Abstract
Pulmonary emphysema is characterized by alveolar wall destruction, and cigarette smoking is the main risk factor in this disease development. S100A8 is a member of the S100 protein family, with an oxidative stress–related and antiinflammatory role. The mechanisms of human alveolar type II (ATII) cell injury contributing to emphysema pathophysiology are not completely understood. We wanted to determine whether S100A8 can protect ATII cells against injury induced by cigarette smoke and this disease development. We used freshly isolated ATII cells from nonsmoking and smoking organ donors, as well as patients with emphysema to determine S100A8 function. S100A8 protein and mRNA levels were low in individuals with this disease and correlated with its severity as determined by using lung tissue from areas with mild and severe emphysema obtained from the same patient. Its expression negatively correlated with high oxidative stress as observed by 4-hydroxynonenal levels. We also detected decreased serine phosphorylation within S100A8 by PKAα in this disease. This correlated with increased S100A8 ubiquitination by SYVN1. Moreover, we cultured ATII cells isolated from nonsmokers followed by treatment with cigarette smoke extract. We found that this exposure upregulated S100A8 expression. We also confirmed the cytoprotective role of S100A8 against cell injury using gain- and loss-of-function approaches in vitro. S100A8 knockdown sensitized cells to apoptosis induced by cigarette smoke. In contrast, S100A8 overexpression rescued cell injury. Our results suggest that S100A8 protects ATII cells against injury and cigarette smoke–induced emphysema. Targeting S100A8 may provide a potential therapeutic strategy for this disease.
Keywords: alveolar type II cell, apoptosis, cigarette smoke, lung injury, pulmonary emphysema
Clinical Relevance
Our results suggest that S100A8 protects ATII cells against injury and cigarette smoke–induced emphysema. Targeting S100A8 may provide a potential therapeutic strategy for this disease.
Emphysema is a form of chronic obstructive pulmonary disease (COPD) (1, 2). It is a progressive and debilitating disease that is recalcitrant to medical interventions (3, 4). The pathogenesis of emphysema is still not well understood, and there is no effective treatment against this disease. For patients with COPD and severe emphysema, both lung volume reduction surgery and lung transplantation are potential treatment options. Emphysema is characterized by alveolar wall destruction and loss of surface area in the lung parenchyma involved in gas exchange. This leads to irreversible weakening respiratory airflow and shortness of breath (1).
Cigarette smoke (CS) contains high concentrations of reactive oxygen species (ROS) (5). Oxidative and carbonyl stress induced by this exposure contributes to alveolar epithelial cell injury (6). Alveolar type II (ATII) cells secrete pulmonary surfactant to decrease the surface tension. They proliferate to restore the epithelium after damage to the more sensitive ATI cells (7, 8). Exposure to CS can induce an increase in epithelial permeability, the proinflammatory response, and cell senescence, and decrease surfactant production (6, 9). The most deleterious effect of this exposure on alveolar epithelial cells is apoptosis or necrosis. Dying cells are replaced through stimulation of proliferation. However, once alveolar cells reach the senescence stage, apoptosis is no longer compensated for by proliferation, which results in a progressive loss of alveolar architecture (9). An inverse correlation between the level of alveolar cellular senescence and proliferation suggests impaired alveolar epithelial cell regeneration. This is supported by the marked imbalance between alveolar epithelial cell apoptosis and proliferation found in the lungs of patients with end-stage emphysema, indicating that apoptotic cells are not adequately replaced by new cells (10).
S100A8 (also known as MRP-8 or calgranulin-A) is a member of the S100 protein family. It has been found in the lungs of patients with disorders such as airway inflammation, cystic fibrosis, asthma, and acute respiratory distress syndrome (11). S100A8 can act independently or form a heterocomplex with S100A9 to mediate cellular responses to ROS generation (12, 13). We have previously shown that the S100A8/S100A9 complex is required for human regulatory T-cell differentiation and may contribute to the maintenance of immune homeostasis (13). Recent studies showed that S100A8 is induced in the absence of S100A9 by oxidative stress or Toll-like receptor agonists in murine keratinocytes (14). Moreover, S100A8 expression was found in the absence of S100A9 in activated macrophages, and in the low-density lipoprotein proteome. This provides strong evidence that S100A8 does not depend on S100A9 for structural stability and has independent functions (15).
In the present study, we hypothesized that S100A8 has a cytoprotective role against CS-induced lung injury and emphysema. We determined S100A8 protein and mRNA levels in primary ATII cells isolated from individuals with this disease compared with nonsmoking and smoking control subjects. We also studied the mechanism of decreased S100A8 levels in ATII cells in patients with emphysema.
Methods
Isolation and Culture of Human Primary ATII Cells
Lungs were obtained from deidentified nonsmoking and smoking organ donors through the Gift of Life Donor Program and from patients with emphysema through the Temple Biobank (Temple University, Philadelphia, PA). ATII cells were isolated from nonsmokers, smokers, and patients with emphysema (n = 4–12 per group, 45–69 years old, females and males) as we previously described (16).
Chest Computed Tomography Scans and Tissue Core Processing
The subjects underwent volumetric computed tomography scans of the chest at full inspiration (standard dose = 200 mA) and at end-tidal expiration (low dose = 50 mA). Detailed computed tomography protocols have been previously published (17).
S100A8 Knockdown and Overexpression
The human alveolar epithelial cell line A549 was transfected with S100A8 siRNA (Santa Cruz Biotechnology) for 48 hours using Lipofectamine RNAiMax Reagent (Invitrogen). We used nontargeting (NT) siRNA (5′ UAGCGACUAAACACAUCAAUU 3′ and 3′ UUAUCGCUGAUUUGUGUAGUU 5′) to confirm the specificity of the inhibition.
For transient S100A8 overexpression, A549 cells were transfected with 2.5 μg of pcDNA3.1-S100A8 construct or empty vector (both from Addgene) for 48 hours using Lipofectamine 3000 (Invitrogen). The mCherry-N1 expression plasmid (Clontech Laboratories, Inc.) was used as a control to evaluate the transfection efficiency by fluorescent protein expression.
CS Extract
CS extract (CSE) was prepared using one 3R4F cigarette with no filter (Kentucky Tobacco Research and Development Center) as we previously described (7). Briefly, 100% CSE was prepared in 12.5 ml of Dulbecco’s modified Eagle’s medium without FBS using a peristaltic pump (Mannostat, 72-310-000, Thermo Fisher Scientific).
Real-Time PCR
Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific). RNA was reverse-transcribed into cDNA by using the Superscript II Reverse Transcriptase kit (Thermo Fisher Scientific). The SYBR Green Master Mix kit (Thermo Fisher Scientific) was used for PCR amplification using the StepOnePlus Real-Time PCR System (Applied Biosystems). Gene-specific primers are listed in the Methods section of the data supplement. Gene expressions were calculated as a ratio of S100A8 to GAPDH levels. Obtained values were normalized to one for a control group. Data were analyzed using the ΔΔCt method (18).
Western Blotting and Immunoprecipitation
Immunoprecipitation and Western blotting analysis using primary and secondary antibodies were performed as described in the data supplement.
Flow Cytometry Analysis
Cell apoptosis was determined using the Alexa Fluor 488 Annexin V/Dead Cell Apoptosis kit (Thermo Fisher Scientific). Briefly, cells were stained with 5 μl of Annexin V conjugated to Alexa Fluor 488 and 1 μg/mL of propidium iodide (PI) diluted in binding buffer for 5 minutes. This method allows us to distinguish between viable (unstained) and apoptotic (Annexin V+) cells. Data were acquired using an LSR-II flow cytometer (BD Biosciences) and analyzed by FlowJo (TreeStar).
Statistical Analysis
To evaluate statistical differences among the experimental groups, one-way ANOVA was used. A value of P < 0.05 was considered significant. Data are shown as the mean ± SD from at least three independent experiments.
The methods used in this work are described in detail in the Methods section of the data supplement.
Results
Decreased S100A8 Expression in Human ATII Cells in Emphysema
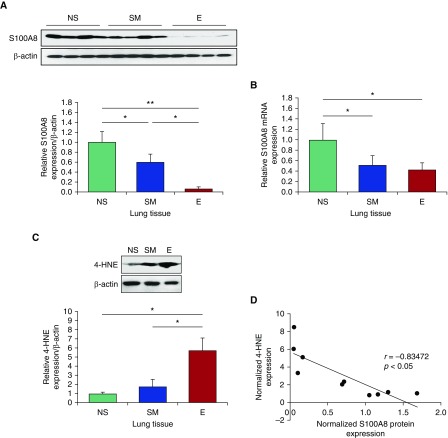
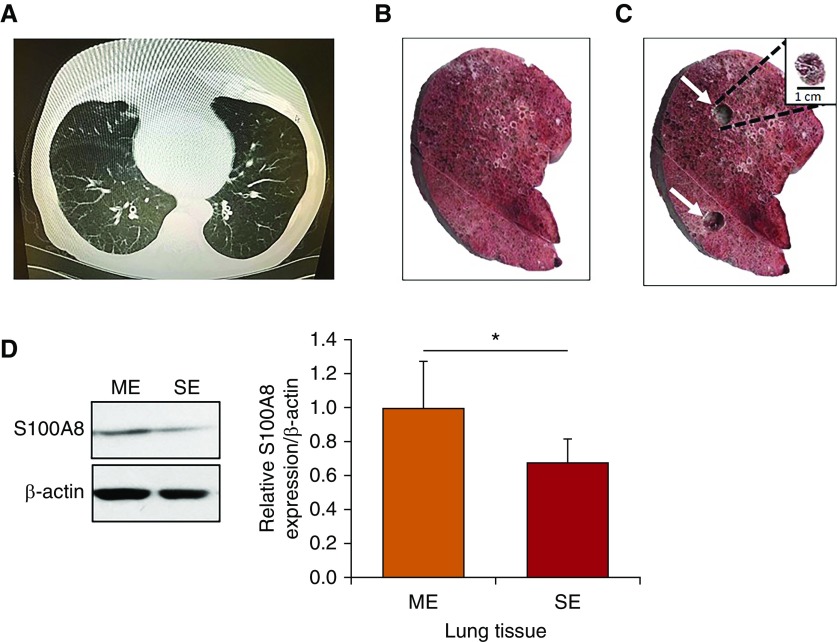
CS-induced oxidative stress can cause ATII cell injury, contributing to emphysema development (1). Here, we wanted to determine S100A8 expression at the gene and protein levels in lung tissue and ATII cells obtained from nonsmokers, smokers, and patients with emphysema. We found significantly lower S100A8 protein expression in lung tissue from patients with emphysema compared with nonsmokers or smokers (Figure 1A). Data obtained from RT-PCR also indicated decreased S100A8 levels in patients with emphysema compared with nonsmokers (Figure 1B). Low S100A8 expression correlated with high oxidative stress in patients with emphysema as detected by the levels of 4-hydroxynonenal (4-HNE), which is a marker of lipid peroxidation products (Figures 1C and 1D). We also found significantly higher ROS generation in smokers and patients with emphysema compared with nonsmokers as detected by dihydroethidium staining (Figure E1 in the data supplement). Next, we sought to gain insights into the correlation between S100A8 expression and the degree of emphysema progression (Figure 2A). We used tissue collected from two regions corresponding to mild and severe disease areas from the same patient (Figures 2B and 2C). Our results indicated that S100A8 was downregulated in areas with severe emphysema compared with mild emphysema as detected by Western blotting (Figure 2D).
Figure 1.
Low S100A8 expression and high oxidative stress in lung tissue from patients with emphysema. (A and B) Western blot images of expression and quantification of S100A8 protein (A) and S100A8 mRNA levels (B) in lung tissue obtained from nonsmokers (NS), smokers (SM), and patients with emphysema (E). Quantification of S100A8 expression was normalized to β-actin and control nonsmokers. (C) Western blot images of expression and quantification of 4-hydroxynonenal (4-HNE) in lung tissue from NS, SM, and patients with E. (D) Correlation analysis of 4-HNE and S100A8 protein expression. *P < 0.05; **P < 0.001. Data are shown as means ± SD.
Figure 2.
Decreased S100A8 levels in lung tissue in severe emphysema. (A) Representative computed tomography scan of a patient with emphysema. The lung slice was obtained from a part of the lower and middle lobes. (B and C) Tissue cores were removed from areas with severe (upper white arrow) and mild (lower white arrow) emphysema from the same patient. A representative core is shown in the upper right corner. (D) Representative Western blot images of expression and quantification of S100A8 in mild and severe emphysema (n = 5). *P < 0.05. Data are shown as means ± SD. ME = mild emphysema; SE = severe emphysema.
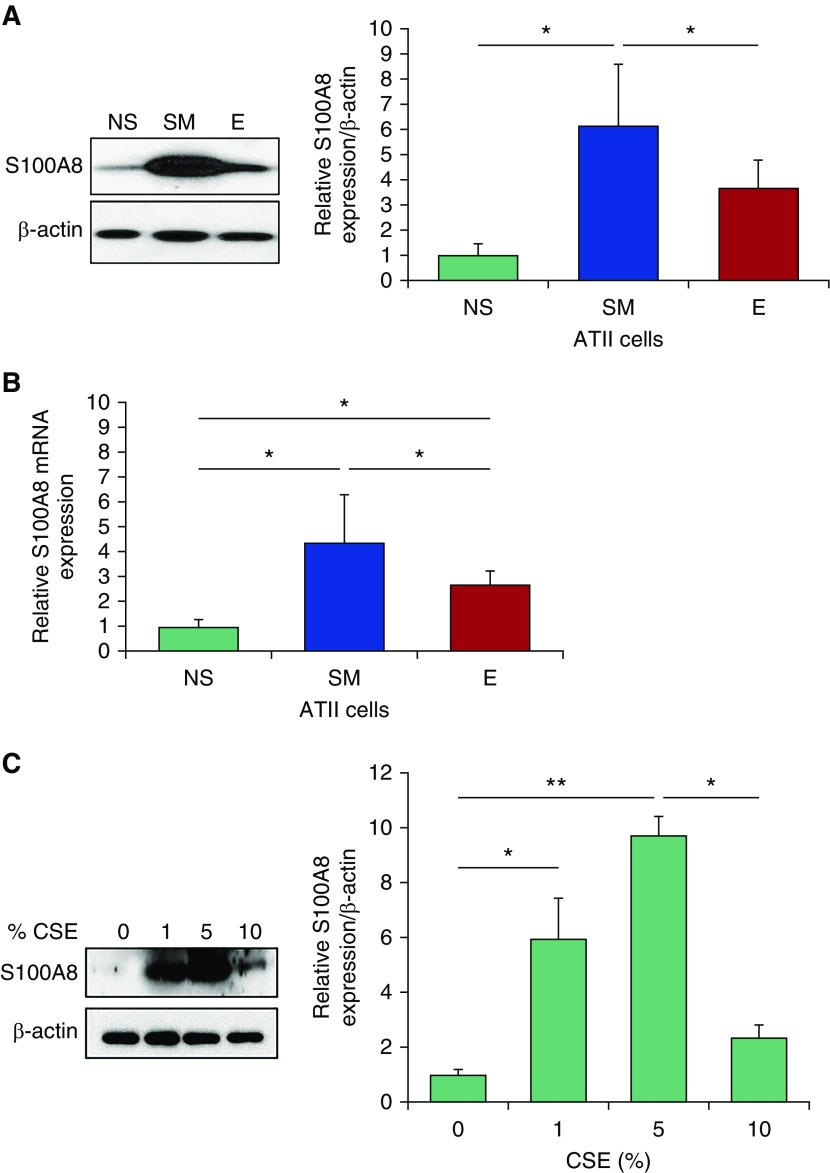
In addition, we found significantly higher S100A8 protein (Figure 3A) and mRNA (Figure 3B) levels in ATII cells from smokers compared with nonsmokers. The discrepancy between S100A8 levels in lung tissue and ATII cells in smokers compared with nonsmokers may be related to the various cell types present in the former samples. Moreover, ATII cells isolated from individuals with emphysema had lower S100A8 expression in comparison with those obtained from smokers. We also cultured human primary ATII cells isolated from nonsmokers followed by exposure to CSE for 24 hours. We found that CSE upregulated S100A8 expression as detected by Western blotting (Figure 3C).
Figure 3.
Low S100A8 expression in ATII cells from patients with emphysema. (A and B) Western blot images of expression and densitometric quantification of S100A8 protein (A) and mRNA levels (B) in freshly isolated ATII cells from NS, SM, and patients with E. Quantification of S100A8 expression was normalized to β-actin and control nonsmokers (n = 4). (C) ATII cells obtained from nonsmokers were treated with 1%, 5%, or 10% cigarette smoke extract (CSE) for 24 hours to determine S100A8 expression by Western blotting and densitometric analysis (n = 3). *P < 0.05; **P < 0.001. Data are shown as means ± SD. ATII = alveolar type II.
It has been reported that S100A8 can act independently or form a heterocomplex with S100A9 (14). We analyzed this interaction in nonsmokers, smokers, and patients with emphysema; however, we did not detect significant differences between these groups (Figure E2). This suggests the independent role of S100A8 in the lung.
Regulation of S100A8 by Phosphorylation and Ubiquitination
Phosphorylation of serine, tyrosine, or threonine residues within S100A8 may regulate its function in many cellular processes (14). Here, we wanted to gain mechanistic insights into the decreased S100A8 levels observed in lung tissue and ATII cells obtained from patients with emphysema. We detected low serine phosphorylation levels within S100A8 in lung tissue (Figure 4A) and ATII cells (Figure 4B) in individuals with this disease, and high expression in smokers. However, we did not detect significant differences in phosphorylation of threonine (Figure E3A) and tyrosine residues (Figure E3B) within S100A8 (P > 0.05). Next, we wanted to identify kinases, which interact with S100A8 and can phosphorylate serine residues in smokers. We screened for 30 kinases by Western blotting (Figure E4A) and selected PKAα, ERK2, casein kinase II α, and PKCα for further analysis (Figure E4B). We observed increased p-PKAα interaction with S100A8 in lung tissue obtained from smokers by immunoprecipitation followed by Western blotting analysis (Figure 4C), which correlated with the levels of serine phosphorylation within S100A8 in these samples. Consistent with these results, we found that p-PKAα expression was increased in ATII cells in smokers and decreased in patients with emphysema (Figure 4D). Although we found S100A8 interaction with p-ERK2, casein kinase II α, and p-PKCα (Figure E4C), we did not detect significant changes in their expression in smokers compared with individuals with this disease (P > 0.05; Figure E4C).
Figure 4.
Decreased S100A8 phosphorylation by PKAα in lung tissue and ATII cells from patients with emphysema. (A) Immunoprecipitation of S100A8 in lung tissue obtained from NS, SM, and patients with E followed by Western blotting to detect phosphorylated (p)-serine expression. Densitometric quantification of p-serine within S100A8 is also shown. The ratio was normalized to control nonsmokers. (B) S100A8 was immunoprecipitated in freshly isolated ATII cells from nonsmokers, smokers, and patients with emphysema, followed by analysis of p-serine expression by Western blotting. Densitometric quantification is also shown. (C) Immunoprecipitation of S100A8 in lung tissue followed by analysis of p-PKAα expression by Western blotting. Densitometric quantification normalized to control nonsmokers is shown. (D) Low p-PKAα levels in ATII cells isolated from patients with emphysema by Western blotting. Densitometric quantification of p-PKAα levels was normalized to control nonsmokers (n = 12). *P < 0.05. Data are shown as means ± SD. IP = immunoprecipitation; ser = serine.
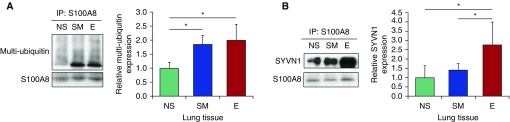
Next, we wanted to check whether decreased phosphorylation of serine within S100A8 in patients with emphysema correlates with protein destabilization and degradation. We found S100A8 ubiquitination in lung tissue obtained from these individuals (Figure 5A). We also detected that SYVN1, an E3 ubiquitin ligase, is responsible for S100A8 ubiquitination (Figure 5B). These results correlated with decreased expression and low phosphorylation levels of S100A8 in ATII cells and lung tissue obtained from patients with emphysema. Our data indicate that S100A8 degradation may contribute to CS-induced emphysema.
Figure 5.
S100A8 ubiquitination in lung tissue from patients with emphysema. (A) IP of S100A8 was performed in lung tissue samples obtained from NS, SM, and patients with E, followed by Western blotting to determine multi-ubiquitin expression. Quantification is also shown. (B) S100A8 was immunoprecipitated in lung tissue obtained from NS, SM, and patients with E to determine SYVN1 expression. Densitometric quantification by Western blotting is shown (n = 4). *P < 0.05. Data are shown as means ± SD.
S100A8 Regulates Cell Susceptibility to Apoptosis
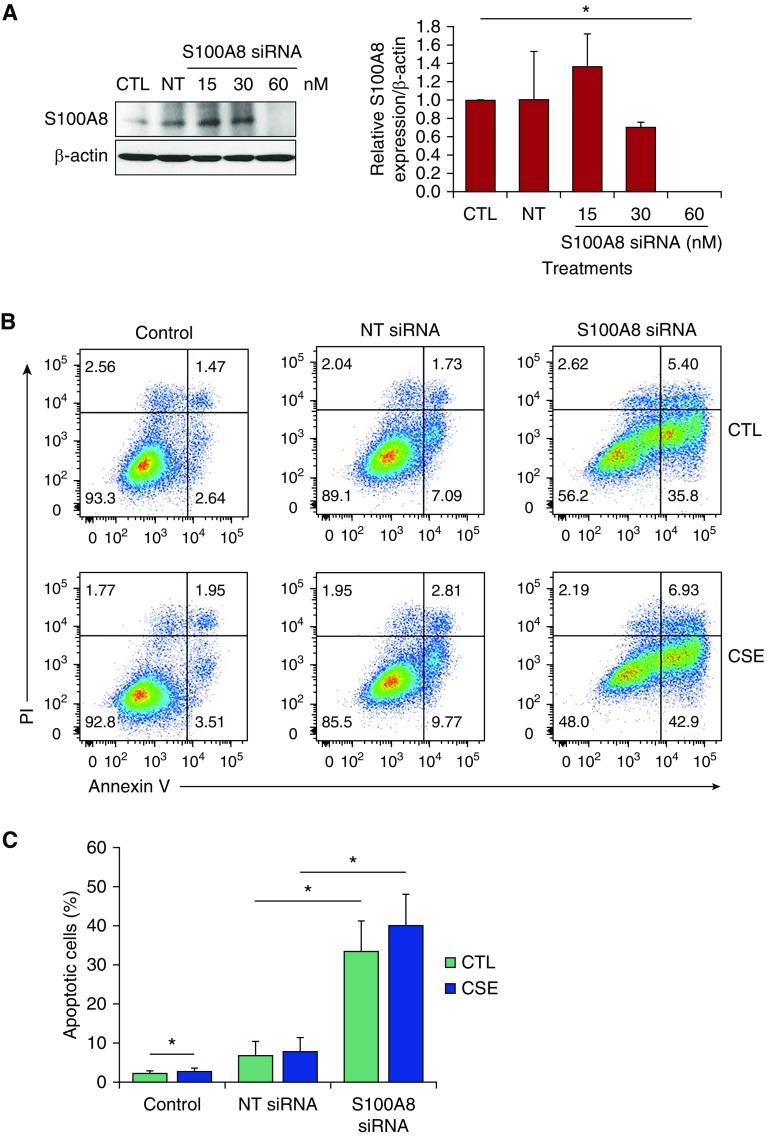
S100A8 deletion causes embryonic lethality in mice (14, 19). To study S100A8 function in vitro, we applied loss- and gain-of-function strategies using the human alveolar epithelial cell line A549 followed by exposure to CSE. We used a siRNA strategy to knock down S100A8 (Figure 6A), and analyzed A549 cell death by double staining with Annexin V and PI (Figure 6B). S100A8 knockdown alone increased the percentage of apoptotic cells (Figure 6C). In addition, we found significantly higher apoptosis in cells treated with S100A8 siRNA followed by exposure to CSE.
Figure 6.
S100A8 knockdown sensitizes cells to apoptosis. (A) A549 cells were transfected with 15, 30, and 60 nM S100A8 siRNA or nontargeting (NT) siRNA for 48 hours. Cell lysates were subjected to Western blotting to determine S100A8 expression. Densitometric quantification of S100A8 expression was normalized to control (CTL). (B and C) A549 cells were transfected with 60 nM NT siRNA or S100A8 siRNA followed by exposure to 20% CSE for 24 hours. Representative images of flow-cytometric analysis using double staining with Annexin V and propidium iodide (PI) and quantification are shown (n = 3). *P < 0.05. Data are shown as means ± SD.
We also used transient overexpression of S100A8 in A549 cells to complement the obtained results (Figures 7A, E5A, and E5B). We found that this protected cells against apoptosis induced by treatment with CSE as detected by Annexin V and PI double staining (Figures 7B and 7C). Our results suggest that S100A8 has a cytoprotective activity against CSE-induced cell injury and may serve as a potential therapeutic target against emphysema development.
Figure 7.
S100A8 overexpression protects A549 cells. (A) A549 cells were transfected with 2.5 μg empty vector or pcDNA3.1-S100A8 for 48 hours followed by analysis of S100A8 expression by Western blotting (n = 3 per group). (B and C) A549 cells were transfected with pcDNA3.1-S100A8 followed by treatment with 20% CSE. Representative flow-cytometry images using Annexin V and PI double staining and quantification (n = 3) are shown. *P < 0.05. Data are shown as means ± SD.
Discussion
The pathogenesis of emphysema is still not well understood (20, 21). This disease is characterized by alveolar wall destruction. The alveolar epithelium consists of ATI and ATII cells (22). ATI cells cover 95% of the lung parenchyma and form a tight epithelial barrier along with ATII cells that helps keep the alveoli dry. ATI cells have special morphological characteristics for efficient gas exchange between the alveolus and the pulmonary capillaries. ATII cells secrete pulmonary surfactant protein to decrease the surface tension (23). It has been reported that the senescence of ATII cells and endothelial cells is accelerated in patients with emphysema. Cellular senescence and apoptosis may explain the abnormal cell turnover that promotes the loss of alveolar epithelial cells in emphysematous lungs (9). The infiltration of inflammatory cells such as macrophages has been also reported. Alveolar macrophages play a unique role in the immune response, pulmonary host defense, and clearance of damaged cells and inhaled particulates during lung injury. Macrophages are activated by CS to release inflammatory mediators. In patients with emphysema, there is an increase in BAL fluid protein concentrations and expression of matrix metalloproteinase (MMP)-1 and MMP-9 in macrophages (24). There is a direct correlation between the presence of parenchymal macrophages and emphysema (25). Previous studies have established a strong relationship between MMPs and COPD (26). MMPs are involved in the inflammatory response and are the regulatory factors of elastin and collagen in the extracellular matrix, which are indispensable for the occurrence of emphysema. Gene-targeted mice deficient in macrophage elastase (MMP-12) are entirely protected from CS-induced emphysema (27). Moreover, macrophages recruit neutrophils and CD8+ lymphocytes, which cause elastolysis and contribute to development of this disease (28, 29). Furthermore, excessive oxidative stress, induced by CS, is also deleterious to alveolar macrophage function, leading to a deficiency in efferocytosis of apoptotic cells, which can be particularly damaging. This indicates that CS-induced oxidative stress results in alteration of cell functions, inflammation, protease–antiprotease imbalance, damage to airspace structure, and disturbance of the normal maintenance of the alveolar wall, which contributes to emphysema pathogenesis (28). Identification of the molecular mechanisms involved in this disease development may lead to novel therapeutic strategies.
In this study, we focused on the mechanism of ATII cell injury by CS and in emphysema. Our previous reports showed primary ATII cell and lung tissue damage in heavy smokers (30) and mice exposed to CS (31, 32). Here we provide evidence that S100A8 has a cytoprotective role against ATII cell injury by CS and emphysema progression.
Deletion of S100A8 gene is embryonic lethal in mice, which suggests its critical function (14, 19). S100A8 expression is increased by oxidative stress, whereas S100A9 does not respond to ROS (14, 33). Recently it has been reported that S100A8 protects against acute murine lung injury by scavenging ROS and reducing proinflammatory mediators and chemokines, leading to suppression of neutrophil infiltration (34, 35). These observations position S100A8 as a potential key factor in protection against lung diseases in which oxidative stress is a part of the pathophysiology. We found S100A8 upregulation at the protein and mRNA levels in freshly isolated ATII cells obtained from smokers and cultured primary ATII cells exposed to CSE. This suggests that oxidative stress induces S100A8 expression and its cytoprotective function. However, we found significantly lower S100A8 levels in ATII cells and lung tissue obtained from patients with emphysema, which correlated with high oxidative stress as detected by 4-HNE expression. We also observed decreased S100A8 levels in severe emphysema compared with mild emphysema. This indicates that S100A8 has a cytoprotective activity and the loss of its function may contribute to emphysema development. A previous study reported that S100A8 expression could be used to predict graft loss due to chronic allograft nephropathy (36). Relatively lower S100A8 mRNA and protein levels were also associated with unfavorable prognosis in that study. Moreover, S100A8/S100A9 deficiency was observed in nonhealing chronic venous wounds (37). These observations suggest that decreased S100A8 levels correlate with cell injury and the proinflammatory response.
PKA is a cAMP-dependent serine/threonine kinase and an important player in many cellular pathways (38). PKA can interact with its targets by direct physical binding or indirect formation of complexes mediated by other linker proteins (39). We found higher serine phosphorylation within S100A8 in ATII cells and lung tissue in smokers. These results suggest activation of the cytoprotective properties of S100A8 against oxidative stress–induced cell injury through its phosphorylation by p-PKAα. Moreover, we detected low S100A8 and p-PKAα levels in emphysema. We analyzed S100A8 ubiquitination to determine the functional consequences of decreased S100A8 phosphorylation by p-PKAα in this disease. Indeed, we found that S100A8 was ubiquitinated in emphysema, which correlated with high oxidative stress. Furthermore, our data suggest that SYVN1, an E3 ubiquitin ligase, is responsible for S100A8 ubiquitination leading to its degradation. The polymerized ubiquitin chain acts as a signal that shuttles the target proteins to proteasome (40). Thus, high oxidative stress and decreased p-PKAα–mediated serine phosphorylation within S100A8 in emphysema may lead to the induction of protein folding stress followed by S100A8 ubiquitination by SYVN1. Our results suggest that S100A8 degradation may contribute to ATII cell injury and CS-induced emphysema development (1).
It has been reported that S100A8 recombinant protein can attenuate airway hyperresponsiveness in ovalbumin-sensitized rats (41). We used loss- and gain-of function studies to determine the role of S100A8 in cells. Interestingly, we found that S100A8 siRNA alone induced cell apoptosis, which indicates the important role of this gene. We also detected significantly higher apoptosis in A549 cells with S100A8 knockdown and treated with CSE compared with CSE alone. On the other hand, S100A8 overexpression had a cytoprotective activity.
In conclusion, our data provide new insight into the cytoprotective function of S100A8 against ATII cell injury induced by CS (Figure E6). Moreover, very high oxidative stress in emphysema correlated with S100A8 degradation. Further studies may focus on its role in susceptibility to CS-induced emphysema. Targeting S100A8 may provide a new therapeutic strategy for this disease.
Supplementary Material
Footnotes
Supported by National Institutes of Health grant R01 HL118171 (B.K.) and Flight Attendant Medical Research Institute grant CIA130046 (B.K.).
Author Contributions: C.-R.L., K.B., and B.K. designed the study and wrote the manuscript. C.-R.L. performed the majority of experiments and analyzed results. G.J.C., N.M., R.M.T., S.K., S.B., and C.M. contributed to the design and interpretation of the study, and provided critical reagents. All authors provided intellectual input and critical feedback.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0144OC on October 2, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Senior RM. Chronic obstructive pulmonary disease—part 2: pathology and biochemistry of emphysema. Thorax. 2002;57:830–834. doi: 10.1136/thorax.57.9.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criner GJ, Mamary AJ. Lung volume reduction surgery and lung volume reduction in advanced emphysema: who and why? Semin Respir Crit Care Med. 2010;31:348–364. doi: 10.1055/s-0030-1254075. [DOI] [PubMed] [Google Scholar]
- 4.Patel N, DeCamp M, Criner GJ. Lung transplantation and lung volume reduction surgery versus transplantation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:447–453. doi: 10.1513/pats.200707-107ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018:5730395. doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoshiba K, Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1:219–226. doi: 10.1186/1617-9625-1-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messier EM, Bahmed K, Tuder RM, Chu HW, Bowler RP, Kosmider B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013;4:e573. doi: 10.1038/cddis.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 10.Calabrese F, Giacometti C, Beghe B, Rea F, Loy M, Zuin R, et al. Marked alveolar apoptosis/proliferation imbalance in end-stage emphysema. Respir Res. 2005;6:14. doi: 10.1186/1465-9921-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes LH, Raftery MJ, Yan WX, Goyette JD, Thomas PS, Geczy CL. S100A8 and S100A9-oxidant scavengers in inflammation. Free Radic Biol Med. 2013;58:170–186. doi: 10.1016/j.freeradbiomed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354. doi: 10.1155/2013/828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CR, Wei TY, Tsai HY, Wu YT, Wu PY, Chen ST. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J. 2015;29:5006–5017. doi: 10.1096/fj.15-273987. [DOI] [PubMed] [Google Scholar]
- 14.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Curr Mol Med. 2013;13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimi F, Hsu K, Endoh Y, Geczy CL. FGF-2, IL-1β and TGF-β regulate fibroblast expression of S100A8. FEBS J. 2005;272:2811–2827. doi: 10.1111/j.1742-4658.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- 16.Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, et al. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13:43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Pel M, van Os R, Velders GA, Hagoort H, Heegaard PM, Lindley IJ, et al. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci USA. 2006;103:1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, et al. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163:2209–2216. [PubMed] [Google Scholar]
- 20.Suzuki M, Sze MA, Campbell JD, Brothers JF, II, Lenburg ME, McDonough JE, et al. The cellular and molecular determinants of emphysematous destruction in COPD. Sci Rep. 2017;7:9562. doi: 10.1038/s41598-017-10126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Berthiaume Y, Voisin G, Dagenais A. The alveolar type I cells: the new knight of the alveolus? J Physiol. 2006;572:609–610. doi: 10.1113/jphysiol.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog EL, Brody AR, Colby TV, Mason R, Williams MC. Knowns and unknowns of the alveolus. Proc Am Thorac Soc. 2008;5:778–782. doi: 10.1513/pats.200803-028HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435. doi: 10.3389/fimmu.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu C, Wang XD, et al. Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. Am J Respir Cell Mol Biol. 2002;26:52–57. doi: 10.1165/ajrcmb.26.1.4253. [DOI] [PubMed] [Google Scholar]
- 26.He S, Xie L, Lu J, Sun S. Characteristics and potential role of M2 macrophages in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3029–3039. doi: 10.2147/COPD.S147144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5:475–477. doi: 10.1513/pats.200708-126ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- 30.Bahmed K, Messier EM, Zhou W, Tuder RM, Freed CR, Chu HW, et al. DJ-1 modulates nuclear erythroid 2-related factor-2-mediated protection in human primary alveolar type II cells in smokers. Am J Respir Cell Mol Biol. 2016;55:439–449. doi: 10.1165/rcmb.2015-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messier EM, Day BJ, Bahmed K, Kleeberger SR, Tuder RM, Bowler RP, et al. N-acetylcysteine protects murine alveolar type II cells from cigarette smoke injury in a nuclear erythroid 2-related factor-2-independent manner. Am J Respir Cell Mol Biol. 2013;48:559–567. doi: 10.1165/rcmb.2012-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimbaldeston MA, Geczy CL, Tedla N, Finlay-Jones JJ, Hart PH. S100A8 induction in keratinocytes by ultraviolet A irradiation is dependent on reactive oxygen intermediates. J Invest Dermatol. 2003;121:1168–1174. doi: 10.1046/j.1523-1747.2003.12561.x. [DOI] [PubMed] [Google Scholar]
- 34.Hiroshima Y, Hsu K, Tedla N, Wong SW, Chow S, Kawaguchi N, et al. S100A8/A9 and S100A9 reduce acute lung injury. Immunol Cell Biol. 2017;95:461–472. doi: 10.1038/icb.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiroshima Y, Hsu K, Tedla N, Chung YM, Chow S, Herbert C, et al. S100A8 induces IL-10 and protects against acute lung injury. J Immunol. 2014;192:2800–2811. doi: 10.4049/jimmunol.1302556. [DOI] [PubMed] [Google Scholar]
- 36.Eikmans M, Roos-van Groningen MC, Sijpkens YW, Ehrchen J, Roth J, Baelde HJ, et al. Expression of surfactant protein-C, S100A8, S100A9, and B cell markers in renal allografts: investigation of the prognostic value. J Am Soc Nephrol. 2005;16:3771–3786. doi: 10.1681/ASN.2005040412. [DOI] [PubMed] [Google Scholar]
- 37.Trøstrup H, Lundquist R, Christensen LH, Jorgensen LN, Karlsmark T, Haab BB, et al. S100A8/A9 deficiency in nonhealing venous leg ulcers uncovered by multiplexed antibody microarray profiling. Br J Dermatol. 2011;165:292–301. doi: 10.1111/j.1365-2133.2011.10384.x. [DOI] [PubMed] [Google Scholar]
- 38.Han B, Poppinga WJ, Schmidt M. Scaffolding during the cell cycle by A-kinase anchoring proteins. Pflugers Arch. 2015;467:2401–2411. doi: 10.1007/s00424-015-1718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X, Jin C, Ren J, Yao X, Xue Y. Proteome-wide prediction of PKA phosphorylation sites in eukaryotic kingdom. Genomics. 2008;92:457–463. doi: 10.1016/j.ygeno.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Huang H, Wang H, Figueiredo-Pereira ME. Regulating the ubiquitin/proteasome pathway via cAMP-signaling: neuroprotective potential. Cell Biochem Biophys. 2013;67:55–66. doi: 10.1007/s12013-013-9628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu YD, Wang Y, Yin LM, Park GH, Ulloa L, Yang YQ. S100A8 protein attenuates airway hyperresponsiveness by suppressing the contraction of airway smooth muscle. Biochem Biophys Res Commun. 2017;484:184–188. doi: 10.1016/j.bbrc.2017.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.