Abstract
During rehabilitation, acute renal failure due to leptospirosis occurred in eight male northern elephant seals (Mirounga angustirostris) that stranded along the central California coast in 2011. Characteristic histologic lesions including renal tubular degeneration, necrosis, and mineralization, and mild lymphoplasmacytic interstitial nephritis were noted in the six animals examined. Immunohistochemistry, bacterial culture, and PCR were positive in 2/3, 2/3, and 3/4 seals, respectively, and 6/8 had high serum antibody titers to Leptospira interrogans serovar pomona. Pulsed-field gel electrophoresis confirmed one isolate as serovar pomona. Variable number tandem repeat (VNTR) analysis showed both elephant seal isolates were identical to each other but distinct from those isolated from California sea lions (Zalophus californianus). The time from stranding to onset of azotemia was 1 to 38 (median=24) days, suggesting some seals were infected at the rehabilitation facility. Based on temporal and spatial incidence of infection, transmission among elephant seals likely occurred during rehabilitation. Molecular (VNTR) analysis of the two isolates indicates there is a unique L. interrogans serovar pomona genotype in elephant seals, and sea lions were not the source of infection prior to or during rehabilitation. This study confirms the susceptibility of northern elephant seals to leptospirosis, indicates intraspecies transmission during rehabilitation, and reports the first isolation and preliminary characterization of leptospires from elephant seals.
Keywords: Acute renal failure, immunohistochemistry, leptospirosis, Mirounga angustirostris, northern elephant seal, pulsed-field gel electrophoresis, serology, variable number tandem repeat
Leptospirosis is a ubiquitous zoonotic disease that affects a variety of animals, including marine mammals (Gulland et al. 1996). Leptospirosis is endemic in California sea lions (Zalophus californianus), though cyclic epizootics occur resulting in high mortalities (Gulland et al. 1996; Colagross-Schouten et al. 2002). However, reports in sympatric phocids, such as northern elephant seals (Mirounga angustirostris) and Pacific harbor seals (Phoca vitulina richardsii), are rare (Stamper et al. 1998; Colegrove et al. 2005). While leptospires have only been isolated from California sea lions, molecular analyses suggest that Leptospira spp. have a pattern of host specificity (Zuerner et al. 2009). Leptospira interrogans serovar pomona exclusively causes disease in otariids, such as California sea lions and northern fur seals (Callorhinus ursinus), whereas the rare reports in phocids have involved Leptospira kirschneri serovar grippotyphosa (Zuerner et al. 2009). From limited cases, leptospirosis in elephant seals manifests similarly to that in sea lions regarding clinical signs, serum biochemical abnormalities, and gross and histologic lesions (Colegrove et al. 2005). We describe additional cases of leptospirosis in northern elephant seals and report the first isolation and initial characterization of leptospires from phocids.
From late March through July, eight (9% of total in 2011) young male northern elephant seals exhibited progressive weight loss and developed acute-onset azotemia with clinical signs of lethargy, anorexia, and dehydration during rehabilitation at The Marine Mammal Center (TMMC), Sausalito, California, USA (37.8592°N, 122.4853°W). Azotemia was characterized by blood urea nitrogen (BUN) and creatinine higher than normal ranges (33–76 mg/dL and 0.2–0.8 mg/dL, respectively) and was often accompanied by hyperphosphatemia (reference range = 6.6–9.9 mg/dL) (Colegrove et al. 2005). Stranding, clinical, and postmortem findings are listed in Table 1.
Table 1.
Stranding, clinical, and postmortem data for eight male northern elephant seals (Mirounga angustirostris) in 2011. California. USA.
| Case no. | Admit date | Onset azotemia | BUNa | Creatb | Phosc | Dispd date | Dispd | Histologic lesionse | IHCf | Culture |
PCRg |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine | Kidney | Urine | Kidney | ||||||||||
| 1 | 25-Jan | 31-Mar | 156 | 3.2 | 19.1 | 3-Apr | Euth | ATN | NAh | NA | + | − | |
| 2 | l-Apr | 22-Apr | 90 | 12.9 | 7.7 | 29-Apr | Euth | ATN | NA | NA | + | − | |
| 3 | 2-Apr | 19-Apr | 153 | 2.4 | 15.4 | 17-Jul | Released | NA | NA | NA | NA | ||
| 4 | 2-Apr | 7-Apr | 31 | 0.9 | 10.8 | 9-Apr | Euth | IN | NA | NA | NA | ||
| 5 | 3-Apr | 28-Apr | 79 | 0.9 | 8.3 | 24-May | Euth | ATN | − | + | + | NA | − |
| 6 | 10-Apr | 6-May | 130 | 1.6 | 11.2 | 15-May | Euth | NA | NA | NA | NA | ||
| 7 | 10-Apr | 18-May | 279 | 4.9 | 11.2 | 23-May | Died | ATN/IN | + | + | NA | + | − |
| 8 | 21-Jul | 21-Jul | 365 | 5.7 | 19 | 21-Jul | Died | IN | + | NA | NA | ||
Blood urea nitrogen (BUN), mg/dL.
Creatinine, mg/dL.
Phosphorus, mg/dL.
Disposition.
ATN = acute tubular necrosis; IN = interstitial nephritis.
Immunohistochemistry, anti-Leptospira spp.
+ = positive; − = negative.
Not available.
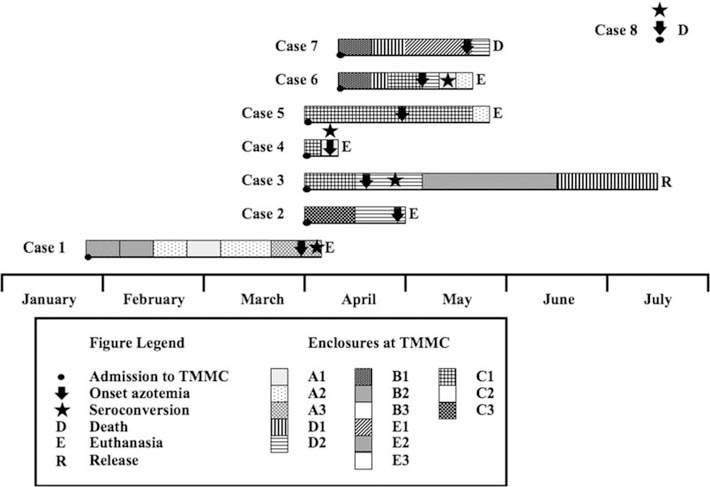
The microscopic agglutination test (MAT) was performed at the California Animal Health and Food Safety Laboratory (Davis, California, USA) on sera from each individual as previously described (Colagross-Schouten et al. 2002) (Table 2). With the exception of case 8, all seals were initially antibody negative (titer ≤100) to serovars bratislava, canicola, grippotyphosa, hardjo, icterohemorrhagiae, and pomona. Case 8 died upon admission and exhibited a strong antibody reaction to all serovars, with a maximum titer of 1:25,600 to serovar icterohemorrhagiae. Of the remaining seals, five were strongly positive to serovar pomona (range 400–25,600); three also seroconverted to hardjo; and two of these were weakly positive to icterohemorrhagiae. Sera from cases 2 and 7 were negative for all serovars based on available tested samples. The three seals with the highest titers to pomona also had titers to hardjo and icterohemorrhagiae, suggestive of cross-reactivity (Colagross-Schouten et al. 2002). Despite treatment, two seals died, and five were euthanized within 1–26 (median=55) days of admission (Fig. 1). Case 3 had a high convalescent titer to pomona (1:3,200) and developed azotemia within 17 days of rehabilitation. Following treatment, this seal had resolution of azotemia, body weight gain, and was released within 89 days. No additional samples were obtained from this individual.
Table 2.
Results of antibody testing for Leptospira serovars on eight male northern elephant seals (Mirounga angustirostris) that stranded along the coast of central California. USA, in 2011.
| Case No. | Microscopic agglutination test (MAT): Serovara | ||||||
|---|---|---|---|---|---|---|---|
| Date | Bratislava | Canicola | Grippotyphosa | Hardjo | Icterohemorrhagiae | Pomona | |
| 1 | 28-Jan | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) |
| 3-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 400 (+) | |
| 2 | 4-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) |
| 12-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | |
| 29-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | |
| 3 | 4-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) |
| 26-Apr | 100 (−) | 100 (−) | 100 (−) | 1,600 (+) | 200 (+) | 3,200 (+) | |
| 9-May | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 3,200 (+) | |
| 17-Jul | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 400 (+) | |
| 4 | 7-Apr | 100 (−) | 100 (−) | 100 (−) | 1,600 (+) | 200 (+) | 25,600 (+) |
| 8-Apr | 100 (−) | 100 (−) | 100 (−) | 800 (+) | 400 (+) | 25,600 (+) | |
| 5 | 12-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) |
| 28-Apr | 100 (−) | 100 (−) | 100 (−) | 1,600 (+) | 100 (−) | 400 (+) | |
| 23-May | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 3,200 (+) | |
| 6 | 12-Apr | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) |
| 6-May | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | |
| 15-May | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 400 (+) | |
| 7 | 19-May | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) | 100 (−) |
| 8 | 21-Jul | 6,400 (+) | 3,200 (+) | 3,200 (+) | 12,800 (+) | 25,600 (+) | 12,800 (+) |
Titers≤1:100 = negative (−); >1:100 = positive (+).
Figure 1.
Temporal and spatial data of northern elephant seals (Mirounga angustirostris) during rehabilitation in 2011, California, USA. Each square in the box at the bottom of the figure represents an enclosure separated by chain-link fence (black line between squares) or intervening corridors (white areas between columns of squares). TMMC = The Marine Mammal Center.
On postmortem examination, seals had fat atrophy and swollen kidneys with pale cortices, congestion along the corticomedullary junction, and loss of renicular differentiation. Samples of major organs from six seals were collected at necropsy, fixed in 10% neutral-buffered formalin, routinely processed, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin and eosin (HE) for histologic evaluation. In contrast to previous reports in pinnipeds, interstitial inflammatory infiltrates were a less prominent histologic feature (Gulland, et al. 1996; Stamper et al. 1998; Colegrove et al. 2005). In 3/6 seals, kidneys had moderate to severe acute tubular degeneration and necrosis with mineralization and minimal inflammation. The remaining seals had mild (n=2) to moderate (n=1) infiltrates of lymphocytes and plasma cells within the cortical interstitium, mild suppurative tubulitis, and variable tubular degeneration and necrosis. Mineralization was noted in some affected renal tubules (cases 1, 2, 4, 5–8), coronary vessels (cases 1 and 2), and adrenal gland (case 4). Case 7 had a mild pulmonary Otostrongylus circumlitis infection and mild multifocal myocardial degeneration suggestive of ischemia. Case 8 had bacterial pneumonia with evidence of sepsis, disseminated intravascular coagulopathy, and necroulcerative gastroenteritis secondary to thrombosis and infarction. Renal failure was considered the major contributing factor to death in all cases.
Immunohistochemistry (IHC) was performed on available formalin-fixed, paraffin-embedded kidney sections from cases 5, 7, and 8 using a streptavidin-biotin method and a Leptospira-specific polyclonal antibody (National Veterinary Services Laboratory, Ames, Iowa, USA) directed against L. interrogans serovars bratislava, canicola, copenhagi (icterohemorrhagiae), hardjo, and pomona and L. kirschneri serovar grippotyphosa (Colegrove et al. 2005). In cases 7 and 8, there was positive antigen staining within degenerate tubular epithelial and inflammatory cells among intratubular necrotic debris. Intact leptospires were not detected in any HE or IHC stained sections.
When available, fresh frozen (−80 °C) urine and kidney samples were analyzed by PCR using species-specific primer sets (Cameron et al. 2008). Leptospiral DNA was detected in 3/3 urine samples and zero of the 4 analyzed kidney samples. Fresh kidney and urine samples were used to inoculate Ellinghausen-McCullough-Johnson-Harris medium supplemented with 10% heat-inactivated rabbit serum and transport medium (Zuerner et al. 2009). Viable bacteria were cultured from urine and kidney samples from case 5 and urine from case 7 and signify the first leptospire isolates from phocids. Using pulsed-field gel electrophoresis (PFGE), the isolate from case 7 was identified as serovar pomona (Galloway and Levett 2010). Both isolates were further analyzed, comparing variable number tandem repeat (VNTR) patterns (Zuerner and Alt 2009). Based on preliminary analysis, the isolates from cases 5 and 7 share identical VNTR patterns that appear to be distinct from all characterized California sea lion isolates. These isolates may represent a new genotype within the pomona group, and further investigation is under way to determine the significance of this unique VNTR pattern.
To our knowledge, this outbreak represents the first documented cases of leptospirosis in elephant seals since initial reports in 1995 (Colegrove et al. 2005). All affected elephant seals were males, ranging in age from approximately 4 wk to 6 mo, and many were recently weaned. Most seals were malnourished upon admission; malnourishment and reduced or insufficient protective immunity have been recognized as factors contributing to epizootics in California sea lions (Gulland et al. 1996) and may have played a role in this outbreak. The significance of the male predominance in this event is unknown, although gender predilection is common in leptospirosis in other mammals (Gulland et al. 1996).
When considering the latency period of 10–14 days and the temporal incidence of disease (Fig. 1), most seals developed disease and seroconverted during rehabilitation (Cameron et al. 2008). With the exception of cases 4 and 8, seals were undergoing rehabilitation for 17–38 days before developing azotemia and clinical signs. Case 4 was in rehabilitation for 5 days prior to exhibiting clinical signs, so it is assumed to have been infected prior to admission. The last case (case 8) presented with severe azotemia and died immediately after arrival at TMMC; thus, this infection occurred in the wild and was unrelated to those in rehabilitation. Based on temporal and spatial incidence (Fig. 1), transmission likely occurred between seals, as affected individuals were cohoused or in proximity to each other during the outbreak. Cases 3, 4, and 5 were cohoused for several days before case 4 was moved to another enclosure, developed azotemia, and was euthanized due to poor prognosis. Within 10 days, case 3 became azotemic and was moved into an enclosure with case 2; soon after, this seal developed azotemia and was euthanized following 1 wk of treatment. Around this time, cases 6 and 7 were together moved to an enclosure neighboring case 2. Over the next month, case 6 was moved to several enclosures and, at one point, was cohoused with case 5. Within 4 wk, cases 5 and 6 became azotemic and were euthanized. Concurrently, case 7 developed severe azotemia and died within 5 days.
Five seals seroconverted to L. interrogans serovar pomona. The isolate from one elephant seal was confirmed as serovar pomona using PFGE, though VNTR patterns of both isolates were identical and distinct from patterns seen in California sea lions (Zuerner and Alt 2009). California sea lions were not considered to be the origin of infection in these two animals. As part of a larger study, these isolates will be further analyzed in attempts to determine other possible sources, including terrestrial mammals. For the remaining elephant seals, sea lions cannot be excluded as a source of infection, because pomona has long been the primary serovar found in California sea lions and rarely implicated in leptospirosis in phocids (Zuerner et al. 2009). California sea lions and northern elephant seals share haul-out areas, and leptospires have been detected via PCR in urine-contaminated sand at these sites (Cameron et al. 2008). After weaning, elephant seals spend their first months of life hauled-out and close to shore. During this period, potential for interspecies transmission is expected to be high, but it has not been confirmed. During the outbreak at TMMC, there were 27 reports of leptospirosis in California sea lions, primarily in the summer (F.M.D.G. unpubl. data). At TMMC, elephant seals and sea lions are never cohoused but are held in closely apposed enclosures, each with a pool containing saltwater supplied by the same closed circulation system, which is treated with sand filtration, ozonation, and chlorination. Despite strict disinfection and biosecurity protocols, contamination between enclosures could have occurred via fomites or humans, as bacterial survival is unlikely in the water circulation system. Together, these findings provide a scenario for transmission between elephant seals, and from sea lions to seals within the rehabilitation facility. Pacific harbor seals were considered an improbable source of infection, as there were no cases of leptospirosis in harbor seals at TMMC in 2011.
The epidemiology of leptospirosis in marine mammals is not fully understood, and our findings highlight the complexity of this disease. There is a paucity of data on antibody prevalence in northern elephant seals, though high titers have been documented in stranded pups (Stamper et al. 1998; Colegrove et al. 2005). We show that elephant seals develop severe renal disease after infection with serovar pomona, the source of which remains elusive. We also provide data indicating intraspecies transmission resulting in an outbreak during rehabilitation. Additional studies are needed to better understand the epidemiology of leptospirosis in pinnipeds, particularly northern elephant seals.
Acknowledgments
We thank Vanessa Fravel, William Van Bonn, Katie Prager, Jamie Lloyd-Smith, Denise Greig, and Rebecca Greene; Lauren Rust, Jennifer Soper, Christine Fontaine, and Carlos Rodriguez; and the staff and volunteers of The Marine Mammal Center for their support. Thanks go to Renee Walker and staff of University of Illinois Histology Laboratory for technical assistance.
LITERATURE CITED
- Cameron CE, Zuerner RL, Raverty S, Colegrove KM, Norman SA, Lambourn DM, Jeffries SJ, Gulland FM. 2008. Detection of pathogenic Leptospira bacteria in pinniped populations via PCR and identification of a source of transmission for zoonotic leptospirosis in the marine environment. J Clin Micro 46:1728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagross-Schouten AM, Mazet JA, Gulland FM, Miller MA, Hietala S. 2002. Diagnosis and seroprevalence of leptospirosis in California sea lions from coastal California. J Wildl Dis 38:7–17. [DOI] [PubMed] [Google Scholar]
- Colegrove KM, Lowenstine LJ, Gulland FM. 2005. Leptospirosis in northern elephant seals (Mirounga angustirostris) stranded along the California coast. J Wildl Dis 41:426–430. [DOI] [PubMed] [Google Scholar]
- Galloway RL, Levett PN. 2010. Application and validation of PFGE for serovar identification of Leptospira clinical isolates. PLoS Negl Trop Dis 4:e824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland FM, Koski M, Lowenstine LJ, Colagross A, Morgan L, Spraker T. 1996. Leptospirosis in California sea lions (Zalophus californianus) stranded along the central California coast, 1981–1994. J Wildl Dis 32:572–580. [DOI] [PubMed] [Google Scholar]
- Stamper MA, Gulland FMD, Spraker T. 1998. Leptospirosis in rehabilitated Pacific harbor seals from California. J Wildl Dis 34:407–410. [DOI] [PubMed] [Google Scholar]
- Zuerner RL, Alt DP. 2009. Variable nucleotide tandem-repeat analysis revealing a unique group of Leptospria interrogans serovar pomona isolates associated with California sea lions. J Clin Micro 47:1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner RL, Cameron CE, Raverty S, Robinson J, Colegrove KM, Norman SA, Lambourn D, Jeffries S, Alt DP, Gulland F. 2009. Geographical dissemination of Leptospira interrogans serovar pomona during seasonal migration of California sea lions. Vet Micro 137:105–110. [DOI] [PubMed] [Google Scholar]