Abstract
Background
We prospectively evaluated the efficacy and toxicity of a non‐platinum triplet regimen for patients with advanced non‐small cell lung cancer (NSCLC) expected to be platinum‐resistant.
Methods
Patients were diagnosed with NSCLC using endobronchial ultrasonography with a guide sheath as a core biopsy. RNA was immediately isolated from unfixed biopsy specimens, and quantitative real‐time reverse transcription‐PCR assays were performed to determine ERCC1 messenger RNA expression. Patients with advanced, untreated NSCLC showing high ERCC1 levels (ΔCt ≧ 6.5) were assigned a non‐platinum triplet regimen of irinotecan and paclitaxel plus bevacizumab. The primary end point was the objective response rate (ORR).
Results
A total of 141 untreated patients were evaluated and 30 patients were entered into this phase II trial. The ORR was 66.7% (95% confidence interval [CI] 47.2–82.7) and median progression‐free survival (PFS) was 215 days. Grade 4 thrombosis occurred in one patient, but other toxicities were mild and controllable. Fifty‐six patients were treated with platinum‐containing regimens and 24 patients responded (ORR 42.8%, 95% CI 29.7–56.7). Twenty‐nine of these patients had high ERCC1 levels, of which 6 patients responded; 27 patients had low ERCC1 levels, 18 patients responded (P = 0.0053 by Fisher’s exact test).
Conclusion
The triplet combination might be effective for patients with advanced, untreated NSCLC overexpressing ERCC1. ERCC1 messenger RNA levels may be a predictive factor for response to platinum‐containing regimens.
Keywords: Bevacizumab, excision repair cross‐complementation group 1, irinotecan, non‐small cell lung cancer, paclitaxel
Introduction
Recently, tyrosine kinase inhibitors, such as gefitinib, erlotinib, afatinib, and crizotinib, have become first‐line chemotherapy regimens for patients with advanced non‐small cell lung cancer (NSCLC) harboring driver mutations,1, 2, 3, 4, 5, 6, 7 although platinum‐based chemotherapy is still considered first‐line treatment for patients without driver mutations.8 However, the toxicities of platinum‐based regimens are still severe and clinical benefit is limited. Thus, predictive biomarkers for response to platinum‐based regimens have been investigated. The ERCC1 protein has shown promise as a predictive biomarker for platinum‐based chemotherapy, especially with cisplatin.9, 10, 11, 12
ERCC1 protein levels in patients with NSCLC have mainly been evaluated by immunohistochemical analysis. However, Friboulet et al. reported that the use of currently available ERCC1 antibodies did not specifically detect the unique functional ERCC1 isoform.13 Immunohistochemistry has limited usefulness to detect ERCC1 for use in guiding therapeutic decisions about cisplatin‐containing regimens.
ERCC1 messenger RNA (mRNA) level has also been studied using reverse transcription (RT)‐PCR assay.14, 15, 16, 17 However, mRNA is unstable, and extraction of mRNA from formalin‐fixed paraffin‐embedded (FFPE) tissue is difficult, suggesting limitations in the usefulness of mRNA to evaluate ERCC1 expression. Fresh core biopsy samples without prior formalin fixation and paraffin embedding are often considered best for evaluating target mRNA. However, obtaining a sufficient unfixed core biopsy from patients with advanced NSCLC, especially non‐squamous NSCLC, can be difficult because tumors are mainly located in the peripheral lung field. Computed tomography (CT)‐guided percutaneous needle core biopsy is usually performed for such patients to obtain a core biopsy. This technique carries a high risk of pneumothorax and sample size is sometimes insufficient for additive biological analysis.18, 19
Endobronchial ultrasonography with a guide sheath (EBUS‐GS) is a new technique to diagnose lung cancer.20, 21 Ultrasonography allows for confirmation that the biopsy samples are actually obtained from within the tumor. We used biopsies obtained by EBUS‐GS as core biopsies and evaluated the mRNA level of ERCC1 in unfixed biopsy samples obtained from patients with suspected advanced non‐squamous NSCLC.
We have previously reported the results of a randomized phase II trial comparing non‐platinum doublets, irinotecan plus paclitaxel (IP) versus irinotecan plus gemcitabine (IG).22 In that trial, the response rate achieved in the IP group was higher than in the IG group, while the toxicities of both regimens were controllable. On the other hand, bevacizumab, a recombinant monoclonal antibody blocking tumor angiogenesis that inhibits vascular endothelial growth factor (VEGF), is now commonly used in combination chemotherapy with irinotecan or paclitaxel for patients with advanced colorectal cancer or non‐squamous NSCLC.23, 24 In the present phase II trial, we evaluated the efficacy and safety of non‐platinum combination chemotherapy consisting of irinotecan plus paclitaxel plus bevacizumab for patients with advanced non‐squamous NSCLC showing high mRNA levels of ERCC1. We also evaluated the relationship between mRNA levels of ERCC1 and the efficacy of platinum‐based chemotherapy.
Methods
Eligibility criteria
The eligibility criteria for this study were as follows: histologically‐confirmed stage IIIB/IV non‐squamous NSCLC (according to the 7th edition of the General Rule for Clinical and Pathological Record of Lung Cancer) with a core biopsy via EBUS‐GS; delta Ct of ERCC1 in biopsy sample ≥ 6.516 the absence of homozygous UGT1A1*6 or UGT1A1*28; aged 20–75 years; no prior chemotherapy with EGFR‐tyrosine kinase inhibitors (TKIs) or ALK inhibitors; no radiation therapy for the primary tumor; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; measurable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; life expectancy of ≥ 12 weeks; and adequate bone marrow, renal, and hepatic function. The major exclusion criteria were: history or presence of hemoptysis or bloody sputum, tumor invading or abutting major blood vessels, a history of radiation therapy to the lung field, or coexistence or history of interstitial lung disease.
This study was performed in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the institutional review boards of the participating institutions and written informed consent was obtained from all patients.
Real time‐PCR
Total RNA was extracted from unfixed frozen tissue using an RNeasy Plus Mini Kit (Qiagen GmbH, Dusseldorf, Germany). Template RNA (approximately 100 ng) was used for complementary DNA synthesis using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Waltham, MA, USA). Complementary DNA (equivalent to 10 ng total RNA) and Platinum qPCR Super Mix (Life Technologies) was used for TaqMan real time‐PCR for ERCC1 and Actin, Beta (ACTB). RT‐PCR was carried out using a Sequence Detection System 9700HT (Life Technologies). Relative expression was calculated as follows: delta‐Ct = Average Ct (ERCC1) − Average Ct (ACTB), Relative Expression = 2−deltaCt. This analysis was independently performed by FALCO Biosystems, Ltd. (Kyoto, Japan).
Treatment
All patients received paclitaxel (150 mg/m2), irinotecan (50 mg/m2), and bevacizumab 15 mg/kg by intravenous infusion on day 1, and irinotecan (50 mg/m2) by intravenous infusion on day 8, repeated every four weeks. Each patient received a minimum of three cycles until the onset of progressive disease or unacceptable toxicity. The maximal number of chemotherapy cycles was six, and bevacizumab maintenance was administered until disease progression, unacceptable toxicity, or patient refusal.
Assessment of treatment
Before treatment, all patients underwent a complete medical history and physical examination, chest radiography, chest and abdominal CT, a radionuclide bone scan or positron emission tomography CT, brain CT or magnetic resonance imaging, and electrocardiography. Complete blood cell counts and blood chemistry studies were also performed and repeated at least twice a week until treatment discontinuation. Scans or radiographs used to assess response were obtained every four to six weeks.
The response was investigator‐determined according to RECIST version 1.1. All adverse events were recorded and classified by grade according to the Common Terminology Criteria for Adverse Events version 3.0.
Additional cohort
To evaluate the efficacy of a platinum‐containing regimen for each ERCC1 of high and low expression, patients that did not show ERCC1 expression (delta‐CT ≥ 6.5) were added to the analysis set as an additional cohort.
Statistical analysis
The primary end point was overall response rate (ORR). A Simon optimal two‐stage design was chosen to determine the total number of patients required for the study.24 Assuming an ORR of 30% for standard therapy, a target response rate of 60% was established. With alpha = 0.05 and beta = 0.10, the estimated number of patients required was 28.
Overall survival (OS) was defined as the interval from the start of treatment to death from any cause. Progression‐free survival (PFS) was defined as the interval from the start of treatment to either progressive disease or death, whichever came first. Survival curves were plotted using the Kaplan–Meier method.
This trial was registered with University Hospital Medical Information Network (UMIN000006514).
Results
Patient characteristics
Between September 2012 and March 2015, the ERCC1 mRNA expression level was evaluated in 141 patients (range of delta‐Ct: 3.9–8.5); 92 patients showed delta‐CT ≥ 6.5. Of those, 30 patients with advanced non‐squamous NSCLC were enrolled in the trial (Fig 1). The patient characteristics are presented in Table 1. Twenty‐seven patients were diagnosed by EBUS‐GS, while three patients were diagnosed by other core biopsy methods, such as thoracoscopic or transbronchial lymph node biopsy. All patients were treated and able to be assessed for toxicities, but two patients refused chemotherapy during the first cycle and asked to receive only supportive care, thus we were unable to evaluate the response in these patients.
Figure 1.
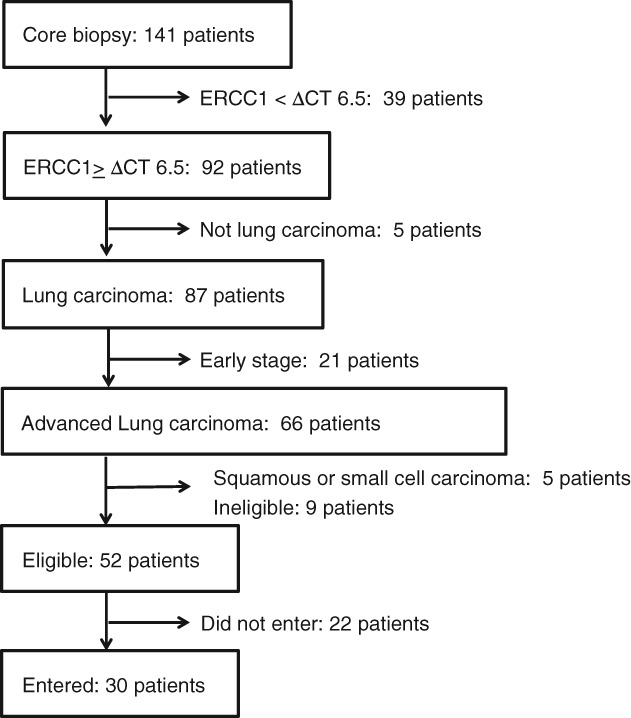
Study scheme.
Table 1.
Patient characteristics
| Characteristics | Number of patients | % |
|---|---|---|
| Age | ||
| Median | 64 | |
| Range | 37–75 | |
| Gender | ||
| Men | 13 | 43 |
| Women | 17 | 57 |
| Stage | ||
| IIIB | 1 | 3 |
| IV | 29 | 97 |
| Histology | ||
| Adenocarcinoma | 30 | 100 |
| Other | 0 | 0 |
| ECOG PS | ||
| 0 | 14 | 47 |
| 1 | 16 | 53 |
| Mutation status | ||
| EGFR deletion 19 | 4 | 13 |
| EGFR L858R | 5 | 17 |
| EGFR others | 1 | 3 |
| EML4‐ALK | 0 | 0 |
| Others | 0 | 0 |
| Diagnosis | ||
| EBUS‐GS | 27 | 90 |
| Others | 3 | 10 |
EBUS‐GS, endobronchial ultrasonography with a guide sheath; ECOG PS, Eastern Cooperative Oncology Group performance status.
Safety
We delivered a total of 106 treatment cycles, with a median of 3 (range 1–6) treatment cycles per patient. The range of bevacizumab maintenance was 0–9 cycles. Table 2 lists the incidence of hematological and non‐hematological toxicities. Neutropenia was the most common grade 3/4 adverse event and occurred in 47% (14/30) of patients. Other grade 3/4 hematological toxicities included leukopenia (27%, 8/30) and anemia (3%, 1/30). Grade 3/4 non‐hematological toxicities included febrile neutropenia (23%, 7/30), hypertension (7%, 2/30), duodenal ulcer, ileus, bleeding, thrombosis, pneumonitis, increased alanine transaminase and aspartate transaminase levels (3%, 1/30 each). No treatment‐related death occurred. In addition, there were no severe complications requiring hospitalization as a result of EBUS‐GS during the study.
Table 2.
Toxicities occurring in all treatment courses
| No. of patients (n = 30) | |||
|---|---|---|---|
| Toxicity | Grade 1–2 (%) | Grade 3 (%) | Grade 4 (%) |
| Hematologic | |||
| Leukopenia | 10 (33%) | 7 (23%) | 1 (3%) |
| Neutropenia | 8 (27%) | 4 (13%) | 10 (33%) |
| Anemia | 7 (23%) | 1 (3%) | 0 |
| Thrombocytopenia | 1 (3%) | 0 | 0 |
| Non‐hematologic | |||
| Febrile neutropenia | 6 (20%) | 6 (20%) | 1 (3%) |
| Anorexia | 10 (33%) | 0 | 0 |
| Nausea | 3 (10%) | 1 (3%) | 0 |
| Vomiting | 1 (3%) | 0 | 0 |
| Diarrhea | 13 (43%) | 0 | 0 |
| Constipation | 5 (17%) | 0 | 0 |
| Hiccups | 2 (7%) | 0 | 0 |
| Duodenal ulcer | 1 (3%) | 1 (3%) | 0 |
| Ileus | 0 | 1 (3%) | 0 |
| Hypertension | 5 (17%) | 2 (7%) | 0 |
| Bleeding | 1 (3%) | 1 (3%) | 0 |
| Thrombosis | 2 (7%) | 0 | 1 (%) |
| Neuropathy | 9 (30%) | 0 | 0 |
| Pneumonitis | 1 (3%) | 1 (3%) | 0 |
| Thrombosis | 2 (7%) | 1 (3%) | 0 |
| Rash | 5 (17%) | 1 (3%) | 0 |
| Pruritus | 1 (3%) | 0 | 0 |
| Fatigue | 5 (17%) | 0 | 0 |
| Arthritis | 1 (3%) | 0 | 0 |
| Myositis | 1 (3%) | 0 | 0 |
| Alopecia | 9 (30%) | 0 | 0 |
| AST | 4 (13%) | 1 (3%) | 0 |
| ALT | 4 (13%) | 1 (3%) | 0 |
| Hypercholesterolemia | 1 (3%) | 0 | 0 |
| Hypoalbuminemia | 3 (10%) | 0 | 0 |
| Hyponatremia | 1 (3%) | 0 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Efficacy
Twenty patients achieved a partial response, 7 achieved stable disease, and one exhibited progressive disease. Figure 2 shows the best tumor changes from baseline in this study. All evaluable patients achieved tumor reduction. As mentioned previously, two patients were not evaluable because of treatment cessation. On the basis of intent‐to‐treat analysis, ORR was 66.7% (95% confidence interval [CI] 47.2–82.7). The median PFS was 229 (95% CI 169–294) days and the median survival time (MST) 857 (95% CI 331–NA) days (Fig 3). The one‐year PFS rate was 15.2%. The one and two‐year OS rates were 69.3% and 54.9%, respectively. OS data were not sufficiently mature at the time of analysis to present results here.
Figure 2.
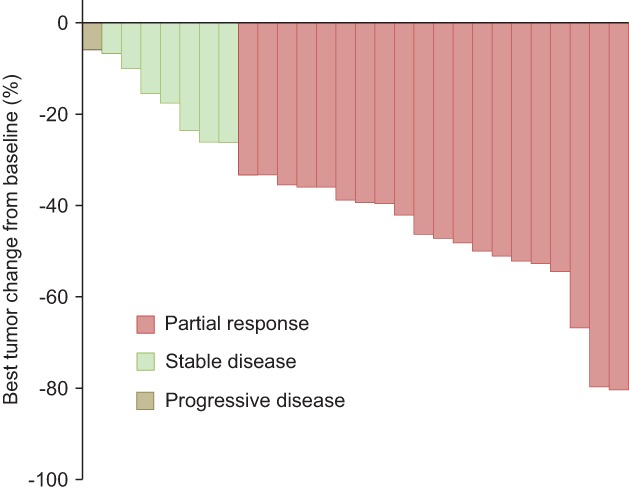
Best tumor change from baseline.
Figure 3.
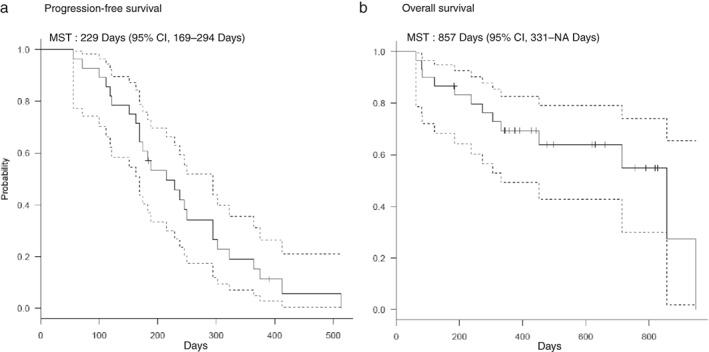
Progresion‐free survival and Overall survival. CI, confidence interval; MST, median survival time.
At the time of this analysis, 56 patients with advanced NSCLC had received platinum‐based chemotherapy. These regimens are summarized in Table 3. Carboplatin plus pemetrexed plus bevacizumab and cisplatin plus pemetrexed were the most commonly used regimens. Platinum‐based chemotherapy was used after triplet therapy immediately in all patients. Responses for the treatment and delta Ct of ERCC1 in these patients are shown in Table 3. Six of 29 patients with high ERCC1 responded to platinum regimens, with an ORR of 20.7%. By comparison, 18 of 27 patients with low ERCC1 responded, with an ORR of 66.7%. The response to the platinum regimens in patients with high ERCC1 was statistically significantly high compared to patients with low ERCC1 (P = 0.0053 by Fisher’s exact test).
Table 3.
Responses to platinum regimens
| ERCC1 high: ORR 20.7% (6/29) | ERCC1 low: ORR 66.7% (18/27) | ||
|---|---|---|---|
| ERCC1 delta Ct | Response | ERCC1 delta Ct | Response |
| 8.5 | PD | 6.4 | PR |
| 8.0 | PD | 6.4 | PR |
| 7.9 | PD | 6.4 | SD |
| 7.8 | PR | 6.4 | PR |
| 7.6 | PR | 6.3 | SD |
| 7.4 | SD | 6.3 | NE |
| 7.4 | SD | 6.2 | PR |
| 7.4 | SD | 6.2 | PR |
| 7.4 | NE | 6.2 | SD |
| 7.3 | PD | 6.2 | PR |
| 7.3 | PD | 6.1 | PR |
| 7.3 | SD | 6.1 | PR |
| 7.2 | SD | 6.1 | PR |
| 7.1 | SD | 6.1 | PR |
| 7.0 | SD | 5.9 | SD |
| 7.0 | PD | 5.9 | SD |
| 7.0 | SD | 5.9 | PR |
| 6.9 | PD | 5.8 | PR |
| 6.8 | PD | 5.8 | PR |
| 6.7 | SD | 5.8 | PR |
| 6.7 | SD | 5.5 | PR |
| 6.7 | PD | 5.4 | PR |
| 6.6 | PD | 5.2 | PD |
| 6.6 | PR | 5.2 | SD |
| 6.6 | PR | 5.0 | PR |
| 6.6 | PR | 5.0 | PD |
| 6.5 | SD | 3.9 | PR |
| 6.5 | PD | ||
| 6.5 | PR | ||
NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
We evaluated the efficacy and safety of a non‐platinum combination regimen – irinotecan and paclitaxel plus bevacizumab – for patients with advanced non‐squamous NSCLC expressing ERCC1 mRNA at a high level. The ORR was 66.7% and met the primary end point of this study. We observed a median PFS of 229 days and an MST of 32.0 months. However, the OS data were insufficient at the time of this analysis, as only two events had occurred. In this trial, grade 3 or 4 neutropenia and leukocytopenia were dominant toxicities (47% and 27%, respectively) in accordance with our previous report about an IP regimen.19 Febrile neutropenia also occurred in 43% of patients. Gastrointestinal toxicities such as nausea, vomiting, and diarrhea were mild and controllable compared to our previous report. Bleeding or thrombosis occurred in patients in this trial but not in our previous study. These adverse events are closely related to the VEGF antibody, thus we believe that bevacizumab might responsible for these toxicities. However, these VEGF antibody‐specific toxicities were also controllable and we have shown that this treatment modality was well tolerated in the eligible patients. In additional analysis, a total of 56 patients with advanced NSCLC were evaluated for ERCC1 mRNA expression and were treated with platinum‐based chemotherapy. Eighteen of 27 patients with low ERCC1 expression responded to platinum regimens (ORR 66.7%), while six of 29 patients with high ERCC1 expression responded (ORR 20.7%). Compared to the high ERCC1 population, the response to platinum‐based chemotherapy was statistically significant in patients with a low ERCC1 level.
The ERCC1 enzyme is thought to be an important enzyme in mediating resistance to platinum by removing platinum‐induced DNA adducts.12 Olaussen et al. were the first to report that patients with ERCC1‐negative NSCLC based on immunohistochemical staining appear to benefit from adjuvant cisplatin‐based chemotherapy,9 and ERCC1 has come to be recognized as a promising predictive factor in platinum‐based chemotherapy. Since then, ERCC1 has been evaluated in many clinical trials and analyses by several immunohistochemical methods.10, 11 Friboulet et al. reported that the currently available ERCC1 antibodies did not specifically detect the unique functional isoform of ERCC1 and concluded that immunohistochemistry may be of limited usefulness to guide therapeutic decision‐making.13
The relationship between ERCC1 mRNA expression and resistance to platinum regimens has been corroborated in patients with advanced NSCLC in previous studies. However, these analyses were almost all conducted using FFPE tissue. The instability of RNA compared to DNA is well recognized, and mRNA is destroyed or damaged in FFPE tissue. Thus, unfixed tissue obtained by core biopsy must be used to evaluate mRNA expression. In advanced lung cancer, percutaneous CT‐guided core biopsy is considered a standard method of obtaining core biopsy samples from primary tumors. However, potential complications, such as pneumothorax or air embolization, are associated with this method.18 EBUS‐GS is a recently developed bronchoscopic method that makes it possible to confirm that samples are obtained from within a tumor based on ultrasonography imaging.20, 21 We adopted this method in our trial and designated the biopsy obtained by this method as a core biopsy. As a result, in this trial we examined the relationship between ERCC1 mRNA levels and the efficacy of platinum chemotherapy. EBUS‐GS is probably one of the safest methods to obtain core biopsy samples from primary tumors in advanced NSCLC.
We did not compare the efficacy of non‐platinum combination chemotherapy to the platinum combination in patients with high levels of ERCC1 because platinum combination therapy is now strongly recommended as first‐line treatment for patients with advanced NSCLC.8 However, our data indicated the possibility that ERCC1 mRNA overexpression extracted from an unfixed core biopsy tissue is a predictive factor for response to platinum‐containing regimens. In addition, as mentioned previously, EBUS‐GS may be a useful method for obtaining a core biopsy. We recommend a randomized, prospective trial to confirm our results using unfixed biopsy specimens collected by EBUS‐GS.
In conclusion, the triplet combination of irinotecan and paclitaxel plus bevacizumab might be effective for patients with advanced, untreated NSCLC overexpressing ERCC1. In addition, ERCC1 mRNA levels extracted from unfixed lung biopsy specimens obtained by EBUS‐GS may also be predictive for a response to platinum‐containing regimens.
Disclosure
No authors report any conflict of interest.
References
- 1. Leighl NB, Rekhtman N, Biermann WA et al Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Guideline. J Clin Oncol 2014; 32: 3673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 3. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 4. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu Y‐L, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 6. Yang JC‐H, Wu Y‐L, Schuler M et al Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–51. [DOI] [PubMed] [Google Scholar]
- 7. Solomon BJ, Mok T, Kim D‐W et al First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 8. Azzoli CG, Temin S, Aliff T et al Focused update of 2009 American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for Stage IV non–small‐cell lung cancer. J Clin Oncol 2011, 2011; 29: 3825–31.21900105 [Google Scholar]
- 9. Olaussen KA, Dunant A, Fouret P et al DNA repair by ERCC1 in non‐small‐cell lung cancer and cisplatin‐based adjuvant chemotherapy. N Engl J Med 2006; 355: 983–91. [DOI] [PubMed] [Google Scholar]
- 10. Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 2007; 356: 800–8. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds C, Obasaju C, Schell MJ et al Randomized phase III trial of gemcitabine‐based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non‐small‐cell lung cancer. J Clin Oncol 2009; 27: 5808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: The role of DNA repair pathways. Clin Cancer Res 2008; 14: 1291–5. [DOI] [PubMed] [Google Scholar]
- 13. Friboulet L, Olaussen KA, Pignon J‐P et al ERCC1 isoform expression and DNA repair in non‐small‐cell lung cancer. N Engl J Med 2013; 368: 1101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bepler G, Williams C, Schell MJ et al Randomized international phase III trial of ERCC1 and RRM1 expression‐based chemotherapy versus gemcitabine/carboplatin in advanced non‐small‐cell lung cancer. J Clin Oncol 2013; 31: 2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cobo M, Isla D, Massuti B et al Customizing cisplatin based on quantitative excision repair cross‐complementing 1 mRNA expression: A phase III trial in non‐small‐cell lung cancer. J Clin Oncol 2007; 25: 2747–54. [DOI] [PubMed] [Google Scholar]
- 16. Lord RVN, Brabender J, Gandara D et al Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non‐small cell lung cancer. Clin Cancer Res 2002; 8: 2286–91. [PubMed] [Google Scholar]
- 17. Rosell R, Danenberg KD, Alberola V et al Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin‐treated advanced non‐small cell lung cancer patients. Clin Cancer Res 2004; 10: 1318–25. [DOI] [PubMed] [Google Scholar]
- 18. Gohari A, Haramati LB. Complications of CT scan‐guided lung biopsy: Lesion size and depth matter. Chest 2004; 126: 666–8. [DOI] [PubMed] [Google Scholar]
- 19. Tomiyama N, Yasuhara Y, Nakajima Y et al CT‐guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006; 59: 60–4. [DOI] [PubMed] [Google Scholar]
- 20. Shien K, Toyooka S, Yamamoto H et al Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell‐like properties in cancer cells. Cancer Res 2013; 73: 3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurimoto N, Miyazawa T, Okimasa S et al Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004; 126: 959–65. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura Y, Soda H, Oka M et al Randomized phase II trial of irinotecan with paclitaxel or gemcitabine for non‐small cell lung cancer: Association of UGT1A1*6 and UGT1A1*27 with severe neutropenia. J Thorac Oncol 2011; 6: 121–7. [DOI] [PubMed] [Google Scholar]
- 23. Hurwitz H, Fehrenbacher L, Novotny W et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 24. Sandler A, Gray R, Perry MC et al Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006; 355: 2542–50. [DOI] [PubMed] [Google Scholar]


