Abstract
The aim of this article is to describe a large mandibular cyst treated with decompression followed by surgical enucleation. Furthermore, we described the utility of cyst volume measurements by using a 3D reconstruction on Cone Beam Computed Tomography (CBCT). The dentigerous cyst is the most common cyst type of epithelial origin, arising from remnants of odontogenic epithelium, asymptomatic and associated with the crown of an unerupted or partially or completely impacted tooth. However, after a long duration and extension of the cyst volume it may provoke significant bone resorption, cortical expansion, tooth displacement and the vitality of neighboring teeth may be affected. The regular treatment of this lesion is enucleation and extraction of the involved tooth. Marsupialization and decompression are proposed when the volume of the cyst is well developed to release the cystic pressure and allow the bone cavity to progressively decrease in volume with the gradual apposition of bone. This report presents a large dentigerous cyst related to impacted mandibular third molar of a 21-year-old male patient. The cyst was treated successfully by decompression and later by surgical enucleation with surgical extraction of the related molar. In conclusion, the combination of decompression and surgical approach showed on the three-dimensional CBCT investigation a significant correlation between the treatment and volume reduction of the cyst. The clinical case described allows us to observe bone formation after decompression and surgical enucleation was performed with less risk on vital anatomic elements.
Key words: Dentigerous cyst, Mandibular, Decompression, Three-dimension, Cone Beam Computed Tomography
Introduction
Odontogenic cysts, in almost all classifications, are divided into two major groups: developmental and inflammatory. Some cysts arise from the proliferation of epithelial residues left behind during odontogenesis; such cysts are generally described as being developmental in origin.1,2 While other types arise from epithelial hyperplasia of the residues due to adjacent foci of inflammation as a consequence of the two major dental diseases, decays and periodontal disease; such cysts are generally described as being inflammatory in origin.3,4
The dentigerous cyst is the second most common odontogenic cyst5,6 and represent 22.3 % of odontogenic cysts.7 Dentigerous cysts have the potential to bone resorption and can expand into the surrounding tissue and tooth roots causing malocclusion or facial asymmetry8 or mandibular paresthesia. 9 The diagnosis is not based only on the radiographic evaluation alone, a histopathologic exam must be conducted to confirm it.10 The keratocysts and unilocular ameloblastomas are commonly misdiagnosed with dentigerous cysts because conventional ameloblastoma show cystic degeneration with no biologic differences. The unilocular ameloblastoma represents an ameloblastoma, which presents as a cyst and has a lower recurrence rate following conservative removal. The histopathologic detection is recognized by luminal, intraluminal, and mural types depending on whether only the cyst lining is affected or not.11 These cysts vary in size; if allowed to enlarge, they may, over time, cause significant bony expansion and destruction of large portions of the bone.12 Plus, they may spread to adjacent anatomical structures such as the maxillary sinus or nasal cavity due to the increasing intra-cystic pressure.12 However, this cyst is asymptomatic in majority9,13 and discovered on dental radiographs usually appearing as a well-defined radiolucency associated with the crown of an unerupted tooth.14 Marsupialization of odontogenic cystic was described by Partsch in 1892, it is a technique where a large window is made in cystic wall and then sutured to the oral mucosa.15 Marsupialization has the advantage of reducing the cyst volume.15 For those cases when no eruption occurs, a period of 3 to 4 months after marsupialization is suggested as critical time for deciding whether to extract or conserve.15 The aim of this paper is to present a case report of the treatment of a large odontogenic cyst in the retro-molar region of the lower jaw by decompression, with a 12 months clinical and radiological follow-up.
Case Report
A 21-year-old male reported to the department of oral surgery at the Saint Joseph University in Beirut, with the main complaint of swelling in his lower left jaw. The swelling was growing for the past 3 weeks with no history of pain, numbing or any other complaints. Patient had no history of systemic or local contraindications, including uncontrolled diabetes, bruxism, smoking, or uncontrolled periodontal disease.
Clinical and radiological examination
The face was asymmetric with a small swelling on the left side of the mandible angle. The overlying skin was normal and light swelling was palpable on the submandibular lymph nodes. The vitality test was negative for the left first and second molars, positive on the first and second mandibular left premolars and the test of paresthesia was negative. Intra-orally, there was a sensitive swelling posterior to the molars with a hard bony on palpation. The color of the overlying mucosa was normal (Figure 1A). Panoramic radiograph showed a radiolucent lesion, unilocular, in contact with the impacted third molar with the crown of the tooth and the roots of the two other molars and the second premolar completely involved in the lesion. Resorption appear on the roots of the second molar. The inferior alveolar nerve is repressed to the basal bone of the lower jaw (Figure 1B). Axial and coronal cuts of the Cone Beam Computed Tomography (CBCT) showed a radiolucency, ball-shaped, measuring 30.29×43.19×37.26 mm (Figure 2A, B). The differential diagnosis was a dentigerous cyst or odontogenic keratocyst tumor or a unicystic ameloblastoma.
Figure 1.
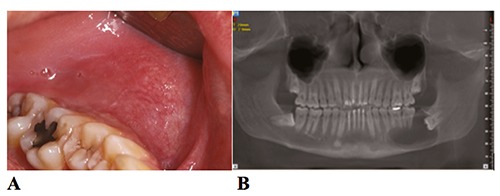
(A) Initial photograph of the left side of the mandible showing posterior swelling and a normal color of the overlying mucosa; (B) Initial panoramic radiograph reconstructed from the cone beam computed tomography (CBCT).
Figure 2.
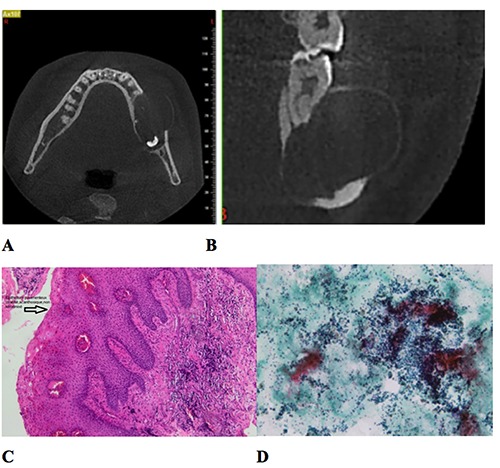
CBCT slices (A) axial (B) coronal to change with a new one with measures. (C, D) Histopathology slides. (C) Cystic membrane: cystic wall composed of fibrous tissue, lined with a non-keratinized stratified squamous epithelium; (D) intra-cystic liquid: a hematologic smear with polymorphous leucocytes as well as foamy or pigmented macrophages.
Biopsy
The importance of the biopsy was discussed with the patient and he gave his approval. Preoperatively, the patient rinsed with 0.12% chlorhexidine gluconate oral rinse (PerioGard; Colgate-Palmolive, Salford, United Kingdom) 5 minutes before the surgery. Local analgesia was achieved by inferior alveolar nerve block 2% articaine with 1:100,000 adrenaline (3M ESPE, Seefeld, Germany). Buccal mucosa was also anesthetized by infiltration close to the second molar. A sample of the intra-cystic fluid was aspired by a fine needle syringe. The aspiration revealed a clear, light-yellow fluid with shiny cholesterol crystals.
Muco-periosteal flap was reflected by sulcular incision from the second premolar to the second molar with a distal buccal releasing incision, and a part of the cyst membrane was dissected and fixed in 10% formaldehyde (Merck, Darmstadt, Germany).
The flap was adjusted and hermetically closed by means of single sutures (Vicryl® 4/0, Johnson & Johnson, medical limited, UK).
Histopathological examination
The cystic border was composed of fibrous tissue, lined with a non-keratinized stratified squamous epithelium. The liquid contained a hematologic smear with polymorphous leucocytes as well as foamy or pigmented macrophages. The results came as a dentigerous cyst with no sign of malignity (Figure 2C, D).
From a practical standpoint, dentigerous cysts are differentiated from keratocysts by the absence of specific features: parakeratosis, palisaded basal layer and corrugated surface.3
Treatment plan
After the histological findings, treatment modalities, enucleation, marsupialization and decompression were discussed with the patient and he accepted our plan to do a decompression followed by enucleation and surgical extraction of the impacted molar after the endodontic treatment of the non-vital first and second left molars. The patient was informed to have a strict soft food diet, to avoid any complication such as mandibular fracture.
Decompression
Under local anesthesia as described before, a triangular distal wedge incision was performed, with the base of the triangle on the distal surface of the second molar. Copious irrigation with physiological saline serum was realized to evacuate all the intracystic fluids. The opening was adjusted to suit the confectioned acrylic stent. The patient was prescribed Amoxicillin + Clavulanic acid 1g, BID for 7 days.
Analgesic medication (400 mg ibuprofen; Abbott Healthcare Products Limited, Vanwall, United Kingdom) was also prescribed, and the patient was advised to rinse his mouth daily with 0.12% chlorhexidine gluconate oral rinse (PerioGard; Colgate- Palmolive, Salford, United Kingdom) during healing. He was instructed to leave the stent in place and visit us twice a week to remove the stent and disinfect it with a 0.12% chlorhexidine solution and thoroughly clean the cavity with saline serum. Through the months, with the bone formation and mesial progression of the third molar the obturator was lifted by the pressure, so every few weeks the obturator part that goes into the cavity was shortened (Figure 3).
Figure 3.
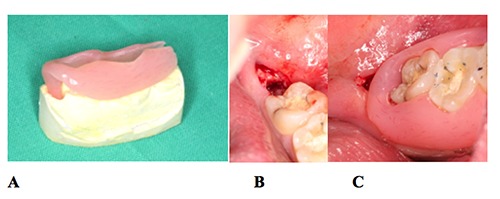
Decompression procedure: (A) acrylic drain, (B) opening of the cyst distally to the second lower left molar, (C) drain stabilized in the mouth without any interference with the occlusion.
After three months, a panoramic radiograph was taken to check the evolution of the lesion. The lesion had retracted but there was not enough bone formation to be able to remove the third molar without damage. Thus, the treatment was resumed for another two months (Figure 4A).
Figure 4.
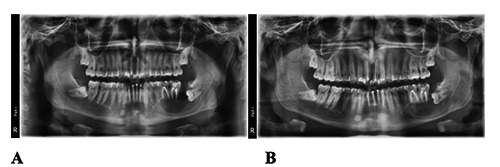
(A) Panoramic radiograph 3 months post-decompression; (B) Panoramic radiograph 5 months post-decompression.
Another panoramic radiograph was taken 5 months post-decompression. It showed mesial displacement of the third molar for about 9 mm, plus a significant bone formation on the basal bone of the jaw which became sufficiently thick to extract the third molar and enucleate the remnants of the cyst, with minimal risk of jaw fracture (Figure 4B).
Enucleation and surgical extraction
The surgical procedure was done under loco-regional analgesia as described for the decompression with the same technique and product. A mucoperiosteal flap was elevated, the third molar was visible. A minimal bone resection was performed, on the buccal and distal side of the molar with a cylindric bur. The fragmentation of the tooth was a must, to avoid a lot of force on the lower jaw. First, the crown of the tooth was cut and removed. Second, the roots were separated and extracted one by one with angulated root elevators. After tooth removal, the enucleation of the remnants of the cyst were slowly dissected and removed. Abundant irrigation was realized to remove all the debris and suturing was performed with a Vicryl® 4/0 (Johnson & Johnson, medical limited, UK). Post-operative medication was the same as for the decompression procedure (Figure 5). Four months post-operatively the panoramic radiograph showed complete bone formation in the cystic cavity (Figure 6).
Figure 5.
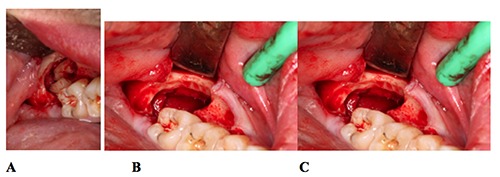
Enucleation surgery: (A) tooth fragmented after osteotomy, (B) cyst enucleation, (C) empty cystic cavity after tooth extraction and enucleation.
Figure 6.
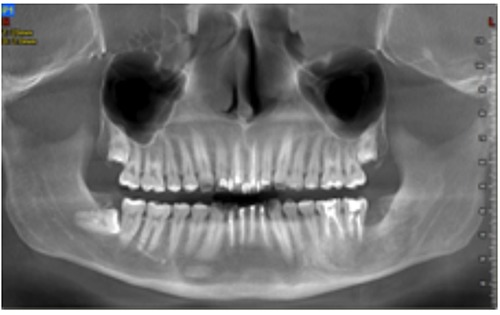
Panoramic radiograph 6 months post-extraction, reconstructed from the CBCT.
Volumetric and linear measurements
The patient underwent two CBCT scans (initial and 4 months after the beginning of the treatment) using the Newtom VGI scanner with a 15×15 cm field of view and 0.3mm voxel size. Scan data were saved in DICOM format and imported to the Simplant Pro 15® (Materialize Dental, Leuven, Belgium) software for further analysis. A semi-automatic segmentation technique16 was used to isolate the affected area from the bone, teeth and soft tissue.
This was done by creating a mask in the Simplant® software. A mask is a selection of voxels within a specified range of gray values. The minimum threshold was set to the lowest (-1024: Empty spaces) while the maximum threshold was adjusted manually in a way that the created mask followed the edge of the surrounding bone structures. The mask was edited in 3D and then manually checked on every single slice using the multi slice editing tool to remove any selection that did not correspond to the cystic cavity or to select and add a missing part of the lesion. A 3D object was created, and the volume of the cyst was calculated automatically by the software in mm3 in the two datasets.
The measured volumes (Figure 7A, B) were:
Figure 7.
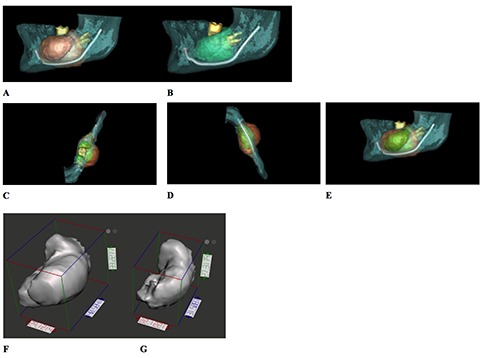
3D reconstruction of the cyst area showing the inferior alveolar nerve, the second and third molars (A) pre-op, (B) 116 days per-op. Superimposition of 2 CBCTs pre-op (orange) and 116 days per-op (green): (C) top, (D) bottom and (E) left views. Maximal linear dimensions in 3D: (F) Pre-op: X: 29.12 Y: 40.58 Z: 33.23, (G) 4 months per-op: X: 22.12 Y: 31.5 Z: 27.32.
16,775 cc pre-operatively;
6,937 cc after 4 months of decompression.
3D models of the cyst were also prepared in the Blue Sky Plan® 4.2.5 (Blue Sky Bio, LLC, Grayslake, IL, USA) software and exported as stereolithography (STL) models. A superimposition of the two datasets was realized in the same software (Figure 7C, D, E). Using the Autodesk® Meshmixer® software, the maximum linear dimensions were measured automatically in the 3 planes in mm for both datasets (Figure 7 F, G):
X: Coronal plane (maximal width/buccal- lingual direction);
Y: Axial plane (maximal length/anteroposterior direction);
Z: Sagittal plane (maximal height/corono- apical direction).
The measured linear dimensions (in mm) were: were calculated as:
X: 29.12 Y: 40.58 Z: 33.23 pre-operatively;
X: 22.12 Y: 31.51 Z: 27.32 after 4 months of decompression.
The percent reduction in maximal linear dimensions were:
X: 24.04% Y: 22.38% Z: 17.79%.
In order to assess the speed of shrinkage and the reduction in volume, four formulas
Absolute speed of shrinkage (ml/day) = (initial detected volume – final volume) / duration of decompression = 16,775-6,937 / 116 = 0.085 ml/day.
Relative speed of shrinkage (/day) = (initial detected volume – final volume) ×100 / initial detected volume × duration of decompression = 9.838 ×100/ 16,775 ×116 days = 0.51/day.
Reduction in volume (ml) = initial detected volume – final volume = 9,838 ml.
Relative reduction in volume (%) = reduction in volume × 100 / initial detected volume = 58,64%.
Discussion
The most common odontogenic cysts are apical and lateral cysts, followed by dentigerous cysts and odontogenic keratocysts. The relative incidence of dentigerous cysts according to several studies and related to different populations are 22.3% in France by Meninguaud et al.,17 33% in Mexico by Mosqueda et al.18 and 35.5% by Ledesma-Montes et al.,19 24% in Canada by Daley et al.,20 27% in Japan by Nakamura et al.,21 16.6% in South Africa by Shear et al.,22 19% in Nigeria by Arotiba et al.,23 21.3% in Germany by Kreidler et al.,24 24.8% in Jordan by Bataineh et al.25
Moreover, dentigerous cysts are the most common odontogenic cysts in children and adolescents.26 The relative incidence of dentigerous cysts in pediatric populations is 76.2% in China by Li et al.,26 59.7% in UK by Jones et al.27 68.2% in Chile by Ochsenius et al.,28 89.7% in Brazil by De Souza et al.29 and 66% in Turkey by Tekkesin et al.30
Adults have high rates of inflammatory cysts, whereas in children developmental cysts are more common.26 The most frequent unerupted teeth involved with dentigerous cysts are respectively the mandibular third molars, the maxillary permanent canines, the mandibular premolars and the maxillary third molars and the first common symptom is progressive swelling, followed occasionally by pain if infected.31
The radiographic feature is an unilocular radiolucent lesion involved with the crown of an impacted tooth. The lesion is well-defined by a radio-opaque margin. These cysts tend to resorb the roots of adjacent teeth due to their dental follicle origin. 31
Large dentigerous cysts are usually treated by marsupialization or decompression, which reduce the pressure inside the cystic cavity and permits new bone to reconstruct the bone defect. Adjacent anatomical structures such as a tooth, the sinus, or the inferior alveolar nerve can be protected from damage.32
The procedure decreased the volume of the cyst before enucleation, and extensive surgery can be avoided, and is considered the best treatment for large dentigerous cyst.33,34 The enucleation after marsupialization will be determined by the changes within the cyst cavity. Only when sufficient bone has been created, the enucleation can be safely achieved. However, some studies regarding the assessment of bone apposition after marsupialization have rarely been reported.35
The determination of the proximity of the cyst to vital adjacent anatomic structures is extremely important in enabling the surgeon to decide between marsupialization, decompression and enucleation.32
Traditionally, 4 to 6 months, after marsupialization or decompression, has been considered as a period in which sufficient bone formation to perform enucleation. However, Bodner et al.35 recommended, that cysts should be enucleated at 3 months after marsupialization, based on CT scans with multiplanar reconstruction on 23 patients with marsupialized. The disadvantages of marsupialization are poor oral hygiene in the opening area, healing time prolonged, cystic remnants left behind and patient’s cooperation. The cooperation of the patient is required for a long period of time.36,37 Pathologic epithelium left in situ may lead to epithelial proliferation and cystic recurrence.37
One other disadvantage regarding the marsupialization in the lower jaw, is the difficulty of cleaning and drainage, whilst in the upper jaw the drainage is gravity dependent.37
In our case, the stent was well fit and stable around the two molars, to avoid food impaction under it. Without being too bulky causing patient’s discomfort and without interfering with the occlusion.
Comparing our results to a previous study by Song et al.,38 compared reduction in volume in cystic diseases by 3-dimensional CT analysis, the absolute speed of shrinkage was 0,048 ml/day, reduction in volume 0,3ml, relative speed of shrinkage 26,71/day and relative reduction in volume 48,5%. So, we had a relatively faster shrinkage of the dentigerous cyst, even though our patient was 21 years old and this same study concluded that age correlated negatively with the speed of shrinkage in dentigerous cysts and the speed of shrinkage is related to the initial size of the cystic lesion.38
In this report, the evolution in the bone apposition was assessed through measurement of bone density and the cyst volume after decompression. Significant increases in bone apposition and a remarkable decrease in the cyst cavity were found 3 months after decompression, in agreement with Bodner et al.35 However, if the cyst volume is larger than 80 mL and the cortex is very thin or even absent before decompression, secondary enucleation should be delayed for another 2 to 3 months. In our case report, the total healing time was 12 months. The patient was well motivated, compliant and had no complaint about the treatment modality.
Conclusions
The decompression can be a valuable treatment for dentigerous cysts despite the need of a second surgery and the prolonged treatment. From a clinical point of view, the case treated in this paper allows us to confirm that decompression of large cyst is a very important step before enucleation and the use of 3D CBCT reconstruction was very useful for evaluation the decrease of the intra-cyst volume and to observe the bone apposition.
Funding Statement
Funding: none.
References
- 1.Browne RM. Investigative pathology of the odontogenic cysts. Boca Raton: CRC Press; 1991. [Google Scholar]
- 2.Mehra P. Benign cysts and tumors of the jaw bones. Stucker FJ, de Souza C, Kenyon GS, et al., eds. Rhinology and facial plastic surgery. Berlin, Heidelberg: Springer Berlin Heidelberg;. 2009, pp 395-429. [Google Scholar]
- 3.Manor E, Kachko L, Puterman MB, et al. Cystic lesions of the jaws- a clinicopathological study of 322 cases and review of the literature. Int J Med Sci 2012;9:20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koseoglu BG, Atalay B, Erdem MA. Odontogenic cysts: a clinical study of 90 cases. J Oral Sci 2004;46:253-7. [DOI] [PubMed] [Google Scholar]
- 5.Gondim JO, Moreira Neto JJS, Nogueira RLM, Giro EMA. Conservative management of a dentigerous cyst secondary to primary tooth trauma. Dental Traumatol 2008; 24:676-9. [DOI] [PubMed] [Google Scholar]
- 6.Dinkar AD, Dawasaz AA, Shenoy S. Dentigerous cyst associated with multiple mesiodens: a case report. J Indian Soc Pedod Prev Dent 2007;128:201-5. [DOI] [PubMed] [Google Scholar]
- 7.Meningaud JP, Oprean N, Pitak-Arnnop P, Bertrand J. Odontogenic cysts: a clinical study of 695 cases. J Oral Sci 2006;48:59-62. [DOI] [PubMed] [Google Scholar]
- 8.Ziccardi VB, Eggleston TI, Schneider R. Using fenestration technique to treat a large dentigerous cyst. JADA 1997;128:201-5. [DOI] [PubMed] [Google Scholar]
- 9.Summer M, Bas B, Yildiz L. Inferior alveolar nerve paresthesia caused by dentigerous cyst associated with three teeth. Med Oral Patol Oral Cir Bucal 2007;12:E388-90. [PubMed] [Google Scholar]
- 10.Robinson RA. Diagnosing the most common odontogenic cystic and osseous lesions of the jaws for the practicing pathologist. Mod Pathol 2017;30:S96-103. [DOI] [PubMed] [Google Scholar]
- 11.Porgel MA. The keratocystic odontogenic tumor. Oral Maxillofac Surg Clin North Am 2013;25:21-30. [DOI] [PubMed] [Google Scholar]
- 12.Kubota Y, Yamashiro T, Oka S, et al. Relation between size of odontogenic jaw cysts and the pressure of fluid with in. Br J Oral Maxillofac Surg 2004;42:391-514. [DOI] [PubMed] [Google Scholar]
- 13.Ertas U, Yavuz S. Interesting eruption of 4 teeth associated with a large dentigerous cyst in mandible by only marsupialization. J Oral Maxilofac Surg 2003; 61:728-30. [DOI] [PubMed] [Google Scholar]
- 14.Shand JM, Heggie AA. Cysts of the jaws and advances in the diagnosis and management of nevoid basal cell carcinoma syndrome. Oral Maxillofac Surg Clin N Am 2005;17:403-14. [DOI] [PubMed] [Google Scholar]
- 15.Miyawaki S, Hyomoto M, Tsubouchi J, et al. Eruption speed and rate of angulation change of a cyst-associated mandibular second premolar after marsupialization of a dentigerous cyst. Am J Orthod Dentofac Orthoped 1999;116:562-78. [DOI] [PubMed] [Google Scholar]
- 16.Ghosn N, Khoury J, Naaman N. Computer-assisted analysis of bone volume for sinus augmentation procedure. IAJD 2016;7:95-108. [Google Scholar]
- 17.Meningaud J-P, Oprean N, Pitak-Arnnop P, Bertrand J-C. Odontogenic cysts: a clinical study of 695 cases. J Oral Sci 2006;48:59-62. [DOI] [PubMed] [Google Scholar]
- 18.Mosqueda-Taylor A, Irigoyen-Camacho ME, Diaz-Franco MA, Torres-Tejero MA. Odontogenic cysts. Analysis of 856 cases. Med Oral Organo Of Soc Espanola Med Oral Acad Iberoam Patol Med Bucal 2007;7:89-96. [PubMed] [Google Scholar]
- 19.Ledesma-Montes C, Hernández-Guerrero JC, Garcés-Ortíz M. Clinicopathologic study of odontogenic cysts in a Mexican sample population. Arch Med Res 2000;31:373-6. [DOI] [PubMed] [Google Scholar]
- 20.Daley TD, Wysocki GP, Pringle GA. Relative incidence of odontogenic tumors and oral and jaw cysts in a Canadian population. Oral Surg Oral Med Oral Pathol 1994;77:276-80. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Ishida J, Nakano Y, et al. A study of cysts in the oral region. Cysts of the jaw. J Nihon Univ Sch Dent 1995;37:33-40. [DOI] [PubMed] [Google Scholar]
- 22.Shear M. Cysts of the oral regions. third. Oxford: Wright Butterworth- Heinemann; 1992. pp 75-98. [Google Scholar]
- 23.Arotiba JT, Lawoyin JO, Obiechina AE. Pattern of occurrence of odontogenic cysts in Nigerians. East Afr Med J 1998;75:664-6. [PubMed] [Google Scholar]
- 24.Kreidler JF, Raubenheimer EJ, van Heerden WF. A retrospective analysis of 367 cystic lesions of the jaw—the Ulm experience. J Cranio-Maxillo-fac Surg Off Publ Eur Assoc Cranio-Maxillo-fac Surg 1993;21:339-41. [DOI] [PubMed] [Google Scholar]
- 25.Bataineh AB, Rawashdeh MA, Al Qudah MA. The prevalence of inflammatory and developmental odontogenic cysts in a Jordanian population: a clinicopathologic study. Quintessence Int Berl Ger 2004;35:815-9. [PubMed] [Google Scholar]
- 26.Li N, Gao X, Xu Z, et al. Prevalence of developmental odontogenic cysts in children and adolescents with emphasis on dentigerous cyst and odontogenic keratocyst (keratocystic odontogenic tumor). Acta Odontol Scand 2014; 72:795-800. [DOI] [PubMed] [Google Scholar]
- 27.Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med 2006;35:500-7. [DOI] [PubMed] [Google Scholar]
- 28.Ochsenius G, Escobar E, Godoy L, Peñafiel C. Odontogenic cysts: analysis of 2,944 cases in Chile. Med Oral Patol Oral Cirugia Bucal 2007;12:E85-91. [PubMed] [Google Scholar]
- 29.de Souza L-B, Gordón-Núñez M-A, Nonaka C-F-W, et al. Odontogenic cysts: demographic profile in a Brazilian population over a 38-year period. Med Oral Patol Oral Cirugia Bucal 2010;15:e583-90. [PubMed] [Google Scholar]
- 30.Tekkesin MS, Olgac V, Aksakalli N, Alatli C. Odontogenic and nonodontogenic cysts in Istanbul: Analysis of 5088 cases. Head Neck 2012;34:852-5. [DOI] [PubMed] [Google Scholar]
- 31.Mortazavi H, Baharvand M. Jaw lesions associated with impacted tooth: A radiographic diagnostic guide. Imaging Sci Dent 2016;46:147-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Liu B, Han QB, et al. Changes in bone density and cyst volume after marsupialization of mandibular odontogenic keratocysts. J Oral Maxillofac Surg 2011;69:1361-6. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura N, Mitsuyasu T, Mitsuyasu Y, et al. Marsupialization for odontogenic keratocysts: Long-term follow-up analysis of the effects and changes in growth characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:543. [DOI] [PubMed] [Google Scholar]
- 34.Tan ZZ, Liu B, Wei JX, et al. Effects of mandibular odontogenic keratocyst surgery and removable partial prostheses on masticatory performance. J Prosthet Dent 2007;97:107. [DOI] [PubMed] [Google Scholar]
- 35.Bodner L, Bar-Ziv J. Characteristics of bone formation following marsupialization of jaw cysts. Dentomaxillofac Radilol 1998;27:166. [DOI] [PubMed] [Google Scholar]
- 36.Enislidis G, Fock N, Sulzbacher I, Ewers R. Conservative treatment of large cystic lesions of the mandible: a prospective study of the effect of decompression. Br J Oral Maxillofac Surg 2004;42:546-50. [DOI] [PubMed] [Google Scholar]
- 37.Soliman MM, Dayem Hassan HA, Elgazaerly H, et al. Marsupialization as a treatment modality of large jaw cysts. World Appl Sci J 2013;21:1752-9. [Google Scholar]
- 38.Song IS, Park HS, Seo BM, et al. Effect of decompression on cystic lesions of the mandible: 3-dimensional volumetric analysis. Br J Oral Maxillofac Surg 2015;53:841-8. [DOI] [PubMed] [Google Scholar]


