To the Editor:
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal disease characterized by parenchymal fibrosis and structural distortion of the lungs. IPF is believed to be a disorder of abnormal wound healing, wherein the initial trigger to the fibrotic response is injury to the alveolar epithelial cells, followed by an exuberant, nonresolving wound-healing response (1). Injury of alveolar epithelial cells results in the elaboration of a fibrinous matrix and activation of several profibrotic mediators, of which transforming growth factor β (TGF-β) is the most established (2). Lung-targeted overexpression of TGF-β results in the development of lung fibrosis in animals (2). Conversely, inhibition of TGF-β can inhibit in vivo fibrogenesis (3).
TGF-β drives the transformation of resident fibroblasts to myofibroblasts characterized by de novo expression of cytoskeletal proteins, including smooth muscle α-actin (SMA), the most established marker for myofibroblast differentiation (4). Myofibroblast transformation is also associated with secretion of extracellular matrix proteins (cellular fibronectin [FN], collagen isoforms, etc.), liberation of profibrotic factors (e.g., connective tissue growth factor [5, 6]), and an increased resistance to apoptosis (7), thus perpetuating ongoing tissue fibrosis. Myofibroblasts are invariably found in histologic sections of lung specimens from patients with pulmonary fibrosis, and they represent an important pathogenic mechanism for the progressive nature of IPF. Therefore, understanding the cellular and molecular mechanisms of myofibroblast transformation in response to TGF-β is of great importance for understanding the pathogenesis of the disease.
In this study, we used primary cultures of human lung fibroblasts (HLFs) at passages 3-9. TGF-β1 and specific inhibitor of Smad3 (SIS3) were obtained from Calbiochem. SB4321542 was obtained from Cayman. The following antibodies were used: SMA (A2547) and tubulin (T6074) from Sigma-Aldrich, Col1A1 (sc-8784-R) from Santa Cruz Biotechnology, FN (610077) from BD Transduction, and Smad2 (L1603) and phospho-Smad2 (13804) from Cell Signaling Technology.
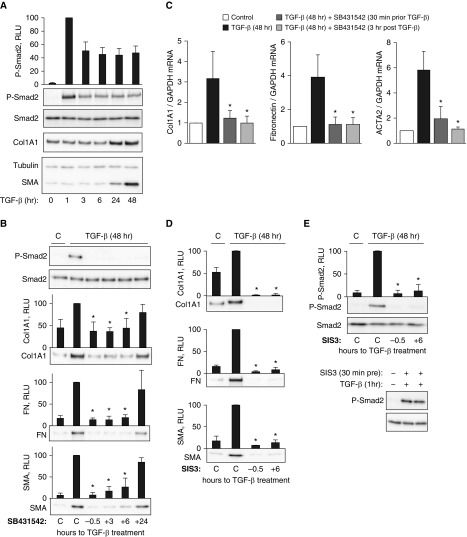
TGF-β signals through activation of TGF-β receptors and subsequent phosphorylation and activation of Smad2/3 transcription factors driving the transcription of target genes. We and other investigators have previously demonstrated that in HLFs, maximal phosphorylation of Smad2 occurs at 30–60 minutes and then declines after 3 hours of TGF-β treatment (8). In the present study, the extended kinetics analysis revealed that Smad2 remained partially phosphorylated (by ∼50%) at 3–48 hours, as compared with the maximum phosphorylation observed at 1 hour after TGF-β treatment of HLFs (Figure 1A). With this finding in mind, we asked whether the sustained partial Smad2 phosphorylation is important for myofibroblast differentiation induced by TGF-β, using a specific inhibitor of TGF-β receptor kinase activity, SB431542. As shown in Figure 1B, pretreatment of HLFs with SB431542 eliminated Smad2 phosphorylation and resulted in a substantial decrease of TGF-β–induced SMA, collagen 1 (Col1A1), and FN expression as expected. However, addition of SB431542 to HLFs 3 hours or 6 hours after TGF-β treatment had a similar effect on myofibroblast differentiation occurring through the 48-hour treatment with TGF-β (Figure 1B). When applied 24 hours after TGF-β treatment for an additional 24 hours (total 48 h), SB431542 had no significant effect on TGF-β–induced expression of SMA, Col1A1, or FN (Figure 1B). Delayed application of SB431542 also resulted in inhibition of TGF-β–induced increases in Col1A1, FN, and SMA (ACTA2 gene) mRNA levels (Figure 1C). To assess the role of Smad3 activity in this process, we used a specific Smad3 inhibitor, SIS3 (9). As shown in Figure 1D, SIS3, applied either before or 6 hours after TGF-β treatment, drastically reduced expression of SMA, FN, and Col1A1 in response to TGF-β. Interestingly, although it had no effect on the initial Smad2 phosphorylation (indicating intact TGF-β receptor activation), SIS3 abolished sustained Smad2 phosphorylation in response to TGF-β (Figure 1E).
Figure 1.
Sustained Smad2 phosphorylation is required for transforming growth factor β (TGF-β)–induced myofibroblast differentiation. Primary cultured human lung fibroblasts were treated with 1 ng/ml TGF-β, 5 μM SB431542, or 10 μM specific inhibitor of Smad3 (SIS3) applied at the times indicated. The cells were then lysed and analyzed for expression or phosphorylation of the desired proteins by Western blotting, or the desired mRNAs by real-time qPCR. (A) Time course of TGF-β–induced Smad2 phosphorylation relative to smooth muscle α-actin (SMA) and collagen 1 (Col1A1) expression. (B) Effect of SB431542 applied 0.5 hour before or 3 hours, 6 hours, and 24 hours after TGF-β treatment on TGF-β–induced Smad2 phosphorylation, and SMA, Col1A1, and fibronectin (FN) expression at 48 hours. (C) Effect of SB431542 applied 0.5 hours before or 3 hours after TGF-β treatment on TGF-β–induced mRNA levels of SMA, Col1A1, and FN at 48 hours. (D) Effect of SIS3 applied 0.5 hours before or 6 hours after TGF-β treatment on TGF-β–induced SMA, Col1A1, and FN expression at 48 hours. (E) Effect of SIS3 applied 0.5 hours before or 6 hours after TGF-β treatment on TGF-β–induced Smad2 phosphorylation at 48 hours or 1 hour of TGF-β treatment as indicated. Data represent the results of at least three independent experiments. *P < 0.01. RLU = relative light units; p-SMAD2 = phospho-SMAD family member 2.
Our data suggest that sustained Smad2 phosphorylation in response to TGF-β is dependent on Smad3 activity and is required for myofibroblast differentiation. While acknowledging the role of a canonical immediate Smad2/3-dependent gene transcription in response to TGF-β, we propose that this is not sufficient for a complete myofibroblast differentiation, as Smad2 phosphorylation has to be sustained through at least 6 hours of TGF-β stimulation. The fundamental importance of this finding may relate to the mechanism of other TGF-β–induced cellular responses, such as epithelial-to-mesenchymal transition, which should be assessed in future studies. The practical application of our finding may relate to studying the mechanisms of antifibrotic interventions: unchanged short-term Smad2 phosphorylation in response to TGF-β by a given intervention may not necessarily indicate the Smad2-independent mechanism, as exemplified by the comparison of the short-term and long-term effects of SIS3 in HLFs (Figure 1E).
The mechanism underlying sustained Smad2 signaling in response to TGF-β represents another important fundamental question. Zi and colleagues demonstrated through mathematical and experimental models that TGF-β–induced acute and long-term Smad2 signaling responses in HaCaT keratinocytes have different consequences for gene transcription (10). They reported that sustained Smad signaling leads to an “ultrasensitive or switch-like” phenotype, wherein even a small increase in TGF-β results in large changes in gene transcription. Translating this idea to our study, the sustained Smad2 phosphorylation in response to TGF-β that we observed may suggest that fibroblasts acquire a switch-like response, which could be a crucial step for myofibroblast differentiation. Possible mechanisms for this sustained Smad2 activation may include positive-feedback regulation of TGF-β signaling, for example, through the expression and/or matrix-dependent liberation of TGF-β (11, 12). The data we obtained using SIS3 suggest that Smad3-dependent gene transcription is required for sustained Smad2 phosphorylation (Figure 1E). This may also point to potentially distinct mechanistic functions of Smad2 and Smad3 in the context of myofibroblast differentiation. The mechanism and relative contributions of a sustained Smad2 and Smad3 activation to myofibroblast differentiation in response to TGF-β will be examined in future studies.
Supplementary Material
Footnotes
Supported by National Institutes of Health award 1R56HL127395 (N.O.D.), National Center for Advancing Translational Sciences/National Institutes of Health award UL1TR000430 (N.O.D.), and the Russian Scientific Foundation (L.V.S. and S.N.O.).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 2.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 6.Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136–142. doi: 10.1007/s11926-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kach J, Sandbo N, La J, Denner D, Reed EB, Akimova O, et al. Antifibrotic effects of noscapine through activation of prostaglandin E2 receptors and protein kinase A. J Biol Chem. 2014;289:7505–7513. doi: 10.1074/jbc.M113.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 10.Zi Z, Feng Z, Chapnick DA, Dahl M, Deng D, Klipp E, et al. Quantitative analysis of transient and sustained transforming growth factor-β signaling dynamics. Mol Syst Biol. 2011;7:492. doi: 10.1038/msb.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Froese AR, Shimbori C, Bellaye PS, Inman M, Obex S, Fatima S, et al. Stretch-induced activation of transforming growth factor-β1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194:84–96. doi: 10.1164/rccm.201508-1638OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.