Abstract
Aims
Although depression has weak associations with several Type 2 diabetes mellitus (DM) outcomes, it is possible that these associations are concentrated within certain patient subgroups that are more vulnerable to their effects. This study tested the hypothesis that depression is related to glycaemic control and diabetes-related quality of life (DQOL) in patients who are prescribed injected insulin, but not those on oral glucose-lowering agents alone.
Methods
Participants (103 on insulin, 155 on oral glucose-lowering agents alone) with Type 2 DM were recruited from a large US healthcare system and underwent assessment of glycaemic control (glycated haemoglobin; HbA1c), medication adherence and diabetes self-care behaviours, DQOL and depression (none, mild, moderate/severe).
Results
There was a significant regimen × depression interaction on HbA1c (P = 0.002), such that depression was associated with HbA1c in patients using insulin (β = 0.35, P < 0.001) but not in patients using oral agents alone (β = −0.08, P = NS). There was a similar interaction when quality of life was analysed as an outcome (P = 0.002). Neither effect was mediated by regimen adherence.
Conclusions
The generally weak association between depression and glycaemic control is concentrated among patients who are prescribed insulin. Similarly, the association between depression and illness quality of life is strongest in patients prescribed insulin. Because this is not attributable to depression-related adherence problems, psychophysiological mechanisms unique to this group ought to be carefully investigated. Clinicians might be especially vigilant for depression in Type 2 DM patients who use insulin and consider its potential impact upon their illness course.
Keywords: depression, diabetes, diabetes regimen, Type 2 diabetes mellitus
Introduction
In patients with Type 2 diabetes mellitus (DM), depression is associated with poor glycaemic control, complications, hospital admission and other poor outcomes [1–4]. While statistically significant, these associations are generally quite small in magnitude, typically explaining only about 2–8% of the variance in diabetes outcomes. As a result, researchers have sought to determine whether linkages with depression might cluster within various patient subgroups defined by demographic or clinical characteristics.
Although age and ethnicity have been examined as factors which may modify the link between depression and glycaemia, the most replicated finding is that depressive symptoms seem to play a role in patients with Type 1 DM, but not Type 2 DM [5,6]. Potential explanations for this pattern include the impact of neurohormonal factors related to both mood state and blood glucose and the possible effects of antidepressant medication on endocrine functioning. To date, there is no clear support for any one mechanism over another.
Another interesting possibility is that this pattern may be primarily attributable to the relative complexity of the Type 1 diabetes regimen relative to the use of oral glucose-lowering agents. Insulin use can be significantly more complex than the use of oral therapies, requiring more frequent glucose self-monitoring, dosage changes in response to fluctuations in food intake or exertion and the use of both long-acting as well as short-acting formulas. Compared with the use of oral medication, the relatively complex behaviours involved in insulin use would probably be more disrupted by depression and some patients experience considerable psychological difficulty with the transition to insulin [7], which may in turn lead to depression. This overall association has received some preliminary support. In unadjusted analyses of patients with either Type 1 or Type 2 diabetes, depressive symptom severity and glycaemic control were significantly associated in a sample of patients who were prescribed at least three daily insulin injections (r = 0.28), but not among those with any of the less intensive regimens [8]. However, it is unclear whether this effect is consistent enough to exist in Type 2 DM per se and analyses were not adjusted for potential confounders.
The study objective was therefore to build upon existing work by testing the hypothesis that depression is related to Type 2 DM outcomes in patients using insulin, but not among those using oral glucose-lowering agents alone. To rigorously test our hypothesis, we adjusted for potential confounders, incorporated criterion-based classification of depression presence and severity, considered subjective quality-of-life outcomes and formally tested the regimen × depression interaction. Finally, we tested whether regimen adherence explained any detected differences in depression–outcome associations.
Methods
Participants
Potential participants were identified using the administrative and clinical databases of a large urban healthcare system in the midwestern USA. Patients were eligible if they had Type 2 DM as indicated by at least one of the following: (i) at least one hospital admission with a diabetes-related ICD-9 code (250.x, 357.2, 362.0 or 366.41); (ii) at least two outpatient visits with a diabetes-related ICD-9 code; or (iii) at least one prescription for an oral glucose-lowering medication or monitoring supplies. In order to be eligible, patients also had to be of either Caucasian or African-American ethnicity, able to complete self-report instruments and not diagnosed with bipolar depression.
Procedures
Eligible patients were mailed a study invitation letter, followed by a recruitment telephone call from research staff (including both African-American and Caucasian recruiters) for further screening and enrolment scheduling. After informed consent, participants attended a research appointment for assessment of depressive symptoms, diabetes regimen adherence and self-care behaviours, glycaemic control, demographic characteristics and medical characteristics. All procedures were approved by our Institutional Review Board.
Measures
The presence of probable depressive disorder was assessed with the Patient Health Questionnaire-9 (PHQ-9) [9], in which respondents use a 4-point scale to indicate: ‘How often, over the last 2 weeks, were you bothered by any of the following problems?’ Its advantages over other available scales include its brevity, reflection of the Diagnostic and Statistical Manual (DSM-IV) criteria and strong evidence of criterion validity. Using established cut-off points, the PHQ-9 has 88% sensitivity and 88% specificity for interview-detected major depression. In the present study, participants were classified as either non-depressed (< 10), mildly depressed (≥ 10 but < 15) or moderately/severely depressed (≥ 15). Diabetes-related quality of life (DQOL) was measured using the Problem Areas in Diabetes instrument [10], a validated instrument that consists of 20 items covering emotional responses to diabetes rated on a 5-point scale from which a total score is calculated. Medication adherence was assessed using the 4-item measure developed by Morisky et al., which elicits information about the presence of various forms of medication non-adherence and has demonstrated concurrent and predictive validity and adequate internal consistency [11]. Additional self-care behaviours (diet, exercise, foot care and blood glucose monitoring) were assessed with the Summary of Diabetes Self-Care Activities (SDSCA) [12], which has adequate test–retest reliability, is sensitive to change and is correlated with other measures of the same constructs. Co-morbid medical illnesses were assessed by abstracting electronic medical records using a checklist of 13 common medical illnesses used in prior primary care studies [13,14]. Glycaemic control (glycated haemoglobin; HbA1c) was measured with the DCA 2000 (GMI Inc., Ramsey, MN, USA), which analyses capillary blood samples through a monoclonal antibody method. Participants classified themselves using US Census racial/ethnic categories. Socio-economic status (SES) was assessed using the US Census Bureau Index of Socioeconomic Status [15] adjusted for the current regional Consumer Price Index.
Data analysis
Descriptive statistics were calculated to summarize study variables. Variables with skewed distributions were rank converted for analysis. Bivariate associations were conducted to identify potential confounders using Pearson correlation and independent-samples Student’s t-tests for continuous variables and χ2-test analysis for categorical variables. Associations between depression and the two outcomes (glycaemic control and quality of life) were analysed using linear multiple regression modelling. Independent variables were entered in three blocks corresponding to illness and demographic control covariates, main effects and interaction terms, evaluated against an alpha criterion of P < 0.05. Interaction scores were calculated by multiplying scores for regimen (0 for oral glucose-lowering agents alone, 1 for insulin) and depression level (non-depressed, mild depression and moderate/severe depression).
Results
Enrolment and retention
Of 1569 patients who received a study announcement by post, 392 could not be reached for telephone solicitation after multiple attempts. Another 695 were discovered to be ineligible because they did not meet entry criteria on the basis of medical (e.g. did not have diabetes) or demographic (i.e. were neither African-American nor Caucasian) factors, leaving 482 patients. Of these, 37 patients declined participation by means of returning the enclosed postcard and 445 were reached for telephone solicitation, 273 of which consented to the study. Combining those who declined by telephone or post, 57% of 482 ostensibly eligible patients were recruited. Consent was unrelated to age and gender, although African-Americans were more likely to consent than Caucasians (62 vs. 52%, P = 0.025). Of those recruited, 258 (95%) provided complete data and were thus analysable cases. Attrition was significantly associated with younger age (P < 0.001) and poor glycaemic control (P = 0.023), but not with ethnicity, gender, depression SES, DQOL or adherence behaviours. The final sample was demographically and medically diverse (Table 1). Half of the participants were female, 55% were African-American, ages ranged from 27 to 88 years, 40% were prescribed insulin, diabetes duration ranged from 1 to 60 years and 21% had at least two significant co-morbid medical conditions.
Table 1.
Demographic and medical characteristics, by regimen
| Mean ± sd or per cent | ||||
|---|---|---|---|---|
| Variable | Pooled (n = 258) | On oral agent alone (n = 155) | On insulin (n = 103) | P |
| Age (years) | 57.2 ± 8.4 | 57.9 | 56.3 | 0.131 |
| Gender (female; %) | 49.2 | 51.3 | 46.1 | 0.415 |
| African-American (%) | 55.4 | 49.7 | 64.1 | 0.023 |
| Socio-economic status index | 65.0 ± 17.5 | 64.3 ± 18.0 | 66.0 ± 16.7 | 0.448 |
| Diabetes duration (years) | 10.9 ± 8.2 | 9.0 ± 7.0 | 13.6 ± 9.1 | < 0.001 |
| Two or more co-morbid medical conditions (%) | 20.9 | 19.1 | 23.8 | 0.371 |
| Medication adherence | 0.8 | 0.8 | 0.8 | 0.999 |
| HbAlc(%) | 7.59 ± 1.62 | 7.38 ± 1.54 | 7.91 ± 1.74 | 0.005 |
| Depressive symptom severity (PHQ-9 total) | 5.5 ± 4.7 | 5.0 ± 4.1 | 6.32 ± 5.4 | < 0.001 |
| Diabetes quality of life (PAID total) | 21.3 ± 16.3 | 19.6 ± 16.8 | 24.0 ± 21.1 | 0.068 |
HbA1c, glycated haemoglobin; PAID, Problem Areas in Diabetes; PHQ-9, Patient Health Questionnaire-9; SD, standard deviation.
Preliminary analysis of bivariate associations
Table 1 shows sample characteristics by regimen. Compared with participants on oral glucose-lowering agents alone, those on insulin were more likely to be African-American (P = NS) and had significantly longer diabetes duration, poorer glycaemic control and more severe depressive symptoms.
Multivariable regression analyses
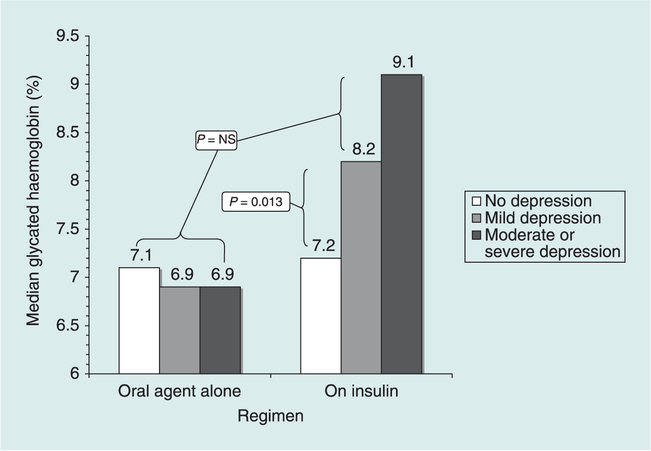
Table 2 contains the results of multivariable regression analyses of glycaemic control. After adjusting for demographics and medical covariates (Block 1), there was a significant association between depression and glycaemic control (Block 2, P = 0.033) in the expected direction. The interaction term (Block 3) also had a significant effect (P = 0.002). Regimen-stratified follow-up analyses indicated that the adjusted association (β coefficient) between depression and glycaemic control was −0.08 (P = 0.356) for participants on oral glucose-lowering agents alone and 0.35 (P < 0.001) for participants on insulin (Z for difference between coefficients = 3.46, P = 0.0003). The interaction effect is depicted in Fig. 1. The same pattern of significant findings emerged when depression was analysed as a continuous dimension of symptom severity rather than as a three-category variable.
Table 2.
Multivariable ordinary least squares (OLS) regression analysis of diabetes outcomes
| Dependent variable | Independent variables | R2 change | P | Standard β | P |
|---|---|---|---|---|---|
| Glycaemic control (HbAlc) | Block 1 | 0.05 | 0.005 | ||
| n = 258 | Gender* | −0.11 | 0.093 | ||
| R2 total = 0.11 | Ethnicity† | 0.13 | 0.042 | ||
| P < 0.001 | Diabetes duration | 0.14 | 0.024 | ||
| Block 2 | 0.03 | 0.018 | |||
| Regimen‡ | 0.10 | 0.144 | |||
| Depression§ | 0.13 | 0.033 | |||
| Block 3 | 0.03 | 0.002 | |||
| Regimen × depression | 0.55 | 0.002 | |||
| Diabetes quality of life (PAID) | Block 1 | 0.04 | 0.010 | ||
| n = 255 | Gender* | 0.21 | 0.001 | ||
| R2 total = 0.28 | Ethnicity† | 0.01 | 0.990 | ||
| P< 0.001 | Diabetes duration | 0.04 | 0.547 | ||
| Block 2 | 0.21 | < 0.001 | |||
| Regimen‡ | 0.04 | 0.493 | |||
| Depression§ | 0.45 | < 0.001 | |||
| Block 3 | 0.03 | 0.001 | |||
| Regimen × depression | 0.52 | 0.001 |
Coded as male = 0, female = 1.
Coded as Caucasian = 0, African-American = 1.
Coded as on oral glucose-lowering agent alone = 0, on insulin = 1.
Coded as non-depressed = 0, mild depression = 1, moderate or severe depression = 2.
HbA1c, glycated haemoglobin; PAID, Problem Areas in Diabetes.
FIGURE 1.
Median glycaemic control by regimen and depression level.
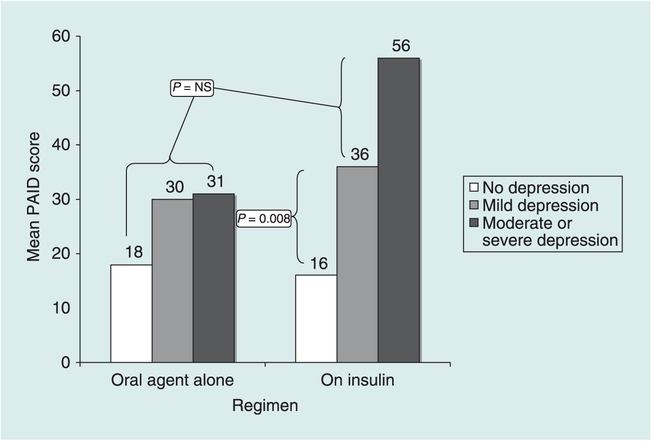
Table 2 also contains the results of the analysis of DQOL as an outcome of the same predictor variables, which showed very similar results. Both the depression main effect and the regimen × depression interaction term were significant. Regimen-stratified follow-up analyses indicated that the adjusted association between depression and quality of life was significant for both groups of participants (P for both < 0.005), but that the coefficient for those on insulin was significantly greater than for those on oral glucose-lowering agents alone (β= 0.64 and 0.22, respectively, Z for difference = 4.15, P < 0.0001). The interaction effect is depicted in Fig. 2.
FIGURE 2.
Mean PAID score by regimen and depression level.
Test for mediation by adherence
Both of the above analyses were then re-conducted to determine whether the depression main effects or interactions were mediated by adherence, by including adherence main effects in both of the above multivariate models. Although medication adherence had significant adjusted associations with depression (β = 0.20, P = 0.002), glycaemic control (β = 0.16, P = 0.014) and quality of life (β = 0.18, P = 0.002), the regime × depression interaction effect persisted in both models (β = 0.53 and 0.50, respectively). Thus, mediation by medication non-adherence was not supported. Exploratory analyses of other adherence behaviours showed similar results.
Because insulin therapy is typically required for patients who are chronically hyperglycaemic despite adequate trials of oral medication, we considered whether the significant associations were because of restricted HbA1c range within the group using oral glucose-lowering agents alone. However, the same pattern of statistically significant interactions emerged when the analyses excluded participants with HbA1c values ≤ 6.0 or ≥ 10.0%.
Discussion
To summarize, the findings indicate that, in patients using insulin, depression was associated with poorer glycaemic control and quality of life, whereas this was not the case for those who were on oral medications alone. Our findings suggest that the relatively weak association between depressive symptoms and Type 2 DM outcomes found in previous studies may be as a result of the tendency for researchers to combine two subgroups in which depression plays a different role; i.e. patients who use insulin with those who do not. There are several plausible explanations for this result, only some of which can be addressed within this study. For example, the observed differences may be because of regimen-specific behavioural mechanisms. First, to the extent that depression disrupts medication adherence [16,17], it seems plausible that this is more likely for patients prescribed insulin, which is a more complex regimen and therefore probably more burdensome than taking oral medication alone. Second, we would speculate that glycaemic control would be more disrupted by a few missed injections than a few missed oral medication doses. However, mediation analyses indicated that, although poor adherence occurred with both regimens, it did not account for the differences by regimen.
A second possible explanation relates to the psychological impact of insulin therapy. Patients ascribe significant negative meaning to the concept of requiring injections [7,18,19], which increases the influence of depressed mood upon outcomes, particularly quality of life. However, the depression–outcome association appears to exist in diabetic patients who do not use insulin [17,20]. If exogenous insulin had some directly depressogenic effect on brain function then this could explain the findings. Once depression is present, pathological processes such as hypercortisolism or activation of inflammatory mediators may further worsen diabetes outcomes.
Third, regimen type is a direct reflection of diabetes severity and duration. Because insulin therapy is typically prescribed when glucose cannot be controlled through oral glucose-lowering agents alone [21], insulin therapy is an obvious marker of more advanced diabetes. Both depression and severe diabetes may be associated with neurohormonal abnormal levels of cortisol and catecholamines [22]. We would further speculate that, as blood glucose becomes more labile, it becomes more strongly related to psychological factors. We previously reported in Type 1 diabetes that psychosocial factors such as daily stress are more strongly related to blood glucose lability than absolute levels of blood glucose [23].
The study results should be generalized with caution because, even although the sample was demographically diverse, enrolment occurred within one health system. Although the measure and its cut-off points used to classify probable depression were originally validated against psychiatric interviews, we did not verify depression presence by structured interview and therefore some psychiatric misclassification may have occurred [24]. The validity of self-reported adherence has often been questioned, because it may lead to inflated estimates. However, self-report measures of medication adherence show moderate-to-high concordance with other measures of medication adherence [25]. For example, in a prior study of antidepressant adherence, we found that self-reported adherence agreed with pharmacy refill data 72% of the time and was unassociated with social desirability bias[13].
The observed associations might be explained by an unmeasured factor such as the absolute level of exposure to exogenous insulin, the subjective burden of using insulin, the personal meaning of requiring insulin for diabetes control or the number of complications [26,27]. Another limitation is that the measures of depression and glycaemic control reflect different time intervals (2 weeks vs. 8–12 weeks respectively), which could weaken observed associations. Finally, the cross-sectional observational study design does not allow causal inference between diabetes and depression and therefore we cannot determine the directionality of associations between mood and diabetes outcomes.
In closing, the findings imply that the association between depression and Type 2 DM outcomes exists only in patients who are prescribed insulin. This effect is not mediated by medication non-adherence, making it unlikely that depression-related insulin omission explains the association. Other potential mechanisms should be carefully studied. These include the potential depressogenic effects of poor glycaemic control and the existence of some common biological, psychological or healthcare factor that might cause poor outcomes in both depression and diabetes. Clinicians who provide diabetes care might be especially vigilant in terms of depression screening and management for diabetic patients who are prescribed insulin, inviting patients to discuss the personal meaning of being on insulin.
Acknowledgements
This study was funded by National Institutes of Health grant R01DK066016 to Dr Aikens and was supported by the Behavioral, Clinical and Health Systems Intervention Research Core of the Michigan Diabetes Research and Training Center (Grant P60DK020572, National Institute of Diabetes and Digestive Kidney Disease).
Abbreviations
- DM
diabetes mellitus
- DQOL
diabetes-related quality of life
- HbA1c
glycated haemoglobin
- PHQ-9
Patient Health Questionnaire
- PAID
Problem Areas in Diabetes
- SES
socio-economic status
Footnotes
Competing interests
Nothing to declare.
References
- 1.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23: 934–942. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of co-morbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2000; 24: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 3.Milano AF, Singer RB. Mortality in co-morbidity (II)—excess death rates derived from a follow-up study on 10 025 subjects divided into 4 groups with or without depression and diabetes mellitus. J Insur Med 2007; 39: 160–166. [PubMed] [Google Scholar]
- 4.Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol 2005; 161: 652–660. [DOI] [PubMed] [Google Scholar]
- 5.Van Tilburg MA, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med 2001; 63: 551–555. [DOI] [PubMed] [Google Scholar]
- 6.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry 2003; 25: 246–252. [DOI] [PubMed] [Google Scholar]
- 7.Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005; 28: 2543–2545. [DOI] [PubMed] [Google Scholar]
- 8.Surwit RS, van Tilburg MA, Parekh PI, Lane JD, Feinglos MN. Treatment regimen determines the relationship between depression and glycemic control. Diabetes Res Clin Pract 2005; 69: 78–80. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. J Am Med Assoc 1999; 282: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 10.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the problem areas in diabetes (PAID) questionnaire. Diabet Med 2003; 20: 69–72. [DOI] [PubMed] [Google Scholar]
- 11.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986; 24: 67–74. [DOI] [PubMed] [Google Scholar]
- 12.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000; 23: 943–950. [DOI] [PubMed] [Google Scholar]
- 13.Aikens JE, Nease DE Jr, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance—phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med 2005; 3: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aikens JE, Rouse ME. Help-seeking for insomnia among adult patients in primary care. J Am Board Fam Pract 2005; 18: 257–261. [DOI] [PubMed] [Google Scholar]
- 15.US Bureau of the Census. Methodology and scores of the socioeconomic status, 1963. Working paper no. 15. Fed Regist 2002; 67: 6931–6933. [Google Scholar]
- 16.Kalsekar ID, Madhavan SS, Amonkar MM, Makela EH, Scott VG, Douglas SM et al. Depression in patients with type 2 diabetes: impact on adherence to oral hypoglycemic agents. Ann Pharmacother 2006; 40: 605–611. [DOI] [PubMed] [Google Scholar]
- 17.Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Res Social Adm Pharm 2005; 1: 508–525. [DOI] [PubMed] [Google Scholar]
- 18.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med 2005; 20: 479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care 2005; 28: 2673–2679. [DOI] [PubMed] [Google Scholar]
- 20.Chao J, Nau DP, Aikens JE. Patient-reported perceptions of side effects of antihyperglycemic medication and adherence to medication regiments in persons with diabetes mellitus. Clin Ther 2007; 29: 177–180. [DOI] [PubMed] [Google Scholar]
- 21.Warren RE. The stepwise approach to the management of type 2 diabetes. Diabetes Res Clin Pract 2004; 65: S3–8. [DOI] [PubMed] [Google Scholar]
- 22.Sachs G, Spiess K, Moser G, Kautzky A, Luger A, Pietschmann P et al. Hormonal and blood glucose responsiveness as an indicator of specific emotional arousal in type 1 diabetics. J Psychosom Res 1993; 37: 831–841. [DOI] [PubMed] [Google Scholar]
- 23.Aikens JE, Wallander JL, Bell DS, Cole JA. Daily stress variability, learned resourcefulness, regimen adherence, and metabolic control in adult type I diabetes mellitus: evaluation of a path model. J Consult Clin Psychol 1992; 60: 113–118. [DOI] [PubMed] [Google Scholar]
- 24.Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007; 30: 542–548. [DOI] [PubMed] [Google Scholar]
- 25.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care 2004; 42: 649–652. [DOI] [PubMed] [Google Scholar]
- 26.Bailey BJ. Mediators of depression in adults with diabetes. Clin Nurs Res 1996; 5: 28–42. [DOI] [PubMed] [Google Scholar]
- 27.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005; 19: 113–122. [DOI] [PubMed] [Google Scholar]