Abstract
Background
Diabetes mellitus (DM) is one of the most common comorbidities in surgically treated non‐small cell lung cancer (NSCLC) patients and has a negative impact on short‐term outcomes. However, the impact of DM on long‐term survival of such patients remains controversial; therefore, we conducted a comprehensive updated meta‐analysis.
Methods
We systematically searched relevant studies in PubMed, Embase, Cochrane Library, and Web of Science up to 6 September 2018. Hazard ratios (HRs) for the impact of DM on overall survival (OS) and recurrence‐free survival (RFS) of patients with surgically treated NSCLC were extracted and analyzed using the STATA 12.0 package.
Results
We included 13 cohort studies consisting of 4343 patients (730 patients with DM and 3613 patients without) with surgically treated NSCLC. Meta‐analysis showed that patients with DM had significantly poorer OS (random effects: HR 1.30, 95% confidence interval 1.05–1.60; P = 0.016) than those without. However, with a limited sample size, there was no significant difference in RFS (random effects: HR 1.06, 95% confidence interval 0.71–1.58; P = 0.786) between patients with and without DM after surgical resection of NSCLC.
Conclusion
DM is an independent unfavorable prognostic factor for patients with surgically treated NSCLC. High‐quality studies with appropriate adjustment for confounding factors are needed to confirm our conclusions.
Keywords: Diabetes mellitus, lung cancer, meta‐analysis, prognosis, surgery
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide and non‐small cell lung cancer (NSCLC) accounts for approximately 85%.1, 2 Lung cancer has a very poor prognosis, with a five‐year relative survival rate of 16%.3 Currently, surgery remains the preferred option for treating resectable NSCLC. In patients with surgically resected NSCLC, in addition to tumor characteristics, such as tumor size and lymph node metastasis, comorbidities are also reported to have an impact on prognosis.4 Diabetes mellitus (DM), one of the most common comorbidities in NSCLC patients,5 is an independent risk factor for developing NSCLC.6 The unfavorable impact of DM on short‐term outcomes of patients with surgically treated NSCLC has been well established.5, 7 However, no definitive conclusion has been made as to the impact of DM on long‐term survival in such patients. Some studies have reported that patients with DM have significantly poorer overall survival (OS) and recurrence‐free survival (RFS) than those without,8, 9, 10 while others found that DM had no significant impact on the prognosis of surgically treated NSCLC patients.11, 12, 13 A previous meta‐analysis of three studies published up to 2013 explored the impact of DM on surgically treated NSCLC patients;8, 12, 13 however, because of the limited sample size no statistically significant impact was observed.14 Since 2013, more and more studies investigating the impact of DM on the survival of patients with surgically treated NSCLC have been published.9, 15, 16, 17 The previous meta‐analysis did not examine the impact of DM on RFS of patients with surgically treated NSCLC, which we deem important in order to draw a relatively objective conclusion about the impact of DM on long‐term prognosis of patients with surgically treated NSCLC. Therefore, we pooled the updated data and conducted a meta‐analysis to explore the actual impact of DM on survival and recurrence in surgically treated NSCLC patients. To our knowledge, this is the most comprehensive meta‐analysis to focus on this topic.
Methods
Search strategies
We conducted a systematic search of PubMed, Embase, Cochrane Library, and Web of Science to identify the relevant studies published to 6 September 2018, using the following search terms: (diabetes OR diabetes mellitus OR hyperglycemia OR high blood glucose OR DM) AND (non‐small cell lung cancer OR nonsmall cell lung cancer OR NSCLC) AND (surgery OR surgical OR resection) AND (survival OR prognosis OR prognostic OR recurrence). We also scanned all reference lists from the studies selected by electronic searching to identify further relevant studies.
Study selections
The study inclusion criteria were as follows: (i) either observational studies or randomized controlled trials (RCTs) comparing survival between patients with and without DM after surgical resection of NSCLC; (ii) sufficient data could be obtained for retrieving hazard ratios (HRs) for OS or RFS; and (iii) if the studies were conducted on the same overlapping patients, the completed or most recent study was included. The exclusion criteria were: (i) studies including patients with small cell lung cancer or patients who were not surgically treated; (ii) studies published in non‐English language; (iii) studies without any relevant data extracted for analysis; and (iv) reviews, case reports, conference abstracts, and experiments.
Data extraction and quality assessment
Two authors extracted data from the included studies and compared the results independently. Discrepancy was resolved by the third author's adjudication to avoid bias. Data were carefully retrieved from full articles by using a standardized data collection form, which consisted of first author, country and year of publication, number of patients, disease stage, age, follow‐up time, and study type. The outcomes for analysis in our meta‐analysis consisted of HRs of OS and RFS. The Jadad scale was used for quality assessment of RCTs,18 while quality assessment and risk‐of‐bias analysis of observational studies was evaluated using the Newcastle–Ottawa Scale (NOS), as previously described,19 which consisted of three factors: patient selection, comparability of the study groups, and assessment of outcome. We assigned a score of 0–9 (allocated as stars) to each study after careful evaluation, with a high quality study defined as a study with a quality score of no less than seven. The name of the first author and year of publication were used for identification in our analysis.
Statistical analysis
We performed all analyses using the STATA 12.0 package (StataCorp, College Station, TX, USA) in accordance to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines.20 HRs of OS and RFS of patients with DM compared to those without with a 95% confidence interval (CI) were extracted from the original studies to evaluate the impact of DM on survival of patients with surgically treated NSCLC. When the HR was not directly reported in the original study, it was estimated as demonstrated by Parmar et al. by using five‐year OS or RFS rates extracted directly from the text or Kaplan–Meier curve.21 The between‐study heterogeneity of each study was assessed using χ2‐based Q statistics and I 2 tests. If high heterogeneity (P < 0.10 or I 2 > 50%) was observed, random effects models were applied; otherwise, fixed effects models were used. We conducted sensitivity analysis by sequential removal of each study. We also used a funnel plot to estimate potential publication bias, the asymmetry of which was tested by Begg's and Egger's tests.22 We set the statistical significance at P < 0.05.
Results
Description of studies
A flow chart showing the process of study evaluation is shown in Figure 1. A total of 791 papers were identified in the initial search. After evaluation, 18 papers qualified for detailed evaluation. A previous meta‐analysis and a cohort study were excluded because they did not focus on surgically treated patients.14, 23 Another study was excluded because no relevant data could be extracted to analyze the impact of DM.5 There were three studies by the same research group based on overlapping patients; therefore, only the study with the most complete data was included.4, 10, 24 Finally, 13 retrospective cohort studies but no RCTs were included, with a total of 4343 patients (730 patients with DM and 3613 patients without).8, 9, 10, 11, 12, 13, 15, 16, 17, 25, 26, 27, 28 The main data extracted from these included studies is listed in Table 1. Almost all of the patients had localized resectable disease and all had undergone relatively long follow‐up. The survival data analyzed in these included studies consisted of OS and RFS. The HRs of OS could be obtained directly from five studies and estimated with five‐year OS rates from another five studies, while the HRs of RFS could be obtained directly from four studies and estimated with five‐year RFS rates from two studies (Table 2).
Figure 1.
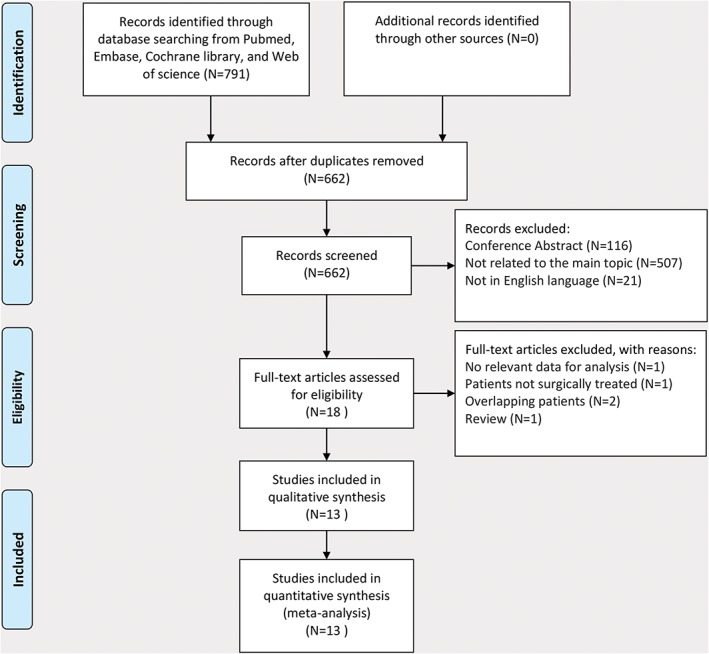
Preferred Reporting Items for Systematic Reviews (PRISMA) flow diagram showing the progress of studies through the review.
Table 1.
Characteristics of the included studies
| Author | Country | Patients | Age (range, years) | Follow‐up | Sample size (n) | DM (n) | Non‐DM (n) | Study design | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Dominguez‐Ventura et al.11 | United States | Pathologic stage I–IV (majority stage I–II) | Median: 82 (80–94) | Median: 2.2 years (range: 1 month–13.6 years) | 288 | 16 | 272 | Cohort study | NOS: 7 stars |
| Win et al.8 | United Kingdom | Pathologic stage I–IIIA | Mean: 69 (42–85) | All patients had at least 3 years and up to 5 years follow‐up | 110 | 12 | 98 | Cohort study | NOS: 7 stars |
| Bartling et al.12 | Germany | Majority T1‐4N0‐1; only 4 had distant metastasis | Median: 68 for DM and 66 for non‐DM group (51–80) | 60 months | 166 | 55 | 111 | Cohort study | NOS: 7 stars |
| Varlotto et al.10 | United States | Stage I–IIIB (majority stage I) | Median: 68 (38–96) | Median: 33 months (range: 3–98) | 537 | 96 | 441 | Cohort study | NOS: 7 stars |
| Fan et al.25 | China | Pathologic T1–3N1M0 | NA | Median: 53.8 months (range: 1.4–81.8) | 199 | 15 | 184 | Cohort study | NOS: 7 stars |
| Nakazawa et al.13 | Japan | NA | NA | NA | 388 | 69 | 319 | Cohort study | NOS: 6 stars |
| Washington et al.26 | United States | Pathological stage I–IIIA | Median: 67 (21–92) | Median: 30 months (range: 1–149) | 957 | 122 | 835 | Cohort study | NOS: 9 stars |
| Dhillon et al.15 | United States | Pathologic stage I | Mean: 68.5 (21–93) | Median: 44 months | 409 | 71 | 338 | Cohort study | NOS: 7 stars |
| Jeon et al.27 | Korea | Pathologic stage I | NA | Median: approximately 40 months | 211 | 29 | 182 | Cohort study | NOS: 6 stars |
| Kuo et al.28 | Taiwan, China | Pathologic stage I | Mean: 63.9 | More than 3 months for each patient | 181 | 48 | 133 | Cohort study | NOS: 6 stars |
| Jeon et al.9 | Korea | Pathologic stage I and II | Median: 64 (32–81) | Median: 40 months | 271 | 42 | 229 | Cohort study | NOS: 6 stars |
| Medairos et al.16 | United States | Pathologic stage I and II | Mean: 69.0 | Median: 19.5 months | 158 | 81 | 77 | Cohort study | NOS: 6 stars |
| Motoishi et al.17 | Japan | Pathological stage I–IIIB | Mean: 70.1 (44–88) | Median: 1136 days (range: 11–3598) | 468 | 74 | 394 | Cohort study | NOS: 7 stars |
DM, diabetes mellitus; NA, not available; NOS, Newcastle‐Ottawa Scale.
Table 2.
Main outcomes extracted from included studies
| Overall survival | Recurrence‐free survival | ||||
|---|---|---|---|---|---|
| Author | Comparison | HR | 95% CI | HR | 95% CI |
| Dominguez‐Ventura et al.11 | DM vs. non‐DM | 1.03‡ | 0.57–1.85 | NA | NA |
| Win et al.8 | DM vs. non‐DM | 2.12 | 1.02–4.38 | NA | NA |
| Bartling et al.12 | DM vs. non‐DM | 1.16‡ | 0.78–1.74 | NA | NA |
| Varlotto et al.10 | DM vs. non‐DM | NA | NA | 2.04 | 1.36–3.06 |
| Fan et al.25 | DM vs. non‐DM | NA | NA | 0.85‡ | 0.26–2.75 |
| Nakazawa et al.13 | DM vs. non‐DM | 1.45‡ | 0.98–2.15 | NA | NA |
| Washington et al.26 | DM vs. non‐DM | 1.08 | 0.80–1.44 | 1.33 | 0.74–2.40 |
| Dhillon et al.15 | DM vs. non‐DM | 1.07‡ | 0.73–1.56 | NA | NA |
| Jeon et al.27 | DM vs. non‐DM | 2.07 | 0.87–4.92 | NA | NA |
| Kuo et al.28 | DM vs. non‐DM | NA | NA | 0.98 | 0.64–1.53 |
| Jeon et al.9 | DM vs. non‐DM | 3.76 | 1.69–8.33 | NA | NA |
| Medairos et al.16 | DM vs. non‐DM | 0.47 | 0.16–1.36 | 0.47 | 0.22–0.89 |
| Motoishi et al.17 | DM vs. non‐DM | 1.23‡ | 0.81–1.86 | 0.94‡ | 0.62–1.43 |
Hazard ratios (HRs) for overall and Recurrence‐free survival were estimated using the five‐year rates, as demonstrated by Parmar et al.21 CI, confidence interval; DM, diabetes mellitus; NA, not available.
Quality assessment and risk of bias
Because all of the included studies were cohort studies, quality assessment and risk‐of‐bias analysis were evaluated using NOS. Quality assessment of the included cohort studies is listed in Table 1. Eight studies were ranked as high quality, while five studies were ranked as low quality, which suggested a potential risk of bias.
Meta‐analysis of the impact of diabetes mellitus on the survival of surgically treated non‐small cell lung cancer patients
Ten studies, including a total of 3426 patients, reported the impact of DM on OS of patients with surgically treated NSCLC. Meta‐analysis showed that patients with DM had significantly shorter OS than those without after surgical resection of NSCLC (random effects: HR 1.30, 95% CI 1.05–1.60; P = 0.016; I 2 = 47.1%) (Fig 2a). Six studies, including a total of 2500 patients, reported the impact of DM on RFS of patients with surgically treated NSCLC. Meta‐analysis showed that there was no significant difference in RFS between patients with and without DM (random effects: HR 1.06, 95% CI 0.71–1.58; P = 0.786; I 2 = 68.5%) (Fig 2b). However, potential heterogeneities were observed during analysis.
Figure 2.
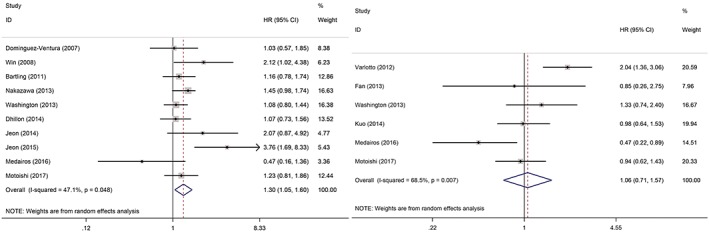
Forest plots of (a) overall and (b) recurrence‐free survival. CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio.
Sensitivity analysis and publication bias
We conducted sensitivity analysis by sequentially removing each study to evaluate the stability of our results based on OS and RFS. Sequential removal of each study did not change the outcomes of primary analysis (Fig 3). Publication bias was tested with a funnel plot to analyze OS, which had a symmetrical appearance (Begg's test: P = 0.421; Egger's test: P = 0.490), suggesting no significant publication bias (Fig 4).
Figure 3.
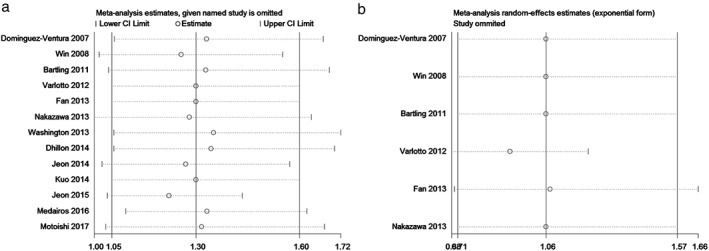
Sensitivity analysis for (a) overall and (b) recurrence‐free survival. CI, confidence interval.
Figure 4.
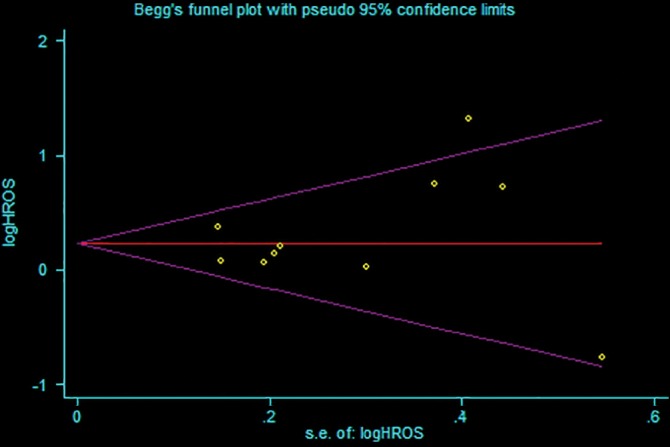
Funnel plot of the included studies for analysis of overall survival. Begg's test: P = 0.421; Egger's test: P = 0.490. HROS, hazard ratio of overall survival; SE, standard error.
Discussion
DM has become a major global health problem causing significant morbidity and mortality, which was estimated at a prevalence of 6.4% in 2010 and is expected to increase to 7.7% in 2030.29 In China, the prevalence of DM is as high as 9.1%, which causes significant disease burdens.30 DM is an independent risk factor in the development of lung cancer6 and is also reported to have a negative impact on the short‐term outcomes of patients with surgically treated NSCLC.5, 7, 26 However, opinions over the impact of DM on long‐term prognosis of patients with surgically treated NSCLC are mixed. A previous meta‐analysis found that DM had no significant impact on the survival of these patients (HR 1.71, 95% CI 0.94–3.08; P = 0.08); however a limited sample was included.14 Therefore, in order to draw a relatively reliable conclusion about the impact of DM on the prognosis of patients with surgically treated NSCLC, we conducted an updated meta‐analysis by pooling all available evidence.
We included 13 studies with a total of 4400 patients with surgically treated NSCLC. Ten studies were included to analyze OS and our results showed that patients with DM had significantly poorer OS than those without (HR 1.30, 95% CI 1.05–1.60; P = 0.016). However, only six studies were included in RFS analysis, and no significant difference in RFS between patients with and without DM after surgical resection of NSCLC was observed (HR 1.06, 95% CI 0.71–1.58; P = 0.786). Regarding the impact of DM on the prognosis of surgically treated NSCLC patients, previous studies have drawn controversial conclusions. Some studies found that DM was significantly correlated with worse OS and RFS in patients with surgically treated NSCLC,8, 9, 10 while others found no significant difference.11, 12, 13 However, it should be noted that almost all previous studies included a very limited sample of patients with DM,8, 9, 10, 11, 12, 13, 15, 16, 17, 25, 26, 27, 28 with only one study including a sample of over 100 DM patients.26 A previous meta‐analysis with a sample of 146 DM patients found that patients with DM tended to have a poor prognosis compared to those without, with marginal statistical significance.14 A larger sample of DM patients may reveal a significant difference in survival between the groups. As a result, we included a total of 730 patients with DM and our study proved that patients with DM had significantly poorer OS than those without after surgical resection of NSCLC. However, similarly, because of a limited sample to analyze RFS, no sufficient evidence of a significant difference in RFS between patients with and without DM was observed. DM is also independently correlated with poor prognosis in patients with various cancers, such as pancreatic cancer,31 renal cell carcinoma,32 and breast cancer.33 These results suggest that DM is an independent unfavorable prognostic factor for NSCLC after surgical resection. As a result, surgically treated NSCLC patients with DM might benefit from better glucose control and closer postoperative follow‐up.10
The potential association between DM and prognosis of patients with surgically treated NSCLC remains complex. Some studies have reported that type 2 DM is characterized by insulin resistance and is associated with chronic hyperinsulinemia, which could enhance growth hormone receptor expression and increase IGF‐1 receptor production and availability, thus leading to IGF‐1 receptor signal pathway activation.34, 35 The activated IGR‐1 receptor signal pathway plays a central role in cell growth, differentiation, transformation, metastasis, and survival.13 Evidence has shown that increased serum hemoglobin A1c, C‐peptide, and IGF‐1 levels are significantly correlated with the development and progression of lung cancer.36 Moreover, high expression of IGF‐1 receptor is also significantly associated with poor prognosis in NSCLC.37 These results may, to some degree, explain why lung cancer patients with DM have a significantly poorer prognosis than those without. However, in contrast, one study reported that DM could delay NSCLC progression by increasing the level of long‐lived proteins modified with advanced glycation end product, which could lead to beneficial effects.12 Moreover, the impact of DM on the prognosis of patients with NSCLC could be complicated with the administration of an anti‐diabetic therapeutic agent, such as metformin, which exerts its anti‐diabetic effect predominantly via liver kinase B1 (LKB1)‐dependent activation of adenosine monophosphate‐activated protein kinase (AMPK).38 Because it regulates the LKB‐1/AMPK signal pathway, metformin has also been proven to significantly inhibit cell proliferation, induce apoptosis, and block cell cycle progression in NSCLC cells,39 thus exerting an antitumor effect in NSCLC patients with DM. Therefore, further studies are needed to elucidate the actual interaction of DM with lung cancer.
Our meta‐analysis had several limitations. First, because of the lack of RCTs, only retrospective cohort studies were included for analysis, which might reduce the statistical power of our results. Second, potential heterogeneities and the low quality of several studies could also affect the validity of our results. Third, RFS data was only available from six studies, and as a result, the impact of DM on RFS should be further verified. Moreover, the impact of DM on the prognosis of patients with NSCLC could be biased by the effect of administration of an anti‐diabetic therapeutic agent, such as metformin. Finally, we could not extract the HRs of OS or RFS directly from some studies and therefore, we could only estimate HRs for these studies based on five‐year OS or RFS rates as previously described.19 Therefore, further well‐conducted RCTs are needed to confirm and update our conclusions.
In this updated meta‐analysis, we attempted to draw an objective conclusion about the impact of DM on long‐term survival of patients with surgically treated NSCLC by including all relevant up‐to‐date studies. We found that patients with DM had significantly poorer OS than those without. Therefore, DM is an independent unfavorable prognostic factor for patients with surgically treated NSCLC. Further studies, however, are needed to confirm and update our conclusions.
Disclosure
No authors report any conflict of interest.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008; 359: 1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 4. Varlotto JM, Recht A, Flickinger JC, Medford‐Davis LN, Dyer AM, Decamp MM. Factors associated with local and distant recurrence and survival in patients with resected nonsmall cell lung cancer. Cancer 2009; 115: 1059–69. [DOI] [PubMed] [Google Scholar]
- 5. Jeong SS, Choi PJ, Yi JH, Yoon SS. Impact of lifestyle diseases on postoperative complications and survival in elderly patients with stage I non‐small cell lung cancer. Korean J Thorac Cardiovasc Surg 2017; 50: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JY, Jeon I, Lee JM, Yoon JM, Park SM. Diabetes mellitus as an independent risk factor for lung cancer: A meta‐analysis of observational studies. Eur J Cancer 2013; 49: 2411–23. [DOI] [PubMed] [Google Scholar]
- 7. Li SJ, Fan J, Zhou J, Ren YT, Shen C, Che GW. Diabetes mellitus and risk of bronchopleural fistula after pulmonary resections: A meta‐analysis. Ann Thorac Surg 2016; 102: 328–39. [DOI] [PubMed] [Google Scholar]
- 8. Win T, Sharples L, Groves AM, Ritchie AJ, Wells FC, Laroche CM. Predicting survival in potentially curable lung cancer patients. Lung 2008; 186: 97–102. [DOI] [PubMed] [Google Scholar]
- 9. Jeon HW, Kim KS, Kim YD et al Lymphatic vessel invasion in pathologic stage I and II non‐small cell lung tumors. Surg Today 2015; 45: 1018–24. [DOI] [PubMed] [Google Scholar]
- 10. Varlotto J, Medford‐Davis LN, Recht A et al Confirmation of the role of diabetes in the local recurrence of surgically resected non‐small cell lung cancer. Lung Cancer 2012; 75: 381–90. [DOI] [PubMed] [Google Scholar]
- 11. Dominguez‐Ventura A, Cassivi SD, Allen MS et al Lung cancer in octogenarians: Factors affecting long‐term survival following resection. Eur J Cardiothorac Surg 2007; 32: 370–4. [DOI] [PubMed] [Google Scholar]
- 12. Bartling B, Simm A, Sohst A, Silber RE, Hofmann HS. Effect of diabetes mellitus on the outcome of patients with resected non‐small cell lung carcinoma. Gerontology 2011; 57: 497–501. [DOI] [PubMed] [Google Scholar]
- 13. Nakazawa K, Kurishima K, Tamura T, Ishikawa H, Satoh H, Hizawa N. Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first‐line therapy. Med Oncol 2013; 30: 367. [DOI] [PubMed] [Google Scholar]
- 14. Zhu L, Cao H, Zhang T et al The effect of diabetes mellitus on lung cancer prognosis: A PRISMA‐compliant meta‐analysis of cohort studies. Medicine 2016; 95 (17): e3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhillon SS, Groman A, Meagher A, Demmy T, Warren GW, Yendamuri S. Metformin and not diabetes influences the survival of resected early stage NSCLC patients. J Cancer Sci Ther 2014; 6: 217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medairos RA, Clark J, Holoubek S et al Metformin exposure is associated with improved progression‐free survival in diabetic patients after resection for early‐stage non‐small cell lung cancer. J Thorac Cardiovasc Surg 2016; 152: 55–61.e1. [DOI] [PubMed] [Google Scholar]
- 17. Motoishi M, Sawai S, Hori T, Yamashita N. The preoperative HbA1c level is an independent prognostic factor for the postoperative survival after resection of non‐small cell lung cancer in elderly patients. Surg Today 2018; 48: 517–24. [DOI] [PubMed] [Google Scholar]
- 18. Jadad AR, Moore RA, Carroll D et al Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 19. Deng HY, Wang YC, Ni PZ et al Radiotherapy, lobectomy or sublobar resection? A meta‐analysis of the choices for treating stage I non‐small‐cell lung cancer. Eur J Cardiothorac Surg 2017; 51: 203–10. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–34. [DOI] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non‐small cell lung cancer (NSCLC) patients. Lung Cancer 2012; 76: 242–7. [DOI] [PubMed] [Google Scholar]
- 24. Varlotto JM, Medford‐Davis LN, Recht A et al Identification of stage I non‐small cell lung cancer patients at high risk for local recurrence following sublobar resection. Chest 2013; 143: 1365–77. [DOI] [PubMed] [Google Scholar]
- 25. Fan C, Gao S, Hui Z et al Risk factors for locoregional recurrence in patients with resected N1 non‐small cell lung cancer: A retrospective study to identify patterns of failure and implications for adjuvant radiotherapy. Radiat Oncol 2013; 8: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Washington I, Chino JP, Marks LB et al Diabetes mellitus: A significant co‐morbidity in the setting of lung cancer? Thorac Cancer 2013; 4: 123–30. [DOI] [PubMed] [Google Scholar]
- 27. Jeon HW, Moon MH, Kim KS et al Extent of removal for mediastinal nodal stations for patients with clinical stage I non‐small cell lung cancer: Effect on outcome. Thorac Cardiovasc Surg 2014; 62: 599–604. [DOI] [PubMed] [Google Scholar]
- 28. Kuo CH, Wu CY, Lee KY et al Chronic obstructive pulmonary disease in stage I non‐small cell lung cancer that underwent anatomic resection: The role of a recurrence promoter. COPD 2014; 11: 407–13. [DOI] [PubMed] [Google Scholar]
- 29. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 30. Yang L, Shao J, Bian Y et al Prevalence of type 2 diabetes mellitus among inland residents in China (2000–2014): A meta‐analysis. J Diabetes Investig 2016; 7: 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen H, Zhan M, Wang W, Yang D, Wang J. Impact of diabetes mellitus on the survival of pancreatic cancer: A meta‐analysis. Onco Targets Ther 2016; 9: 1679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Li H, Gu L et al The impact of diabetes mellitus on renal cell carcinoma prognosis: A meta‐analysis of cohort studies. Medicine 2015; 94: e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: A systematic review and meta‐analysis. Medicine 2016; 95: e5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Humar M, Kern I, Vlacic G, Hadzic V, Cufer T. Insulin‐like growth factor 1 receptor expression in advanced non‐small‐cell lung cancer and its impact on overall survival. Radiol Oncol 2017; 51: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2005; 2: 48–53. [DOI] [PubMed] [Google Scholar]
- 36. Zhang M, Li X, Zhang X, Yang Y, Feng Z, Liu X. Association of serum hemoglobin A1c, C‐peptide and insulin‐like growth factor‐1 levels with the occurrence and development of lung cancer. Mol Clin Oncol 2014; 2: 506–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Shi X, Wang T, Ying C, He S, Chen Y. The prognostic and clinicopathological significance of IGF‐1R in NSCLC: A meta‐analysis. Cell Physiol Biochem 2017; 43: 697–704. [DOI] [PubMed] [Google Scholar]
- 38. Shaw RJ, Lamia KA, Vasquez D et al The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310: 1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo Q, Liu Z, Jiang L et al Metformin inhibits growth of human non‐small cell lung cancer cells via liver kinase B‐1‐independent activation of adenosine monophosphate‐activated protein kinase. Mol Med Rep 2016; 13: 2590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


