Abstract
Chronic kidney disease (CKD) is associated with excess cardiovascular mortality, resulting from both traditional and nontraditional, CKD-specific, cardiovascular risk factors. Nontraditional risk factors include the entity Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) which is characterised by disorders of bone and mineral metabolism, including biochemical abnormalities of hyperphosphatemia and hyperparathyroidism, renal osteodystrophy, and vascular calcification. Increased arterial stiffness in the CKD population can be attributed amongst other influences to progression of vascular calcification, with significant resultant contribution to the cardiovascular disease burden. Pulse wave velocity (PWV) measured over the carotid-femoral arterial segments is the noninvasive gold-standard technique for measurement of aortic stiffness and has been suggested as a surrogate cardiovascular end-point. A PWV value of 10 m/s or greater has been recommended as a suitable cut-off for an increased risk of cardiovascular mortality. CKD is a risk factor for an excessive rate of increase in aortic stiffness, reflected by increases in PWV, and increased aortic PWV in CKD shows faster progression than for individuals with normal kidney function. Patients with varying stages of CKD, as well as those on dialysis or with a kidney transplant, have different biological milieu which influence aortic stiffness and associated changes in PWV. This review discusses the pathophysiology of arterial stiffness with CKD and outlines the literature on PWV across the spectrum of CKD, highlighting that determination of arterial stiffness using aortic PWV can be a useful diagnostic and prognostic tool for assessing cardiovascular disease in the CKD population.
1. Introduction
Chronic kidney disease (CKD) is increasingly recognised globally as a major public health problem. CKD is defined as abnormalities of kidney structure and/or function, present for at least 3 months, the prevalence of which is estimated to be between 8 and 16% worldwide [1]. Patients with CKD have substantially increased morbidity and mortality compared to individuals without CKD, with the increase in disease burden and outcome events largely attributed to cardiovascular disease [2]. Considerable cardiovascular risk in the CKD population results not only from traditional cardiovascular risk factors, but also from atypical, CKD-specific cardiovascular risk factors including abnormalities of mineral and bone metabolism [3, 4].
Cardiovascular disease is the leading cause of death in CKD and a predominant driver for this is increased conduit artery stiffness, in particular of the elastic thoracic aorta. Increased carotid-femoral aortic stiffness, as measured by segmental pulse wave velocity (PWV), is a strong, independent predictor of cardiovascular mortality in this population [5, 6]. Although no current therapies are available, aortic stiffness is a potentially modifiable cause of cardiovascular dysfunction and a useful biomarker in risk stratification for patients with CKD. Previous studies have suggested that therapeutic modification of arterial stiffness may ameliorate cardiovascular mortality [7].
Carotid-femoral PWV is utilised as the noninvasive gold standard measure of arterial stiffness, with assessment of changes in vascular stiffness having been advocated as a suitable surrogate cardiovascular end-point in clinical trials. Development of vascular calcification and arterial stiffness observed with progression of CKD results in a different age-related pattern of PWV change than in the disease-free normal population. In addition, PWV has been demonstrated to characterise the progression of arterial disease in a CKD population [8, 9]. This review briefly outlines the pathophysiology of increased arterial stiffness in patients with CKD and discusses the use of PWV to assess arterial stiffness, as well as PWV changes mediated by interventions in clinical trials, in this population.
2. Pathophysiology of Aortic Stiffness and Cardiovascular Disease
Functional aortic stiffness is a result of both normal nonlinear pressure dependent changes in aortic wall properties and (usually irreversible) intrinsic structural changes. Increased aortic stiffness and its relatively easily measured manifestation, inappropriately increased PWV, result in the pathophysiological consequences of an increase in the magnitude of the aortic pressure waveform as well as other changes in the morphology of this wave including more rapid decay of diastolic blood pressure magnitude resulting in increased pulse pressure measured either centrally or at the brachial artery. These alterations in pressure wave morphology are associated with increased pulsatility exposure in feeding arteries to low impedance vascular beds, such as the kidneys and brain, with organ parenchyma exposed to high levels of mean blood pressure and increased mechanical strain, as well as being atherogenic in coronary arteries and associated with a decreased ability to augment coronary blood flow in hyperemic states.
There remain controversy and uncertainty regarding the predominant cause of these deleterious central blood pressure changes with conflicting hypotheses put forward. The traditional model of central pressure augmentation due to increased pressure wave reflection and from a predominant distal reflection site has been challenged by a more contemporary suggestion. This proposes that increased central blood pressure is predominantly due to increased proximal aortic stiffness and in fact decreased impedance mismatch distally which is caused by increased pulse pressure due to increased central pressure propagating distally [10].
Whatever the predominant underlying mechanism for the effect of increased central blood pressure and other changes in the pressure waveform associated with increased aortic stiffness, the clinical sequelae include increased cardiovascular and cerebrovascular events and deleterious effects on kidney function. Any primary renal effects that increase blood pressure will exacerbate the “vicious cycle” relationship between operating blood pressure and intrinsic aortic wall stiffness. Increased blood pressure causes increased functional stiffness due to the nonlinear volume pressure relationship of the aortic wall. Similarly, CKD or degenerative processes that primarily increase aortic stiffness will increase blood pressure.
3. Arterial Stiffness and Ageing
Arteries demonstrate a gradual, age-related impairment in vascular function likely related to reduction in endothelium-derived nitric oxide bioavailability and increased production of vasoconstrictors [11, 12]. Increased exposure and impaired ability for defence mechanisms to resist oxidative stress and inflammation may contribute to age-related changes in vascular function. Arteries also undergo structural changes with age including gradual thickening of the arterial wall, changes in wall content (advanced glycation end-products and less elastin), and an increase in conduit artery diameter [13]. These changes in structure have important interactive effects on artery function, with increases in small and large arterial stiffness representing a characteristic change with older age.
Increased aortic stiffness with ageing, and in association with disease processes, is often the result of medial degeneration, i.e., the process of arteriosclerosis, in contrast to atherosclerosis. Atherosclerosis is the underlying pathology of cardiovascular disease and is characterised by abnormalities in lipid metabolism leading to lipid-filled macrophage deposition in the subendothelial layers of the arterial wall, which results in chronic inflammation [14, 15]. Atherosclerotic disease contributes to the majority of vascular pathology in the general population with traditional Framingham cardiovascular risk factors, and different nonmodifiable risk factors such as genetic predisposition and ethnicity are also increasingly being understood.
Arteriosclerosis is associated with direct structural changes including elastin fragmentation and medial calcification but is also affected by interaction with cellular and molecular changes in the overlying intimal layer, i.e., the inflammatory atherosclerotic process. Increases in arterial stiffness with arteriosclerosis, independent of mean arterial pressure, result in end-organ damage by imposing hemodynamic stress on vascular beds, particularly vascular beds of low impedance and high flow. Different disease entities, in addition to ageing, contribute to arteriosclerosis and increased arterial stiffness dependent upon risk cohorts.
4. Pathophysiology of Arterial Stiffness and CKD
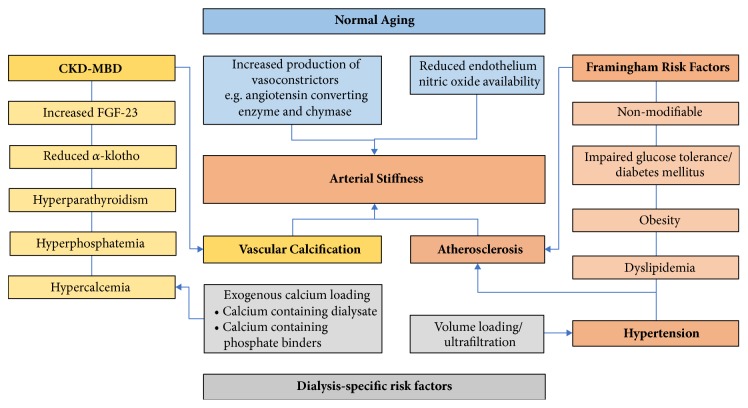
Accelerated ageing is observed in patients with CKD and the mechanisms underlying arterial stiffness in CKD are extremely complex (Figure 1). One of the contributing factors is vascular calcification associated with Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). CKD-MBD is a complex entity comprising multiple mineralisation abnormalities, including hyperphosphatemia, hyper- and hypocalcemia, and hyperparathyroidism. As an active process, and in combination with a reduction in calcification inhibitors, deranged calcium and phosphate metabolism promotes vascular calcification in patients with CKD. Vascular calcification can either take place in the intima or in the media of the vessel wall. Calcification of the intima is a part of atherosclerosis, while medial calcification is the hallmark of arteriosclerosis. Both are prominent in CKD but arteriosclerosis primarily has an important role in the development of arterial stiffness in this population.
Figure 1.
Pathophysiological factors contributing to arterial stiffness in patients with chronic kidney disease. Abbreviations: CKD-MBD, Chronic Kidney Disease–Mineral Bone Disorder; FGF-23, fibroblast growth factor 23.
Elevated serum phosphate is a late manifestation of CKD and has been shown to accelerate mineral deposition in vessel walls leading to increased vascular calcification and arterial stiffness. α-Klotho and fibroblast growth factor 23 (FGF-23) are emerging factors in CKD-MBD and are thought to be involved in the pathogenesis of vascular calcification [16, 17]. There has been conflicting data regarding the relationship between PWV and FGF-23 however, despite the association of FGF-23 with progression in CKD and change in vascular calcification [18]. A recent observational study assessing PWV, aortic calcification, and bone mineral markers over a 12-month period in patients with advanced CKD reported a change in FGF-23 associated with changes in aortic calcification, although no change was seen in α-klotho [19]. There are changes in multiple other inhibitor and promotor proteins associated with the complex process of vascular calcification in patients with CKD, many of which are involved in normal bone formation. These include reduction in fetuin-A (a circulating inhibitor responsible for complexing with insoluble calcium-phosphate complexes), reduction in matrix Gla protein (another important inhibitor which can modify vascular calcification), and an increase in osteoprotegerin (which may inhibit vascular calcification through the RANK ligand pathway, although there is conflicting literature about its role) [20].
In patients with CKD, deleterious mechanisms are operative and lead to the increase in clinical manifestations of cardiovascular disease. The presence of CKD can be considered as both cause and effect of the increased aortic stiffness indicated by increased values of carotid-femoral PWV, which provides a potentially integrative pathophysiological mechanism linking CKD to increases in cardiovascular and cerebrovascular events.
5. Use of PWV to Determine Arterial Stiffness
PWV was initially described as a physiological concept by Crighton Bramwell in the early 1900s. He noted that the velocity of the pulse wave will have a proportional relationship to arterial wall tension and blood pressure [21]. With increasing technological advances in the later part of the century, a number of different techniques for determining vascular compliance were discovered. PWV can now be reliably measured by a combination of different tools which may include electrocardiography, blood pressure cuffs, and carotid tonometry. These methods estimate the time taken for the pulse wave to travel between two spaced arteries and subsequently determine a velocity measurement calculated from the elapsed time and distance between the two points. Most commonly, this is between the carotid and femoral arteries, although brachial ankle PWV has also been determined in studies [22]. Measuring PWV in vessels of closer proximity can be less accurate. Regardless of the site of measurement, correlations exist between different types of PWV measurement, including the use of magnetic resonance imaging (MRI) to determine PWV [23].
Ambulatory PWV determined over a 24-hour period has been validated against both invasive and noninvasive measurements of arterial stiffness [24, 25]. This 24-hour ambulatory method involves measurement of the brachial artery oscillometric blood pressure waves to estimate PWV and correlates to dynamic changes in blood pressure with activity throughout the day [26]. These ambulatory methods have been listed as a viable method for measurement of PWV in a statement by the American Heart Association on standardising vascular research in arterial stiffness [27].
6. PWV and Predictive Value in the Non-CKD Population
Reference values for PWV in a population with normal kidney function have been determined via a subgroup of the “Reference Values for Arterial Stiffness” Collaboration database, measuring PWV in 11,092 subjects [25]. This collaboration determined the difference in PWV according to cardiovascular risk factors, including smoking status, as well as for nonmodifiable risk factors such as age or gender. Of note, there is an observable increase in PWV of 1 m/s for every decade above midlife and PWV also increased by mean tertile of blood pressure [25]. Community-based cohorts such as AGES-Reykjavik and Framingham have also shown that PWV changes in the presence of other cardiometabolic risk factors, such as obesity, dysglycemia, hypertension, and hypercholesterolemia [28].
In the 2018 European Society of Cardiology Guidelines, a consensus PWV threshold of 10 m/s was reported as suggestive of increased cardiovascular risk and appropriate to be utilised to stratify intermediate risk patients (Grade 2b recommendation) [29]. This recommendation is based on the association between higher PWV with increased mortality and cardiovascular events. In a systemic meta-analysis of 17 original studies analysing cardiovascular events and mortality in relation to aortic PWV, the pooled relative risk for all-cause mortality was 1.15 for an increase in 1 m/s and 1.42 for an increase by one standard deviation (SD) [30]. A similar relative risk was also observed for cardiovascular mortality, with a predictive value independent of other traditional cardiac risk factors. Of note, this meta-analysis was all-inclusive, including patients in the general population at risk as well as higher risk subgroups, including patients with end-stage kidney disease (ESKD).
In a more recent meta-analysis of 16 studies (17,635 patients), Ben-Shlomo et al. assessed the predictive validity of aortic PWV for cardiovascular disease events beyond conventional cardiovascular risk factors and reported that PWV may enable better identification of high-risk populations who could benefit from more aggressive cardiovascular risk factor management [31]. After adjusting for conventional risk factors in this systematic review, PWV was a significant predictor of coronary heart disease (HR 1.23 [95% CI, 1.11 - 1.35]), stroke (HR 1.28 [95% CI, 1.16 - 1.42]), and cardiovascular events (HR 1.30 [95% CI, 1.18 - 1.43]).
Numerous other studies have evaluated the cardiovascular risk profile in patients with higher PWV. In a follow-up of the Rotterdam Study, aortic PWV, when separated into different tertiles, had a significant impact on cardiovascular mortality [32]. Coronary heart disease-free survival matched a model of logistic regression in the 3350 patients followed up over ten years in this study. Following from this premise, PWV has been shown to have a similar predictive value for other forms of large vessel atherosclerotic vascular disease, such cerebrovascular disease and peripheral vascular disease, although similar major cardiovascular end-points are utilised in studies [33]. PWV has also been shown to predict mortality and assist in prognostication for determining functional outcomes following an acute ischemic stroke [34], and an association is also seen with peripheral vascular disease patients who demonstrate increased mortality with higher tertiles of PWV. However, there are no studies to date which evaluate the predictive value of PWV on requirement of vascular intervention such as amputation [35].
More recent studies have evaluated the association between PWV and the development of cognitive impairment, independent of cardiovascular risk factors [36]. This relationship may be due to microvascular cerebral damage, associated with pressure effects on cerebral parenchyma in the context of increased central blood pressure secondary to increased aortic stiffness [37]. Increased PWV scores in longitudinal studies of those with cognitive impairment have been associated with poorer scores on verbal and cognitive testing, subclinical vascular brain injury, and in some studies, PWV has been an independent risk factor of cognitive decline [38, 39]. In one longitudinal cohort study, the odds of cognitive impairment increased by 40% in the context of higher tertiles of PWV [40].
7. PWV in the CKD Population
Patterns of PWV progression are different in the CKD population, with clinical manifestations of CKD often related to an accelerated ageing process and an increase in vascular calcification worsening with advancing CKD stages. Vascular calcification is reported to increase in both frequency and severity with deterioration in renal function, with an estimated 80-90% of patients having heavy calcific burden with CKD Stage 5D (on dialysis) [41–43]. Young patients, even those aged under 30 years, with advanced CKD or ESKD demonstrate a considerable prevalence of arterial calcification, which correlates with the significant cardiovascular burden in this population [42]. This accelerated aging process may also have a molecular basis, with reduced levels of soluble α-klotho in patients with CKD which are associated with increased arterial stiffness.
7.1. PWV in Nondialysis CKD Patients
CKD leads to an accelerated increase in PWV compared to the general population. Evaluation of carotid-femoral PWV in 150 patients with varying stages of CKD demonstrated early onset of elevated aortic stiffness and increased rate of progression over a year in CKD patients in contrast to those with normal kidney function, although in this study there was no significant change in PWV between different levels of kidney function [43]. In observational studies, change in progression of PWV by approximately 1 m/s each year is reported in patients with CKD [44], which represents an increased rate in progression of arterial stiffness in comparison to the normal ageing process [19]. Observational studies in the Rotterdam cohort also demonstrated an association between arterial stiffness and decline in kidney function, with an increased relative risk of CKD of 1.08 (95% CI, 1.03 - 1.14) for each SD higher PWV [45]. Increased PWV with greater vascular calcification is associated with higher mortality [46], perhaps due to the intimate relationship between blood pressure, vascular events, and the clinical entity of CKD known as renovascular disease. Table 1 highlights a number of key observational trials in CKD cohorts which utilise PWV as an outcome.
Table 1.
Prospective observational studies assessing PWV in patients with CKD.
| Study | Population and follow-up | Outcome |
|---|---|---|
| Zoungas et al, 2007 (ASFAST) [6] |
315 CKD Stage 4–5, median 42 months follow-up | PWV predictive of composite end point of cardiovascular events; HR for every 1 m/s increase in PWV was 1.18 (95% CI, 1.12 - 1.25) |
| Chen et al, 2012 [50] |
186 CKD Stage 3–5 patients, mean 22 months follow-up | PWV associated with increased rate of CKD progression |
| Tholen et al, 2013 [44] | 70 CKD Stage 2–4, 12 months follow-up | Increase in PWV over 1 year by 1.1 m/s |
| Baumann et al, 2014 [51] | 135 CKD Stage 2–4, 42 months follow-up | Aortic PWV ≥ 10 m/s an independent predictor of mortality; HR 5.1 (95% CI, 1.1 - 22.9) |
| Chandra et al, 2014 [52] | 240 CKD Stage 3–5, up to 48 months follow-up | Low heart rate variability and high PWV associated with increase in first cardiovascular event; HR 1.19 (95%CI, 1.09 - 1.31) |
| Townsend et al, 2015 [9] (CRIC study) |
2800 CKD, 7-10 years follow-up | Increase in PWV according to CKD Stage by up to 1 m/s per year; increasing PWV associated with cognitive impairment, mortality, heart failure admissions. |
| Cseprekal et al, 2016 [53] | 94 CKD Stage 1–5 patients, median 52 months follow-up | PWV related to cardiovascular events; other associations included increasing calciprotein particles |
| Krishnasamy et al, 2017 [19] | 42 CKD Stage 4 vs 40 healthy controls, 12 months follow-up | CKD associated with higher PWV (9.7[7.6-11.7] vs 8.1[7.2-9.7] m/s), with mean progression of PWV 1.3 m/s over 12 months |
| Kim et al, 2017 [54, 55] (KNOW-CKD) |
2238 CKD Stage 1–5, over 10 years follow-up | Higher PWV with increasing CKD stage; also associated with increased aortic calcification and reduced bone mineral density |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease;HR, hazard ratio;PWV, pulse wave velocity.
ASFAST, Atherosclerosis and Folic Acid Supplementation Trial.
CRIC, Chronic Renal Insufficiency Cohort.
KNOW-CKD, KoreaN Cohort Study for Outcomes in patients With Chronic Kidney Disease.
A number of interventional trials have been conducted aiming to reduce the progression of arterial stiffness in patients with CKD and using PWV as a measure of arterial stiffness. The majority of studies aim to attenuate existing Framingham risk factors or proteinuria to improve arterial stiffness. Interventions have included eplerenone, enalapril, and candesartan in combination and atorvastatin (Table 2) [47–49]. Dual ACE/ARB inhibition demonstrated a small change in PWV over time, however counteracted by issues with progression of kidney disease and significant hyperkalemia leading to almost 15% of patients being withdrawn from this trial due to safety events. Other vascular interventional studies have only recruited small numbers of participants and are likely to be underpowered to demonstrate benefits on PWV.
Table 2.
Interventional trials in the non-dialysis CKD population using PWV as a reported outcome.
| Study | Population and follow up | Intervention | Outcome |
|---|---|---|---|
| Lipid-lowering trials | |||
| Fassett et al, 2010 [47] (LORD) |
37 CKD Stage 2–4, >3 years follow-up | Atorvastatin 10mg daily vs placebo | 48% slower increase in PWV with atorvastatin (not significant) |
| Morita et al, 2014 [56] | 37 CKD Stage 2–5, >24 weeks follow-up | Ezetimibe 10mg daily | Significant reduction in brachial ankle PWV (1,770.4±590.3 cm/s to 1,702.5± 519.9 cm/s), but no correlation with LDL levels |
| Anti-hypertensive trials | |||
| Edwards et al, 2009 [57] | 112 CKD Stage 2–3, 40 weeks follow up | Spironolactone 25mg daily vs placebo | Improvement in PWV -0.8 +/- 1.0m/s vs -0.1 +/- 0.9m/s |
| Frimodt-Møller et al, 2012 [48] | 67 CKD Stage 3–5, 24 weeks follow-up | Monotherapy with ACEI or ARB, then randomised to dual therapy | Improved arterial stiffness in dual RAS blockade cohort (PWV -0.3m/s) |
| Boesby et al, 2013 [49] ALBLOCK-2 |
54 CKD Stage 3–4, >24 weeks follow-up | Open label trial of eplerenone vs placebo | No effect on PWV; reduction in pulse wave reflection with eplerenone |
| CKD-MBD Modulation trials | |||
| Chitalia et al, 2014 [58] | 26 CKD Stage 3–4, 18 weeks follow up | Vitamin D supplementation 300,000 units at baseline and 8 weeks | No significant effect on arterial stiffness; change in endothelial biomarkers with reductions in ICAM-1, VCAM-1 |
| Kumar et al, 2017 [59] | 120 CKD Stage 2–4, 16 weeks follow-up | Cholecalciferol 300,000 IU at baseline and 8 weeks vs placebo | Significant reduction in PWV with cholecalciferol (-1.24 m/s over 16 weeks) |
| Levin et al, 2017 [60] | 87 CKD Stage 3b-4, 6 months follow-up | Vitamin D 5000 IU daily, calcitriol vs placebo | Non-significant change in PWV with fixed dose cholecalciferol |
| Seifert et al, 2013 [61] | 38 CKD Stage 3, 12 months follow-up | Lanthanum carbonate vs placebo | Non-significant change in PWV |
| Chue et al, 2013 [62] (CRIB-PHOS) |
120 non-diabetic CKD Stage 3, 40 weeks follow-up | Sevelamer carbonate vs placebo | No change observed in PWV |
| Non-pharmacological trials | |||
| Van Craenenbroek et al, 2015 [63] | 48 CKD Stage 3–4, 3 months follow-up | Parallel group design, 3-month home based aerobic training program (4 daily training sessions of cycling) vs control | No significant change in PWV |
Abbreviations: CKD, chronic kidney disease;ICAM-1, Intercellular Adhesion Molecule 1; LDL, low-density lipoprotein;PWV, pulse wave velocity; VCAM-1, Vascular Cell Adhesion Molecule 1.
ALBLOCK-2, Aldosterone Blockade in CKD.
LORD, Lipid Lowering and Onset of CKD trial.
One randomized controlled trial involving patients with CKD Stages 3b to 5 assessed the effect of vitamin D supplementation on arterial stiffness measured by PWV [60]. Patients were randomised to placebo, calcitriol, or calcifediol. In advanced CKD, there appeared to be a trend towards improvement over a 6-month period with vitamin D supplementation when adjusted for baseline, particularly in patients who were vitamin D deficient. However, there was also a significant reduction in parathyroid hormone, an expected normal physiological response to vitamin D supplementation, which may have affected the results. A smaller cohort evaluating the efficacy of two large pulsed doses of cholecalciferol (300,000 units) over 16 weeks did not demonstrate a significant change in PWV [58]. However, reductions were seen in biomarkers of endothelial function such as E-selectin, Intercellular Adhesion Molecule 1 (ICAM-1), and Vascular Cell Adhesion Molecule 1 (VCAM-1), which may suggest alterations to vascular remodelling. No significant change in FGF-23 was noted and limitations of this study included a short follow-up period and the study was underpowered to demonstrate significant alterations in PWV.
In an attempt to improve cardiovascular disease, the use of phosphate binders in nondialysis CKD patients has also been studied in randomised trials with assessment of changes in PWV as a surrogate end-point. These studies however, which have varied from 38 to 120 patients with CKD Stages 3 and 4 and involved varying phosphate binder treatment (lanthanum, sevelamer, and calcium-based binders) over 9- to 12-month periods, have not shown any change in PWV with this intervention [61, 62].
7.2. PWV in Dialysis Patients
Long-term cardiovascular outcomes of patients undertaking either peritoneal dialysis or conventional satellite hemodialysis are similar, but overall they are significantly worse compared to age- and gender-matched individuals in the general population [64]. Longer hours hemodialysis however has been associated with improved cardiovascular outcomes in observational trials, when compared to conventional hemodialysis, perhaps related to improved ultrafiltration rates and potentially better middle molecule clearance on dialysis [65].
Conflicting reports exist in observational studies regarding the impact of dialysis modality on arterial stiffness as measured by PWV [66, 67]. Studies present disparate results, likely in the setting of small patient numbers, although different dialysis modalities certainly all affect arterial stiffness. In a longitudinal study analysing differences in PWV over 12 months between patients on peritoneal dialysis and hemodialysis, increased progression of arterial stiffness was observed with hemodialysis [68]. The most significant difference between the cohorts was in residual renal function, with patients on hemodialysis predominantly anuric in contrast to more urine output in those on peritoneal dialysis (mean urine output recorded at 546.1 ± 365 mL daily). There is limited data otherwise regarding outcomes related to arterial stiffness in peritoneal dialysis. Reports of mortality outcomes for patients on satellite hemodialysis and peritoneal dialysis have been similar at a registry level and are likely due to the significant distribution of cardiovascular comorbidity in patients with ESKD rather than dialysis modality specific attenuation [69].
Hemodialysis causes significant changes in volume loading and blood pressure as a function of ultrafiltration. In observational studies, PWV is relatively stable during nondialysis days [70], although, at an individual level, PWV exhibits cyclical change with dialysis sessions including reduction after dialysis [71]. Higher PWV is particularly demonstrated after a three-day intradialytic break, in contrast to the two-day break [72]. One hypothesis is that excess volume amplifies aortic blood pressure and stiffness. Reduced mortality has been observed with quotidian home hemodialysis in contrast to satellite dialysis and daily dialysis dosing has been associated with a reduction in PWV, potentially by eliminating excess fluid load and electrolyte imbalances associated with dialysis breaks [71].
From a cardiac perspective, inappropriately increased PWV is associated with increased left ventricular mass in patients on hemodialysis, highlighting the mechanistic contribution of increased arterial stiffness to the development of left ventricular hypertrophy in this cohort [73]. Hemodiafiltration, postulated to reduce the stress of ultrafiltration and contribute to improved cardiac outcomes, has not been correlated with improvements in vascular stiffness in contrast to conventional hemodialysis [74]. A case-controlled study of 148 patients on hemodialysis and 141 on hemodiafiltration showed no change in brachial ankle PWV [75].
Vascular calcification in patients on dialysis is common, often extensive and progressive, and the causes are multifactorial. Contributing factors include the use of calcium-containing phosphate binders as well as higher calcium dialysate. Exogenous calcium loading has been associated with an increase in arterial stiffness in dialysis patients, independent of age, blood pressure, and dialysis vintage [76]. Higher calcium dialysate concentrations have also been associated with an increase in PWV, although patient numbers in these studies are small [77]. One small observational trial with a reduction of dialysate calcium from 1.75mmol/L to 1.5mmol/L in 20 patients on hemodialysis demonstrated improvement in PWV as well as serum calcification markers [78]. In fact, this dialysate calcium concentration is higher than most international practices and therefore it would be interesting to assess the effect of PWV with lower calcium concentrations, although this has not been studied. A small randomised unblinded study determined an interaction between time and dialysate calcium concentration in hemodialysis, again with increased PWV in those on higher dialysate calcium [79].
Several small studies have also assessed the impact of exercise programs in patients undertaking dialysis. Exercise is hypothesised to improve overall cardiovascular risk and potentially mitigates the impact of hypertension upon arterial stiffness. One cross-over study of 19 patients showed a trend towards improvement in PWV in patients who undertook interdialytic exercise, with subsequent deterioration in PWV after cessation of exercise [80]. Other trials in hemodialysis patients have failed to demonstrate any significant difference in PWV with exercise [81]. However, nonpharmacological interventions may potentially be beneficial to improve PWV in dialysis, as have been studied in other CKD cohorts with varying results [63].
7.3. PWV in Kidney Transplant Patients
Kidney transplantation improves cardiovascular prognosis, but its impact on arterial stiffness is still controversial. Kidney transplantation may potentially mitigate the development of further worsening of arterial stiffness [82], and donor age, living kidney donation, and mean blood pressure appear to be the main determinants of improvement in arterial stiffness after kidney transplantation. The predictive value of PWV for cardiovascular mortality has also been reported in kidney transplant patients, and there appears to be an attenuation of the usual increased PWV seen with advanced CKD [83]. In a prospective cohort of 66 kidney transplant patients, there was no significant change in aortic PWV in the first year posttransplantation, in contrast to the majority of studies demonstrating progression of arterial stiffness in patients with CKD over the same timeframe [82]. Several small studies suggest an improvement in carotid intima media thickness as well as PWV following kidney transplantation in relation to age-matched dialysis controls. PWV values in transplant recipients correlate with previous dialysis vintage and changes in PWV with any intervention, consistent with lack of reversal of structural changes, do not reduce to values seen in the general population [84].
8. Further Research
The prognostic significance and change in PWV in the CKD population need further investigation. The influence of dialysis modality upon PWV requires further evaluation, including the assessment of progression of arterial stiffness with different dialysis modalities. The contribution of residual kidney function, arterial turbulence, and fluctuations in volume state to changes in PWV also warrant further investigation. It has been hypothesised that peritoneal dialysis causes less hemodynamic and cardiovascular stress than hemodialysis; however, differences in mortality and cardiovascular outcomes have not been shown. Changes in PWV measured in carefully conducted trials would be helpful to determine the contribution of arterial stiffness in patients with ESKD.
Additionally, improvement in arterial stiffness in kidney transplantation should be investigated further. Kidney transplantation is the gold standard therapy for ESKD and is associated with reduced mortality, in part by mitigating the rate of vascular calcification due to restored calcium phosphate homeostasis. However, kidney transplant recipients have multiple cardiometabolic risk factors which still contribute to development of cardiovascular disease. PWV could potentially be utilised as a study end-point to assess the effect of interventions in improving overall cardiovascular outcomes in the context of graft dysfunction and posttransplant diabetes in this cohort. Of interest would be to study the use of newer hypoglycaemic agents, such as SGLT-2 inhibitors, in posttransplant diabetes with measurement of PWV as a surrogate cardiovascular end-point, although these medications would be used with caution in kidney transplant recipients with impaired kidney function.
Recently, new oscillometric methodologies using simple brachial cuffs, such as Mobil-O-Graph, have been introduced in patients with CKD to measure parameters of 24-hour arterial stiffness including PWV. This enables study of the 24-hour variability of these parameters, which will hopefully lead to better cardiovascular risk stratification and improved cardiovascular outcomes of CKD patients [85].
In conclusion, PWV is increased in CKD compared to healthy age-matched populations. It provides a unique, noninvasive clinical tool for determining arterial stiffness and hypertensive end-organ damage. Multiple studies have reported the predictive value of heightened PWV in the development of any major vascular disease. In the CKD cohort, studies utilising PWV have demonstrated greater progression of arterial stiffness, in contrast to the general population with normal kidney function, corresponding to an increase in cardiovascular mortality. Factors such as CKD stage, exogenous calcium load, dialysate calcium, volume status, and dialysis modality may all contribute to progression of PWV. Further interventions to reduce the burden of cardiovascular disease in the CKD population are essential and could be studied initially using PWV measurement as a surrogate marker.
Conflicts of Interest
Nicole Lioufas, Carmel M. Hawley, James D. Cameron, and Nigel D. Toussaint have no conflicts of interest.
References
- 1.Jha V., Garcia-Garcia G., Iseki K., et al. Chronic kidney disease: global dimension and perspectives. The Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.URDSU. Besthesda, Md, USA: The National Institute of Diabetes and Digestive and Kidney Diseases; 2017. Annual Report. [Google Scholar]
- 3.Salgueira M., Del Toro N., Moreno-Alba R., Jiménez E., Aresté N., Palma A. Vascular calcification in the uremic patient: A cardiovascular risk? Kidney International Supplements. 2003;63(85):S119–S121. doi: 10.1046/j.1523-1755.63.s85.28.x. [DOI] [PubMed] [Google Scholar]
- 4.McGovern A. P., de Lusignan S., van Vlymen J., et al. Serum Phosphate as a Risk Factor for Cardiovascular Events in People with and without Chronic Kidney Disease: A Large Community Based Cohort Study. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074996.e74996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blacher J., Guerin A. P., Pannier B., Marchais S. J., Safar M. E., London G. M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.CIR.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 6.Zoungas S., Cameron J. D., Kerr P. G., et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. American Journal of Kidney Diseases. 2007;50(4):622–630. doi: 10.1053/j.ajkd.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Toussaint N. D., Kerr P. G. Vascular calcification and arterial stiffness in chronic kidney disease: Implications and management. Nephrology. 2007;12(5):500–509. doi: 10.1111/j.1440-1797.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 8.Jablonski K. L., Decker E., Perrenoud L., et al. Assessment of vascular function in patients with chronic kidney disease. Journal of Visualized Experiments. 2014;(88) doi: 10.3791/51478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend R. R. Arterial stiffness and chronic kidney disease: lessons from the Chronic Renal Insufficiency Cohort study. Current Opinion in Nephrology and Hypertension. 2014;24(1):47–53. doi: 10.1097/mnh.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell G. F., Conlin P. R., Dunlap M. E., et al. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51(1):105–111. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 11.Napoli C., de Nigris F., Williams-Ignarro S., Pignalosa O., Sica V., Ignarro L. J. Nitric oxide and atherosclerosis: an update. Nitric Oxide: Biology and Chemistry. 2006;15(4):265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Wang M., Jiang L., Monticone R. E., Lakatta E. G. Proinflammation: The key to arterial aging. Trends in Endocrinology & Metabolism. 2014;25(2):72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thijssen D. H. J., Carter S. E., Green D. J. Arterial structure and function in vascular ageing: Are you as old as your arteries? The Journal of Physiology. 2016;594(8):2275–2284. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber C., Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nature Medicine. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 15.Lu H., Daugherty A. Recent Highlights of ATVB Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(3):485–491. doi: 10.1161/ATVBAHA.115.305380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasrallah M. M., El-Shehaby A. R., Salem M. M., Osman N. A., El Sheikh E., El Din U. A. S. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrology Dialysis Transplantation . 2010;25(8):2679–2685. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 17.NasrAllah M. M., El-Shehaby A. R., Osman N. A., et al. The Association between Fibroblast Growth Factor-23 and Vascular Calcification Is Mitigated by Inflammation Markers. Nephron Extra. 2013;3(1):106–112. doi: 10.1159/000356118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desjardins L., Liabeuf S., Renard C., et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporosis International. 2012;23(7):2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 19.Krishnasamy R., Tan S., Hawley C. M., et al. Progression of arterial stiffness is associated with changes in bone mineral markers in advanced CKD. BMC Nephrology. 2017;18(1):p. 281. doi: 10.1186/s12882-017-0705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rennenberg R. J. M. W., Schurgers L. J., Kroon A. A., Stehouwer C. D. A. Arterial calcifications. Journal of Cellular and Molecular Medicine. 2010;14(9):2203–2210. doi: 10.1111/j.1582-4934.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghasemzadeh N., Zafari A. M. A Brief Journey into the History of the Arterial Pulse. Cardiology Research and Practice. 2011;2011:14. doi: 10.4061/2011/164832.164832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messas E., Pernot M., Couade M. Arterial wall elasticity: State of the art and future prospects. Diagnostic and Interventional Imaging. 2013;94(5):561–569. doi: 10.1016/j.diii.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Parikh J. D., Hollingsworth K. G., Kunadian V., Blamire A., MacGowan G. A. Measurement of pulse wave velocity in normal ageing: Comparison of Vicorder and magnetic resonance phase contrast imaging. BMC Cardiovascular Disorders. 2016;16(1, article no. 50) doi: 10.1186/s12872-016-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzardo L., Lujambio I., Sottolano M., et al. 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertension Research. 2012;35(10):980–987. doi: 10.1038/hr.2012.78. [DOI] [PubMed] [Google Scholar]
- 25.Horváth I. G., Németh Á., Lenkey Z., et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. Journal of Hypertension. 2010;28(10):2068–2075. doi: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 26.Omboni S., Posokhov I. N., Rogoza A. N. Evaluation of 24-Hour Arterial Stiffness Indices and Central Hemodynamics in Healthy Normotensive Subjects versus Treated or Untreated Hypertensive Patients: A Feasibility Study. International Journal of Hypertension. 2015;2015:10. doi: 10.1155/2015/601812.601812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend R. R., Wilkinson I. B., Schiffrin E. L., et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/hyp.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell G. F. Arterial stiffness: Insights from Framingham and Iceland. Current Opinion in Nephrology and Hypertension. 2015;24(1):1–7. doi: 10.1097/MNH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 29.Williams B., Mancia G., Spiering W., et al. ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 30.Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shlomo Y., Spears M., Boustred C., May M., et al. Aortic pulse wave velocity improves cardiovascular event prediction; an individual participant meta-analysis of prospective observational data from 17, 635 subjects. Journal of the American College of Cardiology. 2014;63(7):636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verwoert G. C., Elias-Smale S. E., Rizopoulos D., et al. Does aortic stiffness improve the prediction of coronary heart disease in elderly? The Rotterdam Study. Journal of Human Hypertension. 2012;26(1):28–34. doi: 10.1038/jhh.2010.124. [DOI] [PubMed] [Google Scholar]
- 33.Saji N., Toba K., Sakurai T. Cerebral Small Vessel Disease and Arterial Stiffness: Tsunami Effect in the Brain? Pulse. 2016;3(3-4):182–189. doi: 10.1159/000443614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J., Song T.-J., Kim E. H., et al. Brachial-ankle pulse wave velocity for predicting functional outcome in acute stroke. Stroke. 2014;45(8):2305–2310. doi: 10.1161/STROKEAHA.114.005576. [DOI] [PubMed] [Google Scholar]
- 35.Ikura K., Hanai K., Oka S., et al. Brachial-ankle pulse wave velocity, but not ankle-brachial index, predicts all-cause mortality in patients with diabetes after lower extremity amputation. Journal of Diabetes Investigation. 2017;8(2):250–253. doi: 10.1111/jdi.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Lyu P., Ren Y., An J., Dong Y. Arterial stiffness and cognitive impairment. Journal of the Neurological Sciences. 2017;380:1–10. doi: 10.1016/j.jns.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Scuteri A., Wang H. Pulse wave velocity as a marker of cognitive impairment in the elderly. Journal of Alzheimer's Disease. 2014;42:S401–S410. doi: 10.3233/JAD-141416. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi Y., Fujiwara Y., Nofuji Y., et al. Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. Journal of Epidemiology. 2015;25(9):592–599. doi: 10.2188/jea.JE20140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scuteri A., Tesauro M., Guglini L., Lauro D., Fini M., Di Daniele N. Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. International Journal of Cardiology. 2013;169(5):371–377. doi: 10.1016/j.ijcard.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Hazzouri A. Z. A., Newman A. B., Simonsick E., et al. Pulse wave velocity and cognitive decline in elders: The health, aging, and body composition study. Stroke. 2013;44(2):388–393. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raggi P., Boulay A., Chasan-Taber S., et al. Cardiac calcification in adult hemodialysis patients: a link between end-stage renal disease and cardiovascular disease? Journal of the American College of Cardiology. 2002;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 42.Goodman W. G., Goldin J., Kuizon B. D., et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. The New England Journal of Medicine. 2000;342(20):1478–1483. doi: 10.1056/nejm200005183422003. [DOI] [PubMed] [Google Scholar]
- 43.Temmar M., Liabeuf S., Renard C., et al. Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. Journal of Hypertension. 2010;28(1):163–169. doi: 10.1097/HJH.0b013e328331b81e. [DOI] [PubMed] [Google Scholar]
- 44.Tholen S., Klofat K., Pan C. R., et al. Progression of aortic pulse wave velocity in patients with chronic kidney disease. The Journal of Clinical Hypertension. 2013;15(11):833–838. doi: 10.1111/jch.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedaghat S., Mattace-Raso F. U. S., Hoorn E. J., et al. Arterial stiffness and decline in kidney function. Clinical Journal of the American Society of Nephrology. 2015;10(12):2190–2197. doi: 10.2215/CJN.03000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigrist M. K., Taal M. W., Bungay P., McIntyre C. W. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clinical Journal of the American Society of Nephrology. 2007;2(6):1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 47.Fassett R. G., Robertson I. K., Ball M. J., Geraghty D. P., Sharman J. E., Coombes J. S. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. Journal of Atherosclerosis and Thrombosis. 2010;17(3):235–241. doi: 10.5551/jat.2683. [DOI] [PubMed] [Google Scholar]
- 48.Frimodt-Møller M., Kamper A. L., Strandgaard S., Kreiner S., Nielsen A. H. Beneficial effects on arterial stiffness and pulse-wave reflection of combined enalapril and candesartan in chronic kidney disease—a randomized trial. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041757.e41757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boesby L., Elung-Jensen T., Strandgaard S., Kamper A. Eplerenone attenuates pulse wave reflection in chronic kidney disease stage 3-4—a randomized controlled study. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064549.e64549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S.-C., Chang J.-M., Tsai Y.-C., Su H.-M., Chen H.-C. Brachial-ankle pulse wave velocity and brachial pre-ejection period to ejection time ratio with renal outcomes in chronic kidney disease. Hypertension Research. 2012;35(12):1159–1163. doi: 10.1038/hr.2012.114. [DOI] [PubMed] [Google Scholar]
- 51.Baumann M., Wassertheurer S., Suttmann Y., Burkhardt K., Heemann U. Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2-4. Journal of Hypertension. 2014;32(4):899–903. doi: 10.1097/HJH.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 52.Chandra P., Sands R. L., Gillespie B. W., et al. Relationship between heart rate variability and pulse wave velocity and their association with patient outcomes in chronic kidney disease. Clinical Nephrology. 2014;81(1):9–19. doi: 10.5414/CN108020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cseprekál O., Egresits J., Tabák Á., et al. The significance of micro- and macrovascular biomarkers on cardiovascular outcome in chronic kidney disease: A prospective cohort study. Journal of Human Hypertension. 2016;30(7):449–455. doi: 10.1038/jhh.2015.96. [DOI] [PubMed] [Google Scholar]
- 54.Kim H., Yoo T.-H., Choi K. H., et al. Baseline cardiovascular characteristics of adult patients with chronic kidney disease from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) Journal of Korean Medical Science. 2017;32(2):231–239. doi: 10.3346/jkms.2017.32.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim C. S., Bae E. H., Ma S. K., et al. Chronic kidney disease-mineral bone disorder in Korean patients: A report from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) Journal of Korean Medical Science. 2017;32(2):240–248. doi: 10.3346/jkms.2017.32.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morita T., Morimoto S., Nakano C., et al. Renal and vascular protective effects of ezetimibe in chronic kidney disease. Internal Medicine. 2014;53(4):307–314. doi: 10.2169/internalmedicine.53.0649. [DOI] [PubMed] [Google Scholar]
- 57.Edwards N. C., Steeds R. P., Stewart P. M., Ferro C. J., Townend J. N. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. Journal of the American College of Cardiology. 2009;54(6):505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 58.Chitalia N., Ismail T., Tooth L., et al. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0091363.e91363 [DOI] [Google Scholar]
- 59.Kumar V., Yadav A. K., Lal A., et al. A randomized trial of Vitamin D supplementation on vascular function in CKD. Journal of the American Society of Nephrology. 2017;28(10):3100–3108. doi: 10.1681/ASN.2017010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levin A., Tang M., Perry T., et al. Randomized controlled trial for the effect of vitamin D supplementation on vascular stiffness in CKD. Clinical Journal of the American Society of Nephrology. 2017;12(9):1447–1460. doi: 10.2215/CJN.10791016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seifert M. E., De Las Fuentes L., Rothstein M., et al. Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. American Journal of Nephrology. 2013;38(2):158–167. doi: 10.1159/000353569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chue C. D., Townend J. N., Moody W. E., et al. Cardiovascular effects of sevelamer in stage 3 CKD. Journal of the American Society of Nephrology. 2013;24(5):842–852. doi: 10.1681/ASN.2012070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Craenenbroeck A. H., Van Craenenbroeck E. M., Van Ackeren K., et al. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3-4: a randomized controlled trial. American Journal of Kidney Diseases. 2015;66(2):285–296. doi: 10.1053/j.ajkd.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 64.McDonald S. P., Russ G. R., Kerr P. G., Collins J. F. ESRD in Australia and New Zealand at the end of the millennium: A report from the ANZDATA Registry. American Journal of Kidney Diseases. 2002;40(6):1122–1131. doi: 10.1053/ajkd.2002.36943. [DOI] [PubMed] [Google Scholar]
- 65.Jun M., Jardine M. J., Gray N., et al. Outcomes of extended-hours hemodialysis performed predominantly at home. American Journal of Kidney Diseases. 2013;61(2):247–253. doi: 10.1053/j.ajkd.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 66.Chang J. H., Yoon S.-J., Han S. H., et al. The impact of dialysis modality on arterial stiffness in patients with end-stage renal disease. Renal Failure. 2010;32(8):947–953. doi: 10.3109/0886022X.2010.502607. [DOI] [PubMed] [Google Scholar]
- 67.Covic A., Goldsmith D. J. A., Florea L., Gusbeth-Tatomir P., Covic M. The influence of dialytic modality on arterial stiffness, pulse wave reflections, and vasomotor function. Peritoneal Dialysis International. 2004;24(4):365–372. [PubMed] [Google Scholar]
- 68.Mimura T., Takenaka T., Kanno Y., Aoki H., Ohshima J., Suzuki H. Comparison of changes in pulse wave velocity in patients on continuous ambulatory peritoneal dialysis and hemodialysis one year after introduction of dialysis therapy. Advances in Peritoneal Dialysis. 2005;21:139–145. [PubMed] [Google Scholar]
- 69.Marshall M. R., Polkinghorne K. R., Kerr P. G., Agar J. W. M., Hawley C. M., McDonald S. P. Temporal Changes in Mortality Risk by Dialysis Modality in the Australian and New Zealand Dialysis Population. American Journal of Kidney Diseases. 2015;66(3):489–498. doi: 10.1053/j.ajkd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Karpetas A., Sarafidis P. A., Georgianos P. I., et al. Ambulatory recording of wave reflections and arterial stiffness during intra- and interdialytic periods in patients treated with dialysis. Clinical Journal of the American Society of Nephrology. 2015;10(4):630–638. doi: 10.2215/CJN.08180814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Micco L., Torraca S., Sirico M. L., Tartaglia D., Di Iorio B. Daily dialysis reduces pulse wave velocity in chronic hemodialysis patients. Hypertension Research. 2012;35(5):518–522. doi: 10.1038/hr.2011.230. [DOI] [PubMed] [Google Scholar]
- 72.Koutroumbas G., Georgianos P. I., Sarafidis P. A., et al. Ambulatory aortic blood pressure, wave reflections and pulse wave velocity are elevated during the third in comparison to the second interdialytic day of the long interval in chronic haemodialysis patients. Nephrology Dialysis Transplantation . 2015;30(12):2046–2053. doi: 10.1093/ndt/gfv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin S. J., Kim Y. K., Chung S., et al. The impact of the aortic pulse wave velocity on the cardiovascular outcomes of hemodialysis patients. Journal of Korean Medical Science. 2009;24(1):S121–S128. doi: 10.3346/jkms.2009.24.S1.S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Power A., Charitaki E., Davenport A. Haemodialysis and haemodiafiltration lead to similar changes in vascular stiffness during treatment. The International Journal of Artificial Organs. 2016;39(5):228–234. doi: 10.5301/ijao.5000503. [DOI] [PubMed] [Google Scholar]
- 75.Charitaki E., Davenport A. Does hemodiafiltration reduce vascular stiffness measured by aortic pulse wave velocity compared with high-flux hemodialysis? Hemodialysis International. 2014;18(2):391–395. doi: 10.1111/hdi.12119. [DOI] [PubMed] [Google Scholar]
- 76.London G. M., Marchais S. J., Guérin A. P., Boutouyrie P., Métivier F., De Vernejoul M. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. Journal of the American Society of Nephrology. 2008;19(9):1827–1835. doi: 10.1681/ASN.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Charitaki E., Davenport A. Do higher dialysate calcium concentrations increase vascular stiffness in haemodialysis patients as measured by aortic pulse wave velocity? BMC Nephrology. 2013;14(1, article 189) doi: 10.1186/1471-2369-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J.-K., Moon S. J., Park H. C., et al. Effects of lowering dialysate calcium concentrations on arterial stiffness in patients undergoing hemodialysis. Korean Journal of Internal Medicine. 2011;26(3):320–327. doi: 10.3904/kjim.2011.26.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leboeuf A., Mac-Way F., Utescu M. S., et al. Impact of dialysate calcium concentration on the progression of aortic stiffness in patients on haemodialysis. Nephrology Dialysis Transplantation . 2011;26(11):3695–3701. doi: 10.1093/ndt/gfr138. [DOI] [PubMed] [Google Scholar]
- 80.Toussaint N. D., Polkinghorne K. R., Kerr P. G. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodialysis International. 2008;12(2):254–263. doi: 10.1111/j.1542-4758.2008.00262.x. [DOI] [PubMed] [Google Scholar]
- 81.Chan D., Green S., Fiatarone Singh M. A., Barnard R., Bonder C. S., Cheema B. S. Effect of intradialytic resistance training on pulse wave velocity and associated cardiovascular disease biomarkers in end stage renal disease. Nephrology. 2017;23(11):1055–1062. doi: 10.1111/nep.13212. [DOI] [PubMed] [Google Scholar]
- 82.Birdwell K. A., Jaffe G., Bian A., Wu P., Ikizler T. A. Assessment of arterial stiffness using pulse wave velocity in tacrolimus users the first year post kidney transplantation: A prospective cohort study. BMC Nephrology. 2015;16(1):p. 93. doi: 10.1186/s12882-015-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell A., Opazo Saez A., Kos M., Witzke O., Kribben A., Nürnberger J. Pulse wave velocity predicts mortality in renal transplant patients. European Journal of Medical Research. 2010;15(10):452–455. doi: 10.1186/2047-783X-15-10-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z., Qin Y., Du L., Luo X. An improvement of carotid intima-media thickness and pulse wave velocity in renal transplant recipients. BMC Medical Imaging. 2018;18(1):p. 23. doi: 10.1186/s12880-018-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.László A., Reusz G., Nemcsik J. Ambulatory arterial stiffness in chronic kidney disease: A methodological review. Hypertension Research. 2016;39(4):192–198. doi: 10.1038/hr.2015.137. [DOI] [PubMed] [Google Scholar]