Abstract
Background
In epidermal growth factor receptor (EGFR)‐mutant non‐small cell lung cancer (NSCLC), brain metastasis is known as a poor prognosis factor. However, prognostic factors in the patients without brain metastasis remain unclear. In this study, we aimed to clarify the differences between metastatic site and prognosis in common EGFR‐mutant NSCLC patients without brain metastasis.
Methods
Chemotherapy‐naïve, advanced EGFR‐mutant NSCLC patients without brain metastasis diagnosed between January 2010 and March 2016 were enrolled. We evaluated prognosis according to the presence or absence of bone metastases, liver metastasis, and pleural effusion.
Results
A total of 50 EGFR‐mutant NSCLC patients without brain metastasis were enrolled. The median progression‐free survival and overall survival were significantly shorter in patients with pleural effusion than in those patients without (progression‐free survival 7.0 months, 95% confidence interval [CI] 3.7–13.0 vs. 13.0 months, 95% CI 9.1–21.7, hazard ratio [HR] 2.29, 95% CI 1.11–4.73, P = 0.020; overall survival 19.5 months, 95% CI 5.7–28.8 vs. 55.3 months, 95% CI 24.0–not evaluable, HR 3.00, 95% CI 1.35–6.68, P = 0.005). Pleural effusion was an independent factor of poor prognosis for progression‐free survival (HR 3.44, 95% CI 1.50–7.88, P = 0.003) and overall survival (HR 2.34, 95% CI 1.00–5.44, P = 0.049).
Conclusion
Pleural effusion might be a poor prognosis factor for advanced EGFR‐mutant NSCLC patients without brain metastasis treated with first‐generation EGFR‐tyrosine kinase inhibitors. Further precision medicine according to the metastatic site is required.
Keywords: Epidermal growth factor receptor, metastasis, non‐small cell lung cancer, pleural effusion, prognosis factor
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide. Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases.1 Half of lung cancer patients will have several metastases at diagnosis.2 In recent years, several investigations have revealed the oncogenic mechanisms caused by driver gene mutations that are associated with lung cancer. Epidermal growth factor receptor gene (EGFR) mutation, such as exon 19 deletions or exon 21 point mutation (L858R), is one of the driver gene mutations that is present in approximately 50% of NSCLCs in the East Asian populations.3 These mutations increase the kinase activity of EGFR, leading to hyperactivation of downstream survival signaling pathways.4 In a previous study, >85% of EGFR‐mutant NSCLC patients had exon 19 deletion or exon 21 L858R detected,5 which are called “common EGFR mutations.”
NSCLC with common EGFR mutations markedly respond to first‐generation EGFR‐tyrosine kinase inhibitors (EGFR‐TKIs) compared with platinum‐based chemotherapy.6, 7 Despite EGFR‐TKIs being effective clinically, approximately 15% of patients have no survival benefit.6, 8 Although several prognosis factors have been reported, the metastatic site is one of the most important among these factors. Indeed, metastatic sites, such as the brain, bone, and liver, as well as pleural effusion, were reported as predictors of poor prognosis in advanced EGFR‐mutant NSCLC.9, 10, 11, 12, 13 Among these sites, the median overall survival (OS) was significantly shorter in patients with brain metastasis than in those without brain metastasis. Specifically, the presence of brain metastasis has been previously confirmed to be a prognosis factor in a multivariate analysis.9
Although several studies have assessed the correlation between metastatic site and the prognosis of EGFR‐mutant NSCLC patients,11, 12, 13 they often included patients with brain metastasis, which is an independent factor for poor prognosis.9 Therefore, limited information is available regarding the prognosis factor of EGFR‐mutant NSCLC patients without brain metastasis. In this study, we aimed to clarify the impact on prognosis according to the metastatic site in common EGFR‐mutant NSCLC patients without brain metastasis at diagnosis.
Methods
Patients
We retrospectively enrolled chemotherapy‐naïve advanced or postoperative‐recurrent NSCLC patients without brain metastasis who were diagnosed based on histological or cytological examination at the Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan, between January 2010 and March 2016. Distant metastatic sites were screened by positron emission tomography, computed tomography, and gadolinium‐enhanced magnetic resonance imaging for the brain at diagnosis. The eligibility criteria were: (i) patients with common EGFR mutations, such as exon 19 deletion or exon 21 L858R; (ii) patients treated with first‐generation EGFR‐TKI, such as gefitinib or erlotinib, as a first‐line therapy, until disease progression or unacceptable toxicities; and (iii) patients without brain metastasis confirmed by gadolinium‐enhanced magnetic resonance imaging at diagnosis.
Evaluation of patient characteristics
Clinical parameters, such as age, sex, histological subtype, stage, EGFR mutation status, metastatic sites, Eastern Cooperative Oncology Group performance status (PS), smoking status, progression‐free survival (PFS), and OS, were retrospectively collected from medical records. We examined metastatic sites, such as the bone and liver, and pleural effusion confirmed by computed tomography at diagnosis. The study protocol was approved by the ethics committees of our hospital (No. 744). TNM factor was classified using the 7th edition of the TNM stage classification system.
Statistical analysis
Cox proportional hazards modeling, which used several factors of patient profiles, was used. To analyze PFS, the time‐to‐event was estimated using the Kaplan–Meier method and compared by the log–rank test. PFS was defined as the period from the initiation date of EGFR‐TKI treatment to the date of disease progression or death. OS was defined as the period from the initiation date of EGFR‐TKI treatment to the date of death. Prognostic factors for OS were identified using univariate and multivariate logistic regression analyses. We performed the multivariate analysis based on significant factors identified in the univariate analysis in this present study. All statistical analyses were performed using EZR for Windows, version 1.35 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). All P‐values <0.05 were considered statistically significant.
Results
Patients’ characteristics
Of the 63 advanced or postoperative‐recurrent EGFR‐mutant NSCLC patients receiving first‐generation EGFR‐TKI during the study period, 13 patients were excluded because of the presence of brain metastasis. A total of 50 EGFR‐mutant NSCLC patients were analyzed in this study. The median age was 74 years (range 38–88 years), 29 patients (58.0%) were women, 36 (72.0%) were never smokers, and 46 (92.0%) had a good PS, 0 and 1. The histological subtype of most patients was adenocarcinoma (98.0%), and 25 patients (50.0%) had an exon 19 deletion. As shown in Table 1, 17 patients had bone metastases, four had liver metastases, and 13 had pleural effusion. A metastasis in multiple organs was detected in five patients. Of 17 patients with bone metastases, eight (47.1%) received palliative radiotherapy and two (11.8%) received bone‐modifying agent therapy.
Table 1.
Patients’ characteristics by metastatic site
| All patients | Bone metastasis | Liver metastasis | Pleural effusion | |
|---|---|---|---|---|
| n = 50 | n = 17 | n = 4 | n = 13 | |
| Patients’ characteristics | n (%) | n (%) | n (%) | n (%) |
| Age (years) | 74.0 (38.0–88.0) | 72.0 (38.0–83.0) | 71.0 (66.0–80.0) | 80.00 (38.0–88.0) |
| Sex | ||||
| Male | 21 (42.0) | 7 (41.2) | 2 (50.0) | 5 (38.5) |
| Female | 29 (58.0) | 10 (58.8) | 2 (50.0) | 8 (61.5) |
| Smoking status | ||||
| Smoker | 14 (28.0) | 6 (35.3) | 3 (75.0) | 2 (15.4) |
| Non‐smoker | 36 (72.0) | 11 (64.7) | 1 (25.0) | 11 (84.6) |
| Performance status | ||||
| 0 | 19 (38.0) | 5 (29.4) | 0 (0.0) | 1 (7.7) |
| 1 | 27 (54.0) | 11 (64.7) | 3 (75.0) | 9 (69.2) |
| 2 | 4 (8.0) | 1 (5.9) | 1 (25.0) | 3 (23.1) |
| Histological subtype | ||||
| Adenocarcinoma | 49 (98.0) | 17 (100.0) | 4 (100.0) | 12 (92.3) |
| Squamous cell carcinoma | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (7.7) |
| Stage | ||||
| IIIB | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IV | 34 (68.0) | 15 (88.2) | 4 (100.0) | 12 (92.3) |
| Postoperative recurrence | 15 (30.0) | 2 (11.8) | 0 (0.0) | 1 (7.7) |
| EGFR mutation status | ||||
| Exon 19 deletion | 25 (50.0) | 7 (41.2) | 3 (75.0) | 6 (46.2) |
| Exon 21 L858R | 25 (50.0) | 10 (58.8) | 1 (25.0) | 7 (53.8) |
| EGFR‐TKI | ||||
| Gefitinib | 47 (94.0) | 15 (88.2) | 4 (100.0) | 13 (100.0) |
| Erlotinib | 3 (6.0) | 2 (11.8) | 0 (0.0) | 0 (0.0) |
| No. metastatic organs | ||||
| 1 | 45 (90.0) | 15 (88.2) | 2 (50.0) | 13 (100.0) |
| ≥2 | 5 (10.0) | 2 (11.8) | 2 (50.0) | 0 (0.0) |
Patients’ characteristics with pleural effusion
In this study, 13 patients had pleural effusion. Among the 13 patients with pleural effusion, 10 patients acquired resistance to EGFR‐TKI during the period. Of them, seven patients developed disease progression in the pleural effusion, and three patients developed disease progression in the lung metastasis. There was no significant relationship between the presence and absence of pleural effusion in clinical features, including stage, liver metastasis, and bone metastasis (Table 2). There was only one EGFR‐mutant NSCLC patient with pleural effusion in whom an operation was performed; this was a 71‐years‐old woman who had had a right pneumonectomy 2 years previously who developed recurrence due to right malignant pleural effusion diagnosed by pleural effusion cytology. Therefore, we could not statistically analyze the relationship between the site of pleural effusion and the side of the lung among recurrent stage IV cases.
Table 2.
Relationship between the presence of pleural effusion and the associated clinical features
| Pleural effusion | |||
|---|---|---|---|
| Presence, n = 13 | Absence, n = 37 | ||
| Clinical features | n (%) | n (%) | P‐value |
| Stage | |||
| Stage IIIB or IV | 12 (92.3) | 23 (62.2) | 0.076 |
| Postoperative recurrence | 1 (7.7) | 14 (37.8) | |
| Liver metastasis | |||
| Absence | 11 (84.6) | 35 (94.6) | 0.275 |
| Presence | 2 (15.4) | 2 (5.4) | |
| Bone metastasis | |||
| Absence | 11 (84.6) | 22 (59.5) | 0.173 |
| Presence | 2 (15.4) | 15 (40.5) | |
OS and PFS
At the time of analysis, the median follow‐up time for EGFR‐mutant patients was 24.2 months (range 2.2–95.4 months). In all patients, the median PFS was 12.2 months (95% confidence interval [CI] 8.3–13.3 months), and the median OS was 32.5 months (95% CI 23.1–67.4 months; Fig 1). There was no significant difference in PFS and OS between patients with and without bone metastases (Fig 2a, Fig 3a). The median PFS was significantly shorter in patients with liver metastasis than in those without liver metastasis (12.9 months, [95% CI 8.4–16.6] vs. 5.1 months, 95% CI 2.3– not evaluable1 hazard ratio [HR] 4.42, 95% CI 1.45–13.46, P = 0.004; Fig 2b). There was no significant difference in OS between patients with and without liver metastases (Fig 3b). The median PFS was significantly shorter in patients with pleural effusion than in those without pleural effusion (pleural effusion: 13.0 months, 95% CI 9.1–21.7 vs. 7.0 months, 95% CI 3.7–13.0, HR 2.29, 95% CI 1.11–4.73, P = 0.020; Fig. 2c). The median OS was significantly shorter in patients with pleural effusion than in those without pleural effusion (19.5 months, 95% CI 5.7–28.8 vs. 55.3 months, 95% CI 24.0–NE; HR 3.00, 95% CI 1.35–6.68, P = 0.005; Fig. 3c).
Figure 1.
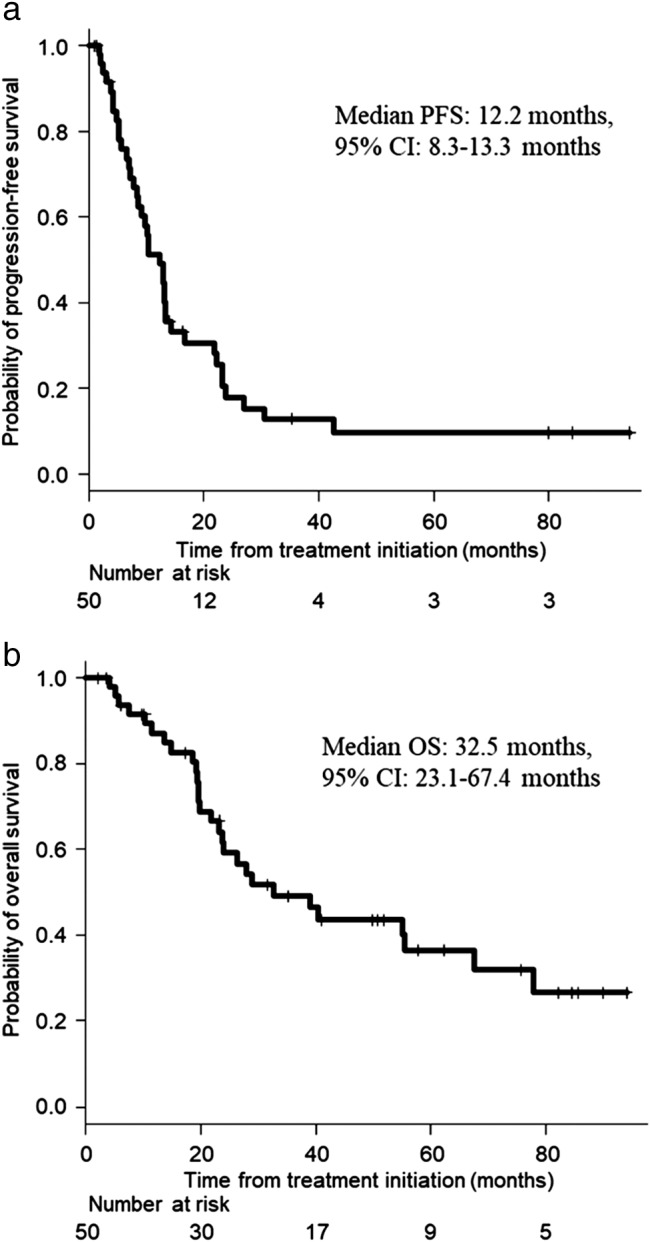
Kaplan–Meier curves for (a) progression‐free survival (PFS) and (b) overall survival (OS) in EGFR‐mutant non‐small cell lung cancer patients. In all patients (n = 50), the median PFS was 12.2 months (95% confidence interval [CI] 8.3–13.3 months), and the median OS was 32.5 months (95% CI 23.1–67.4 months).
Figure 2.
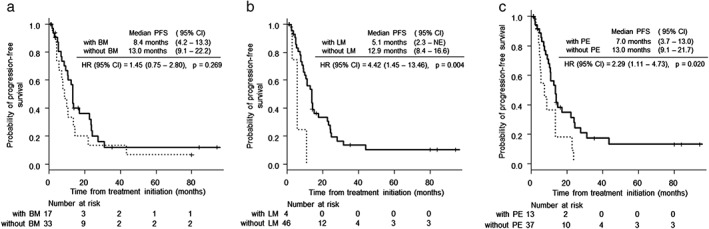
Kaplan–Meier curves for progression‐free survival (PFS) according to the metastatic site in EGFR‐mutant non‐small cell lung cancer patients. (a) There was no significant difference in PFS between patients with and without bone metastasis (BM; P = 0.269). () With BM and () without BM. (b) The median PFS was significantly longer in patients with liver metastasis (LM) than in those without LM (5.1 months, 95% confidence interval [CI] 2.3–not evaluable, vs. 12.9 months, 95% CI 8.4–16.6; hazard ratio [HR] 4.42, 95% CI 1.45–13.46, P = 0.004). () With LM and () without LM. (c) The median PFS was significantly longer in patients with pleural effusion (PE) than in those without PE (7.0 months, 95% CI 3.7–13.0 vs. 13.0 months, 95% CI 9.1–21.7; HR 2.29, 95% CI 1.11–4.73, P = 0.020). ( ) With PE and (
) With PE and ( ) without PE.
) without PE.
Figure 3.
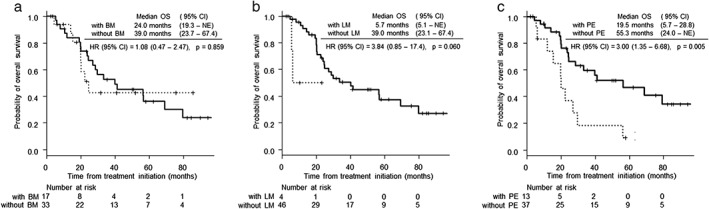
Kaplan–Meier curves for overall survival (OS) according to the metastatic site in EGFR‐mutant non‐small cell lung cancer patients. (a,b) There was no significant difference in OS between patients with and without bone metastasis (BM) and liver metastasis (LM; P = 0.859, 0.600, respectively). () With BM, () without BM, () with LM and () without LM. (c) The median OS was significantly longer in patients with pleural effusion (PE) than in those without PE (19.5 months, 95% confidence interval (CI) 5.7–28.8 vs. 55.3 months, 95% CI 24.0–not evaluable; hazard ratio 3.00, 95% CI 1.35–6.68, P = 0.005). ( ) With PE and (
) With PE and ( ) without PE.
) without PE.
As shown in Table 3, in the multivariate analysis, pleural effusion was an independent prognostic factor for poor PFS (HR 2.98, 95% CI 1.40–6.35, P = 0.005) and OS (HR 2.79, 95% CI 1.14–6.83, P = 0.025), although bone or liver metastases were not independent prognostic factors. Multiple metastatic sites was an independent prognostic factor for PFS (HR 12.60, 95% CI 2.18–72.96, P = 0.005),and poor OS (HR 4.94, 95% CI 1.23–19.87, P = 0.025).
Table 3.
Prognostic factors in EGFR‐mutant non‐small cell lung cancer patients without brain metastasis according to univariate and multivariate analyses
| Progression‐free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Variables | HR, mean (95% CI) | P‐value | HR, mean (95% CI) | P‐value | HR, mean (95% CI) | P‐value | HR, mean (95% CI) | P‐value |
| Age (<75/≥75 years) | 0.70 (0.37–1.34) | 0.281 | — | — | 1.40 (0.66–2.98) | 0.377 | — | — |
| Sex (male/female) | 1.32 (0.70–2.48) | 0.395 | — | — | 1.31 (0.62–2.78) | 0.482 | — | — |
| Performance status (0–1/2) | 1.91 (0.45–7.99) | 0.368 | — | — | 1.94 (0.86–4.36) | 0.104 | — | — |
| Smoking status (smoker/never smoker) | 1.84 (0.91–3.71) | 0.085 | — | — | 0.94 (0.22–4.00) | 0.938 | — | — |
| Histological subtype (adenocarcinoma/squamous cell carcinoma) | 0.17 (0.02–1.38) | 0.059 | — | — | 0.32 (0.04–2.43) | 0.242 | — | — |
| Stage (postoperative relapse/stage IIIB, IVA, IVB) | 0.50 (0.24–1.02) | 0.051 | — | — | 0.32 (0.13–0.83) | 0.014 | 0.49 (0.17–1.38) | 0.175 |
| EGFR mutation status (exon 19 deletion/Exon 21 L858R) | 0.84 (0.45–1.58) | 0.593 | — | — | 0.96 (0.45–2.02) | 0.907 | — | — |
| EGFR‐TKI (gefitinib/erlotinib) | 1.67 (0.40–6.98) | 0.478 | — | — | Not evaluable | 0.080 | — | — |
| No. metastatic organs (≥2/1) | 6.49 (2.31–18.24) | <0.001 | 12.60 (2.18–72.96) | 0.005 | 4.12 (1.14–14.95) | 0.020 | 4.94 (1.23–19.87) | 0.025 |
| Bone metastasis (positive/negative) | 1.45 (0.75–2.80) | 0.269 | — | — | 1.08 (0.47–2.47) | 0.859 | — | — |
| Liver metastasis (positive/negative) | 4.42 (1.45–13.46) | 0.004 | 0.66 (0.11–4.05) | 0.656 | 3.84 (0.85–17.4) | 0.060 | — | — |
| Pleural effusion (positive/negative) | 2.29 (1.11–4.73) | 0.020 | 2.98 (1.40–6.35) | 0.005 | 3.00 (1.35–6.68) | 0.005 | 2.79 (1.14–6.83) | 0.025 |
Discussion
In this study, our multivariate analysis identified pleural effusion and the multiple metastatic organs associated with poorer PFS and OS in EGFR‐mutant NSCLC without brain metastasis.
Correspondingly, previous reports showed that the presence of pleural effusion was a significant poor prognosis factor in EGFR‐mutant NSCLC patients.14, 15 Pleural effusion was reported to be formed through vascular endothelial growth factor (VEGF) activity, which is associated with vascular hyperpermeability.16, 17, 18 In addition, the overexpression of VEGF receptors in tumors reduces the sensitivity to EGFR‐TKI.19 From these findings, EGFR‐TKI has little effect on EGFR‐mutant NSCLC patients with pleural effusion, and the combination therapy of anti‐VEGF therapy and EGFR‐TKI might be theoretically promising for the treatment of EGFR‐mutant NSCLC patients with pleural effusion. Indeed, several clinical trials showed that the combination therapy with erlotinib plus bevacizumab resulted in significantly longer PFS than EGFR‐TKI therapy alone in EGFR‐mutant NSCLC patients, including patients with pleural effusion.20, 21, 22 In addition, the previous study reported that the high levels of VEGF expression in NSCLC tissue were identified as an independent poor prognostic factor.23 Because VEGF levels in malignant pleural effusion caused by lung cancer were significantly higher than VEGF levels in benign exudative pleural effusion,24 EGFR‐mutant NSCLC patients with pleural effusion may have had a worse survival rate, consistent with our study result.
Because patients with brain metastasis were excluded from this study, our study results were different from those of previous studies. In particular, bone metastasis in EGFR‐mutant NSCLC, which was a poor prognosis factor in several reports, did not indicate a poor prognosis in this study.12, 15 Patients with bone metastasis might have prolonged survival due to radiotherapy and bone‐modifying agent therapy.25, 26 However, in the present study, not all patients with bone metastasis received these treatments, and there was no significant difference in prognosis between patients who received these treatments and those who did not (data not shown). As a result, these treatments might have little effect on prognosis in the present study. First‐generation EGFR‐TKI has a limited ability to penetrate the blood–brain barrier; the ratio of first‐generation EGFR‐TKI transformation to the brain was reported to be 1–3%.27 Therefore, the prognosis of EGFR‐mutant NSCLC patients with brain metastasis was considerably poor, as evidenced by the low response rate of 43% in the clinical trial.28 EGFR‐mutant NSCLC patients have a significantly higher incidence of brain and bone metastases.11, 22, 28, 29 Although the proportion of patients with bone and brain metastases were not described in these studies, 92.3% of patients with brain metastases also had bone metastases in the present study. Therefore, bone metastasis might be a factor of poor prognosis, because most EGFR‐mutant NSCLC patients might have both brain and bone metastases at diagnosis.
There were several limitations to our study. This study was a retrospective study conducted at a single institution with a small number of patients. In fact, although previous reports showed PS was the independent prognostic factor,30, 31 PS was not the prognostic factor in this study. However, metastasis to multiple organs was an independent poor prognostic factor in this study, which was consistent with the previous study.32 Therefore, further multi‐institutional studies are required to confirm our study results.
In conclusion, pleural effusion in advanced EGFR‐mutant NSCLC patients without brain metastasis was associated with a poorer PFS and OS. Further precision medicine according to the metastatic site is required.
Disclosure
No author report any conflict of interest.
References
- 1. Govindan R, Page N, Morgensztern D et al Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24: 4539–44. [DOI] [PubMed] [Google Scholar]
- 2. Kocher F, Hilbe W, Seeber A et al Longitudinal analysis of 2293 NSCLC patients: A comprehensive study from the TYROL registry. Lung Cancer 2015; 87: 193–200. [DOI] [PubMed] [Google Scholar]
- 3. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small‐cell lung cancer: Meta‐analyses by ethnicity and histology (mutMap). Ann Oncol 2013; 24: 2371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib‐sensitizing EGFR mutations in lung cancer activate anti‐apoptotic pathways. Science 2004; 305: 1163–7. [DOI] [PubMed] [Google Scholar]
- 5. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer 2006; 118: 257–62. [DOI] [PubMed] [Google Scholar]
- 6. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 7. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 8. Fukuoka M, Wu YL, Thongprasert S et al Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29: 2866–74. [DOI] [PubMed] [Google Scholar]
- 9. Lin JJ, Cardarella S, Lydon CA et al Five‐year survival in EGFR‐mutant metastatic lung adenocarcinoma treated with EGFR‐TKIs. J Thorac Oncol 2016; 11: 556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noronha V, Joshi A, Gokarn A et al The importance of brain metastasis in EGFR mutation positive NSCLC patients. Chemother Res Pract 2014; 2014: 856156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu KL, Tsai MJ, Yang CJ et al Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first‐line gefitinib. Lung Cancer 2015; 88: 187–94. [DOI] [PubMed] [Google Scholar]
- 12. Fujimoto D, Ueda H, Shimizu R et al Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: Importance of bone metastasis. Clin Exp Metastasis 2014; 31: 543–51. [DOI] [PubMed] [Google Scholar]
- 13. Wu SG, Yu CJ, Tsai MF et al Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 2013; 41: 1409–18. [DOI] [PubMed] [Google Scholar]
- 14. Wang TF, Chu SC, Lee JJ et al Presence of pleural effusion is associated with a poor prognosis in patients with epidermal growth factor receptor‐mutated lung cancer receiving tyrosine kinase inhibitors as first‐line treatment. Asia Pac J Clin Oncol 2017; 13: 304–13. [DOI] [PubMed] [Google Scholar]
- 15. Taniguchi Y, Tamiya A, Nakahama K et al Impact of metastatic status on the prognosis of EGFR mutation‐positive non‐small cell lung cancer patients treated with first‐generation EGFR‐tyrosine kinase inhibitors. Oncol Lett 2017; 14: 7589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sack U, Hoffmann M, Zhao XJ et al Vascular endothelial growth factor in pleural effusions of different origin. Eur Respir J 2005; 25: 600–4. [DOI] [PubMed] [Google Scholar]
- 17. Jain RK. Molecular regulation of vessel maturation. Nat Med 2003; 9: 685–93. [DOI] [PubMed] [Google Scholar]
- 18. Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653–60. [DOI] [PubMed] [Google Scholar]
- 19. Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015; 5: 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ichihara E, Hotta K, Nogami N et al Phase II trial of gefitinib in combination with bevacizumab as first‐line therapy for advanced non‐small cell lung cancer with activating EGFR gene mutations: The Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 2015; 10: 486–91. [DOI] [PubMed] [Google Scholar]
- 21. Seto T, Kato T, Nishio M et al Erlotinib alone or with bevacizumab as first‐line therapy in patients with advanced non‐squamous non‐small‐cell lung cancer harbouring EGFR mutations (JO25567): An open‐label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15: 1236–44. [DOI] [PubMed] [Google Scholar]
- 22. Furuya N, Fukuhara T, Saito H et al. Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR‐mutations: NEJ026. ASCO Meeting Abstact, Chicago, Illinois 2018; 36: 9006.
- 23. Lin Q, Guo L, Lin G et al Clinical and prognostic significance of OPN and VEGF expression in patients with non‐small‐cell lung cancer. Cancer Epidemiol 2015; 39: 539–44. [DOI] [PubMed] [Google Scholar]
- 24. Yanagawa H, Takeuchi E, Suzuki Y, Ohmoto Y, Bando H, Sone S. Vascular endothelial growth factor in malignant pleural effusion associated with lung cancer. Cancer Immunol Immunother 1999; 48: 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chou YC, Lin CY, Pai PC et al Dose‐escalated radiation therapy is associated with better overall survival in patients with bone metastases from solid tumors: A propensity score‐matched study. Cancer Med 2017; 6: 2087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scagliotti GV, Hirsh V, Siena S et al Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: Subgroup analysis from a randomized phase 3 study. J Thorac Oncol 2012; 7: 1823–9. [DOI] [PubMed] [Google Scholar]
- 27. Togashi Y, Masago K, Masuda S et al Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non‐small cell lung cancer. Cancer Chemother Pharmacol 2012; 70: 399–405. [DOI] [PubMed] [Google Scholar]
- 28. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378: 113–25. [DOI] [PubMed] [Google Scholar]
- 29. Sekine A, Kato T, Hagiwara E et al Metastatic brain tumors from non‐small cell lung cancer with EGFR mutations: Distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer 2012; 77: 64–9. [DOI] [PubMed] [Google Scholar]
- 30. Inoue A, Yoshida K, Morita S et al Characteristics and overall survival of EGFR mutation‐positive non‐small cell lung cancer treated with EGFR tyrosine kinase inhibitors: A retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol 2016; 46: 462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao D, Chen X, Qin N et al The prognostic role of EGFR‐TKIs for patients with advanced non‐small cell lung cancer. Sci Rep 2017; 7: 40374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eberhardt WE, Mitchell A, Crowley J et al The IASLC lung cancer staging project: Proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2015; 10: 1515–22. [DOI] [PubMed] [Google Scholar]


