Summary
The aim of the present study was to examine the prevalence and causes of adult epilepsy in a general Japanese population. We examined a total of 3333 Japanese residents in the town of Hisayama aged ≥40 years in 2012‐2013. The examination was performed mainly at the municipal center for health promotion, but some subjects were examined in their homes, hospitals, or nursing homes. Twenty‐three subjects had a diagnosis of epilepsy. The prevalence (95% confidence interval [CI]) of epilepsy per 1000 was 6.9 (4.1‐9.7) in total, 4.9 (1.3‐8.5) in men, and 8.4 (4.3‐12.5) in women (P = 0.23 between sexes). The prevalence of epilepsy was significantly higher in the elderly (aged ≥65 years; 10.3 per 1000 [95% CI 5.4‐15.1]) than in the middle‐aged (aged 40‐64 years; 3.6 per 1000 [95% CI 0.7‐6.4]; P = 0.02). The major cause of epilepsy was cerebrovascular diseases (n = 11; 48% of the epilepsy patients). More than half of the epilepsy patients experienced the first episode of seizure in older age (≥65 years; n = 13; 57%). The findings of this study suggest the clinical importance of the prevention of cerebrovascular diseases to reduce the burden of epilepsy in the future.
Keywords: adult epilepsy, cerebrovascular diseases, elderly, middle‐aged, prevalence
1. INTRODUCTION
Epilepsy is one of the common chronic neurologic diseases observed in all age groups from childhood to old age. The Rochester Study in the United States reported that the incidence of epilepsy was higher in infants and the elderly than in other generations,1 and that the prevalence of epilepsy increased with aging.2 Because the proportion of the elderly aged ≥65 years in Japan (25% in 2013) is predicted to increase rapidly (30% in 2025 and 40% in 2060),3 the number of patients with epilepsy may also increase in the future. Therefore, a prevalence study for adult epilepsy is needed to estimate the clinical and social burden of the disease. However, only one population‐based study has estimated the prevalence of adult epilepsy in Japan,4 and no studies in Japan have examined the prevalence and the causes of epilepsy separately in the middle‐aged and elderly. The purpose of the present study was to investigate these issues in a general Japanese adult population.
2. METHODS
The present cross‐sectional study was performed as a substudy of the Hisayama Study, a prospective cohort study of cardiovascular disease in a general Japanese population.5 The town of Hisayama is located in a suburb of the Fukuoka metropolitan area in Japan. The age, occupational distributions, and nutritional intake levels of the residents of Hisayama are similar to those of Japan as a whole.5 A comprehensive health examination for cardiovascular risk factors and epilepsy was performed in this town from June 2012 to November 2013. A total of 4679 residents aged ≥40 years were invited to participate in the examination by letters, telephone, or face‐to‐face invitations. The examination was performed mainly at the municipal center for health promotion in Hisayama. However, some subjects who could not visit the center due to disability, dementia, or severe diseases were interviewed in their homes, hospitals, or nursing homes so as to include as many participants as possible. Thus a final subject group of 3396 subjects participated in the health examination. After excluding 6 subjects who refused to participate in the surveys and 57 subjects for whom information on epilepsy was unavailable, 3333 subjects (71.2% of residents aged ≥40 years) were enrolled in the present study.
In the health examination, a self‐administered questionnaire was used to ask each participant whether he/she had a past history of epilepsy or was currently taking antiepileptic drugs (Appendix S1). This questionnaire was checked by a trained research nurse and a pharmacist through a face‐to‐face interview. In addition, to reduce the possibility of underreporting cases of epilepsy, one of the study physicians directly asked each subject, by face‐to‐face or telephone interview, whether he/she had a history of epilepsy or any episodes of seizures in the past and whether he/she had received antiepileptic drugs. For subjects with possible epilepsy episodes or treatment, including suspected cases, the clinical records—including medical history, brain imaging, and electroencephalography reports, if available—were collected from clinics or hospitals and reviewed by a neurologist (A.T.) to determine the final diagnosis of epilepsy, the age of the first episode of seizure, and the cause of epilepsy. Epilepsy was defined as either having an episode of active epilepsy (having at least one seizure within the last 5 years)6 diagnosed by a local physician or a neurologist or being treated for epilepsy using antiepileptic drugs. The subjects who took antiepileptic drugs for other reasons (eg, essential tremor, neuralgia, and psychiatric disorder) were excluded from the diagnosis of epilepsy. The classification of the causes of epilepsy, the definitions of other variables, and the methods for statistical analyses are described in Appendix S2.
This study was approved by the Kyushu University Institutional Review Board for Clinical Research and informed consent was obtained from all participants.
3. RESULTS
Twenty‐three subjects were diagnosed as having epilepsy, yielding an epilepsy prevalence of 6.9 per 1000 subjects with no statistically significant difference between the sexes (P = 0.23; Table 1). The prevalence of epilepsy was significantly higher in the elderly (≥65 years; 10.3 per 1000) than in the middle‐aged (40‐64 years; 3.6 per 1000; P = 0.02). The subjects who were hospitalized or institutionalized at a nursing home had a significantly higher prevalence of epilepsy (114.3 per 1000) than those living at home (3.4 per 1000; P < 0.001).
Table 1.
Prevalence of epilepsy, the Hisayama Study, 2012‐2013
| Number of subjects with epilepsy/total subjects | Prevalence of epilepsy per 1000 (95% confidence interval) | P‐value between subgroups | |
|---|---|---|---|
| All | 23/3333 | 6.9 (4.1‐9.7) | |
| Age at examination, y | |||
| 40‐64 | 6/1679 | 3.6 (0.7‐6.4) | 0.02 |
| ≥65 | 17/1654 | 10.3 (5.4‐15.1) | |
| Sex | |||
| Male | 7/1430 | 4.9 (1.3‐8.5) | 0.23 |
| Female | 16/1903 | 8.4 (4.3‐12.5) | |
| Residence | |||
| Living at home | 11/3228 | 3.4 (1.4‐5.4) | <0.001 |
| Hospitalized or institutionalized at a nursing home | 12/105 | 114.3 (53.4‐175.1) | |
Among the 23 patients with epilepsy, 13 patients (57%) experienced the first episode of seizure in older age (≥65 years; Table S1). The mean age at examination was significantly older and the mean value of body mass index was significantly lower in subjects with epilepsy than in those without. The subjects with epilepsy were more likely to have a history of cerebrovascular diseases and more likely to be disabled than those without epilepsy.
Except for epilepsy of unknown cause or without focal lesions, the most common cause of epilepsy in subjects overall was cerebrovascular diseases followed by congenital diseases (Figure 1). Among the middle‐aged subjects, the most common cause of epilepsy was congenital diseases, and among the elderly subjects it was cerebrovascular diseases.
Figure 1.
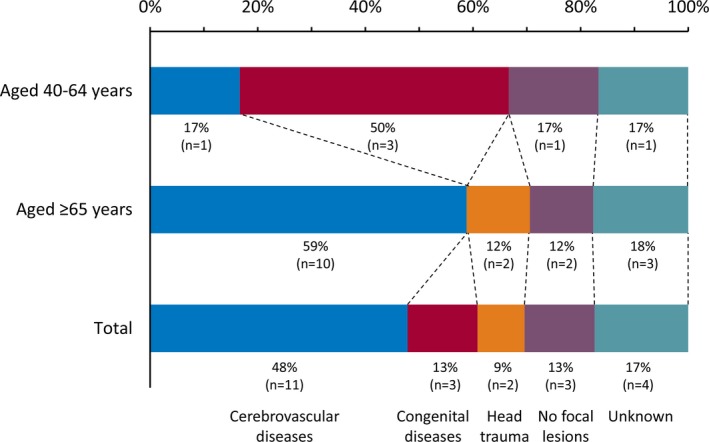
Causes of epilepsy among total subjects, middle‐aged subjects (aged 40‐64 years at examination), and elderly subjects (aged 65 years or older at examination) with epilepsy
4. DISCUSSION
In this study, the prevalence of epilepsy in adults aged ≥40 years was 6.9 per 1000. A meta‐analysis of 67 prevalence studies reported that the prevalence of epilepsy worldwide irrespective of age was approximately 6.4 per 1000.7 A population‐based study in Tottori, Japan reported that the prevalence of epilepsy was 4.4 per 1000 in adults aged ≥20 years.4 A direct comparison of the prevalence of epilepsy among the studies was difficult, because the age ranges varied among the respective study populations. In general, however, the prevalence of epilepsy in the present study seemed to be similar to that in the previously reported studies. Moreover, the present study showed that the most common cause of epilepsy was cerebrovascular disease in the elderly, whereas the main cause of epilepsy was congenital diseases in the middle‐aged. The Veterans Affairs Cooperative Study from the United States also reported that cerebrovascular disease was the major cause of epilepsy in the elderly.8 However, to the best of our knowledge, the present study is the first to estimate the prevalence of epilepsy and its causes separately for middle‐aged and elderly subjects in a general Japanese population. The higher prevalence of epilepsy in the elderly in the present study can be explained mainly by symptomatic seizures after elderly onset cerebrovascular disease. The findings of the present study suggest the clinical importance of the prevention of cerebrovascular disease to reduce the burden of epilepsy in the future.
The present study also found that the prevalence of epilepsy in the elderly was approximately three times as high as that in the middle‐aged, and more than half of subjects with epilepsy experienced the first seizure in older age. In contrast, the above‐mentioned meta‐analysis reported that the prevalence of epilepsy in the elderly (≥ 60 years; 7.17 per 1000) was similar to that in the middle‐aged (30‐59 years; 7.94 per 1000).7 The exact reason for the discrepancy in these findings is unknown, but it may be attributable to a higher prevalence of epilepsy in the Japanese elderly, which in turn could be due to the longer life expectancy in Japan than in other countries.3 In addition, the prevalence of epilepsy in the previous reports might be underestimated as compared with our estimation, because most previous studies did not investigate hospitalized or institutionalized subjects, who have a very high prevalence of epilepsy, whereas not only subjects living at home, but also hospitalized or institutionalized subjects were surveyed in the present study.
The previous observational studies have reported disparate results regarding the sex difference in epilepsy prevalence,9 but a meta‐analysis did not show a clear sex difference.7 In the present study, the prevalence of epilepsy in women seemed to be higher than that in men but the difference did not reach the level of statistical significance. The higher prevalence in women might have been simply a matter of chance, or it might be partially explained by the older age distribution in women (mean age, 65 years) than in men (mean age, 64 years; P = 0.003).
Limitations of the study should be discussed. First, there was a possibility of selection bias, since the participation rate was 71.2% among the residents aged ≥40 years. The prevalence of epilepsy might have been underestimated in the present study, because the nonparticipants could have been less healthy than the participants. However, it is generally agreed that an acceptable participation rate in a population‐based study (a rate that practically eliminates the threat of selection bias attributable to nonparticipants) is >70% of the target population.10 Therefore, we believe that selection bias in the present study, if any, was modest, and thus the prevalence reported in the present study is reliable to some extent. Second, the self‐administered questionnaire was not validated and the completeness of case ascertainment was not assessed. However, we consider that the possibility of underreporting cases of epilepsy was reduced to a minimum through comprehensive screening procedures by the study team. Third, because residents aged ≤39 years were not included in the present study, a direct comparison of epilepsy prevalence with other studies was difficult. Fourth, the small sample size limited the ability to conduct detailed subgroup analyses. Fifth, we cannot deny the possibility that the cases of acute symptomatic seizure without chronic active epilepsy were misclassified into the epilepsy group, despite our detailed review of the clinical information. Sixth, because we did not collect information on epilepsy that was not currently active, lifetime prevalence of epilepsy could not be estimated.
5. CONCLUSIONS
Our study suggests that 6.9 per 1000 individuals have epilepsy in the general Japanese population, and that the prevalence of epilepsy increases with age, probably because of elderly onset cerebrovascular diseases. Intensive efforts for the prevention of cerebrovascular diseases will be needed to reduce the burden of epilepsy in the future.
DISCLOSURE
None of the authors has any conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
This study was supported in part by Grants‐in‐Aid for Scientific Research (A) (JP16H02644, and JP16H02692), (B) (JP16H05850, JP16H05557, JP17H04126, and JP18H02737) and (C) (JP16K09244, JP17K09114, JP17K09113, JP17K01853, JP18K07565, and JP18K09412), and for Early‐Career Scientists (JP18K17925 and JP18K17382) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (H29‐Junkankitou‐Ippan‐003 and H30‐Shokuhin‐[Sitei]‐005); and by the Japan Agency for Medical Research and Development (JP18dk0207025, JP18ek0210082, JP18gm0610007, JP18ek0210083, JP18km0405202, JP18ek0210080, and JP18fk0108075).
Tanaka A, Hata J, Akamatsu N, et al. Prevalence of adult epilepsy in a general Japanese population: The Hisayama study. Epilepsia Open. 2019;4:182–186. 10.1002/epi4.12295
REFERENCES
- 1. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia 1993;34:453–68. [DOI] [PubMed] [Google Scholar]
- 2. Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940‐1980. Epilepsia 1991;32:429–45. [DOI] [PubMed] [Google Scholar]
- 3. Arai H, Ouchi Y, Toba K, et al. Japan as the front‐runner of super‐aged societies: perspectives from medicine and medical care in Japan. Geriatri Gerontol Int 2015;15:673–87. [DOI] [PubMed] [Google Scholar]
- 4. Nakashima K, Yokoyama Y, Shimoyama R, et al. Prevalence of neurological disorders in a Japanese town. Neuroepidemiology 1996;15:208–13. [DOI] [PubMed] [Google Scholar]
- 5. Hata J, Ninomiya T, Hirakawa Y, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: half‐century data from the Hisayama Study (1961‐2009). Circulation 2013;128:1198–205. [DOI] [PubMed] [Google Scholar]
- 6. Commission on Epidemiology and Prognosis, International League Against Epilepsy . Guidelines for epidemiologic studies on epilepsy. Epilepsia 1993;34:592–6. [DOI] [PubMed] [Google Scholar]
- 7. Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta‐analysis of international studies. Neurology 2017;88:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology 2004;62:S24–9. [DOI] [PubMed] [Google Scholar]
- 9. Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy: a review. Epilepsy Res 2009;85:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groves RM. Chapter 4: nonresponse in sample surveys In: Groves RM, editors. Survey errors and survey costs. New York: John Wiley & Sons; 1989:133–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


