Abstract
Milk is a hallmark of mammals that is critical for normal growth and development of offspring. During biosynthesis of lactose in the Golgi complex, H+ is produced as a by-product, and there is no known mechanism for maintaining luminal pH within the physiological range. Here, using conditional, tissue-specific knockout mice, immunostaining, and biochemical assays, we test whether the putative H+/Ca2+/Mn2+ exchanger known as TMEM165 (transmembrane protein 165) participates in normal milk production. We find TMEM165 is crucial in the lactating mammary gland for normal biosynthesis of lactose and for normal growth rates of nursing pups. The milk of TMEM165-deficient mice contained elevated concentrations of fat, protein, iron, and zinc, which are likely caused by decreased osmosis-mediated dilution of the milk caused by the decreased biosynthesis of lactose. When normalized to total protein levels, only calcium and manganese levels were significantly lower in the milk from TMEM165-deficient dams than control dams. These findings suggest that TMEM165 supplies Ca2+ and Mn2+ to the Golgi complex in exchange for H+ to sustain the functions of lactose synthase and potentially other glycosyl-transferases. Our findings highlight the importance of cation and pH homeostasis in the Golgi complex of professional secretory cells and the critical role of TMEM165 in this process.
Keywords: galactosyltransferase, Golgi, calcium transport, mammary gland, manganese, pH regulation
Introduction
Deficiencies in the Golgi transmembrane protein TMEM165 cause a type II congenital disorder of glycosylation in humans (1). Recent reports have shown that this protein, along with its yeast homolog Gdt1, can transport Ca2+ in vitro and promote normal homeostasis of Ca2+, Mn2+, and H+ in the Golgi complex (2–4). These findings suggest that TMEM165 supports glycosylation by maintaining proper pH and supplying Mn2+ and Ca2+ that are required as cofactors in the glycosyltransferases responsible for protein and lipid glycosylation (5, 6). Disruption of the homeostasis of pH or the concentrations of Ca2+ or Mn2+ have been demonstrated to result in deficiencies in glycosylation (6–9). The incidence of identified cases of congenital disorders of glycosylation among humans is increasing, now including more than 125 unique disorders (10).
Transmembrane protein 165 (TMEM165 mRNA)3, formerly Tpar1, is expressed in virtually all tissues and cell types, including the mammary gland (BioGPS). However, TMEM165 mRNA and protein levels increase dramatically, by 7- and 25-fold, respectively, in the mammary gland during the secretory activation phase of lactation (11, 12). TMEM165 protein levels accumulate in the Golgi complexes of alveolar epithelial cells (12) along with α-lactalbumin subunit of lactose synthase, which together with β4-galactosyltransferase I (β4-GALT-I) synthesizes lactose in the lumen from transported UDP-galactose and glucose (13). β4-GALT-I requires Mn2+ as a cofactor for its catalytic activities (13). The secretory pathway Ca2+/Mn2+-ATPase 1 (SPCA1) supplies the Golgi with much of the Mn2+ required for lactose synthase and other glycosyltransferases (14, 15) and much of the Ca2+ that is complexed with Pi and casein proteins to form micelles in the Golgi complex (16). Although there is no obvious requirement for a H+/Ca2+/Mn2+ exchanger in this scheme of milk production in Golgi complex, H+ is produced as a by-product of all glycosylation reactions including lactose biosynthesis, and there is no known mechanism for eliminating excess H+ from the Golgi and maintaining pH within a physiological range. In milk-producing cells, TMEM165 may deacidify the Golgi while supplying Ca2+ and Mn2+ as both enzyme cofactors and nutrients.
In this study, we test the role of TMEM165 in the lactating mammary gland of mice by examining mouse mutants bearing a conditional knockout of TMEM165. Using a Cre-recombinase that is driven by the whey acid protein (WAP) promoter, which is only expressed in milk-producing alveolar epithelial cells during late pregnancy, an 85% depletion of TMEM165 protein was achieved, and strong defects in milk quality were observed. Unexpectedly, calcium, iron, zinc, fat, and total protein concentrations were elevated in the milk of TMEM165-deficient animals. However, lactose accumulation was strongly diminished, and this likely resulted in decreased osmosis and concentrated other components in the milk. We show that the catalytic subunit of lactose synthase, in vitro, is highly sensitive to acidic pH, and we propose that the reduced quality of milk in TMEM165-deficient animals is due to a failure to remove the H+ by-product of lactose biosynthesis, a diminished supply of Mn2+ in the Golgi, or both, which then diminishes lactose synthase activity and osmosis. These findings highlight the importance of ion homeostasis in the Golgi complex of professional secretory cells and the unique role of TMEM165 in exchanging H+, Ca2+, and Mn2+.
Results
Conditional knockout mouse displays normal histology in the lactating mammary gland
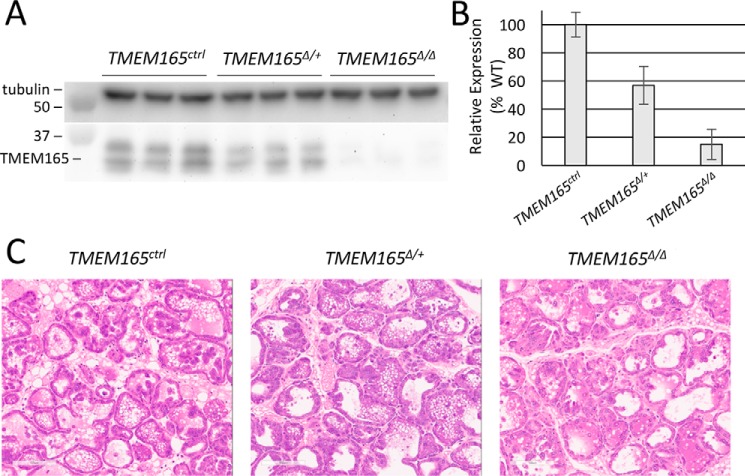
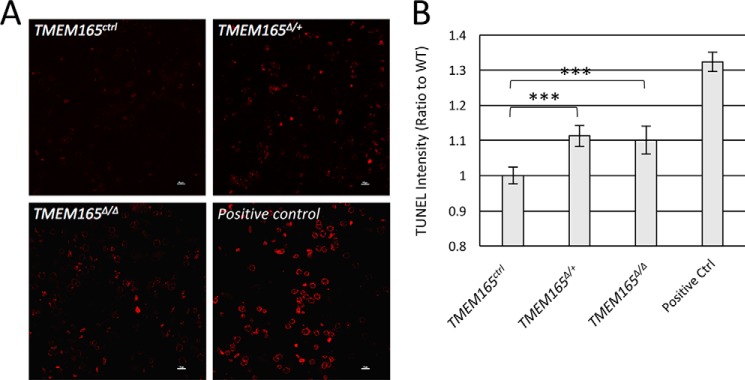
A condition-ready TMEM165 knockout mouse was generated by transfecting embryonic stem cells with a recombinant plasmid from EUCOMM, blastocyst injecting, germ-line screening, and cross-breeding to C57BL/6 WT mice, as described under “Experimental Procedures.” To study the effect of TMEM165 on lactation, we crossed this mouse line to a mouse line expressing Cre recombinase under control by the WAP promoter (17). In the experiments below, all nursing mice were heterozygous for WAP-Cre (i.e. WAP+/Cre) and either homozygous or heterozygous for the floxed allele of TMEM165 (henceforth referred to as TMEM165Δ/Δ and TMEM165Δ/+) or homozygous for the natural allele (TMEM165ctrl). To determine the efficacy of this TMEM165 conditional knockout, Western blots were performed on mammary tissue harvested from mothers after 12 days of lactation. The TMEM165Δ/Δ mice demonstrated an 85% knockdown of TMEM165 expression in mammary tissue, and there was a 43% knockdown in TMEM165Δ/+ mammary tissue, relative to TMEM165ctrl tissue (Fig. 1, A and B). Histological analysis via hematoxylin and eosin sections of mammary tissue from these mothers demonstrated no significant visible difference between genotypes (Fig. 1C). However, TUNEL staining of mammary tissue sections demonstrated a small but significant increase in apoptotic cells in both TMEM165Δ/Δ and TMEM165Δ/+ mice, relative to TMEM165ctrl mice (Fig. 2, A and B). This suggests that even a partial decrease in expression of TMEM165 may occasionally affect cell survival in this tissue.
Figure 1.
TMEM165Δ/Δ mammary glands display normal histology and TMEM165 deficiency. A, Western blots of total protein extracted from mammary glands on day 12 of lactation were performed using anti-TMEM165 and anti-tubulin antibodies. B, the ratios of TMEM165 to tubulin were calculated from quantified bands and averaged among replicates, and the heterozygous and homozygous samples were normalized to TMEM165ctrl animals (± S.D.). C, representative images hematoxylin and eosin–stained tissue from these mothers.
Figure 2.
TMEM165-deficient tissues exhibit elevated cell death. A, TUNEL staining of the tissues identifies cells that have undergone cell death. B, analysis of the intensity shows a statistically significant increase in TUNEL-positive cells in both heterozygous and homozygous-knockout mice, relative to the WT. *, p < 0.05; **, p < 0.01; ***, p < 0.001 using Student's t test.
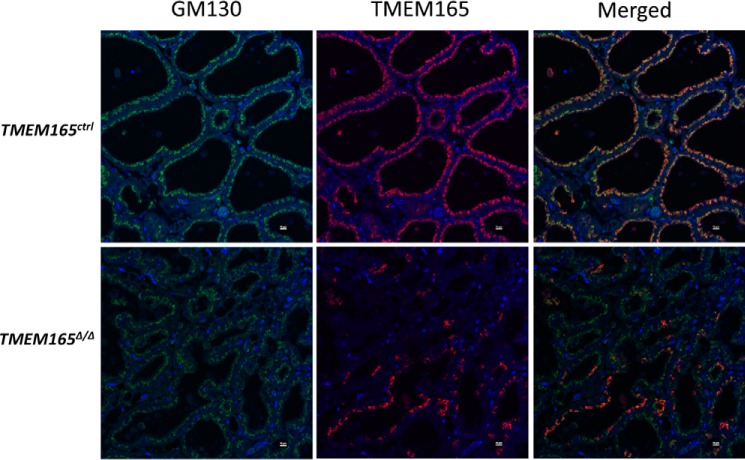
Immunostaining revealed high TMEM165 expression in the Golgi complexes of alveolar epithelial cells that surround the lumen of the TMEM165ctrl mammary tissue (Fig. 3). Interestingly, the tissue from the TMEM165Δ/Δ mouse expressed TMEM165 in a patchy mosaic pattern, where a fraction of the alveolar epithelial cells maintained expression and therefore escaped elimination by WAP-Cre (Fig. 3). One possible reason for the mosaic expression is incomplete deletion of TMEM165 exons from some cells within the developing mammary gland that later populate a small portion of the tissue. Similar mosaic expression of TMEM165 was seen in mice expressing Cre under control of the MMTV promoter (not shown). These findings demonstrated that WAP-Cre produced a large, yet incomplete, decrease in TMEM165 expression in the milk-producing cells of the mammary glands of TMEM165Δ/Δ animals.
Figure 3.
There is mosaic expression of TMEM165 in TMEM165Δ/Δ mammary tissue. In situ immunostaining of fixed and sectioned tissue demonstrates that TMEM165 (red) colocalizes with GM130 (green). 4′,6′-Diamino-2-phenylindole (DAPI; blue) identifies cell nuclei. Some cells within the tissue continue to express TMEM165 in TMEM165Δ/Δ mice, resulting in mosaicism in these mice.
Litters nursed by TMEM165Δ/Δ mothers exhibit low weight gains
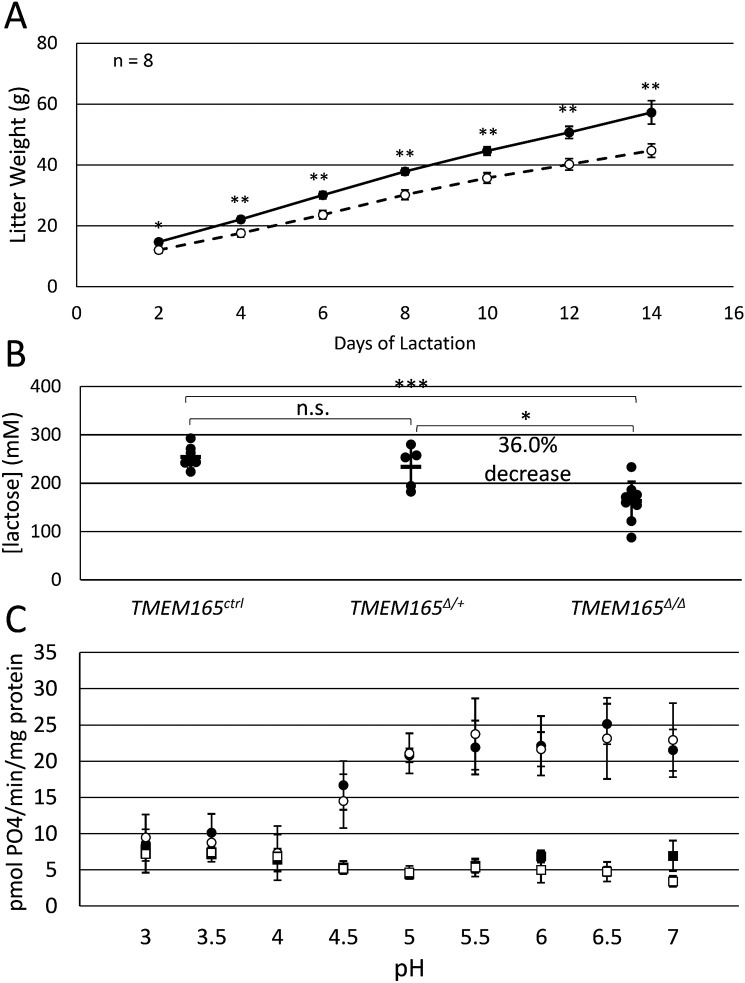
To determine whether TMEM165 deficiency affected the overall quantity or quality of milk production, mothers nursed litters of six pups each, and litter weight was measured every 2 days for 2 weeks. Although all the pups and mothers appeared healthy and behaved normally, pups nursed by TMEM165Δ/Δ mothers displayed significantly lower litter weights than pups nursed by TMEM165ctrl mothers on all days measured (Fig. 4A). By day 14, litters from TMEM165Δ/Δ dams weighed only 78% of those from TMEM165ctrl dams, suggesting a strong defect in milk quality or quantity.
Figure 4.
Milk from TMEM165Δ/Δ mothers exhibits low nutritional quality and low lactose. A, litters of six pups/mother were weighed every 2 days following birth. The data shown are the averages (± S.D.) of litters nursed by eight mothers of TMEM165Δ/Δ and TMEM165ctrl. B, milk collected from mothers of the specified genotype on day 12 of lactation were assayed for lactose as described under “Experimental Procedures.” C, galactosyltransferase activity, representing β4-GALT-1 subunit of lactose synthase, was measured in detergent-solubilized crude membrane extracts prepared from the indicated nursing mothers on day 12 of lactation. The assay method, which measures the concentration of Pi that is produced over time and normalized to the concentration of total protein, is described under “Experimental Procedures.” *, p < 0.05; **, p < 0.01; ***, p < 0.001 using Student's t test.
Lactose deficiency in milk from TMEM165-deficient dams
Because TMEM165 deficiency leads to deficiencies of glycosylation in humans (1), we hypothesized that TMEM165Δ/Δ mice may have a deficiency in lactose biosynthesis. To test this possibility, milk samples were collected from dams after 12 days of lactation with 6 pups/dam, and the lactose concentration was measured. Strikingly, we observed a 36% decrease in lactose concentration in the TMEM165Δ/Δ dams relative to the TMEM165Δ/+ and TMEM165ctrl dams (Fig. 4B). The corresponding decline in osmosis would serve to concentrate the residual lactose and thus partially mask a larger decline in the actual rate of lactose biosynthesis.
To determine whether the decline in lactose biosynthesis is caused by a decline in lactose synthase activity, mammary glands from TMEM165ctrl and TMEM165Δ/Δ dams were dissected and homogenized. Crude membrane fractions were isolated by differential centrifugation, washed to remove lactose, permeabilized with nondenaturing detergent, and assayed in vitro for galactosyltransferase activity using a protocol that monitors liberation of Pi from UDP-galactose in the presence of GlcNAc and added Mn2+ (see “Experimental Procedures”). These conditions measure β4-GALT-I activity directly and do not rely on the potentially unstable association of α-lactalbumin and Mn2+ with the catalytic subunit of lactose synthase. At physiological pH of 6 for the lumen of the Golgi complex, galactosyltransferase activity was indistinguishable in TMEM165ctrl and TMEM165Δ/Δ samples (Fig. 4C). Omission of glucose (Fig. 4C) or UDP-galactose (not shown) resulted in a total loss of galactosyltransferase activity, indicating the specificity of the assay method. Galactosyltransferase activity remained constant as pH of the buffer was increased to 7 or decreased to 5. However, the activity declined to background levels when the pH was lowered to 4 or less (Fig. 4C). Thus, the TMEM165Δ/Δ and TMEM165ctrl samples appeared to contain equivalent activities of the lactose synthase catalytic subunit when assayed in vitro in the presence of excess of Mn2+, and this activity was somewhat sensitive to the acidity of the buffer. These results suggest that lactose synthase enzyme levels are unaffected by TMEM165 deficiency and that the activity of enzyme may be severely diminished in the Golgi complexes of TMEM165Δ/Δ cells. Because TMEM165 is hypothesized to export luminal H+ in exchange for cytoplasmic Mn2+, TMEM165 deficiency may diminish lactose synthase activity by increasing luminal H+, decreased luminal Mn2+, or both, resulting in lower lactose production and diminished milk quality.
Lactose deficiency may concentrate other milk components because of lower dilution by osmosis
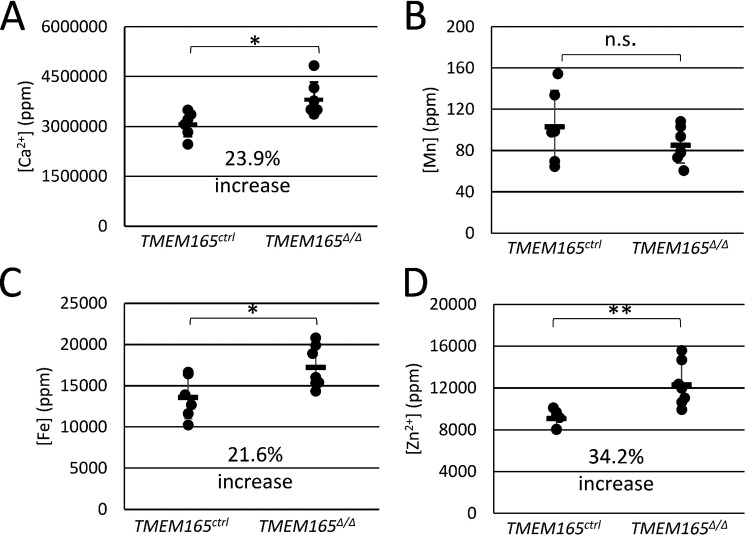
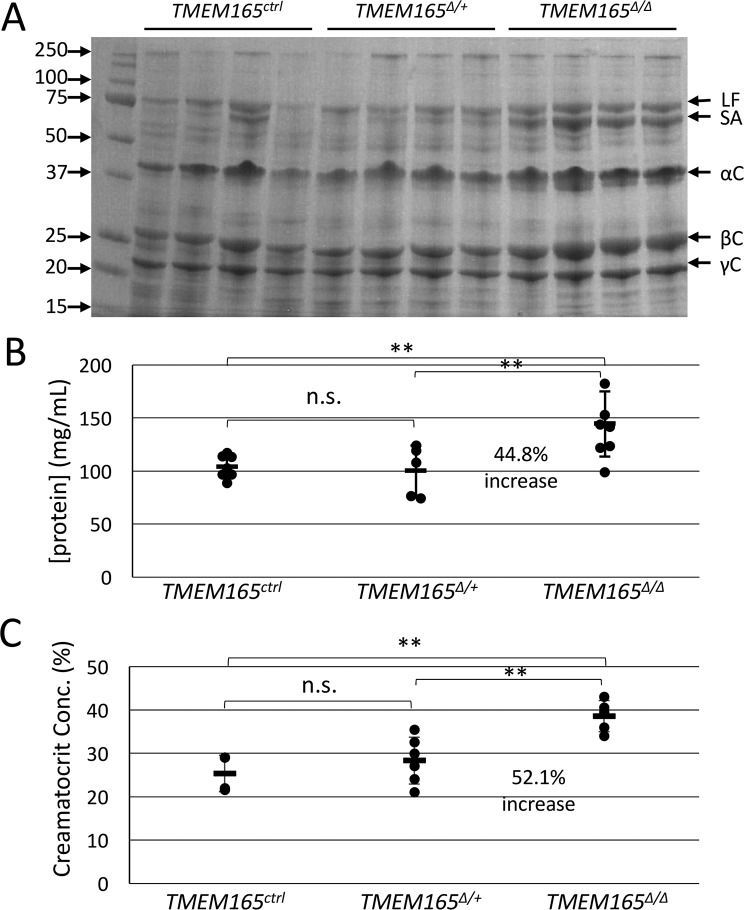
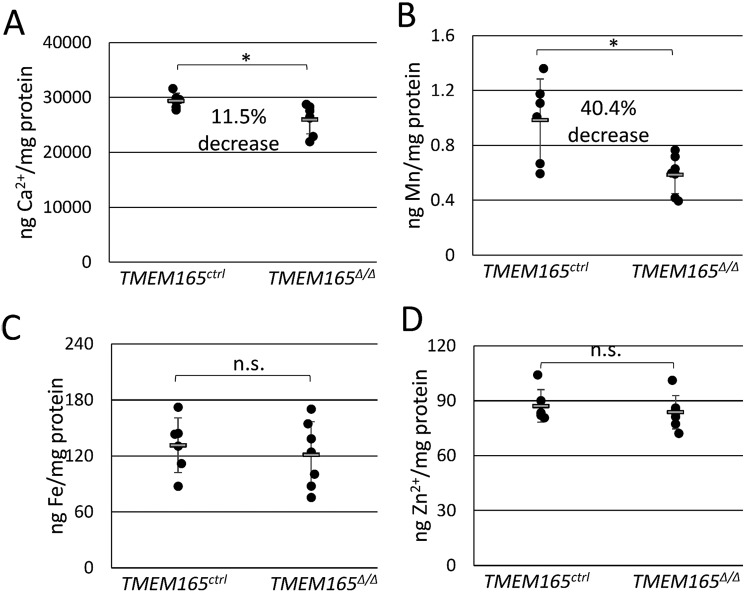
Lactose concentrations in mouse milk can reach 250 mm and thus serves as the major osmolyte of milk and perhaps even in the Golgi complex where lactose is synthesized. To investigate the possibility that the deficiency of lactose secretion in TMEM165-deficient mammary glands produced milk with an osmotic defect, milk samples were treated with concentrated nitric acid to eliminate organic components and then analyzed by inductively coupled plasma MS (ICP-MS) alongside calibration standards (see “Experimental Procedures”). Surprisingly, the concentration of calcium did not decline in milk from TMEM165Δ/Δ dams and actually increased by 23.9% (Fig. 5A), whereas the concentration of manganese remained constant (Fig. 5B). The concentrations of iron and zinc also increased significantly by 21.6 and 34.2%, respectively (Fig. 5, C and D). Because these metals are mostly bound as ions by milk proteins, we also measured the concentration of total protein in untreated portions of the milk samples. Milk from TMEM165Δ/Δ dams contained ∼45% increase in total protein relative to that of TMEM165Δ/+ and TMEM165ctrl dams (Fig. 6B). Analysis of milk proteins by SDS-PAGE and staining with Coomassie Brilliant Blue also suggested increased protein concentration in the milk from TMEM165Δ/Δ dams without any noticeable difference in the pattern of the bands (Fig. 6A). In addition, we estimated the fat composition of the milk by measuring the creamatocrit composition, which is the percentage of milk made up of cream. TMEM165Δ/Δ dams exhibited a 52% increase in creamatocrit concentration, similar to the increase in total protein concentration (Fig. 6C). These findings indicate that milk from TMEM165Δ/Δ dams contains significantly higher concentrations of calcium, iron, zinc, total protein, and lipids, none of which are likely to diminish the milk quality. When the metal concentrations were normalized to total protein instead of volume, iron- and zinc-to-protein ratios were similar in milk from TMEM165Δ/Δ and TMEM165ctrl dams (Fig. 7, C and D). However, milk from TMEM165Δ/Δ dams did exhibit a significant decrease in the calcium- and manganese-to-protein ratios (Fig. 7, A and B), which could lower the activity of Mn2+-dependent glycosyltransferases such as lactose synthase, causing a decrease in lactose synthesis and secretion into milk, lower osmosis, and lower dilution of other milk components, including metals and protein.
Figure 5.
Measurements of metal content in milk from TMEM165Δ/Δ and control mothers were taken. Concentrations of calcium (A), manganese (B), iron (C), and zinc (D) in skimmed milk samples from day 12 of lactation were measured by ICP-MS as described under “Experimental Procedures.” Milk from six control and six TMEM165 homozygous knockout animals were tested. *, p < 0.05; **, p < 0.01; ***, p < 0.001 using Student's t test.
Figure 6.
Measurements show increase of protein and lipid concentrations in milk from TMEM165Δ/Δ mothers. A and B, milk collected from mothers of the specified genotype on day 12 of lactation was skimmed and analyzed by SDS-PAGE and staining with Coomassie Blue (A) and quantified using Bradford stain (B). C, whole milk was also subjected to creamatocrit (lipid) analysis. *, p < 0.05; **, p < 0.01; ***, p < 0.001 using Student's t test. n.s., not significant.
Figure 7.
Normalized metal content in milk indicates significant deficiency of calcium and manganese from TMEM165Δ/Δ mothers. The data from Fig. 5 were normalized to total protein levels measured in Fig. 6B. A, calcium; B, manganese; C, iron; D, zinc. Milk from six control and six TMEM165-homozygous knockout animals were tested. *, p < 0.05; **, p < 0.01; ***, p < 0.001 using Student's t test.
Discussion
TMEM165 is a Golgi-localized transmembrane protein that is expressed in virtually all mammalian tissue and is strongly conserved throughout animals, plants, fungi, and even prokaryotes. The data obtained up to now suggest that TMEM165 and its homologs transport H+, Ca2+, and Mn2+ across the Golgi membrane and therefore directly influence the homeostasis of these ions, which indirectly affects the activities of Golgi-localized glycosyltransferases and other enzymes. The strong up-regulation of TMEM165 expression in the alveolar epithelial cells of the mammary gland immediately before parturition when secretory activation is occurring (11) suggests an important role for this ion exchanger in milk biosynthesis in these prolific secretory cells. Here we show that indeed TMEM165 deficiency in these cells decreases the manganese-to-protein ratio and lactose accumulation in the milk, likely by diminishing Mn2+ transport into the Golgi that is critical for lactose synthase activity. This results in lower osmotic dilution of protein, fat, and other constituents and lower milk quality, as detected by growth rates of litters.
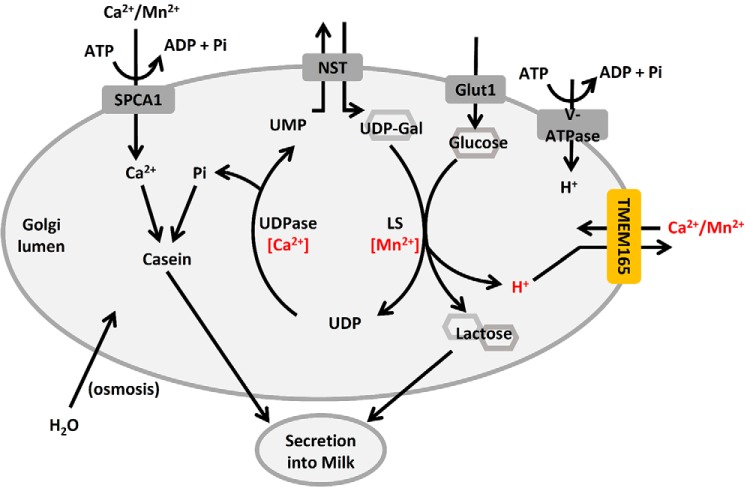
Lactose is the major osmolyte in milk (18). In addition to this, the process of lactose biosynthesis is carried out by a galactosyl-transferase and therefore requires many of the same substrates and conditions as glycosylation (Fig. 8 and Ref. 19). Because TMEM165 deficiency is known to cause congenital disorders of glycosylation (4, 20), it was hypothesized that disruption of expression of TMEM165 during lactation would similarly inhibit the creation of lactose, thereby decreasing the osmotic potential of milk by reducing the lactose composition. This loss of glycosyltransferase activity could result from a decrease in concentration of Mn2+, which is an important cofactor in both lactose synthesis and glycosylation (21), and/or a decrease in pH, resultant from accumulation of protons released during lactose synthesis that would otherwise be released to the cytoplasm by TMEM165.
Figure 8.
A working model of lactose synthesis in the Golgi apparatus demonstrates a possible role for TMEM165. To synthesize lactose, UDP-galactose is transported into the Golgi apparatus in exchange for luminal UMP by a nucleotide-sugar transporter (NST). A Mn2+-dependent lactose synthase (LS) covalently links the galactose to glucose, releasing a proton and UDP, the latter of which is cleaved by a Ca2+-dependent phosphatase (UDPase) into Pi and UMP. The Pi can complex with Ca2+, Mg2+, and casein and then be secreted into milk. Lactose drives osmotic uptake of water in the Golgi, secretory vesicles, and secreted milk to provide volume and reduce viscosity.
Lactose is synthesized from UDP-galactose and glucose in the Golgi lumen by lactose synthase, an enzyme that depends on Mn2+ and is inhibited by acidic pH. Although the expression of the regulatory subunit of lactose synthase (α-lactalbumin) is far greater than the catalytic subunit (β4-GALT-I), the heterozygous knockout mutants of α-lactalbumin exhibited 10–30% declines of lactose concentration in milk, which decreased osmolarity and increased total protein concentration by 20–50% (22, 23). Milk yield also declined in these heterozygotes (23); homozygous knockout mutants produced little milk with no lactose and very high concentrations of protein and viscosity. The consequences of TMEM165 deficiency appeared to be similar to the heterozygous α-lactalbumin deficiency, resulting in moderately decreased lactose and increased total protein, fat, and ion concentrations in the milk.
Upon normalizing Ca2+, Mn2+, Zn2+, and Fe2+ concentrations in milk to total protein, only Ca2+ and Mn2+ appeared to be significantly decreased in the TMEM165Δ/Δ animals. The remaining calcium and manganese in milk is probably transported into the Golgi by the secretory pathway Ca2+/Mn2+-ATPase SPCA1 (6, 14, 24), although SPCA2 may also contribute from a non-Golgi organelle (25). These SPCA pumps contribute ∼40% of the Ca2+ found in milk with the remaining 60% transported by the plasma membrane Ca2+-ATPase PMCA2 (26). Because Ca2+ is ∼30,000 times more abundant in milk than Mn2+, SPCA and TMEM165 likely transport much more Ca2+ than Mn2+ during lactation. The deficiency of Ca2+ transport in TMEM165Δ/Δ animals was not as great as Mn2+ in the milk, possibly because of compensatory effects of the PMCA2 pump, which has been shown to be the primary milk calcium transporter (26). The SPCA pumps appeared unable to fully compensate for the Mn2+ transport defects in the TMEM165Δ/Δ animals, and Mn2+ depletion in the Golgi may lower the activity of lactose synthase and potentially other glycosyltransferases responsible for protein glycosylation despite the fact that both are significantly up-regulated in lactation (15). It is not yet clear whether TMEM165 alters the pH of the Golgi of the milk-producing cells, but a failure to counter-transport H+ from the lumen to the cytoplasm in the knockout animals could lead to hyperacidification and additional loss of lactose synthase and glycosyltransferase activities. H+ accumulation and Mn2+ depletion in the Golgi of these mutant cells could also alter transport of UDP-galactose and other nucleotide sugars instead of, or in addition to, glycosyltransferase activities. Therefore, defects associated with TMEM165Δ/Δ Golgi may be broader yet milder than simple deficiency of α-lactalbumin.
TMEM165 is expressed throughout the body of mammals, and humans with homozygous mutations in TMEM165 exhibit a congenital disorder of glycosylation that ultimately manifests with skeletal and growth abnormalities (1, 27). TMEM165-deficient zebrafish similarly exhibit glycosylation defects and have problems with cartilage development (28). Interestingly, supplementation of culture medium with Mn2+ was found to partially reverse the glycosylation defects of TMEM165-deficient HeLa cells. The supplemental Mn2+ presumably increases Golgi Mn2+ concentration by outcompeting Ca2+ as a substrate for SPCAs. This rationale was first tested in yeast cells lacking a homolog of TMEM165 known as Gdt1. The residual partial defect in glycosylation in gdt1Δ yeast cells was exacerbated by elevated Ca2+ and then ameliorated by supplementation with Mn2+ in a fashion that depended on the SPCA known as Pmr1 (3). Furthermore, when heterologously expressed in bacteria, Gdt1 has been demonstrated to transport Mn2+, although it exhibited a lower affinity for Mn2+ than for Ca2+ (4). Presumably, the mechanism of Mn2+ transport by Gdt1 is similar to that of Ca2+, which has been demonstrated to be coupled to the pH gradient established by the V-ATPase, the deletion of which causes Gdt1 to function in reverse mode, instead releasing Ca2+ from Golgi lumen (29). Transport studies using the purified and reconstituted TMEM165-family proteins will be necessary to ascertain their specific substrate specificities.
In summary, the findings reported here support the hypothesis that TMEM165 exchanges H+, Mn2+, and Ca2+ in the Golgi complex of professional secretory cells and thereby supports glycosyltransferases such as lactose synthase that may be sensitive to H+ and dependent on Mn2+. The extremely high rates of lactose synthesis could generate H+ as a by-product and thus drive Mn2+ and Ca2+ uptake into the Golgi via TMEM165. Imbalanced ion homeostasis in TMEM165-deficient cells leads to defects in lactose biosynthesis through increased inhibition or decreased activation of lactose synthase.
Experimental procedures
Knockout mice and animal care
All procedures relating to animal care and treatment were approved by the Johns Hopkins University Animal Care and Use Committee and conformed to guidelines of the National Animal Disease Center Animal Care and Use Committee and the National Institutes of Health. The mice, maintained in a C57BL/6 or mixed C57BL/6 and 129P background, were housed in a standard 12 h light/12 h dark cycle. Embryonic stem cells (129/SvEv Tac) were transfected with 30 μg of the TMEM165 Knockout First targeting vector (PATHP0001_A_1_B01) from EUCOMM. After insertion of the complete targeting vector was confirmed by PCR of both homology arms, karyotypically normal clones were subjected to blastocyst injection and implantation into pseudopregnant females. The integration of the 5′-homology arm was confirmed using the forward primer 5′-GTGGTGATCAAAGGACACCGTGTGG-3′ and the reverse primer 5′-TGTTAGTCCCAACCCCTTCCTCC-3′, producing a 5,233-bp band, whereas the integration of the 3′-homology arm was confirmed using the forward primer 5′-CAACAGCAAGTGGTTTCAGGG-3′ and the reverse primer 5′-CTCTGGTCTTGATATAGACGTGGTCC-3′ to produce a 5,177-bp band. The resultant chimeric mice were crossed to C57BL/6 WT mice and screened for germ-line transmission of the Knockout First allele by PCR of the resultant progeny, using the forward primer 5′-GTACCGCGTCGAGAAGTTCC-3′ and the reverse primer 5′-GATAGCCTAAGCTCGTGTTACAATC-3′ to produce a 303-bp band. The WT allele was identified by a separate PCR using the same reverse primer but a new forward primer, 5′-CCCTGGATTCTACTTAGTCCCCC-3′, which produced a 362-bp band.
ROSA-Flp mice were a gift from Dr. Rejji Kuruvilla (Johns Hopkins University). Mice containing the ROSA-Flp allele were identified by PCR, using the forward primer 5′-CACTGATATTGTAAGTAGTTTGC-3′ and the reverse primer 5′-CTAGTGCGAAGTAGTGATCAGG-3′. These mice were crossed to the mouse containing the Knockout First allele to remove the portions of the cassette contained within the two Frt sites. This resulted in removal of all modifications except the two loxP sites flanking exon 2 of TMEM165. Backcrossing was then conducted to generate mice that tested positive for the floxed allele of TMEM165, but not the ROSA-Flp allele. Mice were genotyped for the floxed allele of TMEM165 by PCR using the forward primer 5′-AACTTTGGGGAGGCCAGTTAAG-3′ and the reverse primer 5′-GATAGCCTAAGCTCGTGTTACAATC-3′. Using these primers, the floxed allele of TMEM165 produces a 635-bp band, whereas the unfloxed allele produces a 541-bp band. The founder TMEM165fl/+ mice were back-crossed multiple times to C57BL/6 and then inbred to generate TMEM165fl/fl before breeding to C57BL/6 bearing WAP-Cre, which were a gift from Dr. Zhe Li (Harvard). WAP-Cre TMEM165fl/+ males were bred to TMEM165fl/+ and TMEM165fl/fl females, and the resulting females were used in experiments.
For experimentation, females bearing WAP-Cre and either TMEM165fl/fl, TMEM165fl/+, or TMEM165+/+ were bred, respectively, to males bearing TMEM165+/+, TMEM165fl/+, or TMEM165fl/fl, and the resulting litters were equalized to six pups/mouse mother on day 1 of lactation. This breeding program maximized uniformity among the nursing pups. The mice were euthanized at 12 days postpartum for tissue collection, using a 50:50 mix of CO2:O2 followed by cervical dislocation. Mammary tissue was removed, flash-frozen in liquid nitrogen, and stored at −80 °C until membranes were prepared. Some tissue was fixed as described below for microscopy.
Litter weight analysis
On the day of birth, litters were adjusted to six pups/nursing mother. The litters were then weighed every 2 days for the first 14 days of lactation.
Histological, immunofluorescent, and TUNEL stain microscopy
Tissue slides were prepared as described previously (30). Briefly, mammary tissue was fixed in Tellyesniczky's fixative (a 20:2:1 ratio of 70% ethanol, formalin, and glacial acetic acid) for 5 h before being stored in 70% ethanol before paraffin embedding and creation of 4-μm sections. For histological analysis, paraffin-embedded sections were treated with eosin and hematoxylin stain as described by Sheehan and Hrapchak (31). Images were obtained using a Leica Aperio AT2 at 20× magnification. For immunofluorescence, methods were conducted as described previously (12). Briefly, tissue sections were immersed in H-3300 (Vector Laboratories, Burlingame, CA) in a preheated pressure cooker for 20 min after pressure normalized. The sections were cooled and washed three times with distilled, deionized water, followed by PBS for 5 min. Sections were permeabilized and blocked in PBS containing 0.5% Triton X-100, 0.01 g of sodium azide, and 50 mg/ml BSA prior to addition of primary antibodies at 4 °C overnight. The primary antibodies used were anti-TMEM165 N-terminal end (Abcam, Cambridge, MA) and anti-GM130 catalog no. 610822 (BD Transduction Laboratories, San Jose, CA). The sections were washed three times with blocking buffer before incubating at 37 °C for 2 h with secondary antibodies, green antimouse Alexa Fluor A11017 488 F(ab′)2 fragment of goat antimouse IgG (H + L), and red anti-rabbit Alexa Fluor A11070 594 F(ab′)2 fragment of goat anti-rabbit IgG (H + L) (Molecular Probes/Life Technologies, Inc.). The slides were again washed three times with blocking buffer and mounted with VectaShield (Vector Laboratories) and 4′,6′-diamino-2-phenylindole. Finally, TUNEL staining was conducted using the an in situ cell death detection kit, TMR Red (catalog no. 12156792910) from Roche, according to the kit instructions. All confocal microscopic analysis was conducted using a Nikon A1+ confocal scanning microscope with two GaAsP multidetector and two normal PMTs, utilizing NIS-Elements C imaging software (Nikon, Tokyo, Japan). TUNEL stain images captured using the 561-nm laser and GaAsP detector unit, collecting emissions from 570 to 620 nm.
Milk analysis for lactose, creamatocrit, and total protein
Milk collection was done as described previously (26). Briefly, on day 12 of lactation, mothers were separated from their size-normalized litters for 4 h to allow them to produce sufficient milk for collection. The mothers were then anesthetized with avertin prior to being given an intraperitoneal injection of 0.1 IU of oxytocin. After 10 min, milk was collected in a 1.5-ml microcentrifuge tube connected by small tubing to a low vacuum that was pulsed. After collection, the pups were returned to their mother. The milk that was collected was centrifuged for 5 min at 4000 × g, and the floating fat layer was removed. The remaining skimmed milk was frozen and stored at −80 °C for future analysis. The concentration of lactose in the skimmed milk was determined using the Enzychrom lactose assay kit (ELAC-100) from Bioassay Systems, using the defined protocol from the kit. The concentration of protein in the skimmed milk was determined with a Bradford assay, using the Coomassie Plus (Bradford) assay reagent from Pierce, according to the kit instructions.
Determination of metal composition by ICP-MS
A 125-μl aliquot of skimmed milk was prepared for metal composition analysis by conducting acid digestion in 300-mm-long Pyrex digestion tubes using 5 ml of concentrated (70%) HNO3 and 2 ml of 30% H2O2 as a catalyst. Digestion was conducted by gradually heating the samples to 150 °C in a heat block and allowing the reaction to go to completion (sample became clear and colorless). The samples were then allowed to cool to room temperature before normalizing the volume and adjusting the final HNO3 concentration to 6%. The samples were stored in 15-ml metal-free polypropylene tubes from VWR until the compositions of the identified metals were analyzed using an Agilent 7700× inductively coupled plasma mass spectrometer (Agilent Technologies, Santa Clara, CA). Detection of metals was completed using an octopole reaction system cell in helium mode to remove interference. The parameters utilized included a radio frequency power of 1,550 W, an argon carrier gas flow of 1.01 liter/min, an argon make-up gas flow of 0.1 liter/min, a helium gas flow of 4.3 ml/min, an octopole radio frequency of 200 V, and an OctP bias of 18 V. The samples were infused using the model 7700X peristaltic pump with a speed of 0.1 rotations/s and micromist nebulizer. Metal concentrations were derived from a calibration curve generated from a dilution series of the atomic absorption standards of each metal (Fluka Analytical, St. Louis, MO), prepared in the same matrix as the samples. Data analysis was performed using Agilent's Mass Hunter software.
Galactosyltransferase activity assay
Mammary tissue collected from sacrificed mothers on day 12 of lactation was homogenized by 25 passes with Dounce A in 5 ml of a buffer containing 25 mm Tris, 150 mm NaCl, 1% Triton X-100, and 2 mm EDTA at pH 7.5 with a protease inhibitor mixture (Roche). The homogenate was transferred to a 15-ml conical tube and centrifuged at 3000 × g for 10 min to remove cellular debris. The supernatant was then centrifuged in a 10-kDa filtration tube at 3000 × g to remove small molecule substrates and products as well as the EDTA. A total of 50 ml of 25 mm Tris-citrate buffer containing 150 mm NaCl, 1% Triton X-100, 5 mm MgCl2, and 5 mm MnCl2, pH 7.5, with a protease inhibitor mixture (Roche) was added to the samples over multiple spins in the filtration tube. The samples were then removed from the filtration tube and appropriately diluted to normalize the total protein concentration, as determined a Bradford assay. The samples were then aliquoted, and the pH was adjusted in individual aliquots by addition of citrate buffer. The volumes were normalized using Tris-citrate buffer that was previously adjusted to the appropriate pH. Samples were then assayed by the methods described by Wu et al. (32) except UDPase was not added because the samples already expressed excess UDPase activity. Briefly, the samples were transferred to a 96-well plate in 25-μl aliquots. Substrates were then added to achieve a final concentration of 1 mm UDP-galactose and 20 mm GlcNAc. Controls had UDP-galactose alone or neither substrate. After 30 min, the concentration of free phosphate was determined, using the Malachite Green phosphate detection kit from Cell Signaling Technologies, according to the kit instructions.
Gel electrophoresis and Western blotting
Mammary gland microsomes were prepared as previously described (26). Briefly, the tissue was homogenized in 10 volumes of Buffer A (10 mm Tris-HCl, 2 mm MgCl2, 1 mm EDTA, and protease inhibitor mixture (Roche), pH 7.5). The homogenate was mixed with an equal volume of buffer B (buffer A containing 0.3 m KCl) and centrifuged at 10,000 × g for 10 min. The supernatant was collected and adjusted to 0.7 m KCl by the addition of solid KCl, before centrifugation at 100,000 × g for 1 h. The supernatant was discarded, and the pellets were resuspended in buffer C (buffer A containing 0.15 m KCl). Protein concentrations were determined using the Bio-Rad protein assay kit using a BSA standard, and buffer was added accordingly to normalize concentration of protein across samples. Equal volumes of microsomes and 2× SDS sample buffer (0.1 m Tris-HCl, pH 6.8, 4% SDS, 0.2% bromphenol blue, 20% glycerol, 2% β-mercaptoethanol) were combined, and the samples were incubated at room temperature for 30 min. Proteins were processed by separation on a 10% SDS/PAGE gel and Western blotting, as described previously (33). The blots were probed with anti-TMEM165 polyclonal antibodies from rabbit at 1:5,000 dilution (Sigma, HPA038299). Protein standards were probed with anti-α-tubulin polyclonal antibodies from mouse at 1:10,000 dilution (Sigma).
Statistical tests of significance
Student's t tests were implemented on many datasets, as indicated in the figures (*, p < 0.05; *, p < 0.01; ***, p < 0.001).
Author contributions
N. A. S., T. A. R., and K. W. C. conceptualization; N. A. S., M. V. P., and T. A. R. formal analysis; N. A. S. and T. A. R. investigation; N. A. S., T.A.R., and K. W. C. methodology; N. A. S. and T. A. R. writing-original draft; M. V. P., T.A.R., and K. W. C. writing-review and editing; T. A. R. and K. W. C. supervision; K. W. C. funding acquisition; K. W. C. project administration.
Acknowledgments
We acknowledge support and advice provided by Dr. Haiqing Zhao and technical assistance provided by Tera Nyholm and Heather Neu.
This work was supported by grants from the National Institutes of Health Grants R21HD080102 from the NICHD, R21AI115016 from the NIAID, and T32GM007231 from the NIGMS, by United States Department of Agriculture Project 5030-32000-115-00-D, and by funds from Johns Hopkins University. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TMEM165
- transmembrane protein 165
- β4-GALT-I
- β4-galactosyltransferase I
- SPCA
- secretory pathway Ca2+/Mn2+-ATPase
- WAP
- whey acid protein
- ICP
- inductively coupled plasma
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1. Foulquier F., Amyere M., Jaeken J., Zeevaert R., Schollen E., Race V., Bammens R., Morelle W., Rosnoblet C., Legrand D., Demaegd D., Buist N., Cheillan D., Guffon N., Morsomme P., et al. (2012) TMEM165 deficiency causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 91, 15–26 10.1016/j.ajhg.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colinet A. S., Sengottaiyan P., Deschamps A., Colsoul M. L., Thines L., Demaegd D., Duchêne M. C., Foulquier F., Hols P., and Morsomme P. (2016) Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 6, 24282 10.1038/srep24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Potelle S., Morelle W., Dulary E., Duvet S., Vicogne D., Spriet C., Krzewinski-Recchi M. A., Morsomme P., Jaeken J., Matthijs G., De Bettignies G., and Foulquier F. (2016) Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum. Mol. Genet. 25, 1489–1500 10.1093/hmg/ddw026 [DOI] [PubMed] [Google Scholar]
- 4. Thines L., Deschamps A., Sengottaiyan P., Savel O., Stribny J., and Morsomme P. (2018) The yeast protein Gdt1p transports Mn2+ ions and thereby regulates manganese homeostasis in the Golgi. J. Biol. Chem. 293, 8048–8055 10.1074/jbc.RA118.002324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufman R. J., Swaroop M., and Murtha-Riel P. (1994) Depletion of manganese within the secretory pathway inhibits O-linked glycosylation in mammalian cells. Biochemistry 33, 9813–9819 10.1021/bi00199a001 [DOI] [PubMed] [Google Scholar]
- 6. Dürr G., Strayle J., Plemper R., Elbs S., Klee S. K., Catty P., Wolf D. H., and Rudolph H. K. (1998) The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9, 1149–1162 10.1091/mbc.9.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Axelsson M. A., Karlsson N. G., Steel D. M., Ouwendijk J., Nilsson T., and Hansson G. C. (2001) Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 11, 633–644 10.1093/glycob/11.8.633 [DOI] [PubMed] [Google Scholar]
- 8. Kornak U., Reynders E., Dimopoulou A., van Reeuwijk J., Fischer B., Rajab A., Budde B., Nürnberg P., Foulquier F., ARCL Debré-type Study Group, Lefeber D., Urban Z., Gruenewald S., Annaert W., Brunner H. G., et al. (2008) Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat. Genet. 40, 32–34 10.1038/ng.2007.45 [DOI] [PubMed] [Google Scholar]
- 9. Park J. H., Hogrebe M., Grüneberg M., DuChesne I., von der Heiden A. L., Reunert J., Schlingmann K. P., Boycott K. M., Beaulieu C. L., Mhanni A. A., Innes A. M., Hörtnagel K., Biskup S., Gleixner E. M., Kurlemann G., et al. (2015) SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 97, 894–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng B. G., and Freeze H. H. (2018) Perspectives on glycosylation and its congenital disorders. Trends Genet. 34, 466–476 10.1016/j.tig.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein T., Morris J. S., Davies C. R., Weber-Hall S. J., Duffy M. A., Heath V. J., Bell A. K., Ferrier R. K., Sandilands G. P., and Gusterson B. A. (2004) Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 6, R75–R91 10.1186/bcr753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinhardt T. A., Lippolis J. D., and Sa1cco R. E. (2014) The Ca2+/H+ antiporter TMEM165 expression, localization in the developing, lactating and involuting mammary gland parallels the secretory pathway Ca2+ ATPase (SPCA1). Biochem. Biophys. Res. Commun. 445, 417–421 10.1016/j.bbrc.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 13. Fraser I. H., and Mookerjea S. (1976) Studies on the purification and properties of UDP-galactose glycoprotein galactosyltransferase from rat liver and serum. Biochem. J. 156, 347–355 10.1042/bj1560347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ton V. K., Mandal D., Vahadji C., and Rao R. (2002) Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey–Hailey disease. J. Biol. Chem. 277, 6422–6427 10.1074/jbc.M110612200 [DOI] [PubMed] [Google Scholar]
- 15. Reinhardt T. A., Filoteo A. G., Penniston J. T., and Horst R. L. (2000) Ca2+-ATPase protein expression in mammary tissue. Am. J. Physiol. Cell Physiol. 279, C1595–C1602 10.1152/ajpcell.2000.279.5.C1595 [DOI] [PubMed] [Google Scholar]
- 16. Aoki T., Yamada N., Tomita I., Kako Y., and Imamura T. (1987) Caseins are cross-linked through their ester phosphate groups by colloidal calcium phosphate. Biochim. Biophys. Acta 911, 238–243 10.1016/0167-4838(87)90013-6 [DOI] [PubMed] [Google Scholar]
- 17. Wagner K. U., Wall R. J., St-Onge L., Gruss P., Wynshaw-Boris A., Garrett L., Li M., Furth P. A., and Hennighausen L. (1997) Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25, 4323–4330 10.1093/nar/25.21.4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holt C. (1983) Swelling of Golgi vesicles in mammary secretory cells and its relation to the yield and quantitative composition of milk. J. Theor. Biol. 101, 247–261 10.1016/0022-5193(83)90339-9 [DOI] [PubMed] [Google Scholar]
- 19. Berliner L. J., and Robinson R. D. (1982) Structure-function relationships in lactose synthase. Structural requirements of the UDP-galactose binding site. Biochemistry 21, 6340–6343 10.1021/bi00268a003 [DOI] [PubMed] [Google Scholar]
- 20. Dulary E., Potelle S., Legrand D., and Foulquier F. (2017) TMEM165 deficiencies in congenital disorders of glycosylation type II (CDG-II): clues and evidence for roles of the protein in Golgi functions and ion homeostasis. Tissue Cell 49, 150–156 10.1016/j.tice.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Powell J. T., and Brew K. (1976) Metal ion activation of galactosyltransferase. J. Biol. Chem. 251, 3645–3652 [PubMed] [Google Scholar]
- 22. Stinnakre M. G., Vilotte J. L., Soulier S., and Mercier J. C. (1994) Creation and phenotypic analysis of α-lactalbumin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 91, 6544–6548 10.1073/pnas.91.14.6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stacey A., Schnieke A., Kerr M., Scott A., McKee C., Cottingham I., Binas B., Wilde C., and Colman A. (1995) Lactation is disrupted by α-lactalbumin deficiency and can be restored by human α-lactalbumin gene replacement in mice. Proc. Natl. Acad. Sci. U.S.A. 92, 2835–2839 10.1073/pnas.92.7.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lapinskas P. J., Cunningham K. W., Liu X. F., Fink G. R., and Culotta V. C. (1995) Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15, 1382–1388 10.1128/MCB.15.3.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiang M., Mohamalawari D., and Rao R. (2005) A novel isoform of the secretory pathway Ca2+, Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J. Biol. Chem. 280, 11608–11614 10.1074/jbc.M413116200 [DOI] [PubMed] [Google Scholar]
- 26. Reinhardt T. A., Lippolis J. D., Shull G. E., and Horst R. L. (2004) Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J. Biol. Chem. 279, 42369–42373 10.1074/jbc.M407788200 [DOI] [PubMed] [Google Scholar]
- 27. Rosnoblet C., Legrand D., Demaegd D., Hacine-Gherbi H., de Bettignies G., Bammens R., Borrego C., Duvet S., Morsomme P., Matthijs G., and Foulquier F. (2013) Impact of disease-causing mutations on TMEM165 subcellular localization, a recently identified protein involved in CDG-II. Hum. Mol. Genet. 22, 2914–2928 10.1093/hmg/ddt146 [DOI] [PubMed] [Google Scholar]
- 28. Bammens R., Mehta N., Race V., Foulquier F., Jaeken J., Tiemeyer M., Steet R., Matthijs G., and Flanagan-Steet H. (2015) Abnormal cartilage development and altered N-glycosylation in Tmem165-deficient zebrafish mirrors the phenotypes associated with TMEM165-CDG. Glycobiology 25, 669–682 10.1093/glycob/cwv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snyder N. A., Stefan C. P., Soroudi C. T., Kim A., Evangelista C., and Cunningham K. W. (2017) H+ and Pi byproducts of glycosylation affect Ca2+ homeostasis and are retrieved from the Golgi complex by homologs of TMEM165 and XPR1. G3 7, 3913–3924 10.1534/g3.117.300339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cross B. M., Hack A., Reinhardt T. A., and Rao R. (2013) SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS One 8, e67348 10.1371/journal.pone.0067348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheehan D. C., and Hrapchak B. B. (1980) Theory and Practice of Histotechnology, 2nd Ed., C.V. Mosby Company, St. Louis, MO [Google Scholar]
- 32. Wu Z. L., Ethen C. M., Prather B., Machacek M., and Jiang W. (2011) Universal phosphatase-coupled glycosyltransferase assay. Glycobiology 21, 727–733 10.1093/glycob/cwq187 [DOI] [PubMed] [Google Scholar]
- 33. Mehta S., Li H., Hogan P. G., and Cunningham K. W. (2009) Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol. Cell. Biol. 29, 2777–2793 10.1128/MCB.01197-08 [DOI] [PMC free article] [PubMed] [Google Scholar]