Abstract
This review focuses on the role of cyclophilin D (CypD) as a prominent mediator of the mitochondrial permeability transition pore (MPTP) and subsequent effects on cardiovascular physiology and pathology. While a great number of reviews have been written on the MPTP and its effects on cell death, we instead focus more on the biology surrounding CypD itself and the non-cell death physiologic functions of the MPTP as regulated by CypD. A greater understanding of the physiologic functions of the MPTP and its regulation by CypD will likely suggest novel therapeutic approaches for cardiovascular disease, both dependent and independent of programmed necrotic cell death mechanisms.
Keywords: Heart, cyclophilin D, ischemic injury, metabolism, calcium, permeability transition, pore, mitochondria, PPIF
PPIF gene – Domains and Structure
Cyclophilin-D (CypD) is a mitochondrial matrix protein encoded by the peptidyl-prolyl cis-trans isomerase F gene (PPIF), a member of the greater cyclophilin gene family. Cyclophilins are conserved through all eukaryotic and even bacterial kingdoms and all exhibit peptidyl-prolyl cis-trans isomerase (PPIase) activity, with most members directly binding the immunosuppressive drug cyclosporin A (CsA). There are 17 cyclophilins in the human genome and all are primarily implicated in protein folding and chaperone function (1). PPIF, located on human chromosome 10, consists of 6 exons (all coding) and is highly conserved to the yeast homologue (2, 3)). As an aside, the HUGO gene nomenclature committee incorrectly annotated the CypD protein as the PPIF gene (peptidyl-prolyl isomerase F), as it should have been named PPID. The full-length CypD protein consists of 207 AA (22 kDa) with the most prominent feature being a 109 AA cyclophilin domain (cl00197, IPR002130) that imparts prolyl-isomerization activity that is conserved among most cyclophilins (4). To illustrate the importance of the isomerase domain, Baines et al. showed that an isomerase-deficient mutant of cyclophilin D (R96G) was unable to rescue mitochondrial swelling or ROS-induced cell death in Ppif−/− mouse embryonic fibroblasts (MEFs), yet a wildtype (WT) version fully rescued (5). These results suggest that the isomerase domain of CypD is necessary for modulation of the mitochondrial permeability transition pore (MPTP) (detailed more extensively in upcoming sections).
The remaining coding regions impart properties unique to CypD including the first 29 AA that encode the N-terminal mitochondrial targeting sequence and other extra-PPIase regions that may impart isomerase target specificity or binding regions for modulatory partners (1). The crystal structure of human CypD has been elucidated in complex with CsA (6). Kajitani et al. reported that CypD contained eight β-strands, two α-helices, and one 310 helix, representing a structure similar to that of the other known cyclophilin family members (6). The authors also confirmed that CsA binding sites in CypD were analogous among other cyclophilins and that binding did not require a conformational change.
Phylogenetic Conservation
As previously stated the PPIF gene is well conserved across many organisms with predicted homology noted even in plants and fungi (see Table 1, PPIF homology across species) (2, 7, 8). In vertebrates the gene is extremely well conserved with the zebrafish homologous gene (ppifb, Danio rerio) having a 78% protein identity match with H. sapiens and functionally being linked to mitochondrial permeability transition (9). The interest in mitochondrial cyclophilin in other species began with the observation that permeability transition may be a highly conserved phenomenon. Indeed, permeability transition has been noted in Drosophila, yeast, plants, fish and amphibians; for an excellent review on this topic see reference: (10). In addition to academically deciphering the relevance in situ, studying pore function in lower organisms allows high-throughput screening efforts in the search for novel permeability transition inhibitors (drug discovery) and the pursuit of the identity of the elusive ‘pore-forming’ components of the MPTP with genetic screening approaches.
Table 1.
PPIF gene homology across species.
| HomoloGene Pairwise Alignment Scores | Identity % | ||
|---|---|---|---|
| Species | Symbol | Protein | DNA |
| H.sapiens | PPIF | ||
| vs. P.troglodytes | PPIF | 99.5 | 99.5 |
| vs. M.mulatta | PPIF | 97.1 | 96.9 |
| vs. B.taurus | PPIF | 91.4 | 88.3 |
| vs. M.musculus | Ppif | 90.3 | 89 |
| vs. R.norvegicus | Ppif | 89.8 | 88.7 |
| vs. G.gallus | PPIF | 83.1 | 72.6 |
| vs. D.rerio | ppifb | 78.1 | 69.7 |
| vs. D.melanogaster | Cyp1 | 72.4 | 68.1 |
| vs. A.gambiae | AgaP_AGAP000462 | 71.9 | 68.9 |
| vs. S.cerevisiae | CPR1 | 70.4 | 64.6 |
| vs. K.lactis | KLLA0D16676g | 69.8 | 65.2 |
| vs. E.gossypii | AGOS_AGL177C | 70.4 | 67.5 |
| vs. S.pombe | ppi1 | 75.6 | 66.2 |
| vs. M.oryzae | MGG_10447 | 63.9 | 64.3 |
| vs. N.crassa | NCU00726 | 61.1 | 61.4 |
| vs. A.thaliana | CYP20–2 | 72.3 | 61.6 |
| vs. O.sativa | Os05g0103200 | 66.1 | 62.6 |
Mitochondrial Targeting and Import
The mitochondrial targeting sequence for human CypD is encoded by the following N-terminal leader sequence (MLALRCGSRWLGLLSVPRSVPLRLPAARA). The sequence computationally predicts to be a mitochondrial transit peptide recognized at the outer mitochondrial membrane (OMM) where processing allows import into the matrix. This post-translational processing results in the mitochondrial mature form (~18 kDa) that accounts for all CypD function. It had been previously hypothesized that multiple mitochondrial isoforms of cyclophilin exist, but expression of [35S]-labeled CypD by Crompton and colleagues experimentally confirmed cleavage of the predicted mitochondrial localization sequence and mitochondrial import of a single isoform (11). With careful western blotting technique we can visualize both the full-length, cytosolic CypD (~22 kDa) and also the truncated, mitochondrial-localized isoform (~18 kDa) in cardiac protein extracts. Although there is little evidence for transcriptional regulation of CypD, Matas et al. reported alterations in the subcellular distribution of CypD (mitochondrial vs. cytosolic) in rat hearts following volume-overload induced hypertrophy, suggesting another mode of potential regulation (12).
Historical Discovery of the MPTP
The first description of permeability transition can be found in a 1975 manuscript published in The Journal of Biological Chemistry by Douglas Hunter and Robert Haworth where they concluded that Ca2+ addition to isolated cow heart mitochondria induced a “…nonspecific increase in the permeability of the inner membrane, resulting in entry of sucrose into the matrix space and the observed configurational transition (swelling) of mitochondria.” (13). Further they showed that this process required the energized uptake of Ca2+ through a ruthenium red-sensitive mechanism (now known to be the mitochondrial calcium uniporter (MCU), whose genetic identity was recently discovered (14–16)) and that permeability transition resulted in the uncoupling of oxidative phosphorylation. The same group followed this discovery with numerous manuscripts detailing the nature of permeability transition and its role in physiology that have largely held to date. The authors concluded in subsequent studies that the channel is gated in a Ca2+ specific manner and that opening imparts permeability to solutes up to 1.5 kDa in size (17). Hunter and colleagues also noted that MPTP open probability is reduced by ADP, adenine nucleotides, and other divalent cations such as Mg2+ (18–20) (Sr2+, Ba2+ and Mn2+ have also been shown to inhibit permeability transition). Also see an excellent accounting of these seminal findings, including work predating Hunter and Haworth, in the expert reviews of Dr. Paolo Bernardi (21, 22).
Historical Discovery of CypD as a Component of the MPTP
Mitochondrial permeability transition is best defined as the collapse of the chemiosmotic gradient across the inner mitochondrial membrane (IMM) mediated by opening of a large conductance pore that we call the MPTP. This is not to be confused with OMM rupture, which results in the release of cytochrome c and apoptogens from the inner membrane space potentiating apoptotic cell death signaling (see the following in-depth review on OMM permeability (23)). The MPTP has long been postulated to be a channel with finite properties that spans the IMM and OMM simultaneously, although the molecular components that actually constitute this presumed structure remains undefined. Perhaps the most experimentally manipulated feature of the MPTP is that open probability is reduced by the drug cyclosporine A (CsA), which is a potent immunosuppressant originally isolated from a fungus (Tolypocladium inflatum) and found to inhibit calcineurin signaling in complex with CypA (24–27).
Preliminary observations leading to the discovery of CsA’s modulation of MPTP came from electron microscopic analysis of renal biopsies from three different patients treated with CsA, which showed “giant mitochondria with disoriented cristae and paracrystalline inclusions (28).” While this finding is now viewed in opposition to the noted acute effect of CsA blocking MPTP opening and preventing mitochondrial swelling it did focus attention on the mitochondria as a site of action for CsA and was suggestive of matrix Ca2+ overload being induced by CsA, thus prompting further investigation. Two reports examining CsA’s effect on mitochondrial respiration found an inhibitory effect on State 3 respiration and importantly noted that CsA caused a large accumulation of Ca2+ in the matrix (29, 30). This set the stage for Crompton and colleagues who first identified that CsA could inhibit the Ca2+-dependent pore of the inner membrane responsible for mitochondrial permeability transition (31). Shortly thereafter, Pfeiffer’s group confirmed this finding and proposed that CsA bound a modulatory entity specific to pore formation (32). Halestrap and Davidson followed and were the first to identify a matrix-localized PPIase as the target of CsA and further proposed a unified model of interaction with adenine nucleotide translocase (ANT) to modulate permeability transition, which was considered the IMM component of the MPTP (33). Thereafter Crompton and colleagues identified CypD as the likely PPIase controlling opening of the MPTP using a photolabeling technique (34). Genetic confirmation of CypD as a central component of the pore did not come until 2005 when the Molkentin and Tsujimoto Laboratories simultaneously deleted the Ppif gene in the mouse and noted that mitochondria isolated from these animals were resistant to swelling and permeability transition (5, 35). Other genetic loss-of-function models have corroborated these findings solidifying CypD as a bona-fide component of the MPTP (36). However, the in vivo relevance of these data have been questioned (37). Indeed, an alternate hypothesis has been proposed whereby inhibition of CypD by CsA results in the ‘unmasking’ of an as of yet unknown inhibitory binding site for inorganic phosphate (Pi) (to be examined in more detail in later sections) (38).
Biophysical Characterization of the MPTP
Two independent labs employed mitoplast patch-clamping techniques to characterize the biophysical properties of a giant (1–1.5 nanosiemens), non-specific channel of the inner membrane (39, 40). Zoratti and Szabo confirmed the identity of the large conductance channel as the MPTP by verifying it was inhibited by CsA (41). They went on to suggest in another study that the voltage-dependence of this channel was similar to that of the voltage-dependent anion channel (VDAC) showing that alpidem (a ligand of the benzodiazepine receptor, at the time thought to be comprised of VDAC and ANT and a third component) could elicit currents identical to those observed for the MPTP, thus further supporting the contact-site hypothesis whereby the MPTP spans the inner and outer mitochondrial membranes (42, 43). Szabo and Zoratti also hypothesized that the mitoplast recordings attributed to the multiple conductance channel (MCC, also referred to as the mitochondrial megachannel) and those credited to the MPTP were one in the same (44, 45). Numerous sub-conductance states have been recorded for the MPTP indicating a possible physiological relevance for the ‘sub-conductance state’ of the channel outside the well-characterized large-conductance opening associated with cellular demise (44, 46, 47). Zoratti and colleagues also employed single channel recordings of the MPTP in Ppif−/− mitochondria and found that for the most part, recordings were indistinguishable from WT controls; with the exception that CsA-inhibition was lost (48). These findings support the known regulatory role for CypD in MPTP function but that it is not an obligatory component for pore formation, as previously reported (37).
Molecular components of the MPTP (CypD Interactors)
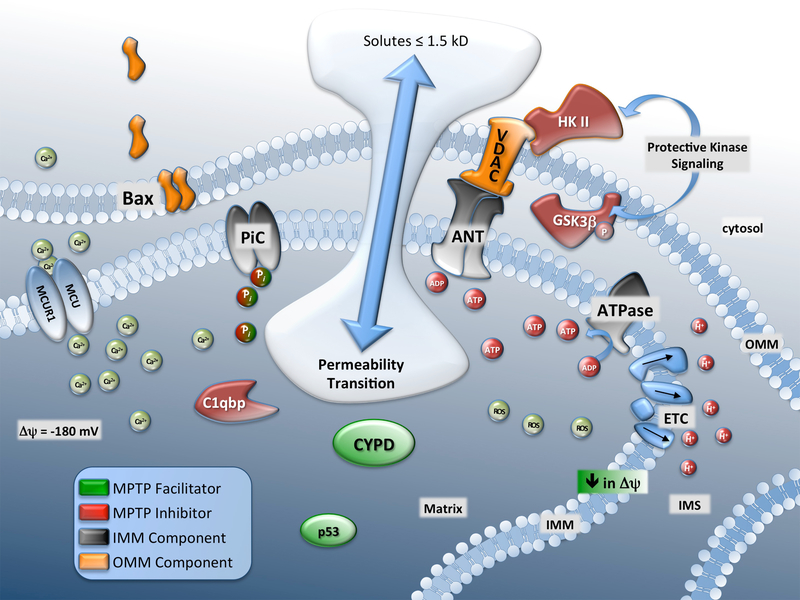
While numerous molecular constituents of the MPTP have been suggested to be necessary for pore formation only CypD has held up to genetic scrutiny. Until fairly recently, the model for the pore included the ANT in the inner membrane with the VDAC in the outer membrane together forming a continuous channel across the intermembrane space under the control of CypD (this is sometimes referred to as the ‘contact-site hypothesis’ and is largely attributed to work by Le-Quoc, Crompton and Halestrap). This model has since been largely discarded given more recent genetic loss-of-function approaches discussed below. However, there remains substantial evidence that numerous components, while not necessary for pore formation, are involved in modulating permeability transition (Figure 1).
Figure 1. CypD interactors and physiological regulators of the MPTP.
Various molecular components of the inner mitochondrial membrane (IMM) and outer mitochondrial membrane (OMM) have been proposed to form the large, non-specific mitochondrial permeability transition pore (MPTP). Upon formation the passage of solutes ≤ 1.5 kD in size cross the IMM resulting in organelle swelling and eventual rupture, a key event in necrotic cell death. Calcium (Ca2+), the most noted mediator of permeability transition, enters the matrix via the mitochondrial calcium uniporter complex (MCU and MCUR1) driven by the highly negative membrane potential (ΔΨ). Reactive oxygen species (ROS) increase MPTP open probability while adenine nucleotides (ADP and ATP) inhibit pore formation. Inorganic phosphate (Pi) while traditionally viewed as an activator of MPTP may have a dual role as an inhibitor under appropriate conditions. (key is lower left). Additional abbreviations not used in text: HK II, hexokinase-II; ATPase, F1/F0 ATPase.
ANT
The finding that bongkrekic acid, an inhibitor of ANT locking it in the “m” confirmation, was capable of delaying permeability transition, while a different inhibitor of ANT that locks it in the “c” confirmation (atractylaside) could sensitize opening in response to Ca2+ suggested that ANT could be a structural component of the MPTP (18, 49, 50). Halestrap and colleagues further developed this hypothesis, venturing that the effects of both CsA and the inhibitory action of ADP on pore opening could be attributed to direct binding of ANT to CypD (33). Using a CypD affinity column both Halestrap and Crompton independently showed molecular interaction with ANT (51, 52). Reconstitution studies by Kroemer’s and Crompton’s groups found that purified isolates of ANT, CypD, and VDAC (also Bcl2 proteins) could form a Ca2+-responsive, CsA sensitive pore in proteoliposomes (52, 53) and work by Brdiczka’s group suggested ANT could itself form a pore-like structure similar to the MPTP (54). The summation of these reports provided strong evidence that ANT was the most likely inner membrane component of the pore. This theory held until mice lacking Ant1 and Ant2 (Ant1/2−/−) were generated and mitochondria isolated from double-null hepatocytes were shown to still undergo permeability transition (CsA sensitive) and remained susceptible to cell death initiated by various agents (55). This singular finding suggested that ANT was not a structural component of the MPTP, although it should be noted that Ant1/2−/− mitochondria required a higher concentration of Ca2+ to induce opening and that ANT ligands (such as ADP) lost inhibitory capacity on pore open probability. These later observations likely suggest that ANT is part of a larger pore complex in the IMM that can directly influence the properties of the MPTP.
VDAC
The first evidence that VDAC (referred to as porin in the older literature) may be the outer membrane pore forming component of the MPTP came in 1985 when the Le-Quoc’s characterized a mitochondrial swelling event that appeared to require the OMM. They found mitoplasts (mitochondria stripped of the outer membrane and IMS constituents by osmotic disruption leaving only the IM and matrix portion of the organelle) did not respond in the classic swelling assay (56). The authors suspected VDAC was involved, possibly by migrating to the inner membrane to permit permeability transition. Crompton and colleagues further suggested VDAC’s involvement by utilizing a CypD-GST affinity column, which showed interaction with VDAC and ANT, as well as that these purified constituents could form a functional pore in proteoliposomes (52). Other reports also supported the VDAC hypothesis through anti-VDAC antibody blockade of permeability transition (57), and inhibition of the MPTP by VDAC phosphorylation (58–60). However, as with ANT, a genetic approach in the mouse eventually dispelled the notion that VDAC was a necessary component of the permeability pore. For example, Baines et al. demonstrated that deletion or knockdown of all three Vdac genes (Vdac1, 2, 3) did not disrupt MPTP function and furthermore did not alter necrotic or apoptotic cell death (61). In fact, deletion of Vdac2 enhanced cell death propensity (61, 62).
F0/F1 ATP synthase
The mitochondrial ATP synthase (complex V) is a multi-subunit complex of the inner membrane that catalyzes the synthesis of ATP via the proton gradient generated by the electron transport chain. Since the earliest characterization of the MPTP, ATP synthase has been postulated to be a possible component of the pore itself (13), although most of this conjecture can probably be attributed to indirect changes in energetic factors that alter open probability (adenine nucleotides, phosphate, Ca2+, etc.). Girogio et al. has shown that CypD directly binds the lateral stalk (subunit d and b) of ATP synthase and alters its activity (63). Another recent report utilizing siRNA-mediated knockdown found the F0 c subunit to be required for permeability transition and the authors speculated that it is a principal component of a supramolecular MPTP complex (64). These findings have been confirmed by others showing that CypD interaction decreases ATP synthesis and hydrolysis rates to modulate both energy production and necrotic cell death (65). The exact mechanism whereby CypD may interact with and modulate complex V is complicated by the previously mentioned interactions of CypD with ANT. For example, Chinopoulos and colleagues suggested that the change in adenine nucleotide exchange associated with ATP synthase binding may be partially attributed to a change in ANT flux rates (66).
Phosphate Carrier (PiC)
The phosphate carrier (PiC, SLC25A3 gene) is the primary transporter of inorganic phosphate (Pi) into the mitochondrial matrix by proton co-transport or in exchange for hydroxyl ions (67). Historically, Pi is a potent modulator of pore opening with countless studies suggesting a definitive relationship between matrix Pi content and MPTP activation (68–73). However, pore regulation by Pi has recently been reappraised, as the entire regulatory action of CypD, and thereby CsA, has been suggested to be Pi dependent (38). However, this hypothesis is in direct contrast to a recent report by Baines et al in which Pi had absolutely no effect in Ppif null or CsA-inhibited pore opening (74), a result that is supported by results from Halestrap’s group who found that Pi actually enhanced MPTP opening, but independent of CsA (73). Despite the potential effect of Pi, the PiC itself has been suggested to be either an effector of the MPTP, or possibly even a structural component (75). However, definitive proof of PiC’s involvement in the MPTP will have to await genetic analysis in Slc25a3 null mice or null cells.
BH3 Proteins: Bax and Bak
Bax and Bak are proapoptotic, BH3-containing proteins of the greater Bcl-2 family that upon conformational change, oligomerize and insert into the OMM to initiate death (76). While there is substantial historical evidence to suggest that OMM permeability may be a required component of permeability transition, how this is regulated and whether or not it is under the control of CypD or matrix constituents remains unknown. Decade-old studies have suggested direct interaction and localization of Bax with previously proposed components of the MPTP including VDAC (77, 78) and ANT (79, 80) and that these interactions may be required for pore formation. However, when these data are reappraised in the light of the genetic studies showing that ANT and VDAC are dispensable for pore formation (described above (55, 61)), Bax’s role in MPTP formation becomes enigmatic. However, a recent study suggested that Bax/Bak is a required component of mitochondrial-dependent necrosis (81). The authors found that mitochondria isolated from Bax/Bak double-null MEFs were resistant to swelling and loss of membrane potential (ΔΨ in response to Ca2+ challenge. The authors proposed that Bax may be altering mitochondrial morphology by influencing mitochondrial fusion as the dynamin-like protein 1 (Drp1) fission inhibitor (Mdivi-1) shifted morphology in Bax/Bak double-null cells to the fused state and largely restored MPTP opening. However, how this functions from the OMM to regulate CypD-dependent IMM opening is uncertain. Thus the role of Bax/Bak in regulating or contributing to the MPTP remains largely undefined.
p53
Recently p53 was reported to be a mediator of pore opening by direct interaction and activation of CypD (82). The authors present this as a new mechanism whereby p53 can modulate cellular necrosis by directly influencing MPTP formation. They suggest that the p53-facilitated increase in pore open probability is directly attributed to the mitochondrial localization of p53 and independent of both transcriptional activity and modulation of Bcl2 proteins (work from the same lab suggested that p53 can activate and oligomerize Bcl2 proteins to permeabilize the OMM (83)). While the authors present a provocative +model there are numerous inconsistencies with the known literature casting doubt on the conclusion that p53 regulates classic MPTP formation and programmed necrotic cell death (84). For example, the authors found no alteration in Ca2+-dependent MPTP opening, which is arguably the most fundamental regulator of permeability transition.
Complement component 1, q subcomponent binding protein (C1QBP)
On the basis of structural and computational analysis, Starkov has suggested C1QBP (also known as gC1qR, p32, gC1QR / 33, SF2, HABP1) is a likely candidate for the pore forming component of the inner membrane (85). C1QBP is well conserved and contains an N-terminal mitochondrial localization sequence. Jiang et al. first speculated it to be an MPTP component given predictions from the crystal structure (86). It is suggested that the β-sheet structure and other biophysical properties impart channel forming potential and a plausible target for CypD (85). Supporting observations include the apoptotic stimulants Hrk and smARF being required for mitochondrial C1QBP-dependent cell death (87, 88). In addition, expression of mitoC1QBP in cell culture induced swelling and cristae derangement of mitochondria, suggestive of MPTP activation (89). In contradiction to these observations, overexpression of C1QBP attenuated ROS-induction of the MPTP and cellular necrosis while knockdown sensitized to MPTP opening (90). In addition, the same study found C1QBP to be a soluble component of the mitochondrial matrix rather than inner membrane localization (90). Thus, while C1QBP appears to be involved with MPTP formation, further studies are required to understand at what level.
Post-translational modification of CypD
Phosphorylation
While many kinase-dependent signaling pathways have been suggested to play a role in the regulation of the MPTP (91) there is only a single report suggesting direct phosphorylation of CypD. Glycogen synthase kinase 3β (GSK3β) has been reported to directly phosphorylate CypD when recombinant proteins were used, although whether CypD is directly phosphorylated by GSK3β in vivo is unknown (92). GSK3β (an often cited modulator of the MPTP) was also shown to translocate to the mitochondrial matrix where it formed a direct interaction with CypD, but not VDAC or ANT (93). However, the role of kinases, in general, as regulators of protein activity/function within the matrix remains a controversial area for several reasons. First, there is often a lack of definitive proof that implicated kinases are actually localized within the matrix, second there is a lack of understanding of what phosphatases are present in the matrix to counteract phosphorylation and provide some sort of dynamic regulation, and third, there is often a lack of associated kinase regulatory proteins or regulatory molecules within the matrix.
Deacetylation
Two independent labs have recently proposed deacetylation as a post-translational modification that regulates CypD. Work from David Sinclair proposed that the NAD+-dependent deacetylase sirtuin-3 (Sirt3) deacetylates CypD on lysine 166, which is adjacent to the binding site for CsA (94). The authors hypothesized that the hypertrophic phenotype associated with aging in Sirt3 deleted mice is mediated by hyperactivation of the MPTP by acetylated CypD (presumably the homeostatic state is maintained by Sirt3 via direct interaction with CypD). While this hypothesis is intriguing it is complicated by previous observations that Ppif null mice are actually predisposed to hypertrophy and heart failure induced by a variety of stressors including swimming (physiological stress) and display an age-associated decrease in contractile reserve (95). This later study suggests that chronic inhibition of the MPTP does not impart a protection against age related hypertrophy but rather the inverse occurs and hypertrophic cardiomyopathy is enhanced. Since the authors failed to perform any experiments to suggest a causal relationship it’s difficult to discern if deacetylation is a true physiologic regulator of CypD. A second proposed mechanism for Sirt3 deacetylation of CypD (acetylation at Lys 145) invoked the dissociation of CypD from ANT that in turn leads to hexokinase-II detachment from the mitochondria to stimulate ATP production (96). While both of these reports suggest that deacetylation of CypD may be a control point for MPTP regulation, causal experiments are needed for confirmation.
S-nitrosylation
Nitric oxide can influence cell signaling by S-nitrosylation (SNO, post-translational thiol modification of a protein cysteine residue by covalent attachment of a NO moiety) of target proteins and multiple studies have observed cardioprotection associated with SNO (97, 98). Pertinent to the current review SNO modification of numerous MPTP modulators has been reported (including: F0F1-ATPase, creatine kinase, thioredoxin, VDAC and CypD) (98–101). To determine if SNO modification of CypD is critical to its regulatory role in controlling the MPTP the Murphy group mutated cysteine 203 (C203S) and reconstituted Ppif−/− MEFs with either WT or mutant protein (102). They found that the mutant cells were resistance to mitochondrial swelling and ROS-induced cell death suggesting that thiol modification at this site may be critical in the regulation of the MPTP. However, the C203S mutant cells behaved similarly to GSNO modified CypD in regards to mitochondrial swelling and Ca2+ retention suggesting that the mechanism of SNO modification in MPTP regulation may be to mask Cys-203 from oxidation (a post-translational redox modification often seen as the antithesis to SNO).
Physiological Function of the MPTP
CypD and the MPTP as a mitoCa2+ Efflux Channel
Given the inseparable nature between Ca2+ and MPTP regulation it was postulated that the pore itself might also affect matrix Ca2+. For example, a mathematical model suggested that under conditions of high intracellular Ca2+ cycling during periods of stress, the known mitoCa2+ efflux pathways mediated by the H+/Ca2+ exchanger and Na+/Ca2+ exchanger, would not be sufficient to prevent Ca2+ overload (103), suggesting that alternate means of removing Ca2+ is needed. The first experimental evidence of the MPTP being involved in mitoCa2+ efflux came in 1992 when Altschuld et al. demonstrated that CsA could inhibit Ca2+ efflux in isolated rat cardiomyocytes (104). This hypothesis was further developed when a biophysical model of Ca2+-induced mitochondrial Ca2+ release was presented that involved low-conductance MPTP opening (105–107). We recently bolstered this proposed physiological function for the MPTP by showing that the genetic loss of CypD results in elevated resting levels of Ca2+ in the matrix and that acute CsA treatment in cultured cardiomyocytes inhibited mitochondrial Ca2+ efflux as shown by an increase in the time rate of decay to baseline following continuous pacing (95). The elevation in matrix Ca2+ was found to be associated with enhanced activity of Ca2+-dependent mitochondrial dehydrogenases and a shift in substrate utilization from fatty acid oxidation to glycolysis in the working heart. These results suggest that the MPTP may be a control point linking mitochondrial metabolism (ATP generation) with myocardial workload (functional demand) through Ca2+ (Figure 2). In support of this study, Korge et al. recently demonstrated that asynchronous transient MPTP opening (presumably in small mitochondrial subpopulations) allowed depolarization to extrude Ca2+, yet recover ΔΨ and ATP generation (108). Additional evidence for CypD/MPTP playing a role in mitochondrial Ca2+ came from adult cortical neurons isolated from Ppif−/− mice where the authors found elevated mitoCa2+ following the activation of plasma membrane Ca2+ channels (109). It is interesting to note that the authors only saw this effect with maximal activation suggesting a threshold of Ca2+ was necessary before invoking extrusion via the MPTP, presumably because of saturation of the traditional Ca2+ efflux channels. Thus, the MPTP, as regulated by CypD, appears to be a physiologic Ca2+ release mechanism that is required for proper metabolic regulation within the mitochondria.
Figure 2. MPTP-mediated mitochondrial calcium (mitoCa2+) Efflux.
The flow chart details a possible physiological function for the MPTP in maintaining mitoCa2+ homeostasis in the heart during times of stress. Low-conductance or transient opening of the pore may represent an important route of Ca2+ extrusion from the matrix. (left side, black arrows = homeostatic conditions, right side, red arrows = pathological conditions).
Metabolic Regulator
Direct evidence for CypD modulation of energy homeostasis can be found in the previously mentioned direct-interactions with complex V (ATP synthase) and modulation by hexokinase II (a regulator of the MPTP that binds within the OMM (110–113)). Both of these proteins are intrinsically linked to ATP production and thus are potential sites of CypD regulation of cellular metabolism. In addition, if transient opening of the pore modulates matrix Ca2+ levels as discussed above, the matrix-localized dehydrogenases and components of the electron transport chain will be directly affected and ATP production impacted. In further support of CypD as a metabolic regulator, Luvisetto et al. reported that Ppif−/− mice develop adult-onset obesity (a finding corroborated by our group, unpublished data) (114). Strikingly the authors found a large increase in body weight at 6 months of age, reaching a maximal difference at 1 year of age as compared to WT controls. This increase in body weight was accompanied by a large increase in white adipose tissue with no apparent change in food intake. This report coupled with our observations of substrate switching in the hearts of Ppif−/− mice, along with significant mRNA and proteomic expression changes in metabolism-associated genes, provides a strong case that CypD may play a fundamental role in global energy homeostasis (95, 115). Whether metabolic control is directly linked to the MPTP’s role in Ca2+ extrusion from the matrix or is mediated by CypD in an MPTP-independent fashion remains to be resolved.
CypD as a Central Regulator of Programmed Necrosis
CypD has been implicated in cell death pathways since it was first identified as the target of CsA (33, 34, 52, 116, 117). The first definitive proof that CypD was critical in necrotic signaling came in 2005 with the previously mentioned reports detailing the genetic deletion of Ppif (5, 35, 36). These studies found that Ppif null cells were highly resistant to cell death induced by cytosolic Ca2+ overload, hydrogen peroxide-mediated ROS stress, and thapsigargin (SERCA inhibitor, ER Ca2+ stress) but died similar to control cells when treated with staurosporine, TNFα, and etoposide (topoisomerase inhibitor, causing DNA fragmentation). The death signature elicited by these various agents suggests that CypD is prominent in necrotic cell death but dispensable in apoptotic signaling pathways. These genetic studies provided some of the first conclusive evidence that necrosis, which has historically been viewed as a non-programmed and an accidental death process, may actually be comprised of distinct molecular signaling events. Ppif null mice have since been employed in numerous animal models, providing an extensive framework to support a central role for the MPTP in pathophysiology (see Table 2, Ppif−/− phenotypes in physiology). The role of the MPTP in programmed necrotic cell death has been extensively covered (118, 119).
Table 2.
Ppif−/− phenotypes in physiology.
| Pathology and Physiology of Ppif gene-deleted Mice | ||
|---|---|---|
| Mouse model | Phenotype as compared to WT controls | Reference |
| Myocardial IR | decreased infarct size | (5, 35) |
| Permanent LCA ligation (myocardial infarction) | increased survival, decreased scar size | (149) |
| transaortic constriction (pressure-overload heart failure) | increased pathology, decreased LV function | (95) |
| CamKIIδc-transgenic model (Ca2+-overload induced cardiomyopathy) | increased pathology, decreased survival | (95) |
| β2a-transgenic model (hyperactivation of L-type Ca2+ channel) | decreased pathology, increased survival | (150) |
| Carotid artery injury model | accelerated thrombosis | (151) |
| Renal IR | cytoprotection | (127, 128) |
| Acetaminophen hepatotoxicity | cytoprotection | (152) |
| Pdx1 haploinsufficient mice (heritable diabetes model) | preservation of pancreatic cells | (153) |
| Cerebral IR | decreased infarct size | (36, 126) |
| Cerebral Neonatal Hypoxia | increased injury | (126) |
| EAE (multiple sclerosis) | axon preservation (cytoprotection) | (154) |
| Amyloid-β mutant model (Alzheimer’s) | cytoprotection, improved learning and memory | (155, 156) |
| SOD1 G93A mutant model (ALS) | delayed disease onset, improved survival | (157) |
| Glutamate-excitotoxicity (ex vivo) | cytoprotection | (158) |
| MPTP neurotoxin administration (Parkinson’s) | acute model cytoprotective, chronic model no change | (159) |
| Sgcd−/− (muscular dystrophy) | reduced skeletal muscle pathology, cytoprotection | (143) |
| Lama2−/− (muscular dystrophy) | reduced pathology, increased survival | (143) |
| Col6a1−/− (muscular dystrophy) | reduced pathology, cytoprotection | (160) |
| Aging | adult-onset obesity | (114) |
| Aging | behavioral defects | (114) |
| Aging | decreased LV contractile reserve | (95) |
| Behavioral testing | impairments in memory | (161) |
| Endurance exercise (swimming) | increased hypertrophy and mortality | (95) |
Role is Ischemia-reperfusion injury
In work predating the identification of CypD it was demonstrated that CsA could reduce hepatic ischemic injury, advocating inhibition of the MPTP as a relevant therapeutic approach in cell death-associated diseases (120–124). In addition to the numerous studies citing cytoprotective actions of CsA in ischemia-reperfusion (IR) injury (please see the following for an extensive review (125)), causative evidence of CypD involvement can be found in studies utilizing Ppif−/− mice, which were protected in numerous IR models including the heart (5, 35), brain (36, 126), and kidney (127, 128). This potent protection is no doubt linked to direct inhibition of the MPTP as the ischemic milieu consists of the classical MPTP activators Ca2+ and ROS.
In addition to the acute effect of inhibiting CypD in IR injury it has also been suggested that pore opening plays a significant role in ischemic-preconditioning (IPC) signaling. IPC is cytoprotective signaling elicited by transient, reciprocal episodes of IR prior to a major ischemic event (129). Interestingly, Ppif−/− mice subjected to IPC or pharmacologic preconditioning were found to be refractory to cytoprotection (130). These results suggest that the MPTP may not only play a role in the catastrophic events leading to necrotic cell death but that sequential activation of the pore (perhaps transient opening) can elicit cytoprotective signaling. While this study did not provide a molecular mechanism to account for this phenomenon it’s plausible that low-conductance opening of the pore could generate superoxide or Ca2+ or modulate a pore-associated component such as GSK3β to activate discrete cytoprotective signaling pathways (some of which has recently been explored (131)). The necessity of CypD for IPC lends further credence to the notion of a physiological function for permeability transition.
Heart failure
We recently showed that Ppif−/− mice have progressively worse cardiac disease than control WT mice when subjected to a model of pressure overload-induced heart failure (95). However, our initial hypothesis was that Ppif−/− mice would be protected from heart failure by reducing the known accumulative effect of myocyte drop out that contributes to heart failure (132, 133). Mice lacking Ppif fared much worse following transaortic constriction displaying a decrease in left ventricular function (LV) and increase in LV dilation, hypertrophy and other markers of disease. Further Ppif−/− mice crossed with the Ca2+/calmodulin-dependent protein kinase IIδ-c overexpressing mouse model of cardiomyopathy and mitochondrial Ca2+ overload, displayed a decrease in survival and large increase in hypertrophy (134, 135). Even more striking Ppif−/− mice subjected to physiological exercise (swimming) also presented with an increase in hypertrophy, lung edema and even mortality (95). Crossing these globally deleted mice with a low-expressing cardiac-specific CypD transgenic line completely rescued the maladaptive phenotype, suggesting a cardiomyocyte driven disease process. Mechanistically, Ppif−/− hearts had a significant shift in substrate utilization (from β-oxidation to glycolysis), suggesting metabolic reprogramming as the likely cause of the worsening heart failure.
CypD-Based Treatment Strategies for CV Disease and Conclusions.
Clinical trials have shown that inhibition of CypD and the MPTP with CsA was protective to patients immediately after MI injury when applied during the revascularization phase (136). Clearly this study and a vast array of animal studies indicate that a MPTP inhibitor should be protective to the heart immediately following ischemic injury, although additional clinical trails are needed to solidify a new standard of care treatment with such inhibitors. However, CsA may not be the best choice of inhibitor as it also blocks the activity of calcineurin, which itself is cardioprotective (137–141). Hence, a more selective CypD inhibitor, such as Debio-025 would represent an ideal choice for acute application during revascularization post-MI injury (142–144). While Debio-025 or similar CypD-inhibiting drugs could certainly be cardioprotective in the short term following ischemic injury by preventing myocyte death, long-term use of such drugs might actually contribute to cardiac disease if a pre-existing cardiac condition is present. Ppif−/− mice were more prone to metabolic-based heart failure when stimulated, even with exercise. Thus, Cyp inhibitors are probably not applicable for chronic use in heart failure patients, even when myocyte drop out is a significant issue. Moreover, prolonged use of Cyp inhibitors in patients being treated for hepatitis C should possibly be evaluated for CV complications (145–148). Regardless of these issues, acute use of an effective Cyp inhibitor should hold great promise for limiting myocardial damage in patients immediately after infarction injury.
Acknowledgements
We thank Dr. Chris Baines at the University of Missouri for his critical reading of the manuscript. This work was supported by grants from the National Institutes of Health of the USA and the Howard Hughes Medical Institute (to JDM).
References
- 1).Davis TL, Walker JR, Campagna-Slater V, Finerty PJ, Paramanathan R, Bernstein G, et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol 2010; 8: e1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, et al. The ncbi biosystems database. Nucleic Acids Res 2010; 38: D492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, et al. Database resources of the national center for biotechnology information. Nucleic Acids Res 2012; 40: D13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, et al. Cdd: Conserved domains and protein three-dimensional structure. Nucleic Acids Res 2013; 41: D348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005; 434: 658–62. [DOI] [PubMed] [Google Scholar]
- 6).Kajitani K, Fujihashi M, Kobayashi Y, Shimizu S, Tsujimoto Y, Miki K. Crystal structure of human cyclophilin d in complex with its inhibitor, cyclosporin a at 0.96-a resolution. Proteins 2008; 70: 1635–9. [DOI] [PubMed] [Google Scholar]
- 7).Scott I, Logan DC. Mitochondria and cell death pathways in plants: Actions speak louder than words. Plant Signal Behav 2008; 3: 475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Scott I, Logan DC. Mitochondrial morphology transition is an early indicator of subsequent cell death in arabidopsis. New Phytol 2008; 177: 90–101. [DOI] [PubMed] [Google Scholar]
- 9).Azzolin L, Basso E, Argenton F, Bernardi P. Mitochondrial ca2+ transport and permeability transition in zebrafish (danio rerio). Biochim Biophys Acta 2010; 1797: 1775–9. [DOI] [PubMed] [Google Scholar]
- 10).Azzolin L, von Stockum S, Basso E, Petronilli V, Forte MA, Bernardi P. The mitochondrial permeability transition from yeast to mammals. FEBS Lett 2010; 584: 2504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Johnson N, Khan A, Virji S, Ward JM, Crompton M. Import and processing of heart mitochondrial cyclophilin d. Eur J Biochem 1999; 263: 353–9. [DOI] [PubMed] [Google Scholar]
- 12).Matas J, Young NT, Bourcier-Lucas C, Ascah A, Marcil M, Deschepper CF, et al. Increased expression and intramitochondrial translocation of cyclophilin-d associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol 2009; 46: 420–30. [DOI] [PubMed] [Google Scholar]
- 13).Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 1976; 251: 5069–77. [PubMed] [Google Scholar]
- 14).De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011; 476: 336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies mcu as an essential component of the mitochondrial calcium uniporter. Nature 2011; 476: 341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, et al. Mcur1 is an essential component of mitochondrial ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 2012; 14: 1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Haworth RA, Hunter DR. The ca2+-induced membrane transition in mitochondria. Ii. Nature of the ca2+ trigger site. Arch Biochem Biophys 1979; 195: 460–7. [DOI] [PubMed] [Google Scholar]
- 18).Hunter DR, Haworth RA. The ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 1979; 195: 453–9. [DOI] [PubMed] [Google Scholar]
- 19).Hunter DR, Haworth RA. The ca2+-induced membrane transition in mitochondria. Iii. Transitional ca2+ release. Arch Biochem Biophys 1979; 195: 468–77. [DOI] [PubMed] [Google Scholar]
- 20).Haworth RA, Hunter DR. Allosteric inhibition of the ca2+-activated hydrophilic channel of the mitochondrial inner membrane by nucleotides. J Membr Biol 1980; 54: 231–6. [DOI] [PubMed] [Google Scholar]
- 21).Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 2006; 273: 2077–99. [DOI] [PubMed] [Google Scholar]
- 22).Bernardi P Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol Rev 1999; 79: 1127–55. [DOI] [PubMed] [Google Scholar]
- 23).Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87: 99–163. [DOI] [PubMed] [Google Scholar]
- 24).Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin a-binding protein cyclophilin. Nature 1989; 337: 473–5. [DOI] [PubMed] [Google Scholar]
- 25).Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 1989; 337: 476–8. [DOI] [PubMed] [Google Scholar]
- 26).Liu J, Farmer JD Jr., Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin a and fkbp-fk506 complexes. Cell 1991; 66: 807–15. [DOI] [PubMed] [Google Scholar]
- 27).Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant fk506 is a cis-trans peptidyl-prolyl isomerase. Nature 1989; 341: 758–60. [DOI] [PubMed] [Google Scholar]
- 28).Mihatsch MJ, Olivieri W, Marbet U, Thiel G, Harder F, Zollinger HU. Giant mitochondria in renal tubular cells and cyclosporin a. Lancet 1981; 1: 1162–3. [DOI] [PubMed] [Google Scholar]
- 29).Jung K, Pergande M. Influence of cyclosporin a on the respiration of isolated rat kidney mitochondria. FEBS Lett 1985; 183: 167–9. [DOI] [PubMed] [Google Scholar]
- 30).Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 1987; 19: 297–303. [DOI] [PubMed] [Google Scholar]
- 31).Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin a of a ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 1988; 255: 357–60. [PMC free article] [PubMed] [Google Scholar]
- 32).Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin a is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 1989; 264: 7826–30. [PubMed] [Google Scholar]
- 33).Halestrap AP, Davidson AM. Inhibition of ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 1990; 268: 153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Tanveer A, Virji S, Andreeva L, Totty NF, Hsuan JJ, Ward JM, et al. Involvement of cyclophilin d in the activation of a mitochondrial pore by ca2+ and oxidant stress. Eur J Biochem 1996; 238: 166–72. [DOI] [PubMed] [Google Scholar]
- 35).Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin d-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434: 652–8. [DOI] [PubMed] [Google Scholar]
- 36).Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin d is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A 2005; 102: 12005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin d. J Biol Chem 2005; 280: 18558–61. [DOI] [PubMed] [Google Scholar]
- 38).Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin a and by cyclophilin d ablation. J Biol Chem 2008; 283: 26307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett 1989; 259: 137–43. [DOI] [PubMed] [Google Scholar]
- 40).Kinnally KW, Campo ML, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr 1989; 21: 497–506. [DOI] [PubMed] [Google Scholar]
- 41).Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin a. J Biol Chem 1991; 266: 3376–9. [PubMed] [Google Scholar]
- 42).Szabo I, Zoratti M. The mitochondrial permeability transition pore may comprise vdac molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett 1993; 330: 201–5. [DOI] [PubMed] [Google Scholar]
- 43).Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise vdac molecules. Ii. The electrophysiological properties of vdac are compatible with those of the mitochondrial megachannel. FEBS Lett 1993; 330: 206–10. [DOI] [PubMed] [Google Scholar]
- 44).Kinnally KW, Lohret TA, Campo ML, Mannella CA. Perspectives on the mitochondrial multiple conductance channel. J Bioenerg Biomembr 1996; 28: 115–23. [DOI] [PubMed] [Google Scholar]
- 45).Szabo I, Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr 1992; 24: 111–7. [DOI] [PubMed] [Google Scholar]
- 46).Pavlov E, Zakharian E, Bladen C, Diao CT, Grimbly C, Reusch RN, et al. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys J 2005; 88: 2614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Loupatatzis C, Seitz G, Schonfeld P, Lang F, Siemen D. Single-channel currents of the permeability transition pore from the inner mitochondrial membrane of rat liver and of a human hepatoma cell line. Cell Physiol Biochem 2002; 12: 269–78. [DOI] [PubMed] [Google Scholar]
- 48).De Marchi U, Basso E, Szabo I, Zoratti M. Electrophysiological characterization of the cyclophilin d-deleted mitochondrial permeability transition pore. Mol Membr Biol 2006; 23: 521–30. [DOI] [PubMed] [Google Scholar]
- 49).Haworth RA, Hunter DR. Control of the mitochondrial permeability transition pore by high-affinity adp binding at the adp/atp translocase in permeabilized mitochondria. J Bioenerg Biomembr 2000; 32: 91–6. [DOI] [PubMed] [Google Scholar]
- 50).Le Quoc K, Le Quoc D. Involvement of the adp/atp carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: Importance of the orientation of the nucleotide binding site. Arch Biochem Biophys 1988; 265: 249–57. [DOI] [PubMed] [Google Scholar]
- 51).Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-d and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 1998; 336 (Pt 2): 287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Crompton M, Virji S, Ward JM. Cyclophilin-d binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 1998; 258: 729–35. [DOI] [PubMed] [Google Scholar]
- 53).Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, et al. The permeability transition pore complex: A target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 1998; 187: 1261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Ruck A, Dolder M, Wallimann T, Brdiczka D. Reconstituted adenine nucleotide translocase forms a channel for small molecules comparable to the mitochondrial permeability transition pore. FEBS Lett 1998; 426: 97–101. [DOI] [PubMed] [Google Scholar]
- 55).Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The adp/atp translocator is not essential for the mitochondrial permeability transition pore. Nature 2004; 427: 461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Le-Quoc K, Le-Quoc D. Crucial role of sulfhydryl groups in the mitochondrial inner membrane structure. J Biol Chem 1985; 260: 7422–8. [PubMed] [Google Scholar]
- 57).Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol 2001; 152: 237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Javadov S, Rajapurohitam V, Kilic A, Zeidan A, Choi A, Karmazyn M. Anti-hypertrophic effect of nhe-1 inhibition involves gsk-3beta-dependent attenuation of mitochondrial dysfunction. J Mol Cell Cardiol 2009; 46: 998–1007. [DOI] [PubMed] [Google Scholar]
- 59).Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, et al. Protein kinase cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res 2003; 92: 873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Bera AK, Ghosh S, Das S. Mitochondrial vdac can be phosphorylated by cyclic amp-dependent protein kinase. Biochem Biophys Res Commun 1995; 209: 213–7. [DOI] [PubMed] [Google Scholar]
- 61).Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 2007; 9: 550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. Vdac2 inhibits bak activation and mitochondrial apoptosis. Science 2003; 301: 513–7. [DOI] [PubMed] [Google Scholar]
- 63).Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, et al. Cyclophilin d modulates mitochondrial f0f1-atp synthase by interacting with the lateral stalk of the complex. J Biol Chem 2009; 284: 33982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, et al. Role of the c subunit of the fo atp synthase in mitochondrial permeability transition. Cell Cycle 2013; 12: 674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Chinopoulos C, Adam-Vizi V. Modulation of the mitochondrial permeability transition by cyclophilin d: Moving closer to f(0)-f(1) atp synthase? Mitochondrion 2012; 12: 41–5. [DOI] [PubMed] [Google Scholar]
- 66).Chinopoulos C, Konrad C, Kiss G, Metelkin E, Torocsik B, Zhang SF, et al. Modulation of f0f1-atp synthase activity by cyclophilin d regulates matrix adenine nucleotide levels. FEBS J 2011; 278: 1112–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Palmieri F The mitochondrial transporter family slc25: Identification, properties and physiopathology. Mol Aspects Med 2012. [DOI] [PubMed] [Google Scholar]
- 68).Crompton M, Costi A. Kinetic evidence for a heart mitochondrial pore activated by ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular ca2+ overload. Eur J Biochem 1988; 178: 489–501. [DOI] [PubMed] [Google Scholar]
- 69).Savage MK, Jones DP, Reed DJ. Calcium- and phosphate-dependent release and loading of glutathione by liver mitochondria. Arch Biochem Biophys 1991; 290: 51–6. [DOI] [PubMed] [Google Scholar]
- 70).Savage MK, Reed DJ. Release of mitochondrial glutathione and calcium by a cyclosporin a-sensitive mechanism occurs without large amplitude swelling. Arch Biochem Biophys 1994; 315: 142–52. [DOI] [PubMed] [Google Scholar]
- 71).Drahota Z, Carafoli E, Rossi CS, Gamble RL, Lehninger AL. The steady state maintenance of accumulated ca++ in rat liver mitochondria. J Biol Chem 1965; 240: 2712–20. [PubMed] [Google Scholar]
- 72).Blaich G, Krell H, Tafler M, Pfaff E. On the state of calcium ions in isolated rat liver mitochondria. Ii. Effects of phosphate and ph on ca2+-induced ca2+ release. Hoppe Seylers Z Physiol Chem 1984; 365: 73–82. [DOI] [PubMed] [Google Scholar]
- 73).Varanyuwatana P, Halestrap AP. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 2012; 12: 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).McGee AM, Baines CP. Phosphate is not an absolute requirement for the inhibitory effects of cyclosporin a or cyclophilin d deletion on mitochondrial permeability transition. Biochem J 2012; 443: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin d and may play a key role in the permeability transition. J Biol Chem 2008; 283: 26312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Tait SW, Green DR. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010; 11: 621–32. [DOI] [PubMed] [Google Scholar]
- 77).Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel vdac. Nature 1999; 399: 483–7. [DOI] [PubMed] [Google Scholar]
- 78).Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, et al. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci U S A 1998; 95: 14681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 1998; 281: 2027–31. [DOI] [PubMed] [Google Scholar]
- 80).Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, et al. Bcl-2 and bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene 2000; 19: 329–36. [DOI] [PubMed] [Google Scholar]
- 81).Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A 2012; 109: 6566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. P53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 2012; 149: 1536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Wolff S, Erster S, Palacios G, Moll UM. P53’s mitochondrial translocation and momp action is independent of puma and bax and severely disrupts mitochondrial membrane integrity. Cell Res 2008; 18: 733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Karch J, Molkentin JD. Is p53 the long-sought molecular trigger for cyclophilin d-regulated mitochondrial permeability transition pore formation and necrosis? Circ Res 2012; 111: 1258–60. [DOI] [PubMed] [Google Scholar]
- 85).Starkov AA. The molecular identity of the mitochondrial ca2+ sequestration system. FEBS J 2010; 277: 3652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Jiang J, Zhang Y, Krainer AR, Xu RM. Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc Natl Acad Sci U S A 1999; 96: 3572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Reef S, Shifman O, Oren M, Kimchi A. The autophagic inducer smarf interacts with and is stabilized by the mitochondrial p32 protein. Oncogene 2007; 26: 6677–83. [DOI] [PubMed] [Google Scholar]
- 88).Sunayama J, Ando Y, Itoh N, Tomiyama A, Sakurada K, Sugiyama A, et al. Physical and functional interaction between bh3-only protein hrk and mitochondrial pore-forming protein p32. Cell Death Differ 2004; 11: 771–81. [DOI] [PubMed] [Google Scholar]
- 89).Chowdhury AR, Ghosh I, Datta K. Excessive reactive oxygen species induces apoptosis in fibroblasts: Role of mitochondrially accumulated hyaluronic acid binding protein 1 (habp1/p32/gc1qr). Exp Cell Res 2008; 314: 651–67. [DOI] [PubMed] [Google Scholar]
- 90).McGee AM, Baines CP. Complement 1q-binding protein inhibits the mitochondrial permeability transition pore and protects against oxidative stress-induced death. Biochem J 2011; 433: 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett 2010; 584: 1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Rasola A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, Bernardi P. Activation of mitochondrial erk protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci USA 2010; 107: 726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur J Pharmacol 2009; 604: 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, et al. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010; 2: 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin d controls mitochondrial pore-dependent ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 2010; 120: 3680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin d induces dissociation of hexokinase ii from the mitochondria. J Cell Sci 2010; 123: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97).Sun J, Murphy E. Protein s-nitrosylation and cardioprotection. Circ Res 2010; 106: 285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Kohr MJ, Aponte AM, Sun J, Wang G, Murphy E, Gucek M, et al. Characterization of potential s-nitrosylation sites in the myocardium. Am J Physiol Heart Circ Physiol 2011; 300: H1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, et al. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: Role of s-nitrosation [corrected]. Proc Natl Acad Sci USA 2004; 101: 11471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Arstall MA, Bailey C, Gross WL, Bak M, Balligand JL, Kelly RA. Reversible s-nitrosation of creatine kinase by nitric oxide in adult rat ventricular myocytes. J Mol Cell Cardiol 1998; 30: 979–88. [DOI] [PubMed] [Google Scholar]
- 101).Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in s-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 2007; 101: 1155–63. [DOI] [PubMed] [Google Scholar]
- 102).Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E. Cysteine 203 of cyclophilin d is critical for cyclophilin d activation of the mitochondrial permeability transition pore. J Biol Chem 2011; 286: 40184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).Bernardi P, von Stockum S. The permeability transition pore as a ca2+ release channel: New answers to an old question. Cell Calcium 2012; 52: 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol 1992; 262: H1699–704. [DOI] [PubMed] [Google Scholar]
- 105).Selivanov VA, Ichas F, Holmuhamedov EL, Jouaville LS, Evtodienko YV, Mazat JP. A model of mitochondrial ca(2+)-induced ca2+ release simulating the ca2+ oscillations and spikes generated by mitochondria. Biophys Chem 1998; 72: 111–21. [DOI] [PubMed] [Google Scholar]
- 106).Ichas F, Mazat JP. From calcium signaling to cell death: Two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta 1998; 1366: 33–50. [DOI] [PubMed] [Google Scholar]
- 107).Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 1997; 89: 1145–53. [DOI] [PubMed] [Google Scholar]
- 108).Korge P, Yang L, Yang JH, Wang YB, Qu ZL, Weiss JN. Protective role of transient pore openings in calcium handling by cardiac mitochondria. Journal of Biological Chemistry 2011; 286: 34851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109).Barsukova A, Komarov A, Hajnoczky G, Bernardi P, Bourdette D, Forte M. Activation of the mitochondrial permeability transition pore modulates ca2+ responses to physiological stimuli in adult neurons. Eur J Neurosci 2011; 33: 831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Fiek C, Benz R, Roos N, Brdiczka D. Evidence for identity between the hexokinase-binding protein and the mitochondrial porin in the outer membrane of rat liver mitochondria. Biochim Biophys Acta 1982; 688: 429–40. [DOI] [PubMed] [Google Scholar]
- 111).Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett 1996; 396: 189–95. [DOI] [PubMed] [Google Scholar]
- 112).Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta 1998; 1368: 7–18. [DOI] [PubMed] [Google Scholar]
- 113).Brdiczka D, Beutner G, Ruck A, Dolder M, Wallimann T. The molecular structure of mitochondrial contact sites. Their role in regulation of energy metabolism and permeability transition. Biofactors 1998; 8: 235–42. [DOI] [PubMed] [Google Scholar]
- 114).Luvisetto S, Basso E, Petronilli V, Bernardi P, Forte M. Enhancement of anxiety, facilitation of avoidance behavior, and occurrence of adult-onset obesity in mice lacking mitochondrial cyclophilin d. Neuroscience 2008; 155: 585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Menazza S, Wong R, Nguyen T, Wang G, Gucek M, Murphy E. Cypd(−/−) hearts have altered levels of proteins involved in krebs cycle, branch chain amino acid degradation and pyruvate metabolism. J Mol Cell Cardiol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin a-sensitive channel. J Biol Chem 1996; 271: 2185–92. [DOI] [PubMed] [Google Scholar]
- 117).Crompton M, Virji S, Ward JM. Cyclophilin-d binding proteins. Biochem Soc Trans 1998; 26: S330. [DOI] [PubMed] [Google Scholar]
- 118).Rasola A, Bernardi P. Mitochondrial permeability transition in ca2+-dependent apoptosis and necrosis. Cell Calcium 2011; 50: 222–33. [DOI] [PubMed] [Google Scholar]
- 119).Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res 2012; 111: 1198–207. [DOI] [PubMed] [Google Scholar]
- 120).Hayashi T, Nagasue N, Kohno H, Chang YC, Nakamura T. Beneficial effect of cyclosporine pretreatment in canine liver ischemia. Enzymatic and electronmicroscopic studies. Transplantation 1991; 52: 116–21. [DOI] [PubMed] [Google Scholar]
- 121).Yamanoi A, Nagasue N, Kohno H, Chang YC, Hayashi T, Nakamura T. Attenuation of ischemia-reperfusion injury of the liver in dogs by cyclosporine. A comparative study with allopurinol and methylprednisolone. Transplantation 1991; 52: 27–30. [DOI] [PubMed] [Google Scholar]
- 122).Kawano K, Kim YI, Kaketani K, Kobayashi M. The beneficial effect of cyclosporine on liver ischemia in rats. Transplantation 1989; 48: 759–64. [DOI] [PubMed] [Google Scholar]
- 123).Hayashi T, Nagasue N, Kohno H, Chang YC, Galizia G, Nakamura T. Evidence that cyclosporine pretreatment protects lysosomal membrane in liver ischemia in dogs. Transplantation 1989; 47: 924–6. [DOI] [PubMed] [Google Scholar]
- 124).Hayashi T, Nagasue N, Kohno H, Chang YC, Nakamura T. Beneficial effect of cyclosporine pretreatment in preventing ischemic damage to the liver in dogs. Transplantation 1988; 46: 923–4. [PubMed] [Google Scholar]
- 125).Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin a and cardioprotection: From investigative tool to therapeutic agent. Br J Pharmacol 2012; 165: 1235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126).Wang X, Carlsson Y, Basso E, Zhu C, Rousset CI, Rasola A, et al. Developmental shift of cyclophilin d contribution to hypoxic-ischemic brain injury. J Neurosci 2009; 29: 2588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127).Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin d gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 2009; 297: F749–59. [DOI] [PubMed] [Google Scholar]
- 128).Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin d and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol 2011; 301: F134–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129).Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986; 74: 1124–36. [DOI] [PubMed] [Google Scholar]
- 130).Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: The essential role of the mitochondrial permeability transition pore. Cardiovasc Res 2007; 75: 530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131).Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM. Mitochondrial cyclophilin-d as a critical mediator of ischaemic preconditioning. Cardiovasc Res 2010; 88: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest 2005; 115: 565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol 2012; 32: 1552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Zhang T, Brown JH. Role of ca2+/calmodulin-dependent protein kinase ii in cardiac hypertrophy and heart failure. Cardiovasc Res 2004; 63: 476–86. [DOI] [PubMed] [Google Scholar]
- 135).Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, et al. Calcium/calmodulin-dependent protein kinase ii contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail 2009; 2: 664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136).Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008; 359: 473–81. [DOI] [PubMed] [Google Scholar]
- 137).Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 2004; 322: 1178–91. [DOI] [PubMed] [Google Scholar]
- 138).Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/nfat coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 2004; 94: 110–8. [DOI] [PubMed] [Google Scholar]
- 139).Lim HW, De Windt LJ, Mante J, Kimball TR, Witt SA, Sussman MA, et al. Reversal of cardiac hypertrophy in transgenic disease models by calcineurin inhibition. J Mol Cell Cardiol 2000; 32: 697–709. [DOI] [PubMed] [Google Scholar]
- 140).De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW 2nd, et al. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res 2000; 86: 255–63. [DOI] [PubMed] [Google Scholar]
- 141).De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, et al. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 2001; 98: 3322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Reutenauer J, Dorchies OM, Patthey-Vuadens O, Vuagniaux G, Ruegg UT. Investigation of debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for duchenne muscular dystrophy. Br J Pharmacol 2008; 155: 574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med 2008; 14: 442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144).Tiepolo T, Angelin A, Palma E, Sabatelli P, Merlini L, Nicolosi L, et al. The cyclophilin inhibitor debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in col6a1−/− myopathic mice. Br J Pharmacol 2009; 157: 1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145).Hopkins S, Gallay P. Cyclophilin inhibitors: An emerging class of therapeutics for the treatment of chronic hepatitis c infection. Viruses 2012; 4: 2558–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146).Flisiak R, Horban A, Gallay P, Bobardt M, Selvarajah S, Wiercinska-Drapalo A, et al. The cyclophilin inhibitor debio-025 shows potent anti-hepatitis c effect in patients coinfected with hepatitis c and human immunodeficiency virus. Hepatology 2008; 47: 817–26. [DOI] [PubMed] [Google Scholar]
- 147).Flisiak R, Feinman SV, Jablkowski M, Horban A, Kryczka W, Pawlowska M, et al. The cyclophilin inhibitor debio 025 combined with peg ifnalpha2a significantly reduces viral load in treatment-naive hepatitis c patients. Hepatology 2009; 49: 1460–8. [DOI] [PubMed] [Google Scholar]
- 148).Birerdinc A, Younossi ZM. Emerging therapies for hepatitis c virus. Expert Opin Emerg Drugs 2010; 15: 535–44. [DOI] [PubMed] [Google Scholar]
- 149).Lim SY, Hausenloy DJ, Arjun S, Price AN, Davidson SM, Lythgoe MF, et al. Mitochondrial cyclophilin-d as a potential therapeutic target for post-myocardial infarction heart failure. J Cell Mol Med 2011; 15: 2443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150).Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 2007; 117: 2431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151).Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, et al. Critical role for the mitochondrial permeability transition pore and cyclophilin d in platelet activation and thrombosis. Blood 2008; 111: 1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152).Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin d deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res 2011; 45: 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153).Fujimoto K, Chen Y, Polonsky KS, Dorn GW 2nd. Targeting cyclophilin d and the mitochondrial permeability transition enhances beta-cell survival and prevents diabetes in pdx1 deficiency. Proc Natl Acad Sci USA 2010; 107: 10214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154).Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, et al. Cyclophilin d inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA 2007; 104: 7558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155).Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin d deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in alzheimer’s disease. Nat Med 2008; 14: 1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156).Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin d deficiency improves mitochondrial function and learning/memory in aging alzheimer disease mouse model. Neurobiol Aging 2011; 32: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157).Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: Involvement in the pathobiology of als mice. Exp Neurol 2009; 218: 333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158).Li V, Brustovetsky T, Brustovetsky N. Role of cyclophilin d-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp Neurol 2009; 218: 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159).Thomas B, Banerjee R, Starkova NN, Zhang SF, Calingasan NY, Yang L, et al. Mitochondrial permeability transition pore component cyclophilin d distinguishes nigrostriatal dopaminergic death paradigms in the mptp mouse model of parkinson’s disease. Antioxid Redox Signal 2012; 16: 855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160).Palma E, Tiepolo T, Angelin A, Sabatelli P, Maraldi NM, Basso E, et al. Genetic ablation of cyclophilin d rescues mitochondrial defects and prevents muscle apoptosis in collagen vi myopathic mice. Hum Mol Genet 2009; 18: 2024–31. [DOI] [PubMed] [Google Scholar]
- 161).Mouri A, Noda Y, Shimizu S, Tsujimoto Y, Nabeshima T. The role of cyclophilin d in learning and memory. Hippocampus 2010; 20: 293–304. [DOI] [PubMed] [Google Scholar]