Abstract
Background
Scutellarin (SCU), a flavonoid isolated from Erigeron breviscapus (Vant.) Hand.‐Mazz., increases autophagy and apoptosis in the adenocarcinoma A549 cell line, which is resistant to cisplatin. However, whether SCU alone has antitumor activity against non‐small cell lung cancer (NSCLC) is unknown.
Methods
Cell Counting Kit‐8, flow cytometry, colony formation, Hoechst 33258 staining, and Western blot analyses were used to examine the proliferation and apoptosis of A549 cells treated with SCU and the possible molecular mechanisms.
Results
The cell viability assay indicated that SCU markedly suppressed the proliferation of A549 cells in concentration and time‐dependent manners. SCU caused significant G0/G1 phase arrest and apoptosis, as evidenced by flow cytometric analyses, Hoechst 33258 staining, and Western blot analyses of cyclin D1, cyclin E, BCL‐2, cleaved‐caspase‐3, and BAX. Furthermore, SCU treatment reduced the level of pan‐AKT, phosphorylated (p)‐mTOR, mTOR, BCL‐XL, STAT3, and p‐STAT3, and increased the level of 4EBP1.
Conclusions
SCU can suppress proliferation and promote apoptosis in A549 cells through AKT/mTOR/4EBP1 and STAT3 pathways. This suggests that SCU may be developed into a promising antitumor agent for treating NSCLC.
Keywords: Apoptosis, cell cycle arrest, non‐small cell lung cancer, scutellarin, STAT3
Introduction
Lung cancer is the leading cause of cancer‐related death, accounting for 25%, although its incidence is decreasing about twice as fast in men as in women.1 Lung cancer is difficult to detect at an early stage, thus numerous patients suffering from lung cancer are only identified with advanced disease.2 Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of the pathological types of lung cancer.3 In recent years, treatment strategies for lung cancer have been limited to surgery, chemotherapy, and radiotherapy. Although much effort has been expended to diagnose and treat lung cancer, the five‐year survival rate remains < 15%.4
The phosphatidylinositol 3‐kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway plays a diverse role in differentiation, adhesion, proliferation, cell survival, motility, and invasion. AKT can be activated by PI3K and then stimulate the mTOR protein.5, 6 A downstream funnel, 4E‐BP1 can be activated by the PI3K/AKT/mTOR pathway and is pivotal for protein synthesis, as dephosphorylated active 4E‐BP1 binds to eIF4E and stops formation of the cap‐dependent translation initiation complex.7, 8 Roh et al. reported that the presence of a high level of phosphorylated (p)‐4E‐BP1 was indicative of poor prognosis in small cell lung cancer.9 Large numbers of potential therapeutics targeting this signaling cascade have been developed, such as perifosine that appears to block AKT‐mediated signaling, thereby suppressing proliferation and inducing apoptosis in NSCLC cells.10 Thus, we suspected that scutellarin (SCU) might be a pathway inhibitor to restrict the growth of NSCLC cells.
STAT3 is recognized as a key target in the progression of various malignancies.11 Aberrantly active STAT3 promotes tumorigenesis, drug resistance, and metastasis in NSCLC.12 Downstream signaling effects of the STAT3 pathway can contribute to lung cancer and currently several STAT3 inhibitors show substantial anticancer and antiangiogenic effects.13, 14 Finding novel agents with STAT3 suppressive activity is vitally important to improve NSCLC treatment.
Substantial evidence has confirmed that SCU, a flavonoid isolated from Erigeron breviscapus (Vant.) Hand.‐Mazz., has antitumor effects on several cancers, including liver, colorectal, breast, and prostate, as well as lymphomas.15, 16, 17, 18, 19 However, little is known about its antitumor mechanisms and whether SCU can inhibit NSCLC when used alone. In the present study, we explored the effects of different concentrations of SCU on proliferation, apoptosis, and tumorigenesis, and the expression of AKT/mTOR/4E‐BP1 and STAT3 signaling pathway proteins in cultured A549 lung cancer cells.
Methods
Chemicals and reagents
Antibodies against caspase‐3, BAX, BCL‐2, cyclin D1, cyclin E, pan‐AKT, 4EBP1, mTOR, p‐mTOR, total (t)‐STAT3, p‐STAT3, BCL‐XL, and β‐actin were obtained from Abcam (Cambridge, MA, USA). Annexin V‐fluorescein isothiocyanate (FITC) and the propidium iodide Apoptosis Detection Kit were purchased from BD Biosciences (San Jose, CA, USA). Immobilon Western Chemiluminescent HRP Substrate was obtained from EMD Millipore (Billerica, MA, USA), F12K medium from M&C Technology Company (Beijing, China), fetal bovine serum from Lonsa Science Srl (Canelones, Uruguay) and SCU (> 98%) powder was purchased from MedChemExpress (Monmouth Junction, NJ, USA). The chemical structure of SCU is shown in Figure 1a.
Figure 1.
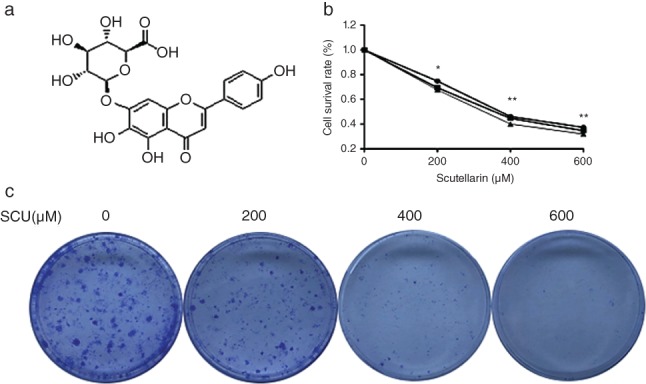
Scutellarin (SCU) suppresses proliferation in non‐small cell lung cancer (NSCLC) A549 cells. (a) The chemical structure of SCU. (b) A549 cells were treated with SCU (0, 200, 400, 600 μM) for 12, 24 and 48 hours. Cell viability was detected by Cell Counting Kit‐8 assay. (c) The proliferative effect of SCU on A549 cells detected by colony formation assay. Data are expressed as means ± standard deviation from three independent experiments. *
P < 0.05, **
P < 0.01 vs. the control group. ( ) 12 hours, (
) 12 hours, ( ) 24 hours and (
) 24 hours and ( ) 48 hours.
) 48 hours.
Cell culture and treatment
A549 cells, an NSCLC cell line, were purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology (Shanghai, China) and cultured in F12K medium supplemented with 10% fetal bovine serum and 1% penicillin‐streptomycin at 37°C with 5% CO2.
Cell viability assay
A549 cells in the logarithmic growth phase were seeded (4 × 103 cells/well) into 96‐well plates at a final volume of 200 μL/well. Different concentrations of SCU (0, 200, 400, 600 μM) were added to the plates. After treatment for 12, 24, and 48 hours, 10 μL of the Cell Counting Kit‐8 (Dojindo, Tokyo, Japan) reagent was added to each well and cultured for another three hours. Absorbance at 450 nm was measured by a multiwell spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Cell cycle analysis by flow cytometry
Based on the cell viability assay results, A549 cells were treated with 0, 200, 400, or 600 μM SCU for 24 hours and then collected and mixed in 70% ethanol at −20°C overnight. After washing twice with phosphate‐buffered saline (PBS) and staining with 400 μL propidium iodide in the presence of 100 μL RNase A for 60 minutes in the dark, the samples were analyzed with a FACSAria flow cytometer (BD Biosciences).
Apoptosis assay
An Annexin V/FITC apoptosis detection kit was used to determine apoptotic cells. In brief, after treatment with 0, 200, 400, or 600 μM SCU for 24 hours, cells were collected, washed twice with ice‐cold PBS, mixed with 200 μL binding buffer and then stained with 5 μL propidium iodide and 5 μL Annexin V/FITC. Fifteen minutes later, the samples were evaluated by flow cytometry.
Colony formation assay
After treatment with 0, 200, 400, or 600 μM SCU for 24 hours, A549 cells were plated into six‐well plates (600 cells/well) and cultured for 14 days in F12K medium containing 30% fetal bovine serum and 1% penicillin‐streptomycin at 37°C with 5% CO2. Paraformaldehyde (4%) was used to fix the colonies for 60 minutes. Cells were stained with crystal violet for 15 minutes and counted.
Hoechst 33258 staining
A549 cells were plated into six‐well plates (5 × 104 cells/well) and treated with 0, 200, 400, or 600 μM SCU for 24 hours. Hoechst 33258 (Solarbio Science & Technology, Beijing, China) was then added into the plate and the cells were incubated for 20 minutes. After washing three times with PBS, the samples were observed under a fluorescence microscope.
Western blot analysis
After treatment with 0, 200, 400, or 600 μM SCU for 24 hours, total protein was obtained by lysing the cells in radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China) containing 1% protease inhibitor cocktail and phenylmethylsulfonyl fluoride. Proteins were separated by 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (EMD Millipore). After blocking in 5% non‐fat milk for one hour, the membranes were incubated with primary antibodies against total (t)‐STAT3, p‐STAT3, pan‐AKT, mTOR, p‐mTOR, BCL‐XL, BCL‐2, cyclin D1, BAX cyclin E, cleaved caspase‐3, eIF4EBP1, and β‐actin. The membranes were then treated with anti‐rabbit or anti‐mouse immunoglobulin G horseradish peroxidase‐conjugated secondary antibodies (Beyotime) for one hour at room temperature. Immunoreactive bands were visualized using Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore). Densitometric analyses were conducted using custom Image J‐based software (NIH, Bethesda, MD, USA).
Statistical analysis
All data are expressed as means ± standard error of the mean. Comparisons between two groups were performed using Prism 6.0 (GraphPad Software, San Diego, CA, USA) with a Student’s t‐test. A P value < 0.05 was considered statistically significant.
Results
Scutellarin (SCU) inhibits A549 cell proliferation
To explore the effect of SCU on A549 cell proliferation, CCK‐8 assay was performed (Fig 1b). The results showed that SCU treatment significantly suppressed the proliferation of A549 cells in time and concentration‐dependent manners (P < 0.05). The long‐term growth inhibitory effect of SCU on A549 cell proliferation was examined by colony formation assay. These results showed that SCU markedly inhibited colony formation in a concentration‐dependent manner (Fig 1c).
SCU induces G0/G1 arrest
To assess the effect of SCU treatment on cell cycle progression, A549 cells were treated with 0, 200, 400, or 600 μM SCU for 24 hours and the percentages of cells in each stage were determined by flow cytometry (Fig 2). The results showed that cells in the G0/G1 phase increased from 63.51% to 87.78%. Furthermore, levels of cyclins E and D1, the G0/G1 cell cycle regulatory proteins, decreased in response to SCU treatment (Fig 3).
Figure 2.
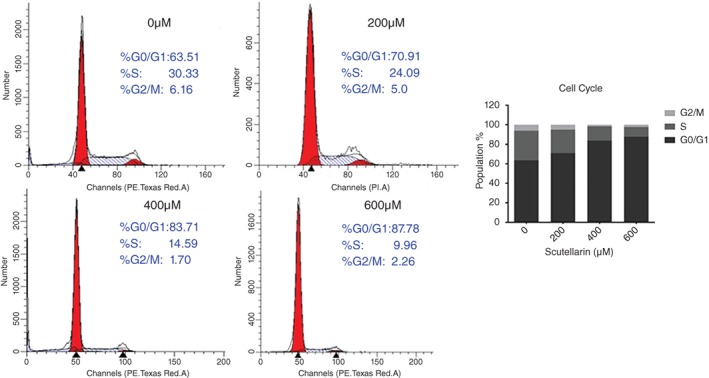
Scutellarin (SCU) induces A549 cell G0/G1 cell cycle arrest. Cells were treated with SCU (0, 200, 400, 600 μM) for 24 hours and the cell cycle was detected by flow cytometry. ( ) G2/M, (
) G2/M, ( ) S and (
) S and ( ) G0/G1.
) G0/G1.
Figure 3.
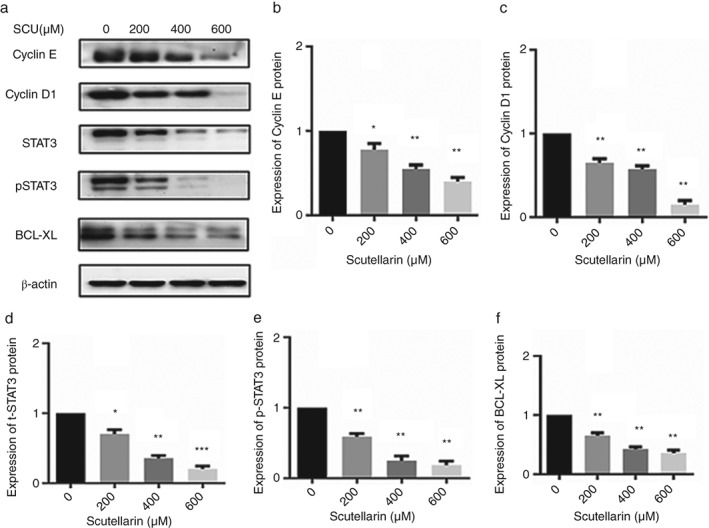
A549 cells were treated with scutellarin (SCU) (0, 200, 400, 600 μM) for 24 hours. Western blotting analyses were used to detect the expression of proteins in the STAT3 pathways, cyclin D1, and cyclin E (a). Data are expressed as means ± standard deviation from three independent experiments. * P < 0.05, ** P < 0.01 vs. the control group (b, c, d, e, f).
SCU induces A549 cell apoptosis
To explore the antitumor mechanism and to evaluate whether the growth repressive effect of SCU was related to apoptosis, we treated A549 cells with 0, 200, 400, and 600 μM SCU for 24 hours, and then added Hoechst 33258 and observed the cells under a fluorescence microscope (Fig 4a). Increasing numbers of Hoechst 33258‐positive cells were observed in a concentration‐dependent manner. This indicated that SCU may induce A549 cell apoptosis. To confirm the presence of apoptosis, flow cytometry with Annexin V/FITC staining was used (Fig 4b). The results showed that the percentage of apoptotic cells significantly increased with SCU treatment compared to the control group.
Figure 4.
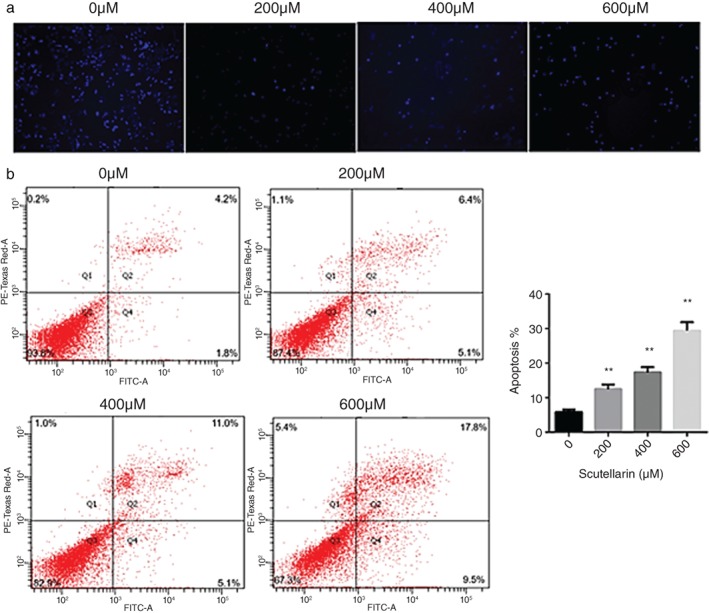
Scutellarin (SCU) treatment induces A549 cell apoptosis. (a) Morphology of nuclei in A549 cells treated with SCU (0, 200, 400, 600 μM), stained by Hoechst 33258, and observed under a fluorescence microscope (magnification, ×10). (b) Cells were treated with SCU (0, 200, 400, 600 μM) for 24 hours. The proportion of apoptotic cells increased significantly in a concentration‐dependent manner. Data are expressed as means ± standard deviation from three independent experiments. * P < 0.05, ** P < 0.01 vs. the control group. FITC fluorescein isothiocyanate.
To explore the molecular mechanism underlying the induction of apoptosis in A549 cells treated with SCU, Western blot assays were performed. The results showed that expression of the anti‐apoptotic BCL‐2 protein was downregulated, while the pro‐apoptotic BAX protein was upregulated, in concentration‐dependent manners. Furthermore, exposure to SCU for 24 hours induced cleavage of caspase‐3 (Fig 5). These findings suggested that SCU induced apoptosis of A549 cells through the pathway activated by cleavage of caspase‐3 and an increased BAX/BCL‐2 ratio.
Figure 5.
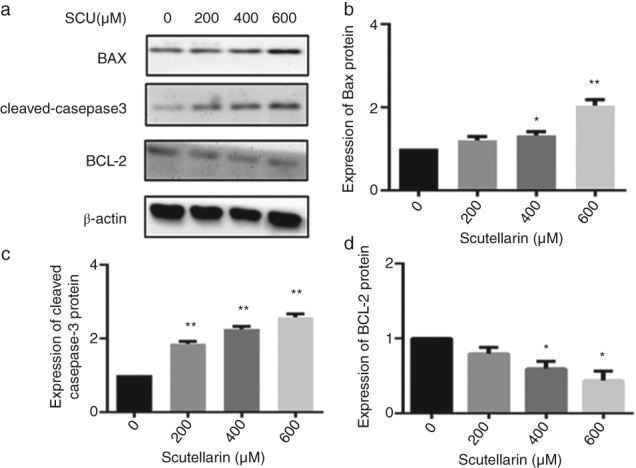
A549 cells were treated with scutellarin (SCU) (0, 200, 400, 600 μM) for 24 hours. Western blotting analyses were used to detect the expression of proteins in BCL‐2, BAX, and cleaved‐caspase‐3 (a). Data are expressed as means ± standard deviation from three independent experiments. * P < 0.05, ** P < 0.01 vs. the control group (b, c, d).
SCU inhibited the AKT/mTOR/4EBP1 and STAT3 signaling pathways
To further explore the regulatory mechanism of apoptosis induced by SCU, proteins in the AKT/mTOR/4EBP1 and STAT3 signaling pathways were examined by Western blot. SCU significantly reduced the levels of pan‐AKT, mTOR, p‐mTOR, t‐STAT3, p‐STAT3, BCL‐XL proteins in a concentration‐dependent manner, while the 4EBP1 protein level was increased (Figs 3, 6). This suggested that SCU inhibited the AKT/mTOR/4EBP1 and STAT3 pathways to exert its effects on A549 cells.
Figure 6.
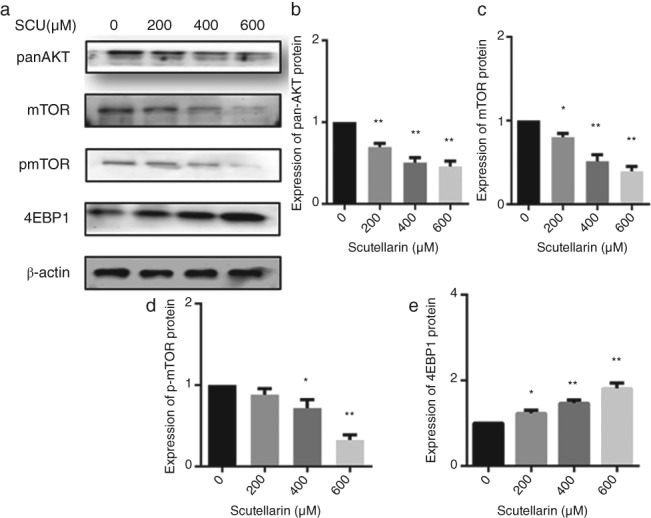
A549 cells were treated with scutellarin (SCU) (0, 200, 400, 600 μM) for 24 hours. Western blotting analyses were used to detect the expression of proteins in the AKT/mTOR/4EBP1 pathways (a). Data are expressed as means ± standard deviation from three independent experiments. * P < 0.05, ** P < 0.01 vs. the control group (b, c, d, e).
Discussion
SCU exhibits activity against several cancer cells.20, 21 Sun et al. reported that SCU enhanced cisplatin‐induces apoptosis and autophagy via the extracellular signal‐regulated kinase (ERK)/P53 or c‐met/AKT signaling pathways.22 This group also revealed that SCU induces apoptosis and autophagy in NSCLC cells through ERK1/2 and AKT signaling pathways in vitro and in vivo.23 Deng et al. showed that SCU inhibits human renal cancer cell proliferation and migration via upregulation of phosphatase and tensin homolog,24 while Ke et al. reported that SCU effectively inhibits hepatocellular carcinoma metastasis in vivo and the migration and invasion of hepatocellular carcinoma cells in vitro.21 Other researchers have reported that SCU induces apoptosis and G2/M arrest, inhibits cell proliferation of prostate cancer PC3 cells, and sensitizes the cells to chemotherapy.16
In this study, we first investigated the toxicity of SCU to A549 lung adenocarcinoma cells. Using CCK‐8 and colony formation assays, our results show that SCU effectively suppresses the proliferation of A549 cells. At the same time, treatment with SCU promotes A549 cell apoptosis and arrest in the G0/G1 phase in concentration‐dependent manners. Furthermore, the BAX/BCL‐2 ratio and cleaved (active) caspase‐3 were increased, and SCU activated the AKT/mTOR /4EBP1 and STAT3 pathways.
Apoptosis is a controlled form of cell death that can lead to various morphological changes, such as nuclear pyknosis, cell surface changes, and chromosome condensation.25, 26 We examined morphological changes by Hoechst 33258 staining and show that A549 cells treated with SCU display specific apoptotic changes in a concentration‐dependent manner. Flow cytometry was used to externalize phosphatidylserine (cell surface changes) in A549 cells and our results show that the percentage of apoptotic cells is markedly increased in a concentration‐dependent manner. Decreasing the level of the anti‐apoptotic BCL‐2 protein and/or increasing the level of the pro‐apoptotic BAX protein plays an important role in disrupting mitochondrial homeostasis, which can lead to apoptotic cell death.27, 28 Thus, the BAX/BCL‐2 ratio is a key regulator of apoptosis in lung cancer.29 Furthermore, caspase‐3, a member of the cysteine protease family, catalyzes crucial steps in apoptotic cell death.27 In this study, SCU increased the BAX/BCL‐2 ratio and the amount of cleaved caspase‐3 in A549 cells.
Cyclins D1 and E are important regulators of cyclin‐dependent kinases that are involved in controlling the G0/G1 checkpoint and proliferation of cancer cells. Some anticancer drugs induce G0/G1 arrest by decreasing the levels of cyclins D1 and E.30, 31 Our results revealed that A549 cells treated with SCU underwent G0/G1 arrest, which was mainly the result of the downregulation of cyclin D1 and E expression.
STAT3, a member of the STAT family, is involved in oncogenic transformation and progression in some cancer types, including lung cancer.11, 12 The antiapoptotic protein, BCL‐XL, is a key protein downstream of the STAT3 signaling pathway.18 In the current study, we found that t‐STAT3, p‐STAT3, and BCL‐XL were significantly decreased in A549 cells treated with SCU in concentration‐dependent manners. This shows that the STAT3 oncogenic pathway contributes to SCU‐induced apoptosis of A549 cells.
The AKT/mTOR pathway is a major anti‐apoptotic pathway that controls cell differentiation, survival, and malignant transformation. Inhibition of this pathway is been regarded as a potential anti‐cancer therapy. mTOR contains two distinct multiprotein signaling complexes: mTOR complex(C)1 and mTORC2. AKT can indirectly activate mTORC1.5, 6, 10 4E‐BP1 is a negative regulator of protein translation in mammalian cells and can be inactivated by p‐mTOR, which promotes its dissociation from eIF‐4E. Dephosphorylated 4EBP1 combines with eIF‐4E and inhibits the initiation of translation in the presence of mTOR inhibitors.7, 8, 9 Our results show that SCU suppresses pan‐AKT, mTOR, and p‐mTOR, and increases 4EBP1 significantly, indicating that the anticancer effects of SCU might be linked to inactivation of the AKT/mTOR/4EBP1 pathway.
Overall, we identified that SCU inhibits proliferation of A549 cells, and induces G0/G1 phase arrest and apoptosis via the AKT/mTOR /4EBP1 and STAT3 pathways. These findings provide underlying molecular and cellular evidence that SCU may be a promising anticancer drug.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (61671276), Natural Science Foundation of Shandong Province (ZR2014HM050, ZR2018PH032, ZR2018PH033) and Clinical Medical Science and Technology Innovation Program of Jinan (201503042).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Postmus PE, Kerr KM, Oudkerk M et al Early and locally advanced non‐small‐cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017; 28: iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 3. Reck M, Rabe KF. Precision diagnosis and treatment for advanced non‐small‐cell lung cancer. N Engl J Med 2017; 377: 849–61. [DOI] [PubMed] [Google Scholar]
- 4. Han K, Jin C, Chen H, Wang P, Yu M, Ding K. Structural characterization and anti‐A549 lung cancer cells bioactivity of a polysaccharide from Houttuynia cordata. Int J Biol Macromol 2018; 120: 288–96. [DOI] [PubMed] [Google Scholar]
- 5. Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 2014; 90: 197–207. [DOI] [PubMed] [Google Scholar]
- 6. Beck JT, Ismail A, Tolomeo C. Targeting the phosphatidylinositol 3‐kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: An emerging treatment strategy for squamous cell lung carcinoma. Cancer Treat Rev 2014; 40: 980–9. [DOI] [PubMed] [Google Scholar]
- 7. Kim YY, Von Weymarn L, Larsson O et al Eukaryotic initiation factor 4E binding protein family of proteins: Sentinels at a translational control checkpoint in lung tumor defense. Cancer Res 2009; 69: 8455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Musa J, Orth MF, Dallmayer M et al Eukaryotic initiation factor 4E‐binding protein 1 (4E‐BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 2016; 35: 4675–88. [DOI] [PubMed] [Google Scholar]
- 9. Roh MS, Lee JH, Kang KW et al Phosphorylated 4E‐binding protein 1 expression is associated with poor prognosis in small‐cell lung cancer. Virchows Arch 2015; 467: 667–73. [DOI] [PubMed] [Google Scholar]
- 10. Heavey S, O’Byrne KJ, Gately K. Strategies for co‐targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat Rev 2014; 40: 445–56. [DOI] [PubMed] [Google Scholar]
- 11. Tang JC, Ren YG, Zhao J, Long F, Chen JY, Jiang Z. Shikonin enhances sensitization of gefitinib against wild‐type EGFR non‐small cell lung cancer via inhibition PKM2/stat3/cyclinD1 signal pathway. Life Sci 2018; 204: 71–7. [DOI] [PubMed] [Google Scholar]
- 12. Maryam A, Mehmood T, Yan Q, Li Y, Khan M, Ma T. Proscillaridin A promotes oxidative stress and ER stress, inhibits STAT3 activation, and induces apoptosis in A549 lung adenocarcinoma cells. Oxid Med Cell Longev 2018; 2018: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo H, Kuang S, Song QL, Liu M, Sun XX, Yu Q. Cucurbitacin I inhibits STAT3, but enhances STAT1 signaling in human cancer cells in vitro through disrupting actin filaments. Acta Pharmacol Sin 2018; 39: 425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Njatcha C, Farooqui M, Kornberg A, Johnson DE, Grandis JR, Siegfried JM. STAT3 cyclic decoy demonstrates robust antitumor effects in non‐small cell lung cancer. Mol Cancer Ther 2018; 17: 1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng Y, Zhang S, Tu J et al Novel function of scutellarin in inhibiting cell proliferation and inducing cell apoptosis of human Burkitt lymphoma Namalwa cells. Leuk Lymphoma 2012; 53: 2456–64. [DOI] [PubMed] [Google Scholar]
- 16. Gao C, Zhou Y, Jiang Z et al Cytotoxic and chemosensitization effects of Scutellarin from traditional Chinese herb Scutellaria altissima L. in human prostate cancer cells. Oncol Rep 2017; 38: 1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou L, Chen L, Fang L. Scutellarin inhibits proliferation, invasion, and tumorigenicity in human breast cancer cells by regulating HIPPO‐YAP signaling pathway. Med Sci Monit 2017; 23: 5130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Zhang S. Scutellarin‐induced apoptosis in HepG2 hepatocellular carcinoma cells via a STAT3 pathway. Phytother Res 2013; 27: 1524–8. [DOI] [PubMed] [Google Scholar]
- 19. Yang N, Zhao Y, Wang Z, Liu Y, Zhang Y. Scutellarin suppresses growth and causes apoptosis of human colorectal cancer cells by regulating the p53 pathway. Mol Med Rep 2017; 15: 929–35. [DOI] [PubMed] [Google Scholar]
- 20. Chan JY, BKH T, Lee SC. Scutellarin sensitizes drug‐evoked colon cancer cell apoptosis through enhanced caspase‐6 activation. Anticancer Res 2009; 29: 3043–8. [PubMed] [Google Scholar]
- 21. Ke Y, Bao T, Wu X et al Scutellarin suppresses migration and invasion of human hepatocellular carcinoma by inhibiting the STAT3/Girdin/Akt activity. Biochem Biophys Res Commun 2017; 483: 509–15. [DOI] [PubMed] [Google Scholar]
- 22. Sun CY, Zhu Y, Li XF et al Scutellarin increases cisplatin‐induced apoptosis and autophagy to overcome cisplatin resistance in non‐small cell lung cancer via ERK/p53 and c‐met/AKT signaling pathways. Front Pharmacol 2018; 9: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun C, Li C, Li X et al Scutellarin induces apoptosis and autophagy in NSCLC cells through ERK1/2 and AKT Signaling Pathways in vitro and in vivo. J Cancer 2018; 9: 3247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng W, Han W, Fan T et al Scutellarin inhibits human renal cancer cell proliferation and migration via upregulation of PTEN. Biomed Pharmacother 2018; 107: 1505–13. [DOI] [PubMed] [Google Scholar]
- 25. Xing SF, Liu LH, Zu ML et al The inhibitory effect of gypenoside stereoisomers, gypenoside L and gypenoside LI, isolated from Gynostemma pentaphyllum on the growth of human lung cancer A549 cells. J Ethnopharmacol 2018; 219: 161–72. [DOI] [PubMed] [Google Scholar]
- 26. Guo CL, Wang LJ, Zhao Y et al A novel bromophenol derivative BOS‐102 induces cell cycle arrest and apoptosis in human A549 lung cancer cells via ros‐mediated PI3K/Akt and the MAPK signaling pathway. Mar Drugs 2018; 16: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan Y, Ye C, Tian Q et al miR‐145 suppresses the proliferation, invasion and migration of NSCLC cells by regulating the BAX/BCL‐2 ratio and the caspase‐3 cascade. Oncol Lett 2018; 15: 4337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Liu F, Jiang S et al Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non‐small cell lung cancer via the suppression of the MMP2 and Bcl‐2/Bax signaling pathways. Oncol Lett 2018; 15: 7409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu S, Gong LS, Li NF, Pan YF, Zhang L. Galangin (GG) combined with cisplatin (DDP) to suppress human lung cancer by inhibition of STAT3‐regulated NF‐kappaB and Bcl‐2/Bax signaling pathways. Biomed Pharmacother 2018; 97: 213–24. [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Liu Y, Yan L et al Increased levels of the long noncoding RNA, HOXA‐AS3, promote proliferation of A549 cells. Cell Death Dis 2018; 9: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Liu H, Wang J et al Curcumol induces cell cycle arrest and apoptosis by inhibiting IGF‐1R/PI3K/Akt signaling pathway in human nasopharyngeal carcinoma CNE‐2 cells. Phytother Res 2018; 32: 2214–25. [DOI] [PubMed] [Google Scholar]


