Abstract
The putative strong anti-nociceptive properties of the antidepressant phenelzine (PLZ) have not been widely explored as a treatment for pain. Antinociceptive effects of PLZ were identified in the formalin model of tonic pain (Mifflin et al. 2016) and in allodynia associated with experimental autoimmune encephalomyelitis, (EAE) a mouse model of multiple sclerosis (Potter et al. 2016). Here, we further clarify the specific types of stimuli and contexts in which PLZ modulates nociceptive sensitivity. We examined the effects of PLZ on transient nociception, and found that PLZ did not change sensitivity to mechanical or noxious heat stimulation. We also reexamined PLZ in the formalin assay, as the effects of PLZ were variable in the Mifflin et al. study. When administered 3h before the intraplantar injection of formalin, PLZ reduced pain-like behavior during the 2nd, but not first phase of the assay. These findings indicate that PLZ selectively inhibits ongoing inflammatory pain while sparing transient reflexive and acute nociception. We also investigated the cellular mechanisms of action of PLZ in the dorsal horn, and as expected of a monoamine-oxidase inhibitor, PLZ increased serotonin (5HT) immunoreactivity. We next used two approaches to test the hypothesis that PLZ inhibits the activation of spinal nociresponsive neurons. First, we evaluated the formalin-evoked protein expression of the immediate early gene, c-fos. PLZ reduced Fos expression in the superficial dorsal horn. Second, we evaluated the effects of PLZ on intracellular calcium responses to superfusion of glutamate (0.3–1.0 mM) in an ex vivo lumbar spinal cord slice preparation. Superfusion with PLZ (100–300 μM) reduced 1 mM glutamate-evoked calcium responses. This was blocked by pretreatment with the 5HT1A-receptor antagonist WAY-100,635, but not the alpha-2 adrenergic antagonist idazoxan. We conclude that PLZ exerts antinociceptive effects through a 5-HT/5HT1AR-dependent inhibition of neuronal responses within nociceptive circuits of the dorsal horn.
Keywords: MAOI, pain, phenelzine, dorsal horn, spinal cord, formalin, plasticity, calcium imaging, c-Fos
Introduction
Monoamine oxidase inhibitors such as phenelzine (PLZ) were once commonly prescribed for the treatment of depression (Fiedorowicz and Swartz, 2004). Concerns over the potential for precipitating a hypertensive crisis when used in the presence of dietary tyramine led to the eventual supplanting of PLZ and other MAOIs by newer drugs, such as the selective-serotonin reuptake inhibitors (SSRIs) (Fiedorowicz and Swartz, 2004). Critical reviews of the literature ultimately revealed that these fears were overstated, and suggested that the MAOIs were widely underutilized (Fiedorowicz and Swartz, 2004, Rapaport, 2007, Shulman et al., 2009). Today, PLZ is occasionally prescribed as a second- or third-line option for depressed patients who do not respond to the more commonly used SSRIs/SNRIs (serotonin/norepinephrine-reuptake inhibitors) (Fiedorowicz and Swartz, 2004, Shulman et al., 2009).
PLZ is unique amongst the MAOIs in that, apart from producing an elevation in CNS levels of the monoamine neurotransmitters through irreversible inhibition of both isoforms of their major degradative enzyme (MAO-A/B), it also reversibly inhibits GABA transaminase, leading to an elevation in CNS GABA content (McManus et al., 1992, Parent et al., 2000). This secondary action, mediated through the active metabolite phenylethylidenehydrazine (PEH), gives PLZ an additional anxiolytic effect in animal models (Paslawski et al., 1996, Parent et al., 2002). PLZ possesses other pharmacological characteristics including inhibition of neuronal glutamate release (Michael-Titus et al., 2000), and also direct chemical scavenging of reactive aldehyde molecules and inhibition of reactive-oxygen species (ROS) production by MAO, perhaps leading to neuroprotective effects (MacKenzie et al., 2010, Song et al., 2010). This broad range of biological activities has led to a renewed interest in novel therapeutic uses for PLZ (Song et al., 2013).
Early studies indicated that PLZ may exert stronger and longer-lasting antinociceptive effects than tricyclic antidepressant (TCA) and perhaps even than the opioids (Emele et al., 1961, Lee et al., 1983), although these studies were limited in scope. Two more recent publications have renewed focus on the potential application of PLZ for pain control, and have extended this to its derivatives PEH and N2-Acetyl-PLZ. Potter et al. 2016 (Potter et al., 2016), found that chronic PLZ treatment prevented allodynic behaviors in the MOG35–55 /female/C57BL6 form of EAE, an animal model commonly employed in the study of MS (Ransohoff, 2012). Furthermore, in the mouse formalin model of ongoing chemogenic pain, Mifflin et al. 2016 (Mifflin et al., 2016) reported that a single injection of PLZ (or PEH) reduced nociceptive sensitivity. However, the mechanism of analgesic action remained undetermined. To test the hypothesis that PLZ exerts its analgesic actions at the level of the dorsal horn of the spinal cord (SCDH), we employed two methods to assess neuronal activity. First, we used immunohistochemistry to evaluate Fos expression in SCDH. Second, we used an ex vivo adult spinal cord slice preparation to bulk-load the ratiometric calcium indicator fura-2, thus allowing real time intracellular glutamate-evoked calcium imaging within the SCDH (Doolen et al., 2012). We imaged responses both before and after superfusion with PLZ, and we used pharmacological antagonists to distinguish the contribution of alpha-2 adrenergic and 5HT1A receptors.
Methods
Animals and Ethics
Thirty 8–12-week-old female C57/BL6 mice (Charles River–Saint Constant, Quebec, Canada) were used in behavioral and immunohistochemistry experiments. These mice were housed 5 per cage. These experiments were conducted in accordance with the Canadian Council on Animal Care Guidelines and Policies, and with protocols approved by the University of Alberta Health Sciences Animal Care and Use Committee. Ten 4–8-week-old female C57/BL6 mice (Charles River–Indianapolis, IN, USA) were used in calcium imaging experiments. These mice were housed 4 per cage. All mice were fed ad libitum. These experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Drug Treatments
For behavioral and immunohistochemistry experiments, mice were divided into two groups (1 cage each / n=5 for von Frey; 2 cages each / n=10 for hotplate/formalin,) which received an intraperitoneal injection of either vehicle (VEH, bacteriostatic water, 10mL / kg body weight) or phenelzine (PLZ, 15 mg/kg body weight, Sigma-Aldrich—Oakville, ON, Canada). The injections were administered on the day of the testing, 1h before the first von Frey test, or 3h before the formalin assay / (post-injection) hotplate test.
Behavioral Pain Assays
Mechanical Sensitivity
The von Frey (VF) assay assessed the effects of PLZ on mechanical (tactile/punctate pressure) sensitivity. Animals were placed in transparent Plexiglas boxes over a wire mesh that allowed access to the plantar hindpaw. Prior to the start of testing, mice underwent a period of habituation to their boxes (5–10 min/day for 3 days). An additional 5–10 min of habituation to the boxes was given prior to each testing session. After this period, the plantar surface of each hindpaw was stimulated five times with each of an ascending series of six weighted Von Frey monofilaments (0.04–2.0g). An observer, blinded to experimental treatment, monitored and recorded behavioral responses. The bending force of the filament which elicited pain-like behaviors (ie. lifting/guarding, shaking, or licking the paw) in response to 60% or more of the stimuli was recorded as the mechanical withdrawal threshold. Left and right paw thresholds were averaged. VF testing was conducted at 1h and 4h post-injection because a single I.P. injection of PLZ significantly elevates monoamine neurotransmitters (5-HT/NA/dopamine) and GABA within 1–4h post-injection, with maximal elevation by 4–6h (Griebel et al., 1998, Parent et al., 2000, Musgrave et al., 2011, Mifflin et al., 2016).
Thermal sensitivity
The hot plate assay was used to assess the effects of PLZ on noxious thermal (heat) sensitivity. The plate was warmed to 520C (a temperature at which nociceptive C fibres are activated (Dubin and Patapoutian, 2010)). Mice were placed onto the plate, and a blind observer monitored and recorded the time to the first pain-like response - usually a flick of the hindpaw, though sometimes a lick or sustained lift. Hot plate testing was conducted at an initial time point (without treatment), and then again at 3h post-injection. This assay was repeated twice per animal to obtain an average withdrawal latency at each time point, with each trial separated by 25 min.
Formalin Assay
We tested the effects of PLZ in a model of ongoing chemogenic pain. Animals were injected with VEH or PLZ 3h prior to the beginning of the assay. For habituation, mice were placed within a clear-walled Plexiglas observation chamber for 10 min on the 3 days prior to the study and then again for 10 min prior to the start of the assay. 1% formalin was prepared daily by diluting from a 37% stock to a 0.9% solution w/v saline. 30μL was injected subcutaneously under the plantar surface of the left hindpaw, and the animal was returned to the observation chamber. Over the next 30 min, an observer blinded to treatment recorded the time (in seconds) spent either lifting/shaking/licking the paw. Timepoints were binned into six 5 min intervals. The first two bins (0–10 min post formalin) and the last four bins (10–30 min post formalin) were defined as ‘phase one’ and ‘phase two’, respectively. Time spent licking/lifting/shaking were added together and reported as ‘nociceptive response time’.
Immunohistochemistry/Immunocytochemistry
Tissue Collection / Preparation
Following the 30 min observation period, 1h was allowed to elapse for expression of the Fos protein before sacrificing the animal for tissue collection. Animals underwent transcardiac exsanguination and perfusion with saline (0.9% w/v) followed by fixation with 4% paraformaldehyde (PFA) / 1% glutaraldehyde in 0.1M PB. Lumbar (L1-L5) spinal cord was removed and post-fixed overnight in PFA/glutaraldehyde. Tissues were then cryoprotected by immersion in 30% sucrose for 48h, followed by embedding in TissueTek OCT and snap-freezing on liquid N2. Frozen tissues were stored at −800C until they could be sectioned on a cryostat (20μm sections) and mounted directly onto slides.
Immunohistochemistry Staining
Tissues were stained using standard immunohistochemistry (IHC) / immunofluorescence protocols as described below. The following reagents/antibodies were used:
c-Fos:
Tissues were incubated with rabbit anti-c-Fos (1:1000, Cell Signalling, Danvers, MA, USA) primary antibody overnight, followed by goat anti-rabbit biotin (1:400, 2h RT, Vector Labs, Burlingame, CA, USA), and avidin-biotin complex (ABC 1:200, 1.5h RT, “VectaStain Elite™ ABC/HRP Kit”, Vector Labs), before visualization with 3,3’-diaminobenzidine (Vector Labs) (plus nickel). Slides were coverslipped using Permount.
Immunofluorescence:
Tissues were incubated overnight with rabbit anti-5-HT (1:1000, Sigma-Aldrich, Oakville, ON, Canada) and then visualized with goat anti-rabbit AlexaFluor 488 (1:200, 1h RT, Invitrogen Life Technologies Inc., Burlington, ON, Canada). Slides were coverslipped using Vectashield™ with DAPI (Vector Labs).
IHC Image Acquisition and Quantification
Slides were imaged using a Zeiss AxioCam MRm camera on a Zeiss Observer Z.1 inverted fluorescence microscope equipped with a 20x objective lens. Images of both ipsilateral and contralateral (to stimulus) dorsal horn at the L4-L5 level were captured for quantification. For each animal, 4 sections (2 sections from each of 2 slides) were imaged. Exposure levels were maintained at a consistent setting for each stain/analysis. Staining was quantified using NIH ImageJ/FIJI in Adobe Photoshop (for manual counting). The number of Fos-immunoreactive neurons were manually counted by an observer blinded to treatment groups. Only the dorsal horn ipsilateral to formalin stimulation was quantified for Fos analysis. 5-HT was quantified by integrated density, performed using template ROIs manually adjusted to fit the individual section (but with consistent overall area ~+/− 2%). Ipsilateral and contralateral dorsal horns were averaged together for the 5-HT analysis. All quantitative IHC image analyses were performed on the original unmodified images. Representative photomicrographs presented in figures were additionally adjusted for brightness, contrast, and histogram scaling to improve the overall visibility of the images. These adjustments were performed only on whole images and were applied in a consistent a manner such that the figures accurately reflect the entire contents and relative intensities of the original images.
Calcium Imaging
Preparation of Adult Mouse Spinal Cord Slices:
As described by Doolen et al. 2012 (Doolen et al., 2012), naïve mice were anesthetized with 5% isoflurane and quickly perfused transcardially with 10 mL of ice-cold sucrose-containing artificial cerebrospinal fluid (aCSF) (sucrose-aCSF) that contained (in mM): NaCl 95, KCl 1.8, KH2PO4 1.2, CaCl2 0.5, MgSO4 7, NaHCO3 26, glucose 15, sucrose 50, kynurenic acid 1, oxygenated with 95% O2, 5% CO2; pH 7.4. The lumbar spinal cord was rapidly (within 90s) isolated by laminectomy from the cervical enlargement to the cauda equina, placed in oxygenated ice-cold sucrose-aCSF, cleaned of dura mater and ventral roots, and super-glued vertically to a block of 4% agar (Fisher Scientific, Pittsburgh, PA) on the stage of a Campden 5000mz vibratome (Lafayette, IN). Transverse slices (450 μm) from lumbar segments L3-L4 were cut in ice-cold sucrose-aCSF using minimum forward speed ranging from 0.03 to 1 mm/s and using maximum vibration. The total dissection and slicing time to ensure slice viability was 22 minutes or less.
Ratiometric Ca2+ Measurements:
Lumber slices were incubated for 60 min at 37° C with Fura-2 AM (10 μM), pluronic acid (0.1%) in oxygenated aCSF containing (in mM): NaCl 127, KCl 1.8, KH2PO4 1.2, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 15, followed by a 20 min de-esterification period in normal aCSF. Prior to recording, slices were kept at RT in a chamber containing approximately 150 mL of oxygenated aCSF. Slices were perfused at 1–2 mL/min with normal aCSF in an RC-25 recording chamber (Warner Instruments, Hamden, CT) mounted on a Nikon FN-1 upright microscope fitted with a 79000 ET FURA2 Hybrid filter set (Nikon Instruments, Melville, NY) and a Photometrics CoolSNAP HQ2 camera (Tucson, AZ). Relative intracellular Ca2+ levels were determined by measuring the change in ratio of fluorescence emission at 510 nm in response to excitation at 340 and 380 nm (200 ms exposure). Paired images were collected at 1 second/frame during the peak of Ca2+ response and 1.5 seconds/frame before and after the peak. Relative changes in Ca2+ levels were evaluated using Nikon Elements software by creating a region of interest over the cell body and then calculating the peak change in ratio. Each slice was used for only one treatment condition. Approximately 10 cells were analyzed in each slice. The peak magnitude of Ca2+ transients were expressed as the difference in ratio following exposure to exogenous glutamate compared to baseline before glutamate. The criterion for a Ca2+ response was a 10% increase above the baseline 340/380nm ratio.
For the initial PLZ(-only) study, Ca2+ transients were in response to a 10s exposure to 0.3mM or 1mM glutamate in aCSF. Eeach slice was stimulated twice with each glutamate concentration (with several min between stimuli to allow calcium levels to return to baseline), prior to superfusion with oxygenated aCSF/PLZ at the given concentration (1 slice per PLZ concentration for 0, 10, 30, 100, and 300μM) for 30 min. Following PLZ superfusion, the slice was re-stimulated two times with each glutamate concentration. For each PLZ concentration, the average glutamate-evoked change in ΔF340/380 ratio before and after PLZ exposure was reported, as well as the change in peak glutamate-evoked Ca2+ response by calculating the post-PLZ magnitude as a percentage of the pre-PLZ response (at each glutamate concentration / average of two stimuli). This change was also expressed as a ‘percent inhibition’ of the pre-PLZ response.
For PLZ plus antagonist studies, only the 1mM glutamate concentration was used. The slice was stimulated twice with 1mM glutamate, then perfused with a receptor antagonist (30μM idazoxan to block adrenergic alpha-2 receptors, 10μM WAY-100,635 to block 5HT1A receptors) in oxygenated aCSF for 10 min prior to and during 2 additional glutamate stimuli. This was done to assess the effect of each antagonist on intracellular calcium responses in the absence of PLZ. Following the antagonist exposure, the slice was perfused with 200μM PLZ plus antagonist in oxygenated aCSF for 30 min, and then re-stimulated twice with 1mM glutamate.
Statistics
For behavioral/immunohistochemistry experiments, statistical analyses were performed with Student’s t-test, or by the Mann-Whitney rank sum test for non-parametric data sets - or by two-way RmANOVA, with post-hoc testing by the Holm-Sidak method. For the calcium imaging experiments, a repeated measures/within-animal design was used. Statistical analyses were performed by within subject t-tests, or Wilcoxon signed rank-test for non-parametric data, or by one-way RmANOVA, with post-hoc testing by the Holm-Sidak method. Significance was set at P < 0.05.
Results
PLZ does not change mechanical or heat sensitivity.
We first asked whether PLZ changes mechanical or heat sensitivity. To this end, we measured mechanical withdrawal thresholds and hotplate withdrawal latencies of VEH and PLZ pretreated animals. VF thresholds were assessed at baseline (prior to VEH/PLZ injection), and at T+1h and T+4h following VEH/PLZ injection. PLZ did not change VF threshold at either time point as compared to the VEH-treated group (FIG 1A; 2-way RmANOVA, effect of treatment NS; F(1,8,2,2)=0.499 (treatment), 0.581 (time), 0.276 (treatment x time), p≤0.500 (treatment)). For the hotplate assay, we examined responses in the baseline condition, and at T+3h post VEH/PLZ injection. Although hot plate latency was slightly reduced at the T+3h time point in both treatment groups, PLZ did not change the withdrawal latency at T+3h post-treatment as compared to the VEH control group. (FIG 1B; 2-way RmANOVA, effect of treatment NS; F(1,18,1,1)=2.516 (treatment), 3.723 (time), 0.125 (treatment x time), p≤0.130 (treatment))
Figure 1. Acute effects of PLZ treatment on basal mechanical and thermal nociceptive sensitivity.
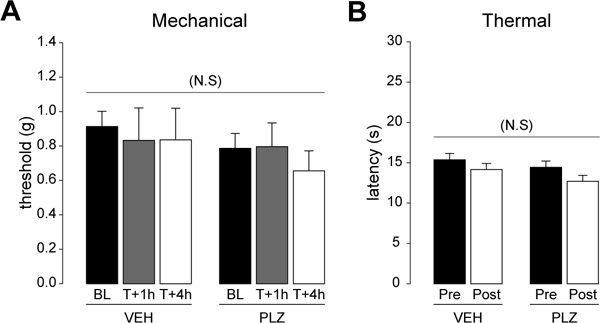
(A) Mechanical withdrawal thresholds to von Frey hairs before and after a single i.p, administration of VEH (n=5) or PLZ (n=5). (2-way RmANOVA, effect of treatment NS p=0.500) (B) Heat withdrawal latency (hotplate at 520C), time to first response (hindpaw flick/lift/lick), before and 3h after acute VEH (n=10) and PLZ (n=10) treatment. (2-way RmANOVA, effect of treatment NS p=0.130)
PLZ inhibits the second phase of responses to intraplantar formalin.
To determine whether PLZ could decrease nociceptive responses to an inflammatory stimulus, we examined responses to intraplantar injection of formalin. Mifflin et al. Mifflin et al. (2016) previously reported that PLZ did not change formalin-evoked nociceptive behaviors in female C57/BL6 mice; however, PLZ did produce a strong trend (~40% reduction) in the second phase (Mifflin et al., 2016). Here, we found that PLZ produced a statistically significant (~50%) reduction of nociceptive responses in the second phase, but not the first phase (phase one, t-test, NS, t(18)=1.065, p≤0.31, FIG 2A; phase two, t-test, t(18)=2.308, *p≤0.04, FIG 2B).
Figure 2. Acute effects of PLZ treatment on formalin-evoked nociceptive behavior.
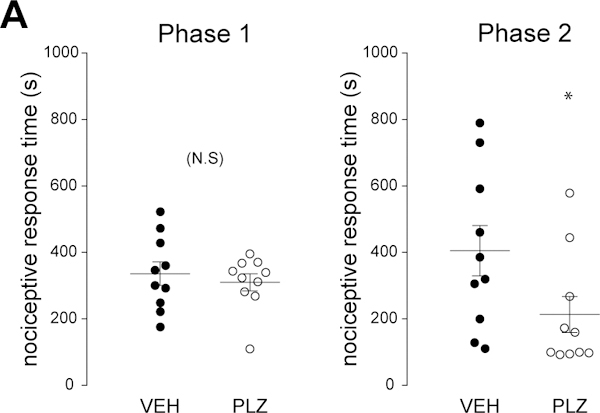
(A) Dot plot of total nociceptive response duration for phase 1 of the formalin response in VEH- (n=10) and PLZ- (n=10) treated animals (0–10 min post-formalin, t-test, p>0.05). Mean indicated by horizontal line. (B) Dot plot of total nociceptive response times for phase 2 (10–30 min post-formalin, t-test, *p=0.033) of the formalin response in acute VEH- and PLZ-treated animals. Mean indicated by horizontal line.
PLZ reduces Fos immunoreactivity in the superficial dorsal horn ipsilateral to intraplantar formalin.
c-Fos is a nuclear transcription factor that is commonly used as an immunohistochemical marker of neuronal activation (Gao and Ji, 2009). Noxious stimulation of the hindpaw with intraplantar formalin reliably evokes a strong c-Fos signal in the ipsilateral superficial dorsal horn of the lumbar SC (Abbadie et al., 1997, Intondi et al., 2010). To test the hypothesis that PLZ reduces spinal neuronal activation, we stained for Fos in the lumbar SCDH of the VEH/PLZ plus formalin-treated animals. As illustrated in FIG 3A, PLZ reduced the number of formalin-evoked Fos+ cells in laminae I-II (FIG 3B; t-test, t(8)=2.9533, *p≤0.02), but not in the deeper laminae III-VI (FIG 3C; Mann-Whitney rank sums test used as equal variance requirement was not met, NS, U(5, 5) = 5.000, p≤0.16).
Figure 3. Effect of PLZ treatment on formalin-evoked c-Fos in the dorsal horn.
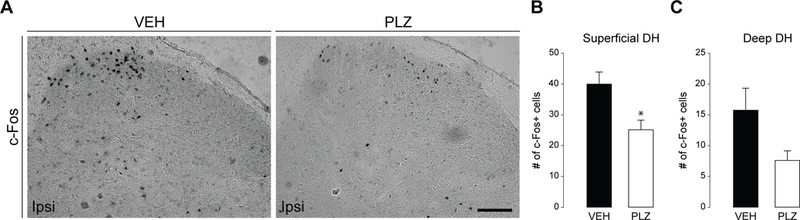
(A) Representative photomicrographs of Fos immunostaining in the ipsilateral lumbar dorsal horn in VEH- and PLZ-treated animals. Scale bar=100μm, applies throughout. (B) Quantification of the number of formalin-evoked Fos+ cells in the ipsilateral superficial (laminae I-II) dorsal horn in VEH (n=5) and PLZ (n=5) treated animals (t-test, *p=0.018). (C) Quantification of the number of Fos+ cells in the ipsilateral deep (laminae III-VI) dorsal horn in acute VEH and PLZ treated animals. (Mann-Whitney Rank Sums, NS p=0.151)
PLZ increases 5–HT+ immunoreactivity in the superficial dorsal horn.
PLZ increases the concentration of monoamine neurotransmitters (5-HT, NA, dopamine) in whole spinal cord of naïve and formalin-treated rodents (Griebel et al., 1998, Parent et al., 2000, Musgrave et al., 2011, Mifflin et al., 2016), but these studies did not localize changes to the dorsal and/or ventral horn. PLZ increased 5-HT+ immunoreactivity within the superficial (laminae I-II) SCDH compared to the VEH treated group (FIG 4A-C; t-test, t(8)=−2.888, *p≤0.02), but had no effect at the ventral horn (FIG 4D-F; t-test, NS, t(8)=−1.365, p≤0.21)
Figure 4. Effect of PLZ treatment on 5-HT+ immunoreactivity in the dorsal horn.
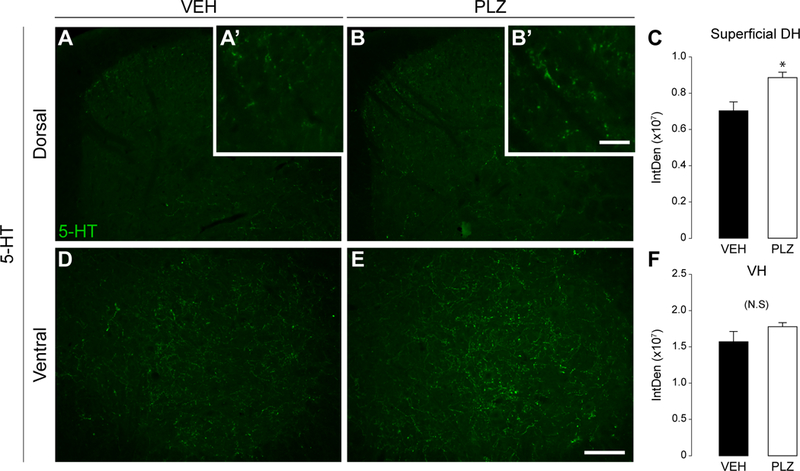
(A,B) Representative photomicrographs of 5-HT immunostaining in the lumbar dorsal horn in VEH (n=5) and PLZ (n=5) treated animals. Scale bar in E=100μm, applies in A-E. (A’,B’ inset) Higher magnification of superficial dorsal horn 5-HT staining. Scale bar=20μm. (C) Quantification (integrated density) of 5-HT+ immunoreactivity in the superficial (laminae I-II) of the bilaterally averaged dorsal horns (t-test, *p=0.020). (D,E) Representative photomicrographs of 5-HT immunostaining in the lumbar ventral horn (VH). (F) Quantification (integrated density) of 5-HT+ immunoreactivity in the (bilaterally averaged) ventral horn. (t-test, NS p=0.209)
PLZ dose-dependently inhibited glutamate-evoked calcium responses in ex vivo lumbar slices.
The preceding results indicate that PLZ decreases inflammatory nociception in vivo and cellular activation/neurotransmission in situ. We next tested the hypothesis that PLZ can inhibit spinal neuronal transmission in ex vivo spinal cord slices. As illustrated in FIG 5A,B, Fura 2-loaded lumbar slices were stimulated by bath application of glutamate at 0.3mM and 1.0mM both before and after superfusion with PLZ. We then compared the post-PLZ intracellular calcium response to glutamate with the pre-PLZ response. This within-subjects design allowed us to directly measure the inhibition produced by PLZ at each concentration in each individual. PLZ did not change the calcium responses to 0.3mM glutamate at any of the tested concentrations (FIG 5C,D). By contrast, PLZ dose-dependently inhibited calcium responses to 1mM glutamate (FIG 5E). This was significant at the 100 and 300μM concentrations of PLZ (FIG 5E; peak (Δ)F340/F380, paired t-test, within slice control, t(4)=3.834, *p≤0.02 at 100μM PLZ, t(4)=6.910, *p≤0.002 at 300μM PLZ). We also analyzed the post-PLZ response as a percent inhibition of the pre-PLZ response. The mean magnitude of inhibition of the peak response to 1mM glutamate was 24.8+/− 4.4% (100μM PLZ) to 36.5+/− 6.2% (300μM PLZ). (FIG 5F)
Figure 5. Dose-dependent inhibition of high-molar glutamate-evoked calcium responses in the dorsal horn by bath-applied PLZ.
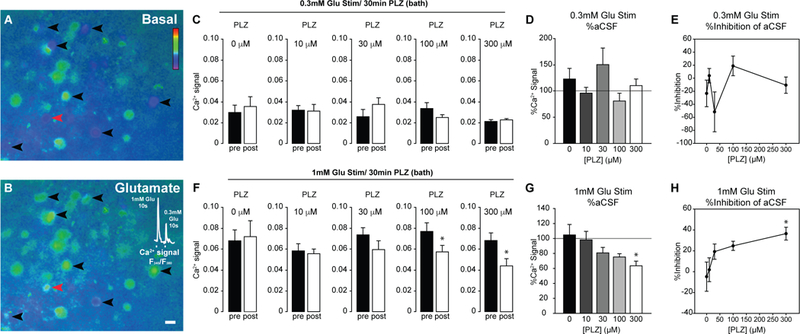
(A,B) Representative pseudocolor images depicting the F340/380 ratio in dorsal horn neurons in an ex vivo lumbar slice (A) before glutamate superfusion, and (B) during glutamate superfusion (1mM, 10s). Pseudocolor scale represents F340/380 ratio. Blue indicates lower, and green/red indicates higher relative Ca2+ levels. Black arrowheads indicate glutamate-responsive cells. Calcium traces from the cell indicated by the red arrowhead are shown in the inlay upon exposure to glutamate at 1 and then 0.3mM (10s each). Scale bar = 10μm. (C) Peak 0.3mM glutamate-evoked Ca2+ responses prior to bath application of PLZ (pre), and 30 min following superfusion of 0, 10, 30, 100, and 300μM concentrations of PLZ, (n=3–5/[PLZ]) (post). (D) Percent signal inhibition produced by PLZ at each tested concentration, using the 0.3mM glutamate stimulus. No significant inhibition was produced at any concentration of PLZ. (E) Peak glutamate-evoked Ca2+ response following 1mM glutamate stimulus prior to application of PLZ (pre) (at each concentration) and following 30 min PLZ superfusion (post). Paired t-test (within slice control) *p=0.019 at 100μM PLZ, *p=0.002 at 300μM PLZ. (F) Percent signal inhibition produced by PLZ at each tested concentration, using the 1.0mM glutamate stimulus. Asterisk denotes significant inhibition (in paired t-test on peak F340/380 post-PLZ vs. pre-PLZ).
Of note, a gradual upward drift in the baseline F340/380 ratio was observed over the course of the experiments in the slices treated with 300μM, but not 100μM, PLZ. To avoid nonspecific effects of the 300μM concentration, we used the lower concentration of PLZ – 200μM – in subsequent experiments.
PLZ inhibition of glutamate-evoked calcium responses is prevented with the 5HT1AR antagonist WAY-100,635, but not the adrenergic antagonist idazoxan.
After confirming that PLZ inhibits neuronal (SCDH) responses in isolated spinal cord slices, we next assessed its neurotransmitter/receptor mechanism. Previous studies including those from our own laboratory ((Mifflin et al., 2016)) indicate that 5-HT can act at the 5HT1A receptor (5HT1AR) to inhibit neuronal activity in the SCDH (Bardin et al., 2000, Bardin et al., 2001, Jeong et al., 2004, Abe et al., 2009, Xie et al., 2012), while NA can act through the alpha-2(A/C) receptor to inhibit SCDH neurons (Sonohata et al., 2004, Fairbanks et al., 2009). For these reasons, we tested the hypothesis that PLZ acts indirectly by enhancing 5-HT or NA transmission in the SCDH. To address this question, we pre-treated lumbar slices with the 5HT1AR antagonist WAY-100,635 (10μM), or the alpha-2 antagonist idazoxan (30μM), prior to and during superfusion with 200μM PLZ. Concentrations of antagonists are based on Kis and working concentrations reported in the literature (Bourgoin et al., 1993, Hirono et al., 2008, Abe et al., 2009). We used the 1mM concentration of glutamate because PLZ did not change the response to 0.3mM glutamate (FIG 5C, above). As illustrated in FIG 6A, 200μM PLZ for 30 min produced a ~20% inhibition of intracellular calcium response to 1mM glutamate (peak (Δ)F340/380, paired t-test, t(4)=3.297, *p≤0.030). No drift in baseline F340/380 was observed over the 30 min period of PLZ superfusion.
Figure 6. Pretreatment with the 5HT1AR antagonist WAY-100,635, but not the adrenergic antagonist idazoxan, blocks the inhibition of intracellular calcium responses in the SCDH by PLZ.
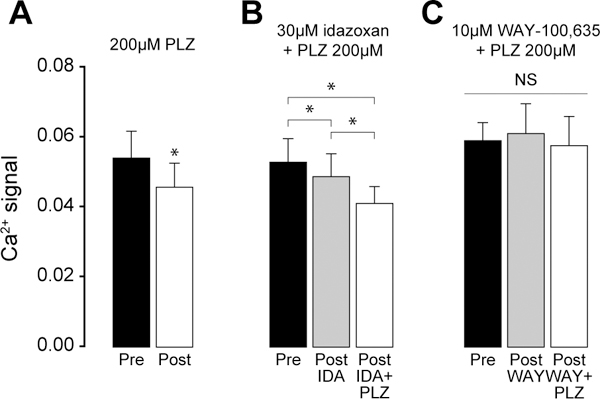
(A) Peak Ca2+ response following 1mM glutamate stimulus, prior to application of 200μM PLZ (pre) (n=5), and following 30 min PLZ superfusion (post). Paired t-test, within slice control, *p=0.030. (B) Peak Ca2+ response following 1mM glutamate stimulus, prior to application of 30μM idazoxan (pre), following 10 min 30μM idazoxan superfusion (post-IDA), and following 30 min application of 200μM PLZ + 30 μM idazoxan (post-IDA+PLZ) (n=4). One-way RmANOVA, *p=0.003, all pairwise post-hoc comparisons by Holm-Sidak, post-IDA vs. pre, *p<0.05, post-IDA+PLZ vs. post-IDA, *p<0.05, post-IDA+PLZ vs. pre, *p<0.05). (C) Peak Ca2+ response following 1mM glutamate stimulus, prior to application of 10μM WAY-100,635 (pre), following 10 min 10μM WAY-100,635 superfusion (post-WAY), and following 30 min application of 200μM PLZ + 10uM WAY-100,635 (post-WAY+PLZ) (n=3). One-way RmANOVA, NS p=0.675.
Idazoxan by itself slightly inhibited glutamate-evoked Ca2+ mobilization. Superfusion of with idazoxan with PLZ produced additional inhibition as compared to the response to idazoxan alone (FIG 6B; one-way RmANOVA, F(3,2)=17.486 between-treatments, *p≤0.003, all pairwise post-hoc comparisons by Holm-Sidak, post-idazoxan vs. pre, t=3.025, *p≤0.05, post-PLZ + idazoxan vs. post-idazoxan, t=2.888, *p≤0.05, post-PLZ + idazoxan vs. pre, t=5.913, *p≤0.05). Therefore, idazoxan did not prevent the expected inhibition of responses to the 1mM glutamate stimulus.
WAY-100,635 did not change glutamate-evoked Ca2+ mobilization. Importantly, co-superfusion of WAY-100,635 prevented PLZ-induced inhibition glutamate-evoked Ca2+ mobilization (one-way RmANOVA, NS, F(2,2)=0.435 between-treatments, p≤0.70). (FIG 6C)
Discussion
Despite promising early reports ((Emele et al., 1961), (Lee et al., 1983)), PLZ and other MAOIs have not been widely studied as potential treatments for pain. In this report, we have assessed the analgesic potential of PLZ in standard assays of acute nociception and in the formalin test, a model of tonic, chemogenic induced pain. Our results demonstrate that PLZ has no effect on basal sensitivity to acutely presented thermal or mechanical stimuli. We did observe however, a significant attenuation of second phase nocifensive behaviours in response to formalin in those mice pre-treated with PLZ. In support of these observations, we also report that high dose glutamate (1.0mM) evoked Ca2+ responses are attenuated in the presence of PLZ with little or no changes observed in responses to lower doses (0.3mM) of applied glutamate.
Recent reports by Potter et al. (Potter et al., 2016) and Mifflin et al. (Mifflin et al., 2016) also confirm that PLZ has antinociceptive and anti-allodynic properties. These reports did not, however, examine the effects of PLZ on neuronal function in the dorsal horn. Furthermore, while Mifflin et al. (Mifflin et al., 2016) observed a significant effect of PLZ in the second phase of the formalin assay in male mice, the effect in females did reach statistical significance. Our results also contrast earlier reports by Lee et al. (Lee et al., 1983) and Emele et al. (Emele et al., 1961). Lee et al. (Lee et al., 1983) examined the antinociceptive effects of PLZ directly compared with meperidine (an opioid) and amitryptaline (a TCA) in the tail flick assay. They found that PLZ produced both a stronger and longer lasting analgesia than either meperidine or amitryptaline. Emele et al. (Emele et al., 1961) compared PLZ with codeine in the hotplate assay, and in phenylquinone-induced writhing in female mice. They also found PLZ to be of equivalent efficacy and greater potency. The discrepancy between our results and those of Lee et al./Emele et al. may be due to the fact that different methods were used to assess heat sensitivity, along with the inherent differences in response profiles when stimuli are present to the tail or hind paws. Animal strain and/or sex differences amongst the different studies may also be a factor. A multitude of previous studies have revealed sex differences in rodents and humans in terms of basal pain sensitivity, functional and behavioral responses to tonic nociceptive stimuli, susceptibility to pathological pain conditions, and response to analgesics including opioids, antidepressants (SNRIs/SSRIs, TCAs, eg: (Zammataro et al., 2017)), anticonvulsants, and adrenergic agonists (Hurley and Adams, 2008). In mice, many of these sex differences are strain/genotype specific (DeLeo and Rutkowski, 2000, Mogil et al., 2000), and may also be specific to the pain modality/model being tested. As noted, Mifflin et al. (Mifflin et al., 2016) demonstrated that male and female C57/BL6 mice exhibit differential responses to PLZ and PLZ derivatives (PEH/N2-Ac-PLZ) in the formalin assay. As these drugs achieved significant elevations of spinal 5-HT/NA and/or GABA in both sexes, it was argued that differential responses to these drugs are likely due to differences in each gender’s functional utilization of the various affected neurotransmitters (ie. 5-HT, NA, DA, and GABA) for endogenous analgesia (antinociception).
Phenelzine inhibits spinal nociceptive neuronal activation
Phenelzine can target spinal and supraspinal sites to exert its analgesic effects. To test spinal sites of action, we evaluated its effects on formalin-induced Fos expression in situ, and glutamate-evoked Ca2+ mobilization ex vivo. We found that PLZ significantly reduced formalin-evoked Fos expression in the superficial laminae of the ipsilateral SCDH. Although the effect of PLZ on Fos expression in the deeper laminae was not statistically significant, we cannot rule out the possibility that this may be a consequence of small sample size and data that was not normally distributed. We conclude that PLZ has a net inhibitory action on neurons within the central nociceptive circuitry of the superficial SCDH. The precise cellular/circuit mechanisms of PLZ cannot be revealed by c-Fos IHC, since Fos-positive interneurons can be either excitatory (i.e glutamatergic) or inhibitory (ie. GABAergic). Nevertheless, the simplest explanation for our results is that PLZ directly inhibited the activation of pronociceptive excitatory neurons in the superficial SCDH.
Using calcium imaging in ex vivo lumbar slices, we studied the effects of PLZ in real time. We found that the 100μM and 300μM concentrations of PLZ inhibited Ca2+ responses evoked by the stronger 1.0mM concentration of glutamate, but not the weaker 0.3mM concentration. Mechanistically, this ‘thresholded’ action raises the possibility that PLZ’s inhibitory effects emerge with the higher (1mM) concentration of glutamate because it is able to engage specific neural circuits and mechanisms within the SCDH that are not activated by the lower glutamate concentration. Cellular mechanisms which may be differentially activated by the stronger concentration of glutamate include the opening of voltage-gated calcium channels, the opening of glutamatergic NMDA receptor (NMDAR) channels, and/or the engagement of polysynaptic excitatory/inhibitory circuits. Central mechanisms, including central glutamate release and the activation of post-synaptic NMDARs in the SCDH, are known to contribute to the 2nd phase of the response to formalin (Coderre and Melzack, 1992b, a). We conclude that PLZ acts to inhibit glutamate-evoked activity in nociceptive neurons in the SCDH. This spinal mechanism likely contributes to the analgesic effects of PLZ.
Monoamine receptors contribute to phenelzine-induced inhibition of spinal nociceptive neuronal activation
Monoaminergic neurons originating in the brainstem project to the SCDH and modulate spinal nociceptive activity (Millan, 2002). These circuits are important for the ‘top-down’ inhibition of pain by higher-level functions, such as attention, emotion, arousal etc. (Millan, 2002, Ossipov et al., 2010). Both 5-HT and NA contribute to the descending modulation of nociception. Activation of specific monoaminergic receptors in the SCDH mediate pain inhibition, while others mediate pain facilitation (Millan, 2002). Certain monoamine receptors are expressed on both inhibitory and excitatory neurons in the SCDH, and so may have functionally opposite effects depending on the specific neuronal circuits engaged (Millan, 2002). The 5HT1A and the alpha 2 adrenergic receptors are associated with antinociception (Millan, 2002).
Serotonin
Using high performance liquid chromatography (HPLC), Mifflin et al. reported that PLZ increases the concentration of monoamine neurotransmitters in the whole spinal cord of formalin treated mice [16]. HPLC is a reliable, sensitive, and fully quantitative method, but lacks the degree of spatial/anatomical specificity that can be achieved with IHC. In the current study, we extend the findings of Mifflin et al. (Mifflin et al., 2016) by determining (with IHC) that 5-HT is specifically elevated in the SCDH following treatment with PLZ.
5HT1A receptor
The 5HT1A receptor is an inhibitory metabotropic G protein coupled receptor which couples intracellularly through Gi/o (Polter and Li, 2010). It is expressed on peripheral afferent terminals in the SCDH (Garraway and Hochman, 2001), as well as on interneurons (Lu and Perl, 2007, Jeong et al., 2012, Xie et al., 2012). The high affinity 5HT1A agonists F-13640 (Buritova et al., 2005), and 8-OH-DPAT (Bardin et al., 2001), have previously been found to inhibit responses in the formalin assay. Mifflin et al. (Mifflin et al., 2016) found that 5HT1A receptor blockade with WAY-100,635 prevented the antinociceptive effects of the PLZ derivative N2-Ac-PLZ in the formalin assay. In the current study, we found that blocking the 5HT1A receptor with WAY-100,635 prevented the inhibitory effects of PLZ in ex vivo lumbar slices. This result suggests that the inhibition produced by PLZ in this paradigm is 5HT1AR-dependent. Although we did not quantify 5-HT levels in the isolated spinal cord slices, we believe a mechanism involving MAO inhibition and subsequent elevation of 5-HT provides a plausible explanation for the effects of PLZ in slice experiments. Apart from the antagonist study presented here, previous studies which quantified the release of [3H]5-HT in superfused transverse spinal cord slice preparations, demonstrated that the terminals of descending serotonergic neurons (which originate in the brain stem) remain functionally intact, and continue to basally release 5-HT in a synaptic/calcium dependent manner (Bineau-Thurotte et al., 1984, Iverfeldt et al., 1989, Franck et al., 1993). Enhanced 5-HT release can also be evoked in these preparations by electrical stimulation or by increasing extracellular [K+]. 5-HT from non synaptic sources (ie. blood) or non synaptic release (ie. membrane disruption) may also be present in isolated spinal cord sections. Generally, in these 5-HT release studies, a micromolar dose of a monoamine oxidase inhibitor (such as pargyline) was required in the superfusate in order to obtain detectable increases in evoked 5-HT release. At the micromolar concentrations used in this study, 30 min. of direct PLZ superfusion should be sufficient to produce extensive MAO inhibition, and elevate levels of 5-HT at the synapse. Therefore, when viewed together, the current study and the experiments by Mifflin et al. (Mifflin et al., 2016) point to a 5-HT/5HT1A receptor-dependent mechanism which contributes to the antinociceptive effects of PLZ. Additional in vivo formalin experiments mirroring those conducted with male mice in Mifflin et al. (ie. involving pretreatment with WAY-100,635 or other monoamine receptor antagonists, prior to PLZ and/or PLZ derivatives) could confirm the role of 5HT/5HT1A in the antinociceptive effect of PLZ in females.
Alpha 2 adrenergic receptor
The alpha 2 adrenergic receptor is also an inhibitory GPCR, which frequently couples through Gi (Lomasney et al., 1991). Three subtypes of the alpha 2 receptor (alpha 2A-C) exist, and are differentially expressed in various CNS regions (Lomasney et al., 1991, Millan, 2002). Like the 5HT1AR, alpha 2 receptors are found on the central terminals of peripheral afferent fibers, and on interneurons in the SCDH (Millan, 2002, Lu and Perl, 2007). The high affinity alpha 2 agonist clonidine has been found to have antinociceptive effects in a variety of assays (Millan, 2002, Pertovaara, 2006), including the formalin test (Kanui et al., 1993). However, Mifflin et al. (Mifflin et al., 2016) found that blocking the alpha 2 receptor with idazoxan did not prevent the antinociceptive effects of N2-Ac-PLZ in the formalin assay in male mice. Similarly, in the current study we find that blocking the alpha 2 receptor with idazoxan did not prevent the inhibitory effects of PLZ in ex vivo lumbar slices. Together, these studies indicate the effects on noradrenaline, mediated through the alpha 2 receptor, are not critical to the observed antinociceptive effects of PLZ.
Summary
In addition to its actions on 5HT/5HT1A pathways, PLZ may exert effects on GABA, other monoamine receptors (ie. adrenergic, dopaminergic), imidazoline receptors, glutamate, and ROS. Each of these may also be relevant to the antinociceptive effects of PLZ, and to the inhibitory actions of PLZ in the spinal dorsal horn. However, our evidence suggests the antinociceptive properties of PLZ are likely mediated by a net 5HT1Aergic inhibition of neuronal activity within central (SCDH) nociceptive networks. Despite the need for further studies designed to elucidate the exact mechanism(s) of PLZ analgesic action, we conclude that PLZ possesses antinociceptive properties which may be of clinical/experimental value.
Acknowledgements:
Funding for this project was provided by operating grants from the Canadian Institutes of Health Research (CIHR) MOP-119338 and MOP-86712, the University of Alberta, Pfizer Canada, and the MS Society of Canada (MSSC). LP was supported by a studentship from the MSSC
List of Abbreviations:
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT1AR
5-HT1A receptor
- aCFS
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- CNS
central nervous system
- DA
dopamine
- EAE
experimental autoimmune encephalomyelitis
- GABA
gamma-aminobutyric acid
- GABA-T
GABA-transaminase
- IDA
idazoxan
- IHC
immunohistochemistry
- I.P.
intraperitoneal
- MAO
monoamine oxidase
- MAOI
monoamine oxidase inhibitor
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NA
noradrenaline
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PEH
phenylethylidenehydrazine
- PFA
paraformaldehyde
- PLZ
phenelzine
- RmANOVA
repeated-measures ANOVA
- ROI
region of interest
- ROS
reactive oxygen species
- RT
room temperature
- S.C.
subcutaneous
- S1
primary somatosensory cortex
- SC
spinal cord
- SCDH
dorsal horn of the spinal cord
- SNRI
selective norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
- VEH
vehicle
- VF/VFH
von Frey (hair)
- WAY
WAY-100,635
Footnotes
Conflict of Interests: The authors declare no competing financial interests
References
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI (1997) Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain 69:101–110. [DOI] [PubMed] [Google Scholar]
- Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M (2009) Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience 159:316–324. [DOI] [PubMed] [Google Scholar]
- Bardin L, Lavarenne J, Eschalier A (2000) Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain 86:11–18. [DOI] [PubMed] [Google Scholar]
- Bardin L, Tarayre J-P, Koek W, Colpaert FC (2001) In the formalin model of tonic nociceptive pain, 8-OH-DPAT produces 5-HT1A receptor-mediated, behaviorally specific analgesia.Eur J Pharmacol 421:109–114. [DOI] [PubMed] [Google Scholar]
- Bineau-Thurotte M, Godefroy F, Weil-Fugazza J, Besson JM (1984) The effect of morphine on the potassium evoked release of tritiated 5-hydroxytryptamine from spinal cord slices in the rat. Brain Res 291:293–299. [DOI] [PubMed] [Google Scholar]
- Bourgoin S, Pohl M, Mauborgne A, Benoliel JJ, Collin E, Hamon M, Cesselin F (1993) Monoaminergic control of the release of calcitonin gene-related peptide- and substance P-like materials from rat spinal cord slices. Neuropharmacology 32:633–640. [DOI] [PubMed] [Google Scholar]
- Buritova J, Larrue S, Aliaga M, Besson J-M, Colpaert F (2005) Effects of the high-efficacy 5-HT1A receptor agonist, F 13640 in the formalin pain model: A c-Fos study. Eur J Pharmacol 514:121–130. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R (1992a) The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci 12:3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R (1992b) The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci 12:3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD (2000) Gender differences in rat neuropathic pain sensitivity is dependent on strain. Neurosci Lett 282:197–199. [DOI] [PubMed] [Google Scholar]
- Doolen S, Blake CB, Smith BN, Taylor BK (2012) Peripheral nerve injury increases glutamate-evoked calcium mobilization in adult spinal cord neurons. Mol Pain 8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A (2010) Nociceptors: the sensors of the pain pathway. J Clin Invest 120:3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emele JF, Shanaman J, Warren MR (1961) The Analgesic Activity Of Phenelzine And Other Compounds. J Pharmacol Exp Ther 134:206–209. [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Wilcox GL (2009) Pharmacological Profiles of Alpha 2 Adrenergic Receptor Agonists Identified Using Genetically Altered Mice and Isobolographic Analysis. Pharmacol Therapeut 123:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Swartz KL (2004) The Role of Monoamine Oxidase Inhibitors in Current Psychiatric Practice.J Psychiatr Pract 10:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck J, Brodin E, Fried G (1993) Differential release of endogenous 5-hydroxytryptamine, substance P, and neurokinin A from rat ventral spinal cord in response to electrical stimulation. J Neurochem 61:704–711. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR (2009) c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? The open pain journal 2:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway SM, Hochman S (2001) Pharmacological characterization of serotonin receptor subtypes modulating primary afferent input to deep dorsal horn neurons in the neonatal rat. Br J Pharmacol 132:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Curet O, Perrault G, Sanger DJ (1998) Behavioral effects of phenelzine in an experimental model for screening anxiolytic and anti-panic drugs: correlation with changes in monoamine–oxidase activity and monoamine levels. Neuropharmacology 37:927–935. [DOI] [PubMed] [Google Scholar]
- Hirono M, Matsunaga W, Chimura T, Obata K (2008) Developmental enhancement of α2-adrenoceptor-mediated suppression of inhibitory synaptic transmission onto mouse cerebellar Purkinje cells. Neuroscience 156:143–154. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Adams MCB (2008) Sex, Gender, and Pain: An Overview of a Complex Field. Anesth Analg 107:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intondi AB, Zadina JE, Zhang X, Taylor BK (2010) Topography and time course of changes in spinal neuropeptide Y immunoreactivity after spared nerve injury. Neuroscience 165:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverfeldt K, Serfozo P, Diaz Arnesto L, Bartfai T (1989) Differential release of coexisting neurotransmitters: frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H]serotonin from tissue slices of rat ventral spinal cord. Acta Physiol Scand 137:63–71. [DOI] [PubMed] [Google Scholar]
- Jeong CY, Choi JI, Yoon MH (2004) Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. Eur J Pharmacol 502:205–211. [DOI] [PubMed] [Google Scholar]
- Jeong H-J, Mitchell VA, Vaughan CW (2012) Role of 5-HT(1) receptor subtypes in the modulation of pain and synaptic transmission in rat spinal superficial dorsal horn. Br J Pharmacol 165:1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanui TI, Tjølsen A, Lund A, Mjellem-Joly N, Hole K (1993) Antinociceptive effects of intrathecal administration of α-adrenoceptor antagonists and clonidine in the formalin test in the mouse. Neuropharmacology 32:367–371. [DOI] [PubMed] [Google Scholar]
- Lee I, Chalon J, Ramanathan S, Gross S, Turndorf H (1983) Analgesic properties of meperidine, amitriptyline and phenelzine in mice. Can Anaesth Soc J 30:501–505. [DOI] [PubMed] [Google Scholar]
- Lomasney JW, Cotecchia S, Lefkowitz RJ, Caron MG (1991) Molecular biology of α-adrenergic receptors: implications for receptor classification and for structure-function relationships. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1095:127–139. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER (2007) Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol 582:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie EM, Song M-S, Dursun SM, Tomlinson S, Todd KG, Baker GB (2010) Phenelzine: An Old Drug That May Hold Clues to The Development of New Neuroprotective Agents. Klinik Psikofarmakoloji Bülteni-Bulletin of Clinical Psychopharmacology 20:179–186. [Google Scholar]
- McManus DJ, Baker GB, Martin IL, Greenshaw AJ, McKenna KF (1992) Effects of the antidepressant/antipanic drug phenelzine on GABA concentrations and GABA-transaminase activity in rat brain. Biochem Pharmacol 43:2486–2489. [DOI] [PubMed] [Google Scholar]
- Michael-Titus AT, Bains S, Jeetle J, Whelpton R (2000) Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex--a possible mechanism of neuroprotection in major depression? Neuroscience 100:681–684. [DOI] [PubMed] [Google Scholar]
- Mifflin KA, Benson C, Thorburn KC, Baker GB, Kerr BJ (2016) Manipulation of Neurotransmitter Levels Has Differential Effects on Formalin-Evoked Nociceptive Behavior in Male and Female Mice. J Pain 17:483–498. [DOI] [PubMed] [Google Scholar]
- Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF (2000) Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav R 24:375–389. [DOI] [PubMed] [Google Scholar]
- Musgrave T, Benson C, Wong G, Browne I, Tenorio G, Rauw G, Baker GB, Kerr BJ (2011) The MAO inhibitor phenelzine improves functional outcomes in mice with experimental autoimmune encephalomyelitis (EAE). Brain Behav Immun 25:1677–1688. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120:3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MB, Habib MK, Baker GB (2000) Time-dependent changes in brain monoamine oxidase activity and in brain levels of monoamines and amino acids following acute administration of the antidepressant/antipanic drug phenelzine. Biochem Pharmacol 59:1253–1263. [DOI] [PubMed] [Google Scholar]
- Parent MB, Master S, Kashlub S, Baker GB (2002) Effects of the antidepressant/antipanic drug phenelzine and its putative metabolite phenylethylidenehydrazine on extracellular gamma-aminobutyric acid levels in the striatum. Biochem Pharmacol 63:57–64. [DOI] [PubMed] [Google Scholar]
- Paslawski T, Treit D, Baker GB, George M, Coutts RT (1996) The antidepressant drug phenelzine produces antianxiety effects in the plus-maze and increases in rat brain GABA. Psychopharmacology 127:19–24. [DOI] [PubMed] [Google Scholar]
- Pertovaara A (2006) Noradrenergic pain modulation. Prog Neurobiol 80:53–83. [DOI] [PubMed] [Google Scholar]
- Polter AM, Li X (2010) 5-HT1A Receptor-Regulated Signal Transduction Pathways in Brain. Cell Signal 22:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LE, Paylor JW, Suh JS, Tenorio G, Caliaperumal J, Colbourne F, Baker G, Winship I, Kerr BJ (2016) Altered excitatory-inhibitory balance within somatosensory cortex is associated with enhanced plasticity and pain sensitivity in a mouse model of multiple sclerosis. J Neuroinflamm 13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM (2012) Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci 15:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH (2007) Dietary restrictions and drug interactions with monoamine oxidase inhibitors: the state of the art. J Clin Psychiat 68 Suppl 8:42–46. [PubMed] [Google Scholar]
- Shulman KI, Fischer HD, Herrmann N, Huo CY, Anderson GM, Rochon PA (2009) Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiat 70:1681–1686. [DOI] [PubMed] [Google Scholar]
- Song M-S, Baker GB, Dursun SM, Todd KG (2010) The antidepressant phenelzine protects neurons and astrocytes against formaldehyde-induced toxicity. J Neurochem 114:1405–1413. [DOI] [PubMed] [Google Scholar]
- Song M-S, Matveychuk D, MacKenzie EM, Duchcherer M, Mousseau DD, Baker GB (2013) An update on amine oxidase inhibitors: Multifaceted drugs. Prog Neuro-Psychoph 44:118–124. [DOI] [PubMed] [Google Scholar]
- Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M (2004) Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol 555:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DJ, Uta D, Feng PY, Wakita M, Shin MC, Furue H, Yoshimura M (2012) Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain 8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammataro M, Merlo S, Barresi M, Parenti C, Hu H, Sortino MA, Chiechio S (2017) Chronic Treatment with Fluoxetine Induces Sex-Dependent Analgesic Effects and Modulates HDAC2 and mGlu2 Expression in Female Mice. Front Pharmacol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


