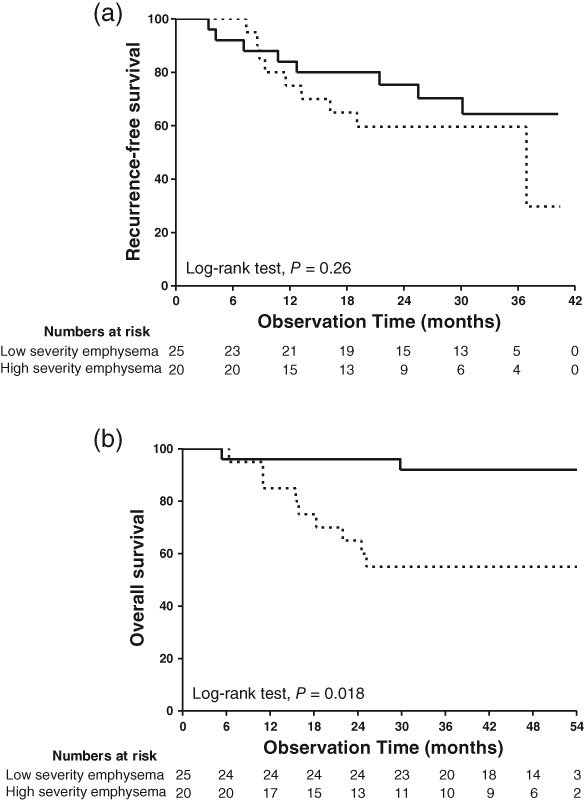
Kaplan–Meier curves for (a) recurrence and (b) overall survival. The patients were dichotomized into two groups according to the quantitative severity of emphysema. ( ) Low severity emphysema group, and (
) Low severity emphysema group, and ( ) High severity emphysema group.
) High severity emphysema group.
Table 3.
Cox proportional hazard model for risk of recurrence
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age, years | 0.97 | 0.92–1.03 | 0.288 |
| Gender | |||
| Female | Ref | ||
| Male | 0.42 | 0.07–2.51 | 0.343 |
| Lung cancer stage | |||
| I | Ref | ||
| II | 1.84 | 0.42–8.17 | 0.421 |
| III | 6.17 | 1.52–25.0 | 0.011 |
| FEV1, % | 1.03 | 0.99–1.06 | 0.144 |
| Smoking status | |||
| Never | Ref | ||
| Former | 0.58 | 0.13–2.69 | 0.487 |
| Current | 4.00 | 0.52–30.8 | 0.183 |
| Emphysema severity | 1.36 | 1.06–1.73 | 0.014 |
Body mass index; CI, confidence interval; FEV1, forced expiratory volume in one second; HR, hazard ratio; ref, reference category.
Discussion
The current study investigated the clinical significance of quantitative emphysema severity of the whole lung and regional lobes in patients with NSCLC who underwent surgical resection. The OS of patients with relatively low emphysema severity was significantly longer than in patients with high severity. Furthermore, the whole‐lung emphysema ratio and advanced lung cancer stage were independently associated with lung cancer recurrence. However, any clinical implications of the lobar emphysema ratio were not evident in this study.
The prediction of prognosis is an indispensable process for the optimal treatment of patients. Prognostic factors of NSCLC can be categorized as tumor‐related, patient‐related, and environmental factors.16 Among dozens of known prognostic factors, tumor stage (tumor‐related factors) and performance status (patient‐related factors) have the strongest impact on the prognosis of NSCLC patients.15 Advanced stage was the most significant factor to predict recurrence in this study. The presence of COPD and higher COPD grades were associated with poorer OS in patients with lung cancer after surgical resection.17, 18 Pulmonary emphysema detected on chest CT is a patient‐related factor and can be regarded as one of the comorbidities. Assessment of pulmonary emphysema can be performed either by automated quantitative CT analysis or visual evaluation. Visual assessment of pulmonary emphysema is subjective and there are some concerns regarding inter‐observer variation. Barr et al. reported a concordance rate between visual and quantitative analysis of emphysema of 75% and showed that the concordance rate was lower in milder COPD.19 On the contrary, automated quantitative CT analysis is an objective method. High reproducibility and accurate comparison can be achieved.20 Visual assessment tends to underestimate emphysema compared to automated quantitative measures in subjects with a low emphysema ratio.21 Thus, visual assessment is limited for evaluating the significance of pulmonary emphysema in patients with low emphysema severity.
Although half of this study population had COPD, the emphysema ratio of study patients was very low. Previous studies using quantitative CT analysis showed clinical significance of pulmonary emphysema in patients with lung cancer.11, 12, 13 Patients were dichotomized into emphysema and non‐emphysema groups according to the degree of whole lung emphysema; the cutoff value of emphysema degree was variable, ranging from 5% to 20%. An attenuation threshold of −910 HU to define pulmonary emphysema was used in previous studies compared to our threshold of −950 HU. A low threshold underestimates emphysema, while a high threshold overestimates emphysema.20 Although an attenuation threshold for the measurement of emphysema has not been established, a threshold of −950 HU has been suggested to correlate best with pathological emphysema.22 The mean whole‐lung emphysema ratio in this study was 1.21% and 42 out of 45 enrolled patients had a whole‐lung emphysema ratio < 5%. While it is uncertain why most of the enrolled patients had considerably low severity emphysema, this study demonstrates that the degree of pulmonary emphysema has prognostic significance, even in lung cancer patients with less severe emphysema.
The association between lobar emphysema ratio in each lobe and lung cancer was recently highlighted. Hohberger et al. reported that the location of lung cancer is associated with the severity of regional emphysema10 and Kinsey et al. showed that the lobar emphysema ratio is associated with size of lung cancer and OS.9 We also attempted to determine the clinical implications of the lobar emphysema ratio. Automatic lobe segmentation by fully automated software was performed, in contrast with the semi‐automatic segmentation used in previous studies.10, 23 Despite the technical merit of lobe segmentation and lobar emphysema measurement, the clinical significance of lobar emphysema severity was not evident in this study.
Some limitations need to be acknowledged. First, a small number of patients with lung cancer were enrolled. Because this study was conducted in a single referral hospital in South Korea, the number of patients undergoing curative‐intent surgery was small. Second, the follow‐up period after surgical resection was relatively short. The median follow‐up interval until the last outpatient visit or death was 45.8 months.
Despite these limitations, some advantages of this study include that patients’ lung function was measured and adjusted through Cox proportional hazard analysis and fully automated lobe segmentation and emphysema quantification were used. Accordingly, a small quantity of pulmonary emphysema of the whole lung and regional lobes could be measured and analyzed.
In conclusion, this study demonstrates that even a low degree of whole lung emphysema impacts on OS and is an independent prognostic factor for recurrence in patients with surgically resected NSCLC. Further studies including a large patient sample with high severity emphysema are required to verify these results.
Disclosure
No authors report any conflict of interest.
Contributor Information
Kyung Nyeo Jeon, Email: knjeon@gnu.ac.kr.
Jong Deog Lee, Email: ljd8611@empal.com.
References
- 1. Rennard SI. COPD: Overview of definitions, epidemiology, and factors influencing its development. Chest 1998; 113 (Suppl. 4): 235S–41S. [DOI] [PubMed] [Google Scholar]
- 2. Vestbo J, Hurd SS, Agusti AG et al Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–65. [DOI] [PubMed] [Google Scholar]
- 3. Schroeder JD, McKenzie AS, Zach JA et al Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 2013; 201: W460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie X, de Jong PA, Oudkerk M et al Morphological measurements in computed tomography correlate with airflow obstruction in chronic obstructive pulmonary disease: Systematic review and meta‐analysis. Eur Radiol 2012; 22: 2085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mets OM, Schmidt M, Buckens CF et al Diagnosis of chronic obstructive pulmonary disease in lung cancer screening computed tomography scans: Independent contribution of emphysema, air trapping and bronchial wall thickening. Respir Res 2013; 14: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johannessen A, Skorge TD, Bottai M et al Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med 2013; 187: 602–8. [DOI] [PubMed] [Google Scholar]
- 7. Oelsner EC, Hoffman EA, Folsom AR et al Association between emphysema‐like lung on cardiac computed tomography and mortality in persons without airflow obstruction: A cohort study. Ann Intern Med 2014; 161: 863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson DO, Weissfeld JL, Balkan A et al Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008; 178: 738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinsey CM, San Jose Estepar R, Wei Y, Washko GR, Christiani DC. Regional emphysema of a non‐small cell tumor is associated with larger tumors and decreased survival rates. Ann Am Thorac Soc 2015; 12: 1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hohberger LA, Schroeder DR, Bartholmai BJ et al Correlation of regional emphysema and lung cancer: A lung tissue research consortium‐based study. J Thorac Oncol 2014; 9: 639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gullon JA, Suarez I, Medina A, Rubinos G, Fernandez R, Gonzalez I. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer 2011; 71: 182–5. [DOI] [PubMed] [Google Scholar]
- 12. Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography‐diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early‐stage lung cancer. Clin Cancer Res 2006; 12: 6730–6. [DOI] [PubMed] [Google Scholar]
- 13. Ueda K, Murakami J, Sano F, Hayashi M, Suga K, Hamano K. Similar radiopathological features, but different postoperative recurrence rates, between stage I lung cancers arising in emphysematous lungs and those arising in nonemphysematous lungs. Eur J Cardiothorac Surg 2015; 47: 905–11. [DOI] [PubMed] [Google Scholar]
- 14. Miller MR, Hankinson J, Brusasco V et al Standardisation of spirometry. Eur Respir J 2005; 26: 319–38. [DOI] [PubMed] [Google Scholar]
- 15. Blanchon F, Grivaux M, Asselain B et al 4‐year mortality in patients with non‐small‐cell lung cancer: Development and validation of a prognostic index. Lancet Oncol 2006; 7: 829–36. [DOI] [PubMed] [Google Scholar]
- 16. Goldstraw P, Ball D, Jett JR et al Non‐small‐cell lung cancer. Lancet 2011; 378: 1727–40. [DOI] [PubMed] [Google Scholar]
- 17. Saji H, Miyazawa T, Sakai H et al Survival significance of coexisting chronic obstructive pulmonary disease in patients with early lung cancer after curative surgery. Thorac Cancer 2018; 9: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sekine Y, Suzuki H, Yamada Y, Koh E, Yoshino I. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg 2013; 61: 124–30. [DOI] [PubMed] [Google Scholar]
- 19. Barr RG, Berkowitz EA, Bigazzi F et al A combined pulmonary‐radiology workshop for visual evaluation of COPD: Study design, chest CT findings and concordance with quantitative evaluation. COPD 2012; 9: 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madani A, Keyzer C, Gevenois PA. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur Respir J 2001; 18: 720–30. [DOI] [PubMed] [Google Scholar]
- 21. Gietema HA, Muller NL, Fauerbach PV et al Quantifying the extent of emphysema: Factors associated with radiologists' estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 2011; 18: 661–71. [DOI] [PubMed] [Google Scholar]
- 22. Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995; 152: 653–7. [DOI] [PubMed] [Google Scholar]
- 23. Gierada DS, Guniganti P, Newman BJ et al Quantitative CT assessment of emphysema and airways in relation to lung cancer risk. Radiology 2011; 261: 950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


