Abstract
The eye contains a circadian system that acts independently from the master circadian clock located in the brain. This circadian system regulates important physiological functions within the eye. Emerging experimental evidence also indicates that disruption of the ocular circadian clock, or its outputs, negatively affects the overall health of the eye. Although previous studies have investigated the effect of aging on the regulation of circadian rhythms, no study has investigated the effects of aging on the circadian rhythm in the ocular system. The aim of the present study was to investigate how aging affects the circadian rhythm of PER2::LUC bioluminescence in the retina, retinal pigment epithelium (RPE) and cornea. Our data suggest that among the three different ocular tissues investigated, the retina appears to be the most affected by aging whereas the RPE and cornea are less affected by aging. Our data, along with studies of other organs and tissues, suggest that reduction in the amplitude of rhythms is probably the most severe effect of aging on the circadian clock.
Keywords: Aging, Bioluminescence, Circadian rhythm, Cornea, PER2::LUC, Retina, RPE
The mammalian eye contains a full circadian system that acts independently from the master circadian clock located in the brain (McMahon et al., 2014). This circadian system regulates important functions ranging from visual processing, susceptibility to light-induced photoreceptor damage, neurotransmitters synthesis and release, disk shedding and phagocytosis, corneal thickness and intraocular pressure (see McMahon et al., 2014 for a recent review). Emerging experimental evidence also indicates that disruption of circadian clock may negatively affect the overall health of the eye. Bmal1 Knock-Out (KO) mice show an increase in cataract and corneal inflammation (Kondratov et al., 2006) and a significant reduction in the number of photoreceptors during aging (Baba et al., 2018). Clock mutant mice also developed cataract during aging (Kondratov et al., 2010) and Clock/Npas2 KO mice also show a significant reduction in the photoreceptor layer (Baba et al., 2018).
Previous studies have shown that aging affects the circadian rhythms in locomotor activity, suprachiasmatic nucleus of hypothalamus (SCN) and peripheral organs (Aujard et al., 2001; Yamazaki et al., 2002; Davidson et al., 2008; Sellix et al., 2012; Nakamura et al., 2015; Tahara et al., 2017), No previous study has investigated the effects of aging on the ocular circadian system. The aim of the present study was to investigate how aging affects the circadian rhythm of bioluminescence in the retina, retinal pigment epithelium (RPE) and cornea in PERIOD2::LUCIFERASE mice (PER2::LUC, Yoo et al., 2004).
PER2::LUC mice raised at Morehouse School of Medicine (water and food ad libitum, 12 h light and 12 h dark, fluorescent tubes 200 to 400 lux at cage level) at the age of 3 and 12 months were used in this study. Mice were sacrificed at ZT 8–10 and eyes were removed (only one eye from each mouse was used in this study) and placed in iced Hank’s salt solution, then the retina, the RPE-choroid and the cornea were carefully separated under a dissecting microscope (see Baba et al., 2010; 2015). The retina and eye cup containing the RPE-choroid were flattened by four radical incisions and placed on the culture membrane (Millicell-CM, PICM030–50, Millipore, Billerica, MA) in a 35 mm Petri dish with 1.2 ml of 199 medium (Cambrex, Walkersville, MD) containing 0.1 mM D-Luciferin K salt (Molecular Imaging Products, Bend, OR), 10 mm HEPES (pH 7.2), penicillin/streptomycin cocktail (2.5 ml / l,15140–122; Gibco, Grand Island, NY), and B27 (2%;17504–044; Gibco) (see Baba et al., 2010 and 2017 for details about the specific culture conditions for each tissue). Corneas were prepared and cultured in the same manner as previously described (Baba et al., 2015). Dishes were sealed and kept at 37 °C. Because culture preparation of ocular tissues can be affected by the time of the day at which the cultures are prepared (Evans et al., 2015) all the cultures were prepared under fluorescent light between Zeitgeber Time (ZT) 8–10. All the procedures were performed in accordance to the National Institutes of Health Guide on the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic Vision Research and were approved by the Institutional Animal Care and Use Committees of Morehouse School of Medicine. The bioluminescence emitted from the cornea, retina and RPE-choroid was measured for 1 min at 10-min intervals using a Lumicycle® (Actimetrics, Wilmette, IL). Bioluminescence recordings were detrended using a 24 h moving average-subtraction method and smoothed by a 2 h moving average. Daily peaks were identified by Origin® software (Origin Lab, Northampton, MA) and the period was calculated from the slope of a linear regression line fitted to circadian peak phases (Baba et al., 2010). The daily amplitude of the circadian rhythm was calculated from the peaks to the median line of wave up to seven days. The value of rhythmic power was calculated by the Fast Fourier Transform (FFT) in Lumicycle Analysis program (Ruan et al., 2012) which was used to determine the dominant periodicity in the data fit by the cosine function with the highest amplitude (Herzog et al., 2014).
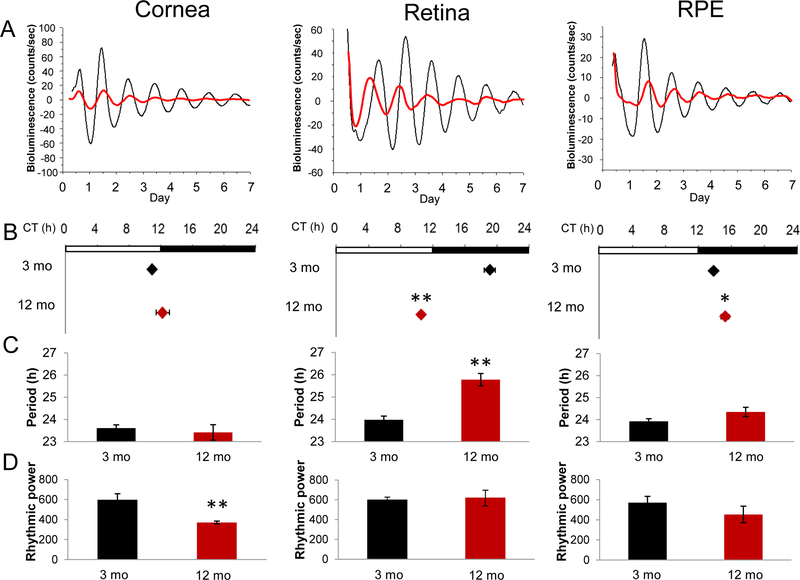
A circadian rhythm in PER2::LUC bioluminescence was observed at both ages and in all the tissues investigated (Fig. 1A), but all tissues from older mice displayed a significant decrease in the amplitude of the PER2::LUC bioluminescence rhythm (p<0.001 to all tissues, Two-way ANOVA, n=6–10). The phase of PER2::LUC rhythm in the retina of old mice was substantially advanced (Fig. 1B, CT 10.52 ± 0.32 h; p<0.01 t-test n=8) relative to young mice (CT 19.10 ± 0.14 h, n=9). The phase of RPE bioluminescence circadian rhythm in 12 months old mice was slightly, but significantly, delayed (approximately 1.5 h) with respect to what observed in the 3 months old mice (3 months CT 13.83 ± 0.23 hr. vs. 12 months CT 15.23 ± 0.48 hr.; p<0.05 t-test n=6–8). No phase change was observed in cornea (n=6–10). The period of the PER2::LUC bioluminescence rhythm in the retina of older mice was approximately 2 hrs. longer that in young mice (Fig. 1C, 3 months.: 23.98 ± 0.16 hr. vs. 12 months: 25.78 ± 0.27 hr.). Interestingly, the power spectrum analysis indicated that the rhythmicity of PER2::LUC rhythm in the cornea was less robust in old mice than in young mice (Fig. 1D, p<0.01, t-test).
Figure 1.
(A) Representative PER2::LUC bioluminescence rhythm in the cornea, retina and RPE dissected from young (3 months (mo.) old; black lines) and old (12 months old; grey lines) mice. (B) PER2::LUC rhythm of old retina showed an approximately 8.5 hrs. of phase-advance and RPE showed a slight delay in old mice. Black diamonds represent data obtained from young mice and grey diamonds represent data obtained from old mice (Mean ± SEM, n=6–10, * p<0.05, ** p<0.01, t-test). White and black bars above the plots indicate the light/dark cycle at which mice were exposed before preparation of organ cultures. (C) The period of PER2::LUC rhythm recorded from old retinas were approximately 2 hrs. longer than those observed in young mice (Mean ± SEM, n=6–10, ** p<0.01, t-test). Black bars represent young mice and grey bars represent old mice. (D) The cornea was the only tissue in which the rhythmic power of PER2::LUC circadian rhythm was affected by aging (Mean ± SEM, n=6–10, ** p<0.01, t-test). Black bars represent young mice and grey bars represent old mice.
Overall, we found that aging affects the circadian rhythms of ocular tissues. The most notable effects can be summarized as follow: 1) the amplitude of PER2::LUC bioluminescence circadian rhythm showed a significant decrease in all the three tissues investigated; 2) the retina and the RPE showed a significant change in the phase of the circadian rhythm; 3) in the retina the period of the circadian rhythm was longer in older mice; and finally, 4) the circadian rhythm was less robust in corneas obtained from older mice.
Our data agree with previous studies which reported that amplitudes of circadian rhythms in the SCN and other tissues decline during aging (Nakamura et al., 2011, Nakamura et al., 2015, Aujard et al., 2001). However, progressive desynchronization of oscilaltors among SCN neurons, not a reduction in clock function, is thought to be the underlying cause of age-dependent reductions in amplitude (Nakamura et al., 2015). A decline in amplitude of the bioluminescence rhythm of xiphoid cartilage explanted from 20–24-month-old PER2::LUC mice and intervertebral disk from 12-month-old PER2::LUC mice also showed a damped circadian amplitude (Gossan et al., 2013; Dudek et al., 2017). Interestingly the amplitude of the bioluminescence rhythm of xiphoid cartilage could be restored to the level observed in young mice by treatment with dexamethasone, thus suggesting that aging may affect the mechanism mediating the synchronization among the cells (Gossan et al., 2013). An additional study also showed that cultured fibroblasts obtained from young and old human subjects did not show any changes in the phase and period of Bmal1::LUC rhythm. However, the Bmal1::LUC circadian rhythm of fibroblasts obtained from young individuals was shorter and phase-shifted when cells were treated with serum obtained from old individuals (Pagani et al., 2011). Hence, these studies may suggest that aging does not affect the capability of the cells to produce a circadian oscillation, but rather aging may prevent the capability of the cells within a tissue to remain synchronized. However, we must be cautious with this interpretation since it is possible that the age-related decrease in the amplitude of bioluminescence rhythms could be due to age-dependent decline in the efficiency of luciferase enzyme.
We found that the phase and period of the retinal circadian PER2::LUC rhythm were significantly affected by aging. While this period/phase relationship would not be predicted from classical circadian entrainment theory, it is consistent with previous investigations in the mouse (Nakamura et al., 2015) (Yamazaki et al., 2002; Sellix et al., 2012). In this context it is important to note that these effects are observed in the peripheral oscillators and not in the SCN, in which aging causes a phase-delay (Asai et al., 2001; Sellix et al., 2012; Davidson et al., 2008; Nakamura et al., 2015; Yamazaki et al., 2002). In contrast, aging did not affect the phase and period of PER2::LUC rhythm in the mouse cornea, consistent with data from rat corneas (Yamazaki et al., 2002). However, we found aging increased the inter-culture variability in the PER2::LUC rhythm (Fig. 1D). Previous studies have reported age-related loss of corneal endothelial cells (Moller-Pendersen, 1997; Bourne & McLaren, 2004; Gipsen, 2013) and we have previously shown that in the cornea the PER2::LUC bioluminescence signal is mostly emitted by the corneal epithelium and endothelium (Baba et al., 2015). Hence it is possible that the increased variability of the PER2::LUC rhythm in this tissue is due to changes in the PER2::LUC rhythmicity between the corneal epithelium and endothelium. Finally, we observed that the effect of aging on the RPE is modest thus suggesting that the circadian clock in this tissue is less sensitive to the aging process.
Previous investigations have shown that many retinal functions are affected by the aging. In the mouse visual processing steadily decreases during aging (Gresh et al., 2003, Li et al., 2001, Baba et al., 2012), photoreceptor viability is also reduced (Gresh et al., 2003, Li et al., 2001, Baba et al., 2009; Gianesini et al., 2017) and dysfunction in the circadian clock leads to photoreceptor loss (Baba et al., 2018). Therefore, it is possible to speculate that the changes in the retinal circadian rhythmicity that occur during aging may negatively influence photoreceptors viability during aging.
In conclusion our study suggests that among the three different ocular tissues investigated, the retina appears to be the most affected by aging whereas the RPE and cornea are less affected by aging. Our data are consistent with previous studies in other organs and/or tissues (Davidson et al., 2008; Sellix et al., 2012; Gossan et al., 2013; Nakamura et al., 2015; Dudek et al., 2017), which have shown that reduction in the amplitude of the rhythms is probably the most severe effect of aging on the circadian clock.
Acknowledgements:
This work was supported by grants from the National Institutes of Health: EY022216, EY026291, T-32 HL103104 to G.T., GM116760 to K.B. and by 5U54NS083932, S21MD000101, G12-RR03034, U54RR026137 to Morehouse School of Medicine;
References
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M and Shibata S (2001) Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66:1133–1139. [DOI] [PubMed] [Google Scholar]
- Aujard F, Herzog ED and Block GD (2001) Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106:255–261. [DOI] [PubMed] [Google Scholar]
- Baba K, Ribelayga CP, Michael Iuvone P, Tosini G (2018) The Retinal Circadian Clock and Photoreceptor Viability. Adv Exp Med Biol 1074:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, DeBruyne JP and Tosini G (2017) Dopamine 2 Receptor Activation Entrains Circadian Clocks in Mouse Retinal Pigment Epithelium. Sci Rep 7:5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Davidson AJ and Tosini G (2015) Melatonin Entrains PER2::LUC Bioluminescence Circadian Rhythm in the Mouse Cornea. Invest Ophthalmol Vis Sci 56:4753–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Mazzoni A, Owino A, Contreras-Alcantara S, Strettoi E and Tosini G (2012) Age-related changes in the daily rhythm of photoreceptor functioning and circuitry in a melatonin proficient mouse strain. PLoS One 7:e37799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM and Tosini G (2009) Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA 106:15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Tosini M, Contreras-Alcantara S and Tosini G (2010) Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis 16:2605–11. [PMC free article] [PubMed] [Google Scholar]
- Bourne WM and McLaren JW (2004) Clinical responses of the corneal endothelium. Exp Eye Res 78:561–72. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M and Block GD (2008) Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging 29:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky YV, Samsa WE and Kondratov RV (2010) Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, Yang N, Ruckshanthi JP, Williams J, Borysiewicz E, Wang P, Adamson A, Li J, Bateman JF, White MR, Boot-Handford RP, Hoyland JA and Meng QJ (2017) The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. Ann Rheum Dis 76:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Suen TC, Callif BL, Mitchell AS, Castanon-Cervantes O, Baker KM, Kloehn I, Baba K, Teubner BJ, Ehlen JC, Paul KN, Bartness TJ, Tosini G, Leise T and Davidson AJ (2015) Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC Biol 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianesini C, Hiragaki S, Laurent V, Hicks D and Tosini G (2016) Cone Viability Is Affected by Disruption of Melatonin Receptors Signaling. Invest Ophthalmol Vis Sci 57:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK (2013) Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci 54: ORSF48–53. [DOI] [PubMed] [Google Scholar]
- Gossan N, Zeef L, Hensman J, Hughes A, Bateman JF, Rowley L, Little CB, Piggins HD, Rattray M, Boot-Handford RP and Meng QJ (2013) The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum 65:2334–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresh J, Goletz PW, Crouch RK and Rohrer B (2003) Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis Neurosci 20:211–220. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Kiss IZ and Mazuski C (2014) Measuring synchrony in the mammalian central circadian circuit. Methods Enzymol 552: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV and Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM, and Tosini G (2014) Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog Retin Eye Res 39C:58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS and Block GD (2015) Age-Related Changes in the Circadian System Unmasked by Constant Conditions. eNeuro 2:ENEURO.0064–15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS and Block GD (2011) Age-related decline in circadian output. J Neurosci 31:10201–10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L, Schmitt K, Meier F, Izakovic J, Roemer K, Viola A, Cajochen C, Wirz-Justice A, Brown SA and Eckert A (2011) Serum factors in older individuals change cellular clock properties. Proc Natl Acad Sci USA 108:7218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Gamble KL, Risner ML, Young LA and McMahon DG (2012) Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS One 7:e38985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M and Davidson AJ (2012) Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci 32:16193–16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Takatsu Y, Shiraishi T, Kikuchi Y, Yamazaki M, Motohashi H, Muto A, Sasaki H, Haraguchi A, Kuriki D, Nakamura TJ and Shibata S (2017) Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. NPJ Aging Mech Dis 3:16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M and Block GD (2002) Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A 99:10801–10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]