Abstract
Chronic cigarette smoking may influence chemosensory function, which in turn, may affect cigarette usage. Because menthol in cigarettes can attenuate nicotine bitterness, choice of menthol/nonmenthol cigarettes may be influenced by ability to perceive bitterness. We examined chemosensory function of chronic smokers, hypothesizing they would show altered function in comparison to non-smokers and by menthol cigarette preference. In laboratory-based measures, chronic smokers (N=135; 84 menthol smokers) self-reported their chemosensory function and participated in smell (identification task with perceived intensity) and taste (quinine and NaCl intensity on tongue-tip and whole mouth) testing. A taste genetics probe (propylthiouracil (PROP) bitterness) also was assessed. Self-reported and measured chemosensory function were compared with nationally-representative 2013-2014 National Health and Nutrition Examination Survey (NHANES) data generated with similar measures. The taste measures also were compared between smokers and age- and sex-matched non-smokers from a laboratory database. Frequencies of self-reported smell and taste alterations among smokers exceeded NHANES prevalence estimates for non-smokers. The rate of measured smell dysfunction also exceeded NHANES prevalence for hyposmia. Compared to non-smokers, smokers reported elevated tongue-tip and whole mouth intensities from 1 M NaCl, with no significant differences in whole mouth quinine or 0.32M NaCl. Inconsistent with previous hypotheses, smokers were not more likely to report depressed PROP bitterness than non-smokers. However, as expected, menthol smokers reported greater PROP bitterness than non-menthol smokers. In conclusion, chemosensory alterations were more frequent among chronic smokers, including hyposmia and heightened intensity from NaCl at an oral-irritant concentration. PROP supertasters were most likely to prefer mentholated cigarettes.
Keywords: hyposmia, oral irritation, smell, taste, tobacco, taste genetics, cigarettes, smoking
1. Introduction
Cigarette smoking is a leading cause of death worldwide. In the United States, 37.8 million adults (15.5%) were considered current cigarette smokers in 2016 [1]. Of broad interest is how chronic smoking influences chemosensory function, which in turn, could impact other health behaviors and smoking cessation efforts. Choice of cigarette flavor, between menthol and non-menthol, also is of interest from a health perspective [2] and has been associated with the TAS2R38 taste receptor genotype [3]. The addition of menthol to cigarettes increases the palatability and appeal of cigarettes by masking the bitterness of tobacco smoke, and by dampening the harshness.
Preference for menthol cigarettes also may be influenced by variation in ability to perceive flavors and taste. Individuals with heightened ability to experience bitterness and irritation from nicotine may prefer mentholated tobacco products to help mask these unpleasant sensations. Conversely, long-term cigarette smoking also may influence chemosensory function and, in turn, the palatability of tobacco products and the success of cessation efforts. However, the effects of smoking on chemosensory function are unclear [4]. Therefore, in this study, we sought to investigate smell and taste function among adults who are current chronic smokers, compare them with non-smokers, and to compare chemosensory function between menthol vs. non-menthol smokers.
Cigarette smoking can alter olfactory processes directly or indirectly. Direct exposure to aqueous cigarette smoke diminishes functional olfactory receptors in animal models [5] and overwhelms the capacity of the olfactory epithelium to regenerate with advancing age [6]. Indirectly, chronic cigarette exposure could increase susceptibility to factors reported to impair olfactory function, including frequent nasal infections, allergies [7], cranial/facial injury [8], and viral infections/tonsillectomy [9]. Smokers appear more susceptible to viral respiratory infections [10, 11], acute and chronic rhinitis, nasal inflammation [12, 13] and traumatic brain injury [14]. In small clinical studies, smokers require higher concentration to detect an odor [15-17]. A dose-response relationship between chronic smoking and diminished odor identification ability has been reported in a community-based study [18].
Population-based studies extend these smaller studies by statistically controlling for demographic factors that can influence the association between smoking and olfactory function, which is usually assessed by odor identification task. The range of measured olfactory dysfunction in population-based studies ranges from as low as 3.8% [19, 20] in the Beaver Dam Offspring Study, to 12.4% in the National Health and Nutrition Examination Survey (NHANES) [9], and up to 19.1% in the Skövde study [21]. Increased rates of olfactory dysfunction are seen in older adults [9, 22, 23], males [23-28], certain ethnic/racial minorities [9, 23] and those with lower income/educational attainment [9, 19].
The association between smoking and olfactory dysfunction in population-based studies is not clear-cut. A population-based sampling from a German city found greater odds of measured olfactory dysfunction among smokers, with greater odds among heavier smokers [29]. The Epidemiology of Hearing Loss Study reported greater odds of olfactory dysfunction among smokers in cross-sectional analyses [22], yet smokers were not more likely to develop olfactory dysfunction over 5-years in the follow-up study [30]. Likewise, the Skövde population-based study failed to find significant associations between odor identification ability and cigarette smoking [21]. Lower risk of olfactory dysfunction and smoking was reported in a large Spanish study with a home-administered odor identification test [31] as well as in the 2012 US National Health and Nutrition Examination Survey (NHANES). The inconsistent association between smoking and olfactory dysfunction may have resulted from defining smoking status using just a single item, which can yield unreliable results.
Similarly, inconsistent findings emerge in studies of self-reported dysfunction. For example, analysis of the 2009 Korean NHANES showed no association between smoking status and a single question about problems with the sense of smell during the previous three months [32]. However, asking a series of questions about olfactory function can improve the self-recognition of olfactory dysfunction [33], which had previously been reported as poor [34, 35]. Analysis of the US NHANES 2012-2014 identified greater odds of self-reported olfactory alteration, defined by a series of questions, among smokers with part of this association explained by risk factors for olfactory dysfunction [36].
Compared with olfactory dysfunction, dysfunction of whole mouth taste perception is less common [37]. This may be partially explained by the redundancy of the three taste-related nerves (cranial nerves VII (facial nerve), IX (glossopharyngeal nerve) and X (vagus nerve)) that carry sensory information from the periphery to the central nervous system. More common is regional taste dysfunction from an area of taste nerve innervation, which in turn, can alter whole mouth taste perception, touch/tactile and pain perception, resulting in dysgeusia and phantom oral pain sensations [38]. One example is viral-related damage of taste from the chorda tympani nerve, depressing taste intensity on the tongue tip. Following the release of inhibition model, loss of taste from the chorda tympani could result in increased touch and pain sensations on the tongue tip, depressed retronasal flavor sensations, and increased whole mouth taste sensations [38]. Damage to multiple taste-related nerves could depress whole mouth taste function, however. Major causes of taste dysfunction include xerostomia (persistent dry mouth), cranial/facial injuries, upper respiratory and middle ear infections, surgeries to the ear, nose or throat, and aging [38]. The prevalence of reported altered taste and retronasal olfactory sensations (recent problems occurring within the last year, loss in taste or flavor with age, or dysgeusia) in adults ≥40 years and older in this NHANES 2011-2012 sample was 19% [39].
Cigarette smoking also may alter taste processes directly or indirectly. Long-term exposure to nicotine in rats decreases fungiform papillae size and changes their anatomical characteristics [40], which could explain lower density of tongue-tip taste papillae (fungiform papillae) [41] or difference in taste bud morphology and vascularization in fungiform papillae [42] among chronic smokers. Smokers have been found to have lower resting salivary flow rates and more frequent reports of xerostomia than non-smokers [43, 44], as well as greater rates of respiratory infections [10, 11]. However, as with olfaction, inconsistent associations have been observed between taste function and cigarette smoking. In comparison to non-smokers, smokers have no significant differences in aqueous taste thresholds [45-47], or only elevated threshold (less sensitivity) to quinine [48], sucrose [49], sodium chloride [50], or elevated thresholds by filter paper method [51] and electrogustometry [42, 52-54] of loci on the tongue tip, foliate papillae and soft palate. Smoking cessation from 2 to 9 weeks was associated with improvements in taste threshold by electrogustometry [54]. Less is understood about smoking associations with suprathreshold taste function, including on regional areas of the tongue. No effects of smoking have been reported on the ability to correctly identify concentrated sweet, salty, sour and bitter sensations [29]. Smoking coupled with obesity has been associated with decreased perceived intensity of sweetness as well as creaminess of dessert-type sugar/fat mixtures independent of olfactory cues [55]. Smoking cessation appear to lead to increases in perceived bitterness [56].
Although nicotine stimulates complex taste, olfactory, and somatosensory sensations [57], variation in bitter taste in particular has been linked to differences in cigarette smoking behaviors. Nicotine stimulates bitter taste through TRPM5-dependent and independent mechanisms [58]. Most studies report intensified oral sensations among phenylthiocarbamide (PTC) and propylthiouracil (PROP) tasters [59], including elevated taste [60] and touch [61] sensations, elevated taste [51] or taste/touch [52] thresholds, and greater risk of oral pain [62]. Research from the 1960s reported that smokers and non-smokers did not differ significantly in PTC/PROP thresholds [48], or that smokers were more likely to be nontasters [63, 64]. More recent studies of bitter taste variation in humans have shown that nontasters of bitterness are more likely to be smokers [65], show greater cigarette dependence [66], and less nicotine aversion [56]. The ability to taste PTC/PROP bitterness relates to polymorphisms in the TAS2R38 receptor gene [67, 68]. Population-based testing indicates that PTC tasters (assessed by taste-strip test or TAS2R38 genotype) may be protected from smoking cigarettes in some racial/ethnic groups [69], whereas non-tasters demonstrate greater motivation to smoke based on sensory cues than do tasters [70]. It should be noted, however, that the association between PTC/PROP bitterness [71] or TAS2R38 genotype and smoking is not always seen [72], and that these effects may instead be race-/ethnic-specific [73]. Menthol in cigarettes blocks some of the negative oral sensations from cigarettes [74, 75], presumably producing a palatable cigarette even to PTC/PROP tasters. Female PTC/PROP tasters identified by TAS238 receptor genotype, for example, were more likely to smoke menthol cigarettes [3].
The purpose of this laboratory-based study was to examine suprathreshold chemosensory function among a group of chronic smokers, including odor identification, perceived odor intensity, and perceived taste intensity with the whole mouth and regionally. The chronic smokers were characterized for cigarette exposure and degree of nicotine dependence with standardized assessment tools. In a case-control design, a primary comparison group of non-smokers was from the 2013-2014 cycle of the US National Health and Nutrition Examination Survey (NHANES), a nationally-representative sample of the U.S. population. A secondary group of non-smokers was from a laboratory-based sample who were age and sex-matched to the chronic smokers for comparison of taste function and the bitterness of PROP. Additionally, we were interested in whether smell and taste function vary between menthol and non-menthol smokers. It was hypothesized that chronic cigarette users would show decrements in smell and taste compared to non-smokers. It was also expected that those demonstrating PROP supertaster status would prefer menthol cigarettes to non-menthol cigarettes.
2. Material and methods
2.1. Subjects
The study was approved by the institutional review board at the UConn Health, where the study took place. The analytic sample of 135 adults (65 males) was obtained from the baseline data of a study on the effects of nicotine and flavorings on the use of electronic cigarettes in regular smokers. Participants were adults from the greater Hartford, Connecticut area, ages 18 to 55 years, who responded to newspaper and radio advertisements between May 2014 and December 2016. A telephone screening protocol determined whether the potential participants met the inclusion and exclusion criteria for initial eligibility. The criteria for inclusion were 1) current use of at least ten cigarettes daily; 2) willing to abstain from cigarette smoking, and to substitute with e-cigarettes, for six weeks; and 3) literacy levels sufficient for reading and signing a consent form in the English language. The criteria for exclusion were: 1) unstable medical or psychiatric disorders, including uncontrolled hypertension (blood pressure>160/100 mm Hg); 2) pregnancy; 3) known hypersensitivity to nicotine or to propylene glycol; 4) previous myocardial infarction(s) or cerebrovascular accident(s); 5) insulin-dependent diabetes; and 6) known chronic obstructive pulmonary disease or asthma. During the initial screening process participants completed the Smoking History Questionnaire (SHQ [76]), a self-report questionnaire used to assess smoking history and patterns. The SHQ includes items pertaining to smoking products, brands preferred/used, and smoking frequency. Based on responses regarding smoking products and brands used, participants were classified as non-menthol or menthol smokers. Participants provided informed and written consent and were compensated $20 for participation in the baseline assessments.
2.2. Study Procedures and Measures
Those persons who met the inclusion/exclusion criteria were invited to participate in the baseline measurements conducted from 10:00AM – 2:00PM at the UConn Health Clinical Research Center. All subjects were asked to refrain from cigarette smoking for at least 3 hours prior to the scheduled time of visit. After the consent process, the participants underwent a brief physical exam, a breath test was conducted to measure exhaled carbon monoxide (CO) (Bedfont Micro+™ Smokerlyzer handheld CO monitor: Harrietsham, Kent, England), and completed smoking-related questionnaires. CO levels are elevated in smokers as well as with recent smoking and a CO breath test with a cut-off value ≤12 ppm can identify smokers who have abstained from smoking for 8 hours or since the previous evening [77]. To characterize nicotine dependence, participants completed the Fagerstrom Test of Nicotine Dependence [78], including time to first cigarette [79] and the Wisconsin Inventory of Smoking Dependence Motives [80]. Because alcohol consumption and cigarette smoking behaviors are associated, smokers were briefly assessed for alcohol consumption by the Alcohol Timeline Followback (TLFB–Alcohol) [81]. Heavy drinking was defined as drinking over the preceding 3 months ≥8 drinks or more per week for females and ≥15 drinks per week for males [82]. Binge drinking was ≥4 drinks during a single occasion for females and ≥6 drinks for males according to the TLFB–Alcohol [81], consistent with the National Institute on Alcohol Use and Abuse definition and US Dietary Guidelines [82].
Following these initial assessment procedures, the chemosensory assessments were carried out.
2.2.1. Self-reported chemosensory function and related risk factors.
Participants first self-reported smell and taste function using a questionnaire similar to that used in the NHANES protocol [39, 83]. Questionnaire items assessed information about smell and taste problems within the past year, sensory losses since age 25 years old, and phantom smell or taste sensations (phantosmia/parosmia, dysgeusia). Consistent with scoring in NHANES [39], Smell Alteration and Taste Alteration were scored 0 if none of these decrements was reported and scored 1 if any of these was reported. In a departure from the NHANES protocol, chronic smokers were asked, beyond the yes/no problem in the past year, to describe their sense of smell or taste as excellent, good, a little trouble, moderate trouble, a lot of trouble, lost sense of smell, refused and don’t know. Thus, a smoker could respond no to a smell problem but also report troubles on the next question. We report this additional description in the text and use the total self-reported alteration in calculation of sensitivity and specificity to the measured olfactory function.
Participants also self-reported risk factors in their health history previously known to be associated with chemosensory alterations, including history of tonsillectomy, persistent cold or flu, nasal congestion, dry mouth (xerostomia) and injury to neck, head, or face. Smokers who reported any of these risk factors were scored as positive-history versus no-history. This history variable was used as a covariate in analyses of self-rated and measured chemosensory function.
2.2.2. Measurement of intensity of taste and olfactory stimuli.
Participants made the intensity ratings on the general Labeled Magnitude Scale (gLMS), a scale with intensity labels spaced in a logarithmic fashion and generalized to measure any kind of psychophysical stimuli [84]. The gLMS scale ranges from 0=nothing to 100=strongest sensation of any kind, with intermediate labels of 1.4=barely detectable, 6=weak, 17=moderate, 35=strong, and 53=very strong. Participants were oriented to using the gLMS by rating the intensity of brightness of three remembered stimuli (for example, the brightness of a well-lit room), using procedures outlined in the NHANES protocol [85]; however, only remembered stimuli were used and not LED-generated brightness stimuli [9]. Previous research has shown that the gLMS generates intensity ratings consistent with magnitude matching [84].
2.2.3. Measured olfactory function.
Olfactory function was measured using a 16-item odor identification task with the addition of an intensity rating of each odor on the gLMS, as described previously [85]. Using a forced choice procedure, the participants were asked to select the correct odor from among three other erroneous distractor odors. The odors were generated by an olfactometer (Osmic Enterprises, Inc., Cincinnati, OH), and included food (cherry, strawberry, lemon, onion, coffee, cinnamon, chocolate, grape, vanilla), warning (gasoline, smoke, menthol) and household (soap, leather, baby powder, rose) odors. Participants correctly identifying zero to twelve items were classified as having one of two dichotomized olfactory dysfunctions (0-7 anosmia/severe hyposmia, 8-12 hyposmia); those correctly identifying ≥13 odors were classified with normosmia. This scoring proportionally follows that for the 40-item University of Pennsylvania Smell Identification Task [86] and consistent with the scoring of olfactory dysfunction with the 16-item Sniffin’ Sticks test® [87].
2.2.4. Measured taste function and PROP tasting.
Taste function was measured according to the NHANES protocol [9, 85]. For the taste testing, participants sampled tastants, served at room temperature and in plastic medicine cups. Following the NHANES protocol, participants used the gLMS to report the intensity of concentrated 1mM quinine hydrochloride (QHCl; SAFC, St. Louis, MO) and 1 M sodium chloride (NaCl; Morton Salt, Chicago, IL) drawn across the tongue apex and then, along with .32 M NaCl, sampled with the whole mouth [85].
As a probe of taste genetics and indicator of variation in oral sensations [68, 88], participants also reported the intensity of 1 mM and 3.2 mM PROP (Tokyo Chemical Industry Co., Ltd., Portland, Oregon) sampled with the whole mouth. Participants expectorated after each sample, rinsed before and after each sample with bottled water, and expectorated the rinse. Consistent with our previous report [88], nontasters, medium tasters, and supertasters of PROP were defined by the perceived intensity of PROP relative to that of NaCl. However here, only a ratio of 1 mM PROP to 0.32 M NaCl was considered (and not also the 3.2 mM PROP to 1 M NaCl in the original definition [88]) because of preliminary findings of higher intensity of response to these stimuli in chronic smokers [89]. Nontasters reported 3.2 mM PROP≤20 (around moderate), 1 mM PROP <17 (less than moderate) and/or the ratio of 1mM PROP to 0.32 NaCl <0.4. Supertasters reported 3.2 mM PROP ≥55 (greater than very strong), 1 mM PROP >35 (greater than strong), a ratio of 1mM PROP to 0.32 NaCl at ≥1, and reported an increase in perceived intensity from 1 mM to 3.2 mM PROP. Medium tasters rated the 3.2 mM PROP as between 20 and 55, or did not meet the criteria for either nontasters or supertasters.
2.3. Statistical Analysis
Statistical analyses were conducted using SPSS version 24 (Armonk, New York) with a significance criterion of p ≤.05. Demographics, smoking history, body mass index (BMI), alcohol drinking, and several health conditions that have been associated with taste and smell function were examined in this sample, as well as subdivided by non-menthol (n=51) and menthol (n=84) smokers. Because recent smoking may have an acute effect on chemosensory function, CO levels were considered in the case-control analyses as well as within the chronic smokers as a covariate. Likewise, problem alcohol drinking behaviors (both heavy and binge drinking) were considered in the analyses. Total smoking exposure (years smoked X average cigarettes smoked per day) showed strong correlation with age in this sample (β=0.61, p<.001) and was not considered separately in the analysis.
For the case-control analysis, Table 1 describes the samples that were compared with the chronic smokers. The NHANES is a nationally-representative sample of adults (ages 40 years to over 80 years old), which was used to compare with the chronic smokers for self-reported function, measured smell and taste, but not PROP bitterness. Since NHANES included older adults, we also compared the smokers with the NHANES sample truncated at age 55 years, the top age of the smoker sample. The NHANES sample for each comparison varies somewhat based on available data. In addition, taste function as well as the intensity of 3.2 mM PROP were compared between the chronic smokers and an age- and sex-match group of reportedly healthy non-smokers without heavy alcohol consumption/binge drinking collected in our laboratory-based studies between 2000 and 2010 (n=260).
Table 1.
Comparison samples to the chronic smokers.
| Source | Description | Cycle | Comparison | Specific sample |
|---|---|---|---|---|
| NHANES | This survey is collected in 2-year cycles collected in participant's home (self-report) and mobile exam unit (smell and taste tests) in various locations throughout the U.S. The sample is statistically weighted to be generalizable to the U.S. population. | 2013/2014 | Self-reported chemosensory function, 8-item odor identification; taste test for quinine and NaCl tongue tip and whole mouth | Total sample (smokers and non-smokers) for self-report and for smell testing. Includes ages 40 to above 80 years. Comparison with chronic smokers also shown for non-smokers and non-smokers between ages 40 and 55 years old. |
| Duffy Laboratory non-smokers | These data were collected in a single research laboratory; a convenience sample recruited from the University community. | 2000-2010 | Taste test for quinine and NaCl tongue tip and whole mouth; propylthiouracil bitterness | 260 age and sex-matched to the sample of chronic smokers |
Self-reported chemosensory function and related risk factors of the sample of chronic smokers were compared to those of the NHANES 2013-2014 sample. Rates of taste and smell alteration within chronic smokers were examined by age decade, gender, menthol smoking status, and history of risk factors (y/n) using the chi-square test. For measured olfactory function, the percent of smokers with olfactory dysfunction was compared to rates for the 2013-2014 NHANES sample (total and nonsmokers) and for the sample truncated at age 55 years (total and nonsmokers). The NHANES classification of olfactory dysfunction, including hyposmia and anosmia/severe hyposmia, was determined using criteria previously reported [9]. Within chronic smokers, the number of odors correctly identified and odor intensity across the 16 odors were compared between males and females, those with/without history of risk factors, and non-menthol versus menthol smokers, using analysis of covariance, controlling for demographics, CO levels, and reported alcohol drinking problems (heavy drinking, binge drinking).
For measured taste function, chronic smokers were compared with NHANES 2013-2014 non-smokers who were between 40 and 55 years of age (for intensity ratings of quinine and NaCl) and a sex and age-matched non-smoker sample (n=260) from our laboratory database (for intensity ratings of quinine, NaCl and PROP). Matching was performed using a propensity score matching procedure in R (the MatchIt procedures) [90]. The propensity score was estimated using the method of nearest to match, matching non-smokers to smokers by age and sex. A greater than 1:1 control to case ratio matching is preferred for increased statistical power and decreased standard errors [91, 92].
Taste function was tested for mean differences between chronic smokers and non-smokers with analysis of covariance, controlling for age- and sex effects (assessing and adjusting for equality of variances), and/or frequency differences across PROP taster group categories or intensity label categories with the chi-square statistic. Taste function and bitterness of PROP between non-menthol and menthol smokers was tested for mean differences with analysis of covariance, controlling for demographic variable that were significantly different between these two groups as well as CO level and reported alcohol drinking problems (heavy drinking/binge drinking). In addition, PROP taster classification of non-tasters, medium tasters and supertasters was compared between menthol and non-menthol preferring chronic smokers.
3.0. Results
Table 2 shows the characteristics of the participants, overall and between non-menthol and menthol preferring smokers, as well as NHANES 2013-2014 nonsmokers aged 40 to 55 years. For nicotine dependence, 84% reported smoking within 30 minutes of waking. For alcohol problems, 17.8% were heavy drinkers, 29.6% were binge drinkers, and 31.1% reported both problems. Forty-six percent of the sample had CO levels about 12 ppm, indicating that they had not abstained from smoking 8 hours prior to the baseline assessment.
Table 2.
Chronic smoker characteristic by menthol smoker status and NHANES 2013-2014 non-smokers
| Characteristics | Total Sample | Non- Menthol |
Menthol | Chi-square between Non- menthol & Menthol |
NHANES 2013-2014 Non- Smokers¥ |
|---|---|---|---|---|---|
| N=135 | N=51 | N=84 | N=919 | ||
| Gender | χ2(1)=3.74* | ||||
| Male | 52% | 21 | 49 | 42% | |
| Female | 48% | 30 | 35 | 58% | |
| Age (years) (mean±SEM) | 37.1±0.91 | 36.7±1.4 | 37.4±1.2 | 46.8±0.2 | |
| Marital Status | χ2(1)=4.11** | ||||
| Single/Divorced/Widowed | 64% | 24 | 25 | 28% | |
| Married/Cohabitating | 36% | 27 | 59 | 72% | |
| Race† | χ2(1)=21.78** | ||||
| Black | 23% | 1 | 30 | 21% | |
| White | 75% | 50 | 50 | 59% | |
| Multi-racial | 2% | 0 | 3 | 20% | |
| Ethnicity | χ2(1)=3.55* | ||||
| Hispanic or Latino | 7% | 1 | 9 | 27% | |
| Not Hispanic or Latino | 93% | 50 | 75 | 73% | |
| Household Income | χ2(1)=8.88** | ||||
| ≤$20,000 | 41% | 12 | 42 | 12% | |
| >$20,000 | 59% | 39 | 40 | 88% | |
| Education Level | χ2(1)=1.29 | ||||
| High School Diploma or less | 41% | 18 | 38 | 36% | |
| Some College or beyond | 59% | 33 | 46 | 64% | |
| Employment Status | χ2(2)=10.28** | ||||
| Full-Time Outside the Home | 38% | 28 | 23 | ||
| Part-Time Outside the Home | 23% | 8 | 23 | ||
| Unemployed/Retired | 39% | 15 | 38 | ||
| Years - Regular Smoker (mean±SEM) | 18.9±0.9 | 19.6±1.3 | 18.5±1.3 | ||
| Age (Years) – Began Regular Smoking (mean±SEM) | 17.3±0.4 | 16.3±0.4 | 17.9±0.6 | ||
| Cigarettes Daily - Regular Smoker (mean±SEM) | 12.0±0.6 | 12.9±0.9 | 11.6±0.7 | ||
| Body Mass Index (mean±SEM) ††† | 28.8±0.6 | 28.2±0.9 | 29.2±0.7 | χ2(2)=4.06 | 28.7±0.34 |
| Underweight (<18.5) | 2% | 0 | 3 | 1% | |
| Normal (18.5-24.9) | 29% | 20 | 19 | 27% | |
| Overweight (25-29.9) | 33% | 16 | 28 | 32% | |
| Obese (>30) | 36% | 15 | 34 | 40% | |
| Heavy Drinking | χ2(2)=1.28 | ||||
| Yes | 18% | 12 | 39 | 15% | |
| No | 82% | 12 | 72 | 85% | |
| Binge Drinking | χ2(2)=4.39* | ||||
| Yes | 30% | 21 | |||
| No | 70% | 19 | |||
| Tonsillectomy and/or adenoidectomy | χ2(1)=0.03 | ||||
| Yes | 19% | 9 | 16 | 15% | |
| No | 81% | 40 | 66 | 85% | |
| Persistent cold/flu in the last 12 months | χ2(1)=1.45 | ||||
| Yes | 7% | 2 | 8 | 5% | |
| No | 93% | 49 | 76 | 95% | |
| Persistent xerostomia | χ2(1)=0.05 | ||||
| Yes | 13% | 6 | 11 | 10% | |
| No | 87% | 45 | 73 | 90% | |
| Frequent nasal congestion/allergies in last 12 months | χ2(1)=0.41 | ||||
| Yes | 27% | 12 | 24 | 27% | |
| No | 73% | 39 | 60 | 73% | |
| Ever suffered neck, face, head injury (concussion) | χ2(1)=1.54 | ||||
| Yes | 20% | 13 | 14 | ||
| No | 80% | 38 | 70 | ||
| Ever suffered neck, face, head injury (lost consciousness) | Fisher exact, p=0.8 |
||||
| Yes | 8% | 7 | 4 | 11% | |
| No | 92% | 44 | 80 | 89% |
Reported as percentage of the NHANES sample or mean±SEM between ages 40 and 55 years old.
(black versus white);
(menthol vs. non, t=2.45, p<0.05);
(normal vs overweight versus obese);
p≤0.1;
p<0.05
More than half of the chronic smokers (73 of 135; 54%) reported at least one of the risk factors in their health history that have been associated with chemosensory alterations. Relative to the NHANES non-smokers, the chronic smokers had comparable rates of tonsillectomy, persistent cold/flu and frequent nasal congestion/allergy, a slightly higher rate (6% higher) of xerostomia, and a slightly lower rate (6% lower) of head trauma with loss of consciousness. The average BMI of the chronic smokers was comparable to that in the NHANES sample (Table 2). Chi-square analyses indicated that, among our sample, menthol cigarettes were preferred over non-menthol cigarettes by chronic smokers who were black, unmarried, had lower incomes, and were unemployed. Because only a single Black participant was classified as non-menthol smoker, analyses by menthol status were conducted among Whites only, as well as the whole study sample. Independent of demographic differences, menthol smokers did not differ significantly on baseline CO levels [F(1, 134)=2.1, p=0.15)]. Among the five risk factors examined, the number of persons endorsing each did not differ between menthol and non-menthol smokers.
3.1. Self-reported Smell and Taste Alteration
The percent of chronic smokers reporting alteration in smell or taste was similar to that for the total NHANES 2013-2014 sample (Table 3). However, the percent of smokers reporting smell or taste alterations exceeded that for the NHANES 2013-2014 sample that was restricted just to non-smokers. To describe these differences, chronic smokers reported lower frequency of phantosmia and dysgeusia yet more endorsements of improved ability to smell and improved ability to taste (compared to when they were 25 years old) than did the NHANES 2013-2014 total sample or the NHANES non-smokers.
Table 3.
Rates of self-reported smell and taste alteration in chronic smokers compared with the NHANES 2013-2014 total sample and never smokers¥
| Percentage (%) of Smoker Sample (n=135) | Prevalence in NHANES 2013-2014 (n=3815) |
Prevalence in NHANES 2013-2014 Never smokers only (n=2025) |
|
|---|---|---|---|
| Smell Alteration† | 21.5 | 21.6 | 17.6 |
| During the past 12 months, had a problem with ability to smell, such as not being able to smell things or things not smelling the way they are supposed to | 10.4 | 8.2 | 6.4 |
| Worse ability to smell now compared to when 25 years old | 17.1* | 15.6 | 12.4 |
| During the past 12 months, smell things when nothing is there (phantom smell) | 2.2 | 7.1 | 5.7 |
| Ability to smell better compared to age 25 years old | 10.8* | 6.5 | 5.4 |
| Taste Alteration† | 18.5 | 16.9 | 14.5 |
| During the past 12 months, had a problem with your ability to taste sweet, sour, salty or bitter foods and drinks | 6.7 | 4.9 | 3.6 |
| Worse ability to taste salt, sweet, sour, or bitter now compared to when 25 years old | 10.0-12.7* | 3.9-4.8 | 3.0-3.9 |
| Ability to taste food flavors such as chocolate, vanilla or strawberry now compared to when 25 years old | 11.7* | 7.9 | 6.3 |
| During the past 12 months, tasted things when nothing was there (dysgeusia) | 0 | 5.5 | 4.7 |
| Better ability to taste since age 25 (salt, sweet, sour, or bitter) compared to age 25 years old | 8.2-11.8* | 4.0-8.2 | 2.6-7.6 |
| Both Smell and Taste Alteration† | 9.6 | 8.6 | 5.7 |
NHANES includes adults ages 40 to over 80 years old.
Sub-scores will not sum to total alteration index because of overlapping responses.
% out of participants who are older than age 25 (n=109 to 111)
In sub-analyses, the self-reported smell alteration frequency (yes/no) among the chronic smokers did not show increases by age decade (χ2(3)=1.88; p=0.60) or vary between males and females (χ2(1)=0.09; p=0.76). However, females were more likely than males to report improved ability to taste since the age of 25 years (versus worse or no change), which was significant for sour (χ2(2)=8.55;p=0.02) and trended for sweet (χ2(2)=5.83; p=0.054) and salty (χ2(2)=5.55; p=0.06) sensations.
Beyond the dichotomous yes/no response and responses allowed in the NHANES protocol, 7 additional smokers reported trouble with their sense of smell (despite a “no” response to the current smell problem question) and 6 additional participants reported problems of inability to smell some things, things don’t smell right, or smells make them anxious. Likewise, with further description of self-reported taste function, 7 smokers reported trouble with the sense of taste (despite reporting “no” current taste problem). Ten additional smokers reported problems, including a little trouble (3 of 10), cannot taste some or most things (3 of 10), some things don’t taste right (2 of 10), or inability to describe the problem (2 of 10). Considering these additional responses and examining for differences shown in Table 2, smell or taste alteration did not vary significantly between menthol smokers versus non-menthol smokers (p’s>0.5). However, chronic smokers who reported a smell alteration were more likely to report a history of risk factors for chemosensory alterations (29 versus 13) than those who did not report a smell alteration (49 versus 44) (χ2(1)=4.66, p<0.05). In a similar analysis, there was no significant association between taste alteration and history of risk factors (χ2(1)=1.03;p=0.31).
3.2. Measured Smell Function
The rate of olfactory dysfunction, particularly hyposmia, among the chronic smokers was 40.7%, exceeding the rate reported in the total 2013-2014 NHANES sample (Table 4), including the subset of non-smokers and those between the ages of 40 and 55 years. Within our sample of chronic smokers, the percentage with olfactory dysfunction was 34.2% among those <40 years of age (n=76), and 49.2% among those ≥40 years (n=59). There was no significant difference in olfactory dysfunction rates between males and females, or between those with versus without self-reported history of risk factors (p’s>0.4).
Table 4.
Rates of measured olfactory dysfunction† among chronic smokers compared with the NHANES 2013-2014 total sample, never smokers, and ages 40 to 55 years old¥
| Chronic smokers matched to all ages in NHANES sample |
Percentage (%) of Smoker Sample (n=135) |
Prevalence in NHANES 2013-2014 (n=3519) |
Prevalence in NHANES 2013-2014 Never smokers only (n=1878) |
|---|---|---|---|
| Anosmia/Severe hyposmia | 2.2 | 2.7 | 2.7 |
| Hyposmia | 38.5 | 10.8 | 10.4 |
| Normosmia | 59.3 | 86.4 | 86.9 |
| Total Dysfunction | 40.7 | 13.5 | 13.1 |
| Chronic smokers matched to 40 to 55 years old from the NHANES sample |
Percentage (%) of Smoker Sample (n=135) |
Prevalence in NHANES 2013-2014 (n=1405) |
Prevalence in NHANES 2013-2014 Never smokers only (n=807) |
| Anosmia/Severe hyposmia | 2.2 | 0.8 | 1.0 |
| Hyposmia | 38.5 | 6.4 | 6.7 |
| Normosmia | 59.3 | 92.9 | 92.3 |
| Total Dysfunction | 40.7 | 7.1 | 7.7 |
By odor identification task (16-item olfactometer-generated odors among smokers; 8-item scratch and sniff booklet among NHANES participants.
NHANES includes adults ages 40 to over 80 years old; this table shows chronic smokers versus all NHANES age groups (top) and restricted to just ages 40 to 55 years.
When examining the sensitivity and specificity of self-report against measured smell dysfunction, the chronic smokers showed much better specificity (correct identification of normal) at 72.5%, than sensitivity (correct identification of dysfunction) at 36.5%. The low sensitivity is expected with the higher frequency of hyposmia versus more severe dysfunction (anosmia/severe hyposmia) [9].
The number of correctly identified odors did not vary significantly between male and female chronic smokers, in analysis of covariance controlling for age and other covariates (12.58±0.24 versus 12.92±0.23, respectively). The odors identified correctly at least 85% of the time were specific foods (100% onion, 94.8% coffee, 92.6% cherry, 88.9% vanilla) and non-foods (93.3% leather, 88.1% baby powder, 85.2% rose). Odors identified incorrectly at least 40% of the time included foods (51.1% chocolate) and non-foods (45.2% gasoline, 40% menthol). In multiple regression analysis independent of demographic variables, CO levels and reported alcohol problems, the number of correctly identified odors did not vary significant between menthol versus non-menthol smokers (β=. 13, p=0.16). The average intensity of the odors ranged from ≤moderate (in order of ascending average intensity—chocolate, menthol), moderate to strong (strawberry, vanilla, smoke, cinnamon, rose, lemon, gasoline, cherry, leather, baby powder), and ≥strong (soap, grape, coffee, onion). In t-tests, the average odor intensity across the 16 odors neither varied significantly between females and males (28.78±1.54 SEM versus 27.75±1.41, respectively; p=.7) nor between non-menthol versus menthol smokers (27.25±1.56 SEM versus 28.92±1.39, respectively; p=.49). Average odor intensity showed weak but significant age-related effects (β=−0.18, p<0.05) in multiple regression analysis independent of demographic variables.
3.3. Taste Function
Relative to the laboratory-based non-smokers, the smokers rated the taste of the 3.2 mM PROP stimulus as more intense when controlling for age, sex, CO level and alcohol drinking effects [F(1, 262)=5.433, p=0.021)]. According to PROP taster classification, the sample of chronic smokers was composed of 21.1% non-tasters, 57.8% medium tasters, and 21.1% supertasters, which is similar to the expected frequency of 25% nontasters, 50% medium tasters, 25% supertasters. In the chronic smokers, the frequency of these taster groups did not vary significantly between females and males (χ(2)2=3.428, p=0.18) or between whites and non-whites (χ(2)2=4.44, p=0.11).
The chronic smokers reported quinine intensity on the tongue tip as between moderate and strong, and significantly less intense than the NaCl stimulus on the tongue tip, which had an average rating between strong and very strong (26.64±1.97 SEM vs. 41.27±1.97, respectively; t=8.679, p<.001). In comparison, water on the tongue tip had average intensity ratings in the barely detectable range (1.64±0.48 SEM). The laboratory-based non-smokers did not report this difference in quinine versus salt intensity on the tongue tip (23.15±1.42 SEM versus 24.29±1.32, t=0.889, p=0.376). Reported history of chemosensory risk factors did not explain significant variation in taste intensity.
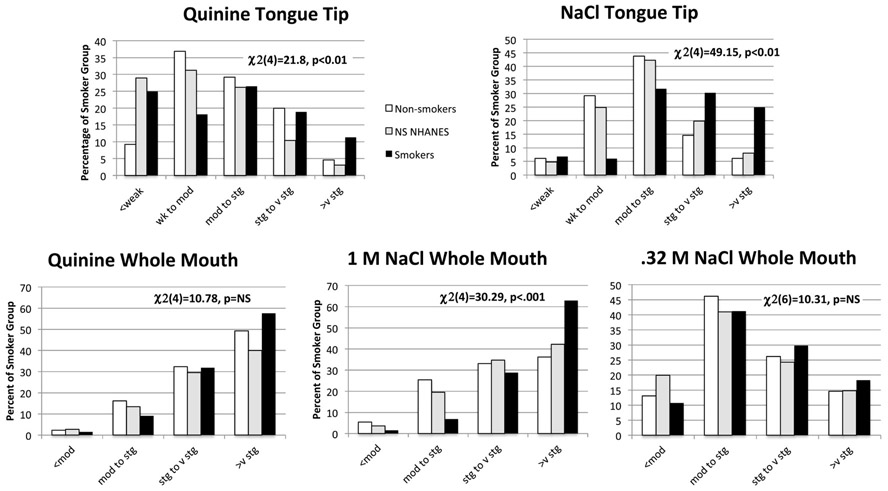
Figure 1 shows the distribution of tongue tip and whole mouth taste intensities by intensity label categories for smokers versus the laboratory-based non-smokers and the NHANES 2013-2014 non-smokers (ages of 40 and 55 years). For quinine on the tongue tip, the smokers had significantly higher variance in distribution by F-test than either non-smoker group (p’s<0.01), with greater frequency in lower and/or higher intensity categories by chi-square analysis. For NaCl on the tongue tip (controlling for age, sex, and examining for CO levels and alcohol drinking problems), smokers reported significantly higher mean intensities than either group of non-smokers [F(2,1027)=23.62, p<0.01)] and higher frequencies in the strong and very strong intensity categories. Likewise, with the whole mouth, quinine intensity did not show mean differences between the non-smoker groups [F(2,1027)=1.51, p=0.22)] or distribution across the intensity categories. However, smokers reported greater intensity in response to whole mouth 1 M NaCl than did either non-smoker group for mean intensity [F(2,1027)=9.75, p<0.01)], and higher frequency of participants in the very strong intensity category with chi-square testing. The intensity of 0.32 M NaCl was a trend for mean intensity [F(2,1027)=2.486, p=0.09)] with smokers tending to report greater intensity than non-smokers in NHANES. The distribution of 0.32 M NaCl across the intensity categories was not different between smokers and non-smoker groups.
Figure 1.
Percent of non-smokers (laboratory-based sample, National Health and Nutrition Examination Survey (NHANES) 2012-2014 sample) and chronic smokers that fall into categories for taste intensity for quinine and NaCl painted on the tongue tip) and quinine and NaCl sampled with the whole mouth. Percentages in non-smokers and smokers were tested by the chi square statistic.
Taste function between menthol and non-menthol preferring smokers
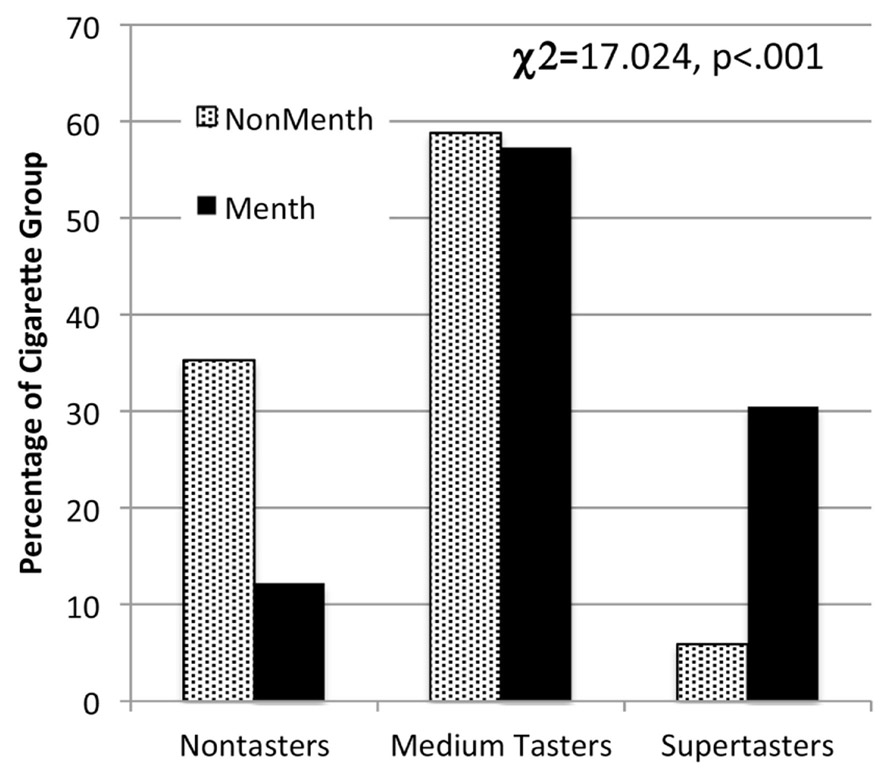
Because of the race difference in menthol and non-menthol smokers in the present study (Table 2) and in the literature [93], taste function was examined in both White, Not Hispanic/Latino participants, as well as in the whole sample with race as a covariate. In analysis of covariance (controlling for demographic, CO, and alcohol variables), the menthol and non-menthol smokers did not vary significantly in mean intensities of tongue tip quinine or NaCl, or whole mouth quinine or NaCl (1M, .32 M) (p’s range between 0.27 for quinine tongue tip and between 0.51 and 0.81 for the other taste probes). However, menthol smokers reported significantly higher average intensity to 1 mM PROP (42.48±3.11 versus 24.74±4.11, F(1,134)=10.42, p<0.01) and 3.2 mM PROP (50.05±3.19 versus 38.44±4.17, F(1,134)=4.34, p=0.03) than did non-menthol smokers. Figure 2 shows the percentage of PROP nontasters, medium tasters, and supertasters within the non-menthol and menthol smoker groups. There were significantly fewer PROP nontasters but more supertasters among the menthol smokers than non-menthol smokers for the whole sample (χ(2)2=17.024, p<0.001) and among the white Not Hispanic/Latino participants (χ(2)2=12.58, p<0.002).
Figure 2.
Percent of non-menthol and menthol smokers who fall into the categories of propythiouracil (PROP) taster categories tested with the chi square statistic.
4.0. Discussion
This observational study involved chemosensory phenotyping of a sample of chronic adult smokers (ages 18 to 55 year) recruited for an electronic cigarette intervention. In case-control comparison, smell and taste function in these chronic smokers differed from non-smokers in the 2013-2014 National Health and Nutrition Examination Survey (NHANES) and/or from a laboratory-based sample of non-smokers. Approximately 41% of the smokers had measured olfactory dysfunction, which was up to 7-fold higher than the non-smokers from 2013-2014 NHANES. The smokers also reported elevated taste intensities from NaCl sampled on the tongue tip or with the whole mouth in comparison to non-smokers, not at concentrations where salt is primarily a taste (0.32 M), but at levels in which salt is an oral irritant (1 M)[94]. In testing the ability to taste the bitterness of concentrated PROP, we did not find that chronic smokers were taste-insensitive or nontasters in comparison to non-smokers. However, among the chronic smokers, those who reported preferring menthol cigarettes were more likely to report elevated bitterness from PROP or more likely to be classified as supertasters.
The findings in the present study are consistent with those of studies linking chronic smoking with greater risk of olfactory impairment in animal models [5, 6], tested by threshold [15-17], odor identification [18, 22, 29] and self-report [36]. Chronic smoking exposure may directly damage the peripheral and central olfactory system. In animal models, chronic exposure to cigarette smoking is associated with changes to the peripheral olfactory system, including olfactory epithelial neuron death, undermining the regeneration capacity to maintain functional tissues [6, 95], as well as decreased functional olfactory receptors [5]. Analysis of the NHANES data support direct associations between chronic smoking on self-reported olfactory alteration, as well as identified indirect associations [36]. That is, chronic smoking was associated indirectly with olfactory alteration through greater frequency of risk factors associated with this alteration, including sinonasal problems, xerostomia and head trauma [36]. Here, we found that chronic smokers who reported a history of these risk factors were more likely to self-report a smell alteration. However, we did not find that our chronic smokers self-reported greater frequencies of sinonasal problems and head trauma than the NHANES non-smokers of comparable age. Chronic smokers are reported to have greater level of sinonasal problems that may remediate with smoking cessation [96].
Of the additional risk factors, the presence of xerostomia was associated with increased odds of measured smell dysfunction in the 2012 NHANES data, but not when fully controlling other demographic and health variables such as poverty, low-education, heavy drinking, physical activity [9]. The slightly higher frequency of self-reported xerostomia in our chronic smokers relative to the NHANES non-smokers (13% versus 8%, respectively) could not explain the elevated measured olfactory dysfunction. Our sample of smokers did report lower rates of phantosmia. Querying about “smelling things when nothing is there” may reflect a phantom sensation and possibly a distortion in quality or parosmia [97], which has been reported more frequently in women, and in those with nasosinus disease, but not among smokers [97].
Of note, we did not observe the usual sex difference in olfactory function within the chronic smokers (females>males) either for number of odors identified correctly or for perceived odor intensity. Chronic smoking may counter the advantage that women show in performance on olfactory tests; females may suffer greater negative effects of smoking on olfactory dysfunction than males [19]. Males also showed higher prevalence of olfactory dysfunction than females in the 2012 NHANES dataset [9], a sex difference that was most pronounced in the most severe dysfunction category (anosmia/severe hyposmia) and nearly equal prevalence in the less severe category (hyposmia). The present study of chronic smokers had fewer cases of severe olfactory dysfunction (anosmia/severe hyposmia) than NHANES, which also could explain a lack of sex differences in olfactory function.
Lower olfactory abilities among our chronic smokers also may relate to broader social issues. Our chronic smokers had lower household income (≤$20,000) than the NHANES non-smokers, as well as lower educational attainment than the NHANES non-smokers, both of which have been associated with greater odds of olfactory dysfunction [9]. The odor identification task employed in the present study requires the ability to detect and correctly label an odor, the latter requiring cognitive processes [98]. In studies with chronic smokers older than those in the present study, poorer ability in odor identification has been associated with mild cognitive impairment [24] and a greater number of cigarettes smoked per day has been associated with poorer cognitive function [99]. Among participants closer in age to those in the present study, variation in olfactory impairment on an odor identification task in the Beaver Dam Offspring Study (mean age=49 years) was explained by three tests of cognitive function (attention, processing speed and executive function) [100]. In the present study, we incorporated perceived odor intensity in the odor identification test, a task that does not require the cognitive burden of odor labeling. In data analysis not shown, the average odor intensities rated by the smokers in the present study were similar to those of non-smokers from a previous study from our laboratory, controlling for age- and sex-effects [85]. Thus, our findings suggest a complexity to olfactory dysfunction among chronic smokers that extends beyond the toxic effects of smoking on the olfactory system to negative effects of low socio-economic status on health and well-being.
Despite the high level of smell alteration among our chronic smokers, awareness of the problem among those with measured dysfunction (sensitivity of self-report) was low, which is to be expected. As we have reported previously [9], adults are unaware of olfactory dysfunction unless it reaches the level of anosmia/severe hyposmia. Perceiving a problem without meeting a diagnostic criterion also has been reported [101]. Conversely, perceiving an improvement in smell ability since age 25 may be specific to the sense of smell or broader “optimistic bias” that has been studied to understand why smokers do not respond to messages about health risks of cigarette smoking [102]. We uncovered a greater level of self-reported smell and taste alteration by providing participants chance to report beyond a dichotomous response (yes/no) to a current problem and changes associated with aging.
Our results did not indicate that smokers have depressed taste perception, either in ability to taste regionally on the tongue tip or with whole mouth experiences, as measured by perceived intensity of concentrated salt and bitter solutions. Much of the previous scientific literature on taste and smoking has involved threshold perception, reporting that smokers have no difference [45-47] or elevated threshold to one or all qualities by solution [48-50], filter paper method [51] and electrogustometry [42, 52-54]. However, threshold may not predict suprathreshold perception [103], with individuals with lowest thresholds showing large variation in perceived intensities.
In the present study, we found that, relative to non-smokers, the smokers reported greater intensities to 1 M NaCl, an oral chemical irritant [94], which is consistent with the elevated oral irritation and pain observed in previous studies [62, 104], and escalated levels of oral health problems [105], periodontitis [106], oral pain [107] and xerostomia [43, 44] among smokers. Other components of somatosensation, however, do tend to be depressed in smokers including thermal sensitivity [61]. The elevated intensity of NaCl irritation could not be explained by loss of taste, as seen with history of tympanic infections or taste nerve damage [38]. Fewer chronic smokers in the present study reported dysgeusia (2.2%) than the non-smokers in the NHANES 2012 [39] or in the NHANES 2013-2014 reported here (5 to 6%). Dysgeusia can result from something tasted in the mouth or from taste nerve damage and production of a central phantom taste sensation [38]. Thus, the results of the taste testing and self-report suggest that the elevated taste intensity in response to 1 M NaCl resulted from an irritation response instead of a pattern of taste dysfunction that has been associated with dysgeusia. Interestingly, we found that a greater majority of our smokers reported improved retronasal olfactory sensations with aging. Enhanced retronasal olfaction could result from elevated taste and oral somatosensory sensations [108].
We did not observe more PROP nontasters among our sample of chronic smokers than the frequency theoretically expected (21% versus 25%, respectively), which is inconsistent with previous findings of higher prevalence of nontasters among smokers [63, 65]. In fact, a recent crowdsourced study reported that higher bitterness of PROP (delivered by filter paper) was reported by current smokers than never smokers [71]. Our testing employed aqueous solutions of concentrated PROP, which is more intense than commercially available PTC strips but roughly equivalent laboratory-made PROP papers [71]. Frequently, PROP taster categories are defined in terms of the PROP-to-NaCl intensity ratio (including 1 M NaCl), with nontasters having the lowest ratios (<0.4) and supertasters the highest (>1.2) [88, 109]. However, our findings with elevated intensities of 1 M NaCl suggest that this ratio could falsely shift the PROP/NaCl ratio toward more nontasters and fewer supertasters. Thus, our method to define taster status relied on perceived intensities of two concentrations of PROP and the relative intensity of PROP compared with .32 M NaCl, as reported in an earlier study [88], but, to avoid erroneously characterizing individuals as nontasters, we did not use the comparison of PROP bitterness to the intensity of 1 M NaCl in our taster classification
Consistent with findings that female tasters by TAS2R38 receptor genotype were more likely to smoke menthol cigarettes [3], the present study found that, relative to non-menthol smokers, menthol smokers were skewed toward reporting that PROP had greater bitterness. This finding did not generalize to the bitterness of quinine, only PROP, which may indicate a stronger taste genetic component to this relationship. Menthol may improve the ability to tolerate the unpleasant sensations of nicotine by providing a minty odor [110] and oral and nasal cooling [111], especially for younger smokers and minorities [112]. Our findings did not support the hypothesis that smokers with a greater level of measured taste alteration would be more likely to report smoking menthol cigarettes. Thus, the pattern of menthol versus non-menthol cigarettes appears more closely associated with a genetic propensity to experience greater bitterness and irritation in foods and beverages [113] and nicotine [56], associated with the phenotypic ability to taste the bitterness of PROP.
This study had limitations, including a convenience sample of chronic smokers, limited representativeness, and reliance on self-reported history. The taste protocol did not include all taste qualities and did not involve testing a wider range of somatosensory sensations. Nonetheless, the sample was well characterized for smoking status and phenotyped with standardized suprathreshold measures that could be directly compared with the new NHANES self-reported and measured smell functioning [9, 39], as well as controlling for the temporal effects of smoking and alcohol drinking problems in the analyses.
5.0. Conclusion
This sample of smokers showed elevated levels of measured olfactory dysfunction but no evidence of taste dysfunction. The smokers reported heightened irritation from a concentrated NaCl solution. Menthol smokers were more likely to be bitter tasters of PROP, the probe for genetic variation in taste. The findings have implications for regulation of tobacco products. Elimination of menthol from cigarettes could have a significant effect on palatability for significant numbers of smokers who are sensitive to bitterness. On the other hand, the use of menthol in nicotine replacement products, including nicotine gum and electronic cigarettes, could help cessation efforts in bitter-sensitive smokers.
Highlights.
Chronic smokers had greater level of mild olfactory dysfunction than non-smokers.
Chronic smokers reported greater intensity from concentrated NaCl than non-smokers.
Chronic smokers were not more likely to be genetic non-tasters of propylthiouracil.
Menthol cigarette smokers were more likely to be genetic supertasters.
Acknowledgements
The authors would like to acknowledge Diane Wilson, Eileen Leonard, and Abigail Young for their work in the conduct of this project.
Funding
This work was supported by Grant 1 R01 DA036492 from the National Institute on Drug Abuse, and in part by General Clinical Research Center Grant M01-RR06192 from the National Institutes of Health.
Footnotes
Declaration of Interests: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR. 2018,67:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB Menthol cigarettes and the public health standard: a systematic review. BMC Public Health. 2017,17:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oncken C, Feinn R, Covault J, Duffy V, Dornelas E, Kranzler HR, et al. Genetic Vulnerability to Menthol Cigarette Preference in Women. Nicotine Tob Res. 2015,17:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Da Re AF, Gurgel LG, Buffon G, Moura WER, Marques Vidor DCG, Maahs MAP Tobacco Influence on Taste and Smell: Systematic Review of the Literature. Int Arch Otorhinolaryngol. 2018,22:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ueha R, Ueha S, Kondo K, Sakamoto T, Kikuta S, Kanaya K, et al. Damage to Olfactory Progenitor Cells Is Involved in Cigarette Smoke-Induced Olfactory Dysfunction in Mice. Am J Pathol. 2016,186:579–86. [DOI] [PubMed] [Google Scholar]
- [6].Ueha R, Ueha S, Kondo K, Kikuta S, Yamasoba T Cigarette Smoke-Induced Cell Death Causes Persistent Olfactory Dysfunction in Aged Mice. Front Aging Neurosci. 2018,10:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stuck BA, Hummel T Olfaction in allergic rhinitis: A systematic review. J Allergy Clin Immunol. 2015,136:1460–70. [DOI] [PubMed] [Google Scholar]
- [8].Coelho DH, Costanzo RM Posttraumatic olfactory dysfunction. Auris, Nasus, Larynx. 2016,43:137–43. [DOI] [PubMed] [Google Scholar]
- [9].Hoffman HJ, Rawal S, Li CM, Duffy VB New chemosensory component to the U.S. National Health and Nutrition Examination Survey (NHANES), first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016,17:221–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP Smoking, alcohol consumption, and susceptibility to the common cold. Am J Pub Health. 1993,83:1277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bagaitkar J, Demuth DR, Scott DA Tobacco use increases susceptibility to bacterial infection. Tob Induced Dis. 2008,4:12-9625-4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benninger MS The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999,13:435–8. [DOI] [PubMed] [Google Scholar]
- [13].Nicola ML, Carvalho HB, Yoshida CT, Anjos FM, Nakao M, Santos Ude P, et al. Young "healthy" smokers have functional and inflammatory changes in the nasal and the lower airways. Chest. 2014,145:998–1005. [DOI] [PubMed] [Google Scholar]
- [14].Ilie G, Adlaf EM, Mann RE, Ialomiteanu A, Hamilton H, Rehm J, et al. Associations between a History of Traumatic Brain Injuries and Current Cigarette Smoking, Substance Use, and Elevated Psychological Distress in a Population Sample of Canadian Adults. J Neurotrauma. 2015,32:1130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosenblatt MR, Olmstead RE, Iwamoto-Schaap PN, Jarvik ME Olfactory thresholds for nicotine and menthol in smokers (abstinent and nonabstinent) and nonsmokers. Physiol Behav. 1998,65:575–9. [DOI] [PubMed] [Google Scholar]
- [16].Hayes JE, Jinks AL Evaluation of smoking on olfactory thresholds of phenyl ethyl alcohol and n-butanol. Physiol Behav. 2012,107:177–80. [DOI] [PubMed] [Google Scholar]
- [17].Ozmen S, Dulger S, Coban S, Ozmen OA, Guzelsoy M, Dikis OS, et al. Olfactory and erectile dysfunction association in smoking and non-smoking men. Physiol Behav. 2016,160:1–5. [DOI] [PubMed] [Google Scholar]
- [18].Frye RE, Schwartz BS, Doty RL Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990,263:1233–6. [PubMed] [Google Scholar]
- [19].Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, et al. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 2012,37:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein R, Tsai MY, et al. Carotid Intima Media Thickness, Atherosclerosis, and 5-Year Decline in Odor Identification: The Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci. 2015,70:879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bramerson A, Johansson L, Ek L, Nordin S, Bende M Prevalence of olfactory dysfunction: the Skövde population-based study. Laryngoscope. 2004,114:733–7. [DOI] [PubMed] [Google Scholar]
- [22].Murphy C, Schubert C, Cruickshanks K, Klein B, Klein R, Nondahl D Prevalence of olfactory impairment in older adults. JAMA. 2002,288:2307–12. [DOI] [PubMed] [Google Scholar]
- [23].Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK The Rate of Age-Related Olfactory Decline Among the General Population of Older U.S. Adults. J Gerontol A Biol Sci Med Sci. 2015,70:1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, et al. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2016,73:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schubert CR, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Fischer ME Association of exercise with lower long-term risk of olfactory impairment in older adults. JAMA Otolaryngol Head Neck Surg. 2013,139:1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Menon C, Westervelt HJ, Jahn DR, Dressel JA, O'Bryant SE Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: a Project FRONTIER study. Clin Neuropsychol. 2013,27:946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundstrom JN Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinol. 2011,49:324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Doty RL, Petersen I, Mensah N, Christensen K Genetic and environmental influences on odor identification ability in the very old. Psychol Aging. 2011,26:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vennemann M, Hummel T, Berger K The association between smoking and smell and taste impairment in the general population. J Neurol. 2008,255:1121–6. [DOI] [PubMed] [Google Scholar]
- [30].Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 2011,121:873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mullol J, Alobid I, Marino-Sanchez F, Quinto L, de Haro J, Bernal-Sprekelsen M, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012,2: 10.1136/bmjopen-2012-001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee Y-M, Prescott J, Kim K-O PROP taster status and the rejection of foods with added tastants. Food Sci Biotechnol. 2008,17:1066–73. [Google Scholar]
- [33].Rawal S, Hoffman HJ, Chapo AK, Duffy VB Sensitivity and Specificity of Self-reported Olfactory Dysfunction in a Home-based Study of Independent-living, Healthy Older Women. Chemosens Percept 2014,7:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nordin S, Monsch A, Murphy C Unawareness of smell loss in normal aging and Alzheimer's disease: discrepancy between self-reported and diagnosed smell sensitivity. J Gerontology B Psychol Sci Soc Sci. 1995,50:P187–92. [DOI] [PubMed] [Google Scholar]
- [35].Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011,26:260–9. [DOI] [PubMed] [Google Scholar]
- [36].Glennon S-G, Huedo-Medina T, Rawal S, Hoffman H, Litt MD, Duffy V Chronic Cigarette smoking associates directly and indirectly with self-reported olfactory alterations: Analysis of the 2011–2014 National Health and Nutrition Examination Survey (NHANES) Nicotine & Tobacco Research. 2017: 10.1093/ntr/ntx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deems D, Doty R, Settle R, Moore-Gillon V, Shaman P, Mester A, et al. Smell and taste disorders: an analysis of 750 from the University of Pennsylvania Smell and Taste Center. Arch Otolaryng Head Neck Surg. 1991,117:519–28. [DOI] [PubMed] [Google Scholar]
- [38].Snyder DJ, Bartoshuk LM Oral sensory nerve damage: Causes and consequences. Rev Endocr Metab Disord. 2016,17:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB Prevalence and risk factors of self-reported smell and taste alterations: Results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 2016,41:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tomassini S, Cuoghi V, Catalani E, Casini G, Bigiani A Long-term effects of nicotine on rat fungiform taste buds. Neurosci. 2007,147:803–10. [DOI] [PubMed] [Google Scholar]
- [41].Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein R, Pankratz N, et al. Factors Related to Fungiform Papillae Density: The Beaver Dam Offspring Study. Chem Senses. 2013,38:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pavlidis P, Vasilios N, Antonia A, Dimitrios K, Georgios K, Georgios A Evaluation of young smokers and non-smokers with Electrogustometry and Contact Endoscopy. BMC Ear Nose Throat Disord. 2009,9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rad M, Kakoie S, Niliye Brojeni F, Pourdamghan N Effect of long-term smoking on whole-mouth salivary flow rate and oral health. J Dent Res Dent Clin Dent Prospects. 2010,4:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dyasanoor S, Saddu SC Association of xerostomia and assessment of salivary flow using modified Schirmer Test among smokers and healthy individuals: A preliminary study. J Clin Diag Res. 2014,8:211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McBurney D, Moskat L Taste thresholds in college-age smokers and nonsmokers.Percept Psychophys. 1975,18:71–3. [Google Scholar]
- [46].Gromysz-Kalkowska K, Wojcik K, Szubartowska E, Unkiewicz-Winiarczyk A Taste perception of cigarette smokers. Annales Universitatis Mariae Curie-Sklodowska.Sectio D: Medicina. 2002,57:143–54. [PubMed] [Google Scholar]
- [47].Sawada M A study of measurements of and factors influencing threshold levels of taste perception. Kokubyo Gakkai zasshi J Stomatolog Soc, Japan: 2005,72:28–41. [DOI] [PubMed] [Google Scholar]
- [48].Krut LH, Perrin MJ, Bronte-Stewart B Taste perception in smokers and non-smokers. Br Med J. 1961,1:384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pepino MY, Mennella JA Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007,31:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jackson JA Heavy smoking and sodium chloride hypogeusia. J Dent Res. 1967,46:742–4. [DOI] [PubMed] [Google Scholar]
- [51].Sato K, Endo S, Tomita H Sensitivity of three loci on the tongue and soft palate to four basic tastes in smokers and non-smokers. Acta oto-laryngologica.Suppl 2002,(546):74–82. [DOI] [PubMed] [Google Scholar]
- [52].Pavlidis P, Gouveris C, Kekes G, Maurer J Changes in electrogustometry thresholds, tongue tip vascularization, density and form of the fungiform papillae in smokers. Eur Arch Otorhinolaryngol. 2014,271:2325–31. [DOI] [PubMed] [Google Scholar]
- [53].Khan AM, Narayanan VS, Puttabuddi JH, Chengappa R, Ambaldhage VK, Naik P, et al. Comparison of Taste Threshold in Smokers and Non-Smokers Using Electrogustometry and Fungiform Papillae Count: A Case Control Study. J Clin Diagn Res. 2016,10:ZC101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cheruel F, Jarlier M, Sancho-Garnier H Effect of cigarette smoke on gustatory sensitivity, evaluation of the deficit and of the recovery time-course after smoking cessation. Tob Induc Dis. 2017,15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pepino MY, Mennella JA Cigarette smoking and obesity are associated with decreased fat perception in women. Obesity (Silver Spring). 2014,22:1050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ahijevych K, Tepper BJ, Graham MC, Holloman C, Matcham WA Relationships of PROP Taste Phenotype, Taste Receptor Genotype, and Oral Nicotine Replacement Use. Nicotine Tob Res. 2015,17:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gyekis JP, Dingman MA, Revitsky AR, Bryant BP, Vandenbergh DJ, Frank ME, et al. Gustatory, trigeminal, and olfactory aspects of nicotine intake in three mouse strains. Behav Genet. 2012,42:820–9. [DOI] [PubMed] [Google Scholar]
- [58].Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, et al. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci. 2009,106:1596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tepper BJ, Melis M, Koelliker Y, Gasparini P, Ahijevych KL, Tomassini Barbarossa I Factors Influencing the Phenotypic Characterization of the Oral Marker, PROP. Nutrients. 2017,9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Klein BE, Klein R, et al. Taste intensity in the Beaver Dam Offspring Study. Laryngoscope. 2013,123:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yekta SS, Luckhoff A, Ristic D, Lampert F, Ellrich J Impaired somatosensation in tongue mucosa of smokers. Clinical Oral Investig. 2012,16:39–44. [DOI] [PubMed] [Google Scholar]
- [62].Riley JL 3rd, Tomar SL, Gilbert GH Smoking and smokeless tobacco: increased risk for oral pain. J Pain. 2004,5:218–25. [DOI] [PubMed] [Google Scholar]
- [63].Fischer R, Griffin F, Kaplan AR Taste Thresholds, Cigarette Smoking, and Food Dislikes. Med Exp Int J Exp Med. 1963,210:151–67. [DOI] [PubMed] [Google Scholar]
- [64].Kaplan AR, Glanville EV, Fischer R Taste Thresholds for Bitterness and Cigarette Smoking. Nature. 1964,202:1366. [DOI] [PubMed] [Google Scholar]
- [65].Enoch MA, Harris CR, Goldman D Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted? Addict Behav. 2001,26:399–404. [DOI] [PubMed] [Google Scholar]
- [66].Snedecor SM, Pomerleau CS, Mehringer AM, Ninowski R, Pomerleau OF Differences in smoking-related variables based on phenylthiocarbamide "taster" status. Addict Behav. 2006,31:2309–12. [DOI] [PubMed] [Google Scholar]
- [67].Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003,299:1221–5. [DOI] [PubMed] [Google Scholar]
- [68].Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008,33:255–65. [DOI] [PubMed] [Google Scholar]
- [69].Risso DS, Kozlitina J, Sainz E, Gutierrez J, Wooding S, Getachew B, et al. Genetic Variation in the TAS2R38 Bitter Taste Receptor and Smoking Behaviors. PLoS One. 2016,11:e0164157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cannon DS, Baker TB, Piper ME, Scholand MB, Lawrence DL, Drayna DT, et al. Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res. 2005,7:853–8. [DOI] [PubMed] [Google Scholar]
- [71].Baker AN, Miranda AM, Garneau NL, Hayes JE Self-reported smoking status, TAS2R38 variants, and propylthiouracil phenotype: an exploratory crowdsourced cohort study. Chem Senses. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gorovic N, Afzal S, Tjonneland A, Overvad K, Vogel U, Albrechtsen C, et al. Genetic variation in the hTAS2R38 taste receptor and brassica vegetable intake. Scand J Clin Lab Invest. 2011,71:274–9. [DOI] [PubMed] [Google Scholar]
- [73].Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet. 2008,45:578–82. [DOI] [PubMed] [Google Scholar]
- [74].Kreslake JM, Wayne GF, Alpert HR, Koh HK, Connolly GN Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. Am J Public Health. 2008,98:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wickham RJ How Menthol Alters Tobacco-Smoking Behavior: A Biological Perspective. Yale J Biol Med. 2015,88:279–87. [PMC free article] [PubMed] [Google Scholar]
- [76].Brown RA, Lejuez CW, Kahler CW, Strong DR Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002,111:180–5. [PubMed] [Google Scholar]
- [77].Sandberg A, Skold CM, Grunewald J, Eklund A, Wheelock AM Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS One. 2011,6:e28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fagerstrom KO, Heatherton TF, Kozlowski LT Nicotine addiction and its assessment. Ear Nose Throat J. 1990,69:763–5. [PubMed] [Google Scholar]
- [79].Fagerstrom K Time to first cigarette; the best single indicator of tobacco dependence? Monaldi Archives Chest Dis. 2003,59:91–4. [PubMed] [Google Scholar]
- [80].Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). J Consult Clin Psychol. 2004,72:139–54. [DOI] [PubMed] [Google Scholar]
- [81].Sobell LC, Sobell MB Alcohol Timeline Followback Users’ Manual. Toronto, Canada: 1995. [Google Scholar]
- [82].Dept US Health Human Services and U.S. Dept of Agriculture. 2015 – 2020 Dietary Guidelines for Americans, 8th edition. 2015. Available at: http://health.gov/dietaryguidelines/2015/guidelines/, accessed Dec 3, 2018. [Google Scholar]
- [83].Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES). 2011 – 2012 Data Documentation, Codebook, and Frequencies: Taste and Smell Disorders [Internet]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/CSQ_G.htm, accessed Dec 3, 2018.
- [84].Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004,82:109–14. [DOI] [PubMed] [Google Scholar]
- [85].Rawal S, Hoffman HJ, Honda M, Huedo-Medina TB, Duffy VB The Taste and Smell Protocol in the 2011-2014 U.S. National Health and Nutrition Examination Survey (NHANES): Test-Retest Reliability and Validity Testing. Chemosens Percept. 2015,8:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Segura B, Baggio HC, Solana E, Palacios EM, Vendrell P, Bargallo N, et al. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013,246:148–53. [DOI] [PubMed] [Google Scholar]
- [87].Rumeau C, Nguyen DT, Jankowski R How to assess olfactory performance with the Sniffin' Sticks test®. Eur Ann Otorhinolaryngol Head Neck Dis. 2016,133:203–6. [DOI] [PubMed] [Google Scholar]
- [88].Bartoshuk LM, Duffy VB, Miller IJ Jr. PTC/PROP Tasting: Anatomy, psychophysics, and sex effects. Physiol Behav. 1994,56:1165–71. [DOI] [PubMed] [Google Scholar]
- [89].Glennon SG, Litt M, Duffy VB. Olfactory but not taste dysfunction among chronic smokers: Baseline results from an e-cigarette intervention. Association for Chemoreception Sciences Meeting. Bonita Bay, FL: Chemical Senses; 2016; 41(7) (abstract). [Google Scholar]
- [90].Ho DE, Imai K, King G, Stuart EA MatchIt: Nonparametric preprocessing for parametric causal inference. J Statistical Software. 2011,42:1–27. [Google Scholar]
- [91].Hennessy S, Bilker WB, Berlin JA, Strom BL Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol. 1999,149:195–7. [DOI] [PubMed] [Google Scholar]
- [92].Kang M, Choi S, Koh I The effect of increasing control-to-case reatio on statistical power in a simulated case-control SNP association. Genomics & Informatics. 2009,7:148–51. [Google Scholar]
- [93].Substance Abuse and Mental Health Services Administration,. Recent Trends in Menthol Cigarette Use. 2011. Retrieved from: www.samhsa.gov/data/sites/default/files/WEB_SR_088/WEB_SR_088/WEB_SR_088.htm. Accessed Dec 3, 2018.
- [94].Green B, Gelhard B Salt as an oral irritant. Chem Senses. 1989,14:259–71. [Google Scholar]
- [95].Vent J, Robinson AM, Gentry-Nielsen MJ, Conley DB, Hallworth R, Leopold DA, et al. Pathology of the olfactory epithelium: smoking and ethanol exposure. Laryngoscope. 2004,114:1383–8. [DOI] [PubMed] [Google Scholar]
- [96].Phillips KM, Hoehle L, Bergmark RW, Caradonna DS, Gray ST, Sedaghat AR Reversal of Smoking Effects on Chronic Rhinosinusitis after Smoking Cessation. Otolaryngol Head Neck Surg. 2017,157:737–42. [DOI] [PubMed] [Google Scholar]
- [97].Sjolund S, Larsson M, Olofsson JK, Seubert J, Laukka EJ Phantom Smells: Prevalence and Correlates in a Population-Based Sample of Older Adults. Chem Senses.2017,42:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010,32:1062–7. [DOI] [PubMed] [Google Scholar]
- [99].North TL, Palmer TM, Lewis SJ, Cooper R, Power C, Pattie A, et al. Effect of smoking on physical and cognitive capability in later life: a multicohort study using observational and genetic approaches. BMJ Open. 2015,5:e008393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein R, Pankratz N, et al. Odor identification and cognitive function in the Beaver Dam Offspring Study. J Clin Exp Neuropsychol. 2013,35:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lenderink AF, Zoer I, van der Molen HF, Spreeuwers D, Frings-Dresen MH, van Dijk FJ Review on the validity of self-report to assess work-related diseases. Int Arch Occup Environ Health. 2012,85:229–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Szklo AS, Coutinho ES Vulnerability and self-perceived health status among light and heavy smokers: the relationship to short-term fear appeal tobacco control messages. Cad Saude Publica. 2009,25:1534–42. [DOI] [PubMed] [Google Scholar]
- [103].Duffy V, Hayes J, Bartoshuk L, Snyder D Taste: Vertebrates – Psychophysics In: Walsh V, ed. Reference Module in Neuroscience and Biobehavioral Psychology, Chemosensory Systems – Taste. Available at: http://www.sciencedirect.com/science/article/pii/B97801280932450290722017. [Google Scholar]
- [104].Logan HL, Fillingim RB, Bartoshuk LM, Sandow P, Tomar SL, Weming JW, et al. Smoking status and pain level among head and neck cancer patients. J Pain.2010,11:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Csikar J, Kang J, Wyborn C, Dyer TA, Marshman Z, Godson J The self-reported oral health status and dental attendance of smokers and mon-smokers in England. PLoS One. 2016,11:e0148700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bergstrom J, Eliasson S, Dock J A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000,71:1338–47. [DOI] [PubMed] [Google Scholar]
- [107].Hicks TA, Wilson SM, Thomas SP, Dennis PA, Neal JM, Calhoun PS Low income as a multiplicative risk factor for oral pain and dental problems among U.S. Veteran smokers. Int J Behav Med. 2018,25(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lim J, Johnson MB Potential mechanisms of retronasal odor referral to the mouth. Chem Senses. 2011,36:283–9. [DOI] [PubMed] [Google Scholar]
- [109].Zhao L, Kirkmeyer SV, Tepper BJ A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 2003,78:625–33. [DOI] [PubMed] [Google Scholar]
- [110].Goyert HF, Frank ME, Gent JF, Hettinger TP Characteristic component odors emerge from mixtures after selective adaptation. Brain Res Bull. 2007,72:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cliff MA, Green BG Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav. 1994,56:1021–9. [DOI] [PubMed] [Google Scholar]
- [112].Caraballo RS, Asman K Epidemiology of menthol cigarette use in the United States. Tob Induc Dis. 2011,9 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Prescott J, Soo J, Campbell H, Roberts C Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol Behav. 2004,82:459–69. [DOI] [PubMed] [Google Scholar]