Summary
Objective
Because outcome data inform and drive healthcare decisions and improvement of patient care, this study aimed to gain a deep understanding of sociodemographic profiles and treatment outcomes of newly presenting and recently diagnosed persons living with epilepsy (PwE) at a tertiary epilepsy center in Rwanda.
Methods
In June 2016 (T1), as a first stage of this single‐center cohort study, an ambispective chart review was conducted on baseline sociodemographic and disease characteristics of PwE using a structured questionnaire. Missing data were obtained by phone interview. In 2017, follow‐up data were collected by phone interview on treatment outcomes after 15‐months (T2).
Results
Of 406 PwE screened, 235 were included at T1 and outcomes on 166 PwE were obtained at T2. More than 70% were <20 years of age, with a male preponderance. A high number of patients were aged ≥20 years, were single (67.4%), unemployed (41.5%), and had no formal education or primary level education (53.9%), possibly reflecting stigma. A mean delay in diagnosis since first seizure increased with age at first seizure, amounting to 3 years for patients aged ≥20 years. At T2, 69.6% of 166 patients who could be contacted reported no seizures in the previous month. Valproate monotherapy was the most frequently prescribed treatment. At T2, 47% had discontinued treatment, which was often not recommended by a physician, despite medical insurance coverage in >90% of patients. Only 19% reported an adverse event. Marked and partial improvement in quality of life (QoL) was reported by, respectively, 50.9% and 32.7% of patients.
Significance
Encouraging results on improved seizure control and QoL were observed at follow‐up. The treatment gap remains high due to loss to follow‐up and treatment discontinuation. In this article, we discuss needs and recommendations for improving patient care, requiring concerted efforts of stakeholders at all levels of the healthcare system.
Keywords: demographics, epilepsy care, patients with epilepsy, Rwanda, treatment outcome
Key Points.
Low education (54%) and high unemployment (41%) were observed in PwE
After 15 months of observation (follow‐up), nearly 70% of patients reported at least 30days of seizure freedom
More than 80% of patients reported marked and partially restored QoL
Treatment gap and dropout rate are high
Recommendations for future epilepsy care in Rwanda
1. INTRODUCTION
In sub‐Saharan Africa, epilepsy is associated with a particularly high burden of disease.1, 2 This is not only due to a greater prevalence of the disease, but also to a poor healthcare infrastructure with limited resources and limited access to antiepileptic drugs (AEDs), and to cultural barriers leading to stigma.3, 4 The greater prevalence of epilepsy in sub‐Saharan populations compared to Latin American and Asian populations, is due mainly to the presence of a higher number of combined risk factors such as cerebral malaria, neurocysticercosis, meningitis, human immunodeficiency virus (HIV) infection, toxocariasis, perinatal events, and traumatic brain injury.1, 5, 6
In Rwanda, the prevalence of active epilepsy was estimated to be 4.9% in 2005, one of the highest in sub‐Saharan Africa.7 Since then, there have been concerted efforts to increase the recognition and understanding of the disease and to reduce the treatment gap through mobile teams educating about epilepsy diagnosis and treatment in rural areas, the creation of the Rwandan Organization Against Epilepsy, capacity building, and several nongovernmental organization (NGO) activities.8 One of the main hospitals that provides care for persons living with epilepsy (PwE) is the CARAES Neuro‐Psychiatric Hospital in Ndera (NPH), on the outskirts of the capital city, Kigali, founded in 1968 by the Brothers of Charity. Over the years, NPH has evolved to provide specialized psychiatric and neurological services and also acts as the tertiary referral center for epilepsy for the entire country.
This study was conducted to gain insight into the sociodemographic profiles of patients attending the NPH and the impact of care and treatment provided by the hospital on epilepsy and the day‐to‐day lives of PwE.
2. METHODS
2.1. Study setting
This single‐center ambispective cohort study was conducted at the NPH between January 2016 and September 2017. The study was approved by the ethics board of the NPH and institutional review board of the University Hospital Center—Kigali (CHU‐K). Full details of the study, including objectives and methodology, were conveyed orally to all participants. Given the large number of participants with poor literacy, only verbal consent was obtained from most of patients and recorded in their medical records. For patients ≤18 years of age, verbal consent was obtained from their parents/caregivers.
2.2. Study participants
Medical charts of all patients attending the NPH neurology department for the first time between January and June 2016 were screened for a diagnosis of epilepsy. Epilepsy was defined as a history of 2 or more unprovoked seizures according to recommendations by the International League Against Epilepsy (ILAE).9, 10 Only patients presenting for the first time to the clinic, with a first seizure or diagnosis since less than a year were included. All diagnoses were established or confirmed by a trained neurologist, psychiatrist, or family physician with experience in epilepsy. Seizure classification was based on information from medical records and is using the ILAE 2011 classification.11
2.3. Data collection
In the first stage of the study (T1), in June 2016, a retrospective chart review of the first visit was conducted. Baseline sociodemographic data and information on disease characteristics, including age at epilepsy onset, seizure type(s), seizure frequency, diagnostic tests, and treatment history were obtained from medical charts, using a predefined paper‐based questionnaire. Patients were also contacted by telephone to obtain additional information.12, 13
In the second stage of the study performed in 2017, 15 months after first visit (T2), patients or caregivers were contacted again for follow‐up using a predefined structured phone interview, performed by the nursing staff of the neurology department. Nurses were trained in the fundamentals of epilepsy and in handling conversations using a paper‐based questionnaire. Patients were asked about their treatment status, seizure frequency over the last 30 days, adverse events (AEs), and quality of life (QoL).
Given the lack of validated instruments in the local language Kinyarwanda, the impact of treatment on QoL could not be assessed directly. Therefore, we asked patients about disease‐related comorbidities and whether they had noticed any difference in their day‐to‐day life since their first visit at the center, using a 4‐item scale: marked improvement, some improvement, no change, and deterioration. For patients unable to respond adequately due to age or disease severity, parents/caregivers were asked to respond.
2.4. Data entry and analysis
Data from the first stage were entered by an independent data entry specialist, and from the second stage by nurses and the lead author (F.V.S.). All entered data were subject to 2‐pass verification.
We defined 2 populations for analysis: a safety set (SS, all patients at T1, first visit) and total analysis set (TAS, all patients with outcome at T2). Data on sociodemographic and disease‐related variables are presented on the SS and data on treatment outcomes are presented on the TAS.
All analyses of the entered data are descriptive and were conducted using an Excel (Microsoft Corp.) and SPSS version 25 (IBM).
3. RESULTS
3.1. Patient population
From January to June 2016, 406 patients newly presenting at the NPH were screened, and 235 patients with an established diagnosis of epilepsy or reporting a first seizure in the preceding year were included. A total of 59 patients (25%) attended the NPH directly, whereas the remaining 176 patients (75%) were referred from other healthcare centers for specialist epilepsy care.
At 15‐month follow‐up, T2, nurses at NPH obtained information on 166 of 235 (70.6%) patients, including 5 deaths. Sixty‐nine patients were lost to follow‐up, predominantly because of disconnected telephone numbers.
3.2. Sociodemographic data
More than 70% of the enrolled patients were aged <20 years. There was a 56% male preponderance (Table 1). Nearly two‐thirds of patients lived outside the city of Kigali in rural areas. Most patients had private or social health insurance coverage, reducing their personal contribution for all medical care to 10% of the total cost. There were no important differences between SS and TAS in baseline demographics.
Table 1.
Sociodemographics of the patient populations (SS and TAS) and epilepsy disease characteristics at baseline T1
| Demographics | SS | TAS |
|---|---|---|
| n = 235 | n = 166 | |
| Age (y) | ||
| Mean | 15.6 | 14.77 |
| Range | 0‐78 | 0‐63 |
| Age group, n (%) | ||
| 0‐4 | 63 (26.8) | 46 (27.7) |
| 5‐9 | 36 (15.3) | 29 (17.5) |
| 10‐19 | 64 (27.2) | 42 (25.3) |
| 20‐39 | 55 (23.4) | 39 (23.5) |
| ≥40 | 16 (6.8) | 10 (6.0) |
| Sex, n (%) | ||
| Male | 132 (56.2) | 93 (56) |
| Female | 103 (43.8) | 73 (44) |
| Health insurance status, n (%) | ||
| Social health care | 164 (69.8) | 120 (72.3) |
| Private insurance | 42 (17.9) | 28 (16.9) |
| Not insured | 12 (5.1) | 6 (3.6) |
| Unknown | 17 (7.2) | 12 (7.2) |
| Setting, n (%) | ||
| Rural | 152 (64.7) | 107 (64.5) |
| Urban (Kigali) | 78 (33.2) | 59 (35.5) |
| Abroad | 5 (2.1) | 0 (0) |
| Seizure type, n (%) | ||
| Generalized tonic‐clonica | 189 (80.4) | 131 (78.9) |
| Focal aware motor/non‐motor | 11 (4.7) | 20 (12.0) |
| Focal impaired awareness motor/non‐motor | 2 (0.9) | 3 (1.8) |
| Focal to bilateral tonic‐clonic | 19 (8.1) | 1 (0.6) |
| Not classified | 14 (6.0) | 10 (6.0) |
| Alternative treatment use (reported >1%), n (%) | ||
| Never used for epilepsy | 158 (67.2) | 102 (61.4) |
| Prayers | 10 (4.3) | 38 (22.9) |
| Herbal medicine | 16 (6.8) | 12 (7.2) |
| Missing data | 51 (21.7) | 11 (6.6) |
SS, safety set at study start T1; TAS, total analysis set at follow‐up T2.
Due to limited diagnostic resources, seizure type requires cautious interpretation; generalized tonic‐clonic may include focal onset, generalized onset, and unknown origin.
There was a low spontaneous reporting of HIV‐positive serology, psychiatric comorbidity, and malnutrition: 1.7%, 5.95%, and 1.28% respectively. Two‐thirds of patients had an unknown HIV status.
The majority of patients aged ≥20 years, were single (67.4%), unemployed (41.5%), and had no formal education or a primary level education only (53.9%) (Table 2).
Table 2.
Demographics and disease specifics by age group at T1, SS
| Age groups | 0‐4 | 5‐9 | 10‐19 | 20‐39 | ≥40 |
|---|---|---|---|---|---|
| N [unknown/missing] | 63 | 36 | 64 | 55 | 16 |
| Marital status, n (%) | |||||
| Under age to get married | 63 (100) | 36 (100) | 45 (70.3) | NA | NA |
| Single/co‐living | NA | NA | 18 (28.1) | 41 (74.6) | 3 (18.8) |
| Married | NA | NA | 1 (1.6) | 13 (23.6) | 8 (50) |
| Widowed | NA | NA | 0 (0) | 0 (0) | 2 (12.5) |
| Divorced | NA | NA | 0 (0) | 1 (1.8) | 3 (18.8) |
| Education, n (%) [22]a | |||||
| Had no education | 0 (0) | 0 (0) | 4 (6.3) | 5 (9.1) | 3 (18.8) |
| Receives no education | 60 (95.2) | 14 (38.9) | 5 (7.8) | 0 (0) | 0 (0) |
| Primary | 3 (4.8) | 21 (58.3) | 28 (43.8) | 22 (40) | 4 (25) |
| Secondary | 0 (0) | 0 (0) | 19 (29.7) | 18 (32.7) | 2 (12.5) |
| Higher | 0 (0) | 0 (0) | 0 (0) | 3 (5.5) | 1 (6.3) |
| Employment, n (%) [10]a | |||||
| Agricultural worker/farmer | NA | NA | 2 (3.1) | 16 (29.1) | 8 (50) |
| Commercial/state employee | NA | NA | 1 (1.6) | 14 (25.5) | 3 (18.8) |
| Unemployed/pensioned | NA | NA | 13 (20.3) | 4 (7.3) | 2 (12.5) |
| Student | 3 (4.8) | 19 (52.8) | 45 (70.3) | 16 (29.1) | 1 (6.3) |
| Age at epilepsy onset | |||||
| 59 [4] | 33 [3] | 62 [2] | 53 [2] | 13 [3] | |
| Mean (y) | 1.21 | 3.76 | 11.15 | 18.98 | 36 |
| Range (min‐max) | 0‐4 | 0‐9 | 0‐19 | 0‐37 | 0‐78 |
| Age at diagnosis | |||||
| 59 [4] | 34 [2] | 59 [5] | 54 [1] | 13 [3] | |
| Mean (y) | 1.73 | 5.3 | 13.54 | 23.92 | 40.23 |
| Range (min‐max) | 0‐4 | 0‐9 | 0‐19 | 2‐38 | 2‐38 |
| Monthly seizure frequency at baseline, n (%) [36]a | |||||
| No seizures | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1‐2 | 12 (18.8) | 9 (25) | 15 (23.4) | 22 (40) | 3 (18.8) |
| 3‐5 | 13 (20.3) | 8 (22.2) | 21 (32.8) | 10 (18.2) | 5 (31.3) |
| 6‐10 | 9 (14.1) | 9 (25) | 8 (12.5) | 5 (9.1) | 3 18.8) |
| 11‐30 | 12 (18.8) | 2 (5.6) | 5 (7.8) | 7 (12.7) | 0 (0) |
| >30 | 9 (14.1) | 3 (8.3) | 4 (6.3) | 4 (7.3) | 0 (0) |
NA, not applicable; SS, safety set, includes all patients at T1.
Indicates number of patients with missing data on this demographic.
3.3. Epilepsy characteristics and diagnosis
More than 80% of patients reported GTCS (generalized tonic‐clonic seizures) and an additional 8% reported focal to generalized onset seizures. However, due to limited diagnostic resources, our seizure‐type classification requires cautious interpretation. GTCS may include focal and generalized onset seizures and GTCS from unknown origin. More than half of patients reported 1‐5 seizures a month (50.7%). Twenty patients (8.5%) reported >30 seizures a month (Table 1).
The mean age of patients at onset of seizures was 10.8 years and 13.4 years at diagnosis, indicating a diagnostic delay of >2.5 years. Yet, for patients aged ≥20 years, this delay increased to 4 years. Patient self‐reported delay in diagnosis occurred in >33% (Table 2).
A total of 73% of patients underwent electroencephalography (EEG) and with normal findings reported in 59.8%. Other diagnostic techniques were rarely used; computed tomography (CT), magnetic resonance imaging (MRI), and blood tests were conducted in 10, 7, and 8 patients, respectively, with mostly normal results being reported.
3.4. Treatment profile at T1 and T2
At T1, 16 (6.8%) and 10 (4.2%) of patients in the SS indicated using traditional medicine or using prayers as epilepsy treatment. Because many patients were referred to NPH from other medical centers, 53 patients (31%) were already receiving treatment at baseline. The most frequently used treatment schedule was valproate monotherapy in all age groups, followed by carbamazepine with a low percentage of patients using phenobarbital.
At T2, 5 of 166 patients (3%) had died and treatment data could not be validated in 4 patients. Of 157 remaining patients, 81 (52%) were taking AEDs, whereas 76 (48%) had stopped taking AEDs. Among those continuing AEDs, the majority were taking monotherapy (n = 63; 78%); 15 (18%) were taking duotherapy, and 3 (4%) triple therapy. The most frequently prescribed AED at T2 was valproate, part of the regimen in 79% of both monotherapy and combination treatments. The second most frequently prescribed AED was carbamazepine in 22% (n = 19) of treatment schedules, followed by phenobarbital in 14.1% (n = 11) of patients.
Among the most frequently self‐reported reasons for treatment discontinuation were seizure cessation (31.7%), lack of money (30.4%), and insufficient information on treatment (20.7%). Given the large number of young patients in the study, reasons for treatment discontinuation were analyzed separately according to age (0‐19 vs ≥20 years). Notable differences were that lack of money and stating a lack of willpower led to more discontinuation among adults than children, whereas seizure cessation and insufficient information were more frequently cited as reasons for discontinuation in the younger age group. Notably, only one patient reported treatment discontinuation due to AEs.
3.5. Treatment outcomes
3.5.1. Seizure frequency evolution from T1 to T2
In the TAS population, seizure frequency per month, from baseline to follow‐up, was available from 166 patients. A substantial reduction in seizure frequency relative to baseline (study start) was reported by all (Figure 1). Notably, 69.6% of patients reported that they had not experienced any seizures in the previous month.
Figure 1.
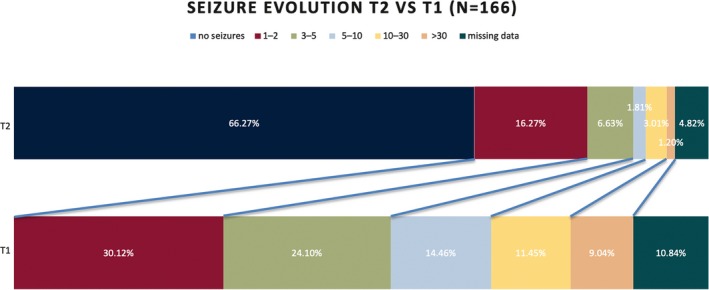
Change in seizure frequency after 15 months (T2) of treatment compared to baseline T1
Because nearly half of patients had discontinued treatment, seizure frequency was analyzed separately for patients who continued or had discontinued treatment with AEDs. Results were found to be similar in the 2 groups (Figure 2). Timing of discontinuation of treatment was not documented.
Figure 2.
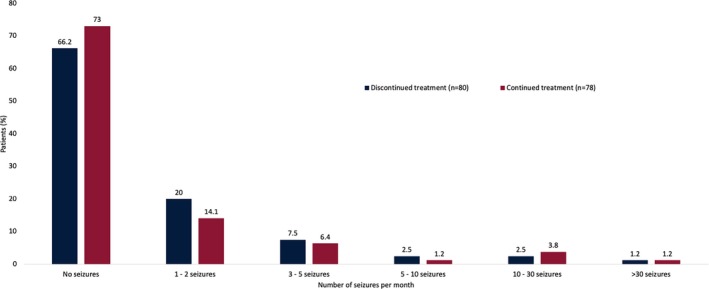
Change in seizure frequency after 15 months of treatment among patients who had continued or discontinued therapy
3.5.2. Adverse events
Most patients (80.6%) reported no AEs (Table 3). The most frequently reported AEs were general weakness/fatigue (8.1%), somnolence (5.0%), and weight gain (3.1%). Analyzed separately for patients who continued or discontinued treatment, AEs occurred slightly less frequently in patients who discontinued compared to those who continued treatment (74.3% vs 86.5%). Conversely, those remaining on treatment reported more frequently fatigue (12.8% vs 3.6%), somnolence (8.9% vs 1.2%), and hypersalivation (5.1% vs 0%).
Table 3.
Incidence of adverse events (AEs) reported at T1
| AEs occurring in >2.5% of all patients | |||
|---|---|---|---|
| AEs, n (%) | Discontinued treatment (n = 82) | Continued treatment (n = 78) | All patients (n = 160) |
| No AEs | 71 (86.5) | 58 (74.3) | 129 (80.6) |
| General weakness/fatigue | 3 (3.6) | 10 (12.8) | 13 (8.1) |
| Somnolence | 1 (1.2) | 7 (8.9) | 8 (5.0) |
| Weight gain | 2 (2.4) | 3 (3.8) | 5 (3.1) |
| Hypersalivation | — | 4 (5.1) | 4 (2.5) |
| Dizziness | 3 (3.6) | 1 (1.2) | 4 (2.5) |
Data are n (%).
At T2, 5 of 235 patients had died, resulting in a mortality rate of 2.12% in our cohort or a mortality rate of 21.27 per thousand patient years. By use of the World Health Organization Verbal Autopsy Questionnaire,14 we identified the cause of death as brain tumor in 1 of 5 , blunt head trauma after a seizure in 1 of 5, and 3 of 5 of unknown origin. The latter may include possible sudden unexpected death in epilepsy (SUDEP) as no other concomitant disease was observed.
3.5.3. Complications/comorbidities T2 compared to T1
Patients/caregivers were asked for additional health problems/complications occurring during the follow‐up period. Responses were obtained in 160 patients, with the majority not reporting any disease complications or comorbidities (Table 4). Age‐related differences in the type of complication were apparent: gait problems and growth/developmental delay were noted only in the youngest age group, whereas minor injuries and having to stop studies/job were frequently reported in the older age groups.
Table 4.
Incidence of comorbidities reported at T2
| Complications occurring in >2.5% of all patients | |||
|---|---|---|---|
| Complications, n (%) | 0‐4 y (n = 76) | 5‐19 y (n = 41) | ≥20 y (n = 43) |
| No complications mentioned | 60 (78.9) | 31 (75.6) | 26 (60.4) |
| Minor injuries | 2 (2.6) | 5 (12.1) | 7 (16.2) |
| Stopped studies/job | 1 (1.3) | 2 (4.8) | 8 (18.6) |
| Behavioral problems | 2 (2.6) | 1 (2.4) | 2 (4.6) |
| Burns | 2 (2.6) | — | 2 (4.6) |
| Growth/developmental delay | 4 (5.2) | — | — |
| Gait problems | 1 (1.3) | — | — |
| Study‐related issues | 2 (2.6) | 1 (2.4) | — |
| Road accident | — | 1 (2.4) | — |
| Social isolation | — | 1 (2.4) | — |
| Fall with fracture | — | — | 2 (4.6) |
3.5.4. Self‐reported change in QoL at T2 compared to T1
Using a 4‐item scale, we measured the impression of change of QoL compared to baseline since the initial visit at the NPH. Of 159 responses obtained, most patients reported an improvement in their day‐to‐day life. Marked, partial improvement, no improvement, and deterioration were reported by 50.9%, 32.7%, 7.5%, and 8.8%, respectively. Of 76 patients who discontinued AEDs, 42 seizure‐free patients without AEs at T2 reported a marked or partial improvement of QoL. On the other hand, of 22 patients who discontinued AEDs and reported AEs and comorbidities, none reported a marked improvement.
4. DISCUSSION
To our knowledge, this is the largest study to date in Rwanda in an epilepsy cohort, reporting demographics, seizure management, and outcomes in a national referral center for epilepsy. Because research in 2005 demonstrated a high prevalence of epilepsy in Rwanda, up to 49 in 1000, 10‐fold the rate in developed countries, there is a need for understanding the needs of patients and gaps in patient care in Rwanda.7
Our cohort of 235 patients had a broad age distribution, ranging from 1‐ to 78‐years‐old, with a substantial proportion younger than 20 years: up to 42% of the children included in our study were younger than10 years old and 24% were even younger than 2 years. This very young age representation, although not uncommon in epilepsy, needs further attention. Indeed, limited information is available on the etiology and exact diagnosis of seizure type or syndrome. In future studies, we need to address the etiology of epilepsy using imaging and lab tests as well as a detailed review of the perinatal records. This recommendation applies also to our broader study population. Indeed, the number of patients with known HIV status, number of further investigations using a broad infectious disease screening or imaging is low. To improve quality of care we recommend expanding the use of EEG by trained personnel. Also, increasing the use of imaging techniques, such as MRI, and increasing the availability of lab tests should improve the quality of care. Recommendations and views for future laboratory deployments have been provided by Nkengasong et al.15 However, in a resource‐constrained environment, expanding these activities may be challenging and will require substantial healthcare investment. For example, the price of a magnetic resonance imaging (MRI) study in Rwanda is 150 USD or 15 USD for insured patients, whereas the average monthly salary in Rwanda is about 160 USD.16
It is encouraging that the majority of PwE had health insurance coverage, enabling them to cover their treatment costs. Still, 26%‐41% of those who stopped their treatment reported that they could not cover the costs of the treatment, including non‐medical costs, such as transportation. Possible contributing factors to this lack of financial power include a lower schooling degree, unemployment, or ongoing schooling. Indeed, only 61% of patients above 20 years of age, reported an economic activity and 48% had no education or had attended only primary school. It is unclear whether this is due to stigma or cognitive decline in our patients.17, 18, 19
Several findings point to stigmatization of PwE. Sebera et al20 reported in 2005 that more than two‐thirds of the Rwandan population considered PwE not apt to get married. In our study, only 1 in 4 PwE was married. Low schooling and employment rate are indirect measures of stigma, as PwE may not get similar chances as nonaffected patients. To enlarge the support from the general population for PwE, additional awareness campaigns are needed, in particular for traditional healers and community workers.
Another reassuring finding was the seizure evolution in our study. Up to 62% of 166 PwE from the T2 group, or 46,8% of the T1 group, were seizure free at T2. Most patients were taking valproate monotherapy. A high percentage of patients who stopped treatment reported 30‐day seizure freedom at T2. Although 30‐day seizure freedom does not qualify for inactive epilepsy, this number has been reported elsewhere. In a study in children with epilepsy in Kenya, a high number of inactive epilepsy was also found in 80% of nontreated patients.21 A possible reason may be regional factors with low risk of recurrence, which needs to be elucidated by better etiologic research. On the other hand, from a cultural perspective, short‐term seizure freedom is often considered a motive to stop treatment by patients; this reinforces the need for better patient education regarding compliance with treatment. Although we need to be cautious at interpreting these high seizure‐freedom rates, those numbers might represent a legitimate good outcome, considering the resource constraint environment and the need for a pragmatical approach in clincal assessments and treatments.
The AED treatment prescribed in a majority of patients was valproate. This is surprising, as the first‐line AED of choice in Rwanda is phenobarbital. However, from a pragmatic standpoint, with valproate having a broad spectrum and good availability in the center, it is a good choice in cases of difficult seizure classification and lacking adequate etiologic assessment. The low rate of use of traditional healing practices is surprising and in contrast to the findings in 2005, possibly explained by underreporting or by the fact that the clinic is a referral center.7
The self‐reported marked improvement of QoL parallels the seizure‐frequency evolution. In addition, of those who discontinued their AEDs and remained seizure‐free, the improvement remained stable.
Our reported outcome data may be biased because only the PwE who could be contacted were included (TAS). Given that the patients who were lost to follow‐up might experience worse seizure control, this study may overestimate seizure control and outcome.
We reported 5 deaths in this study, which suggests a high mortality rate of 21.2 per 1000 patient years. In a post‐ hoc follow‐up, we identified 3 unknown causes of death, using World Health Organization (WHO) verbal autopsy methodology. Although this methodology is straightforward, it proved to be labor‐ and cost‐intensive. We advocate further use of the verbal autopsy methodology for all deaths due to epilepsy as the best available technique to evaluate mortality in PwE when autopsy is not available.1, 11 Population‐based studies have shown that long‐term mortality rate in PwE is up to double the mortality rate of the general population.22 Because the young age of our population, the rather short period of follow‐up, and the type and severity of the seizures may be confounding factors, further studies on mortality rates of epilepsy in sub‐Saharan populations are clearly needed.23, 24, 25
A high number of patients was lost to follow‐up. The main reason for this loss was our inability to reach enrolled patients due to disconnected phone numbers. The high drop‐out rate may be an indicator that PwE lack the incentive of any kind to seek follow‐up. Considering that epilepsy is a disease with high burden, this gap highlights the need for an adjusted approach to PwE in Rwanda.4, 6, 26 In a poststudy intervention, a trained nurse made field trips to visit patients in their own homes, which resulted in retrieving 62 of 69 patients previously lost to follow‐up; these results will be published separately.
Not only follow‐up was difficult in our cohort, also treatment compliance needs attention in PwE. Up to 46.1% of the 166 PwE at T2 had stopped their medication without the consent of their treating clinicians. Many patients mentioned lack of sufficient information on epilepsy and treatment and lack of financial means, due to high non‐medical costs (transportation). We conclude that enhanced patient and family education is needed as well as ensuring attention to the economic status of patients. Adequate ways of communicating medical information will need to be created and a one‐fits‐all format will probably not apply.27
The pragmatic approach of clinicians in Rwanda that is focused on seizure reduction is also reflected in the limited availability of clinical data on seizure type and syndromic classification in our population. Indeed, up to 80% of seizures were classified as generalized tonic‐clonic seizure (GTCS), which is unexpectedly high when compared to other series in developed countries, in which 51%‐64% of seizures are reported to be focal.28 The probability of seizure type misclassification is likely. To improve adequate classification that may lead to better treatment, increased capacity building and improving availability to diagnostic testing specific to neurology and epilepsy is needed. Training of a larger number of neurologists is of paramount importance but will take time. In 2005, 3 neurologists were available in Rwanda to serve a population of 11 million inhabitants. Currently, Rwanda counts 4 active neurologists, or less than 1 per 2 million inhabitants. In addition, suggested interventions include keeping standardized medical records, creating and implementing local AED treatment guidelines, using validated QoL scales and registries, and setting up a more streamlined referral system.
Our ambispective cohort study has other limitations. First, the retrospective analysis of medical records proved to be difficult as medical notes were not standardized. This contributed to a high volume of missing data, poor seizure classification skewing toward GTCS not otherwise specified, and undocumented etiologic or syndromal assessments. Second, we already pointed toward the lack of validated scales and limited follow‐up possibilities. Efforts for standardization and capacity building are ongoing. Third, potential reporting bias is possible due to the choice of telephone consultation and the issues in translation between author and nurse, and toward patients.
In conclusion, encouraging results on seizure control and improved QoL have been achieved in Rwanda. However, a high number lost to follow‐up suggests that epilepsy remains difficult to tackle. Our most important finding is that the epilepsy treatment gap is still important, based on patient‐centered, medical, institutional, and healthcare‐related factors. The key to improvements lies in all factors, with a better understanding of the patients’ cultural concepts of epilepsy and its treatment, optimal drug supply and availability, and improved education among general practitioners and other primary care medical personnel, supported by solid research data from the community. Further studies using improved questionnaires need to be conducted on the prevalence of epilepsy and its risk factors, economic analysis of epilepsy treatment, QoL, SUDEP, and other epilepsy‐associated (co‐) morbidity and mortality. Some interventions, such as improved and standardized patient record keeping, may be achievable in the short term. Concerted efforts of different stakeholders at all levels of the healthcare system will be needed and may take time before a transformational change can be achieved.29
Therefore, this paper is an invitation to all readers to engage with the authors in Rwanda, sharing their expertise at the patient level, hospital level, and/or healthcare level. Needless to say, the energy and passion of the locals and local professionals involved in the field is heroic and should be praised.
DISCLOSURE OF CONFLICTS OF INTEREST
Frank Van Steenkiste received travel funding from UCB Biopharma. Beni Uwacu and Fidèle Sebera have received research grants from UCB. Peter Dedeken is a consultant for UCB Biopharma. Dirk Teuwen is an employee of UCB Biopharma. Paul Boon and his institution have received support from UCB Pharma in the form of research grants and speaker and consultancy fees. The remaining author reports no conflicts of interest relating to this study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTION
All authors state that the material described in the manuscript is the work of the author(s), the manuscript has not been previously published, or in abstract form, and it is not simultaneously under consideration by any other journal. Frank Van Steenkiste, acknowledges that: (a) all co‐authors have been substantially involved in the study and/or the preparation of the manuscript; (b) no undisclosed groups or persons have had a primary role in the study and/or in manuscript preparation (ie, there are no “ghost‐writers”); and, (c) all co‐authors have seen and approved the submitted version of the paper and accept responsibility for its contents.
ACKNOWLEDGMENTS
We acknowledge the contribution of Azita Tofighy for the critical re‐appraisal of the data and wording suggestions. We acknowledge the patients who responded to the telephone interviews, the full study team of nurses at NPH for their commitment during sometimes complex phone interviews, and Fanny Mbungira for her dedication to data entry.
Van Steenkiste F, Fidèle S, Nsanzabaganwa W, et al. An ambispective cohort study on treatment outcomes of patients with epilepsy in a tertiary epilepsy center in Rwanda and recommendations for improved epilepsy care. Epilepsia Open. 2019;4:123–132. 10.1002/epi4.12304
REFERENCES
- 1. Ba‐Diop A, Marin B, Druet‐Cabanac M, et al. Epidemiology, causes, and treatment of epilepsy in sub‐Saharan Africa. Lancet Neurol. 2014;13:1029–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mbuba CK, Ngugi AK, Newton CR, et al. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Preux PM, Ratsimbazafy V, Bhalla D, et al. Methodology of neuroepidemiological studies in tropical countries: a challenge? Rev Neurol (Paris). 2012;168:211–5. [DOI] [PubMed] [Google Scholar]
- 4. Wilmshurst JM, Birbeck GL, Newton CR. Epilepsy is ubiquitous, but more devastating in the poorer regions of the world… or is it? Epilepsia. 2014;55:1322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yemadje LP, Houinato D, Boumediene F, et al. Prevalence of epilepsy in the 15 years and older in Benin: a door‐to‐door nationwide survey. Epilepsy Res. 2012;99:318–26. [DOI] [PubMed] [Google Scholar]
- 6. Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet. 2012;380:1193–201. [DOI] [PubMed] [Google Scholar]
- 7. Sebera F. Prévalence de l’épilepsie au Rwanda. Dakar, Senegal: Mémoire du DES Neurologie, Université Cheikh Anta Diop de Dakar; 2006. [Google Scholar]
- 8. Ba‐Diop A. ILAE Annual report 2013. ILAE Annual report 2013, International League Against Epilepsy[cited 2018 Aug]. Available from https://www.ilae.org/regions-and-countries/regions/ilae-africa/reports. Online Report.
- 9. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. [DOI] [PubMed] [Google Scholar]
- 10. Meinardi H, Scott RA, Reis R, et al. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2008;42:136–49. [DOI] [PubMed] [Google Scholar]
- 11. Berg AT, Scheffer IE. New concepts in classification of the epilepsies: entering the 21st century. Epilepsia. 2011;52:1058–62. [DOI] [PubMed] [Google Scholar]
- 12. Uwacu BH, Sebera F, Niyongira E, et al. A six‐month follow‐up of persons living with epilepsy, newly diagnosed at the CARAES tertiary neurology reference centre at Ndera, Kigali (Rwanda). Eur J Neurol. 2017;24(suppl 1):630. [Google Scholar]
- 13. Uwacu BH. Aspects épidémiologiques, cliniques et evolutifs de l’épilepsie à l'hôpital de CARAES de Ndera, Kigali en 2016. Université Cheikh Anta Diop de Dakar; Dakar, Senegal:48. Unpublished work.
- 14. WHO 2016 verbal autopsy instrument. Geneva, Switzerland: World Health Organization; 2018. [cited 2018 Nov 8]. Available from http://www.who.int/healthinfo/statistics/verbalautopsystandards/en/
- 15. Nkengasong JN, Mbopi‐Keou F‐X, Peeling RW, et al. Laboratory medicine in Africa since 2008: then, now, and the future. Lancet Infect Dis. 2018;18:e362–7. [DOI] [PubMed] [Google Scholar]
- 16. World Bank and various sources. The Gapminder. [cited 2018 Jun]. Available from https://www.gapminder.org/data
- 17. Faure‐Delage A, Mouanga AM, M'Belesso P, et al. Socio‐cultural perceptions and representations of dementia in Brazzaville, Republic of Congo: the EDAC Survey. Dement Geriatr Cogn Dis Extra. 2012;2:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gebrewold MA, Enquselassie F, Teklehaimanot R, et al. Ethiopian teachers: their knowledge, attitude and practice towards epilepsy. BMC Neurol. 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaddumukasa M, Kaddumukasa MN, Buwembo W, et al. Epilepsy misconceptions and stigma reduction interventions in sub‐Saharan Africa, a systematic review. Epilepsy Behav. 2018;85:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sebera F, Munyandamutsa N, Teuwen DE, et al. Addressing the treatment gap and societal impact of epilepsy in Rwanda – results of a survey conducted in 2005 and subsequent actions. Epilepsy Behav. 2015;46:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berrettini WH. Genetics of psychiatric disease. Annu Rev Med. 2000;51:465–79. [DOI] [PubMed] [Google Scholar]
- 22. Lhatoo SD, Johnson AL, Goodridge DM, et al. Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long‐term, prospective, population‐based cohort. Ann Neurol. 2001;49:336–44. [PubMed] [Google Scholar]
- 23. Ding D, Wang W, Wu J, et al. Premature mortality risk in people with convulsive epilepsy: long follow‐up of a cohort in rural China. Epilepsia. 2013;54:512–7. [DOI] [PubMed] [Google Scholar]
- 24. Ding D, Wang W, Wu J, et al. Premature mortality in people with epilepsy in rural China: a prospective study. Lancet Neurol. 2006;5:823–7. [DOI] [PubMed] [Google Scholar]
- 25. Ngugi AK, Bottomley C, Fegan G, et al. Premature mortality in active convulsive epilepsy in rural Kenya: causes and associated factors. Neurology. 2014;82:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Getnet A, Woldeyohannes SM, Bekana L, et al. Antiepileptic drug nonadherence and its predictors among people with epilepsy. Behav Neurol. 2016;2016:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Forsgren L, Beghi E, Oun A, et al. The epidemiology of epilepsy in Europe – a systematic review. Eur J Neurol. 2005;12:245–53. [DOI] [PubMed] [Google Scholar]
- 29. Shorvon SD, Farmer PJ. Epilepsy in developing countries: a review of epidemiological, sociocultural, and treatment aspects. Epilepsia. 1988;29:S36–54. [DOI] [PubMed] [Google Scholar]


