Abstract
Aim:
To provide an update on artificial saliva used to maintain the health of the oral cavity of patients with severe hyposalivation.
Materials and Methods:
A literature search was conducted in April 2018 in three electronic databases (The Cochrane Central Register of Controlled Trials [CENTRAL], PubMed, and Embase) by combining key words and terms related to the population and intervention of the topic.
Results:
The databases search resulted in 455 titles and abstracts. Of these, 21 were judged to meet inclusion criteria and full texts were read. Finally, 10 clinical trials were included for qualitative synthesis.
Conclusion:
Published evidence suggests that all the artificial saliva products tested in included studies reduced symptoms of xerostomia. These products should specifically be selected according to the patients’ concerns and needs. However, the included studies presented a wide range of products and suffered from high risk of bias. Therefore, long-term randomized controlled trials on effects of various products are required.
KEYWORDS: Artificial saliva, salivary substitutes, systematic review, xerostomia
INTRODUCTION
The normal unstimulated Salivary flow (SF) ranges from 0.25 to 0.35 mL/min. Flow of <0.1 mL/min is considered as hyposalivation.[1,2,3,4] However, the values are subject to large biological variation.[2,5]
More than 500 commonly used medications can cause xerostomia such as antidepressants, antihypertensives, opiates, bronchodilators, proton pump inhibitors, antipsychotics, antihistamines, diuretics, and antineoplastics.[6,7,8] The synergistic effects of combinations of medications also contribute to xerostomia so that although SF does not necessarily decrease with increasing age, the elderly are more likely to have dry mouth because of the increased prevalence of chronic illnesses that have pharmacological treatments associated with the adverse effect of dry mouth sensation.[6] Although drug-induced xerostomia is generally reversible, the conditions for which these medications are prescribed are frequently chronic. Another major cause of xerostomia is radiation therapy for cancers of the head and neck.
Xerostomia may also be caused by autoimmune conditions such as Sjögren’s syndrome that damage the saliva-producing cells.
Hyposalivation can have a significant impact on the activities of daily living such as speaking, eating, and sleeping and may cause dry mouth, tingling, decreasing sense of taste, dental caries, periodontal diseases, halitosis, oral health costs, difficulty in wearing dentures, and increase in opportunistic infections such as Candida albicans.[9]
Increasing elderly population and its dependent use of drugs associated with side effects such as disturbed salivary function put the society at risk of this medico-socio-economic problem.[10] Effective management is required to improve the quality of life (QOL) of patients with xerostomia because of any cause.
The main goal of treatment is to relieve clinical symptoms and improve QOL of patients as no curative treatment is available for xerostomia. The therapies available for the management of xerostomia include sialagogues and saliva substitutes, as well as general measures such as sipping water or chewing gum. Symptomatic management of xerostomia is required when saliva production cannot be stimulated effectively. In such cases, saliva substitutes have been considered as treatment alternatives. Artificial saliva preparations are available in different forms such as sprays and gels and are used as a replacement of natural saliva and to mimic its functions.
Several studies have been conducted on artificial saliva till now, but the effectiveness in the management of xerostomia is still controversial. The lubrication of the oral mucosa reduces symptoms although the effects last for short duration.[11] A review conducted in 2009 indicated that there is a need for further clinical studies on the properties of saliva substitutes.[12] Since then, a number of papers related to artificial saliva for xerostomia management that have been published are available. Therefore, an update on this topic is necessary to access the current effectiveness of the lubricant treatment and provide a recommendation based on evidence. This review aims to systematically review clinical trials conducted on human in the past 10 years and provide an update on artificial saliva based on the latest available evidence.
MATERIALS AND METHODS
In this review, preferred reporting items for systematic reviews and meta-analyses and Cochrane Collaboration criteria are used as guideline to formulate review question, identify studies and assess their quality of selected studies, data extraction, and reporting. A protocol was developed before starting the search process for this review.
Identification and selection of relevant studies
Literature search was carried out in electronic databases in April 2018. Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, and Embase were the electronic databases to retrieve the primary studies. In PubMed, filters were used to limit the search to clinical trials conducted on human, published during the past 10 years (from 2009 to 2018).
To obtain additional data, a manual search was performed using the reference lists of included articles. Inclusion and exclusion criteria were also considered in hand-searched articles.
Selection criteria were as follows:
Inclusion criteria
The following criteria were included in the study:
Parallel group and crossover clinical trials.
Articles written in English language only.
Studies on humans only.
Studies published after 2008.
Studies related to artificial saliva substitutes.
Exclusion criteria
The following criteria were excluded from the study:
Review articles.
Letter to editor.
Observational studies.
Studies conducted on animals.
Articles related to interaction of artificial saliva with different dental restorations and metals.
Trials related to systemic medication or sialagogues.
Trials that compared topical treatments with systemic treatments such as oral pilocarpine or oral cevimeline.
Duplicate articles were removed. Articles were initially screened by reading the titles and abstracts. Studies not satisfying the inclusion criteria were excluded from the study. The remaining articles were screened in the final stage by reading the full text, and those not meeting inclusion criteria were excluded from the study.
Data extraction
The review author and a research assistant assessed all randomized controlled trials, which appeared to meet the inclusion criteria for this review, to confirm eligibility, assess risk of bias, and extract data using a data extraction form. Information was then entered into Table 1.
Table 1.
Overview of all included studies
| S. no. | Author | Objective | Study population | Intervention | Results/conclusion |
|---|---|---|---|---|---|
| 1 | Ameri et al., 2016[13] | Compared the efficacy of an herbal compound containing Malva sylvestris and Alcea digitata (Boiss) with artificial saliva (Hypozalix) to improve the symptoms of dry mouth in patients with head and neck cancer | 62 patients of 20–70 years of age with radiation-induced xerostomia, mean age of 51.5 years in experimental group and 50.3 years in the control group | Herbal (M. Sylvestris and A. digitata) and Hypozalix artificial saliva for 4 weeks | The herbal group showed a significant difference between the grade of dry mouth before and after intervention, but no change was observed for grade of dry mouth in the Hypozalix group. Three subjects in the experimental group experienced a decrease in the grade of dry mouth, but there was no change in the grade of dry mouth in the control group. The change of grade was not statistically significant between groups |
| 2 | Dalodom et al., 2016[14] | To investigate the efficacy of OMJ reducing signs and symptoms of dry mouth in elderly patients | 126 patients complaining of xerostomia, diagnosed with hypertension or DM and receiving medical therapies for at least 1 year. Finally, 118 patients completed the study | OMJ and commercial artificial saliva gel for 4 weeks | The effect was not significant at the end of 2 weeks, but continuous daily uses of OMJ for 1 month reduced the signs and symptoms of xerostomia and improved saliva’s properties of the elderly patients. Slight increase in salivary flow and buffering capacity was noticed |
| 3 | Femiano et al., 2011[15] | To compare the efficacy of saliva substitutes and citric acid therapy for oral dryness relief and unstimulated salivary flow in patients with drug-induced xerostomia | 54 patients reporting drug-induced xerostomia | Artificial saliva, 3% citric acid, and distilled water in mouthwash were used four times daily for 30 days | Both artificial saliva and citric acid provided immediate relief from oral dryness. Citric acid also provided a longer lasting feeling of oral moistness at 1 h after use |
| 4 | Montaldo et al., 2010[16] | To assess oral status in a sample of patients with type 2 diabetes before and after therapy with saliva substitutes and oral status in a control group of patients with diabetes who were not given saliva substitutes | 207 with type 2 diabetes were screened and 134 patients were selected for study with mean age of 47.9–2.9 years. Control subjects consisted of 111 healthy volunteers, (mean age of 44.9–5.8 years) | Immunologically active salivary substitute containing lactoperoxidase, lysozyme, glucose oxidase, lactoferrin for 6 months | In type 2 diabetes, in the case of hyposalivation, a therapy with IASS can be of help in reducing the amount of plaque, gingivitis, and positive yeast counts |
| 5 | Skrinjar et al., 2015[17] | To compare the efficiency of Buccotherm, Xeros, and marshmallow root on the QOL in patients with hyposalivation | 60 patients having drug-induced hyposalivation with mean age of 64 years | Buccotherm, Xeros, marshmallow root. Patients were instructed to use the saliva substitutes four times daily during 2 weeks | All tested saliva substitutes were found effective for decreasing the intensity of dry mouth symptoms as well as improvement in the QOL |
| 6 | Sugiura et al., 2010[18] | To assess the effects of Oral balance on oral mucosal total bacterial counts in patients undergoing HCT | 18 patients with neutropenia undergoing HCT and 10 healthy controls. Mean age of the patient’s group was 42.9 ± 16.2 years and 30.5 ± 4.2 years in control group | Oral balance for 1 week | In patients with neutropenia undergoing HCT, the commercially available saliva substitute, Oral balance, did not increase the total counts of oral mucosal bacteria beyond the range found in healthy controls and alleviate the symptoms induced by hyposalivation |
| 7 | Heydarirad et al., 2017[19] | To compare the effect of A. digitata and M. sylvestris with Hypozalix (artificial saliva) on QOL of patients with radiation-induced xerostomia | 60 patients with HNC, aged 20–70 years (50.60 ± 14.53 and 50.26 ± 15.40 years in the intervention group and the control group, respectively), who had xerostomia, finishing radiotherapy at least 2 months before the study | A. digitata, M. sylvestris, and Hypozalix spray for 4 weeks | The intervention group obtained significant lower oral dryness scores as compared to the control group |
| 8 | Vadcharavivad and Boonroung, 2013[20] | Compared clinical effectiveness between two CMC-based saliva substitutes on radiation-induced xerostomia and related QOL | 50 subjects, mean age of 50.6 years with radiation-induced xerostomia | CCMC-based saliva substitute and KCMH artificial saliva for 14 days | Commercially available CMC-based saliva substitute showed more improvement in severity of xerostomia, speech difficulty, taste alteration, and frequency of sipping water compared with KCMH saliva substitute |
| 9 | Gookizadeh et al., 2012[21] | To evaluate whether bioXtra gel would improve radiation-induced xerostomia, lactobacillus colony count, Candida albicans in the saliva cultures, and oral cavity pH | 55 patients with head and neck cancer (except salivary gland cancers) with average age of 54.9 + 15.5 years (range: 19–82 years, 32 males and 23 females) | bioXtra gel | Significant superiority of bioXtra gel for xerostomia during the day and a speech, but it does not indicate significant effect in swallowing xerostomia. 50 patients (90.9%) were satisfied with its taste, and xerostomia of 32 patients (58.2%) was improved |
| 10 | Donath et al., 2016[22] | To evaluate the efficacy, safety, and tolerability of three formulations of DC161 oral spray, a saliva substitute and a comparator Aequasyal in drug-induced xerostomia | 24 subjects, mean age of 66.8 years, 18 years and older with drug-induced xerostomia | An aqueous formulation, DC161-DP0292, compared with Aequasyal during each of the four 1-day study periods, one of the four test products was applied twice a day, washout period was 3 days | The new oral spray, presented as an aqueous formulation, DC161-DP0292, is considered to be a relevant treatment for drug-induced xerostomia, providing fast and long-acting symptomatic relief |
HCT = hematopoietic cell transplantation, HNC = head and neck cancer
Data were recorded under following headings: study title, study author, objective, study population, interventions, and results.
Risk of bias assessment
The recommended approach for assessing risk of bias according to Cochrane handbook was followed. Domains used to assess risk of bias were selection bias, random sequence generation, and allocation concealment; performance bias, blinding participants; detection bias, blinding outcome assessors; attrition bias, incomplete outcome data; reporting bias, selective outcome reporting; and other sources of bias. Sources of bias that cannot be categorized under aforementioned five main categories are discussed under the domain “other sources of bias.”
Each domain includes one or more specific entries in a “risk of bias” table. Studies were categorized into three categories based on the risk of bias: high risk, low risk, and unclear risk of bias. Studies with one or more domain at high risk of bias were considered to be at high risk. Studies with all domains at low risk were considered to be at low risk. Studies with unclear risk of bias for one or more domains were considered to be at unclear risk of bias. Other sources of bias were assessed as a separate domain. The same review author and research assistant independently carried out the risk of bias assessment as part of the data extraction process.
RESULTS
Result of electronic search performed
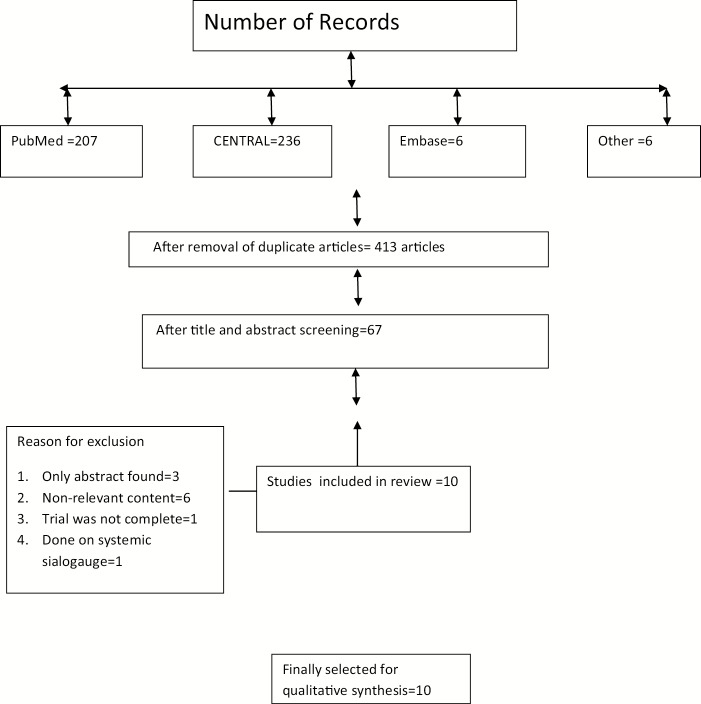
The electronic search identified a total of 455 potential records. A total of 207 articles in PubMed, 236 articles in CENTRAL, and 6 articles were found in Embase. In addition to this, six articles were identified by searching the reference list of relevant studies. Forty-two duplicate articles were found and removed. The same two reviewers who performed electronic search, independently screened titles and abstracts of identified records and selected 67 articles on the basis of inclusion–exclusion criteria. Later, articles published before 2009 were excluded from the study and 21 articles were selected. Full-text copies of these reports were obtained. Of these 21 articles, full text of two articles could not be obtained. Corresponding authors of these two papers were contacted for obtaining full texts but they failed to reply.[23,24] After evaluation of full texts, 11 studies were excluded. Reasons for exclusion are mentioned in flow chart. A total of 10 trials were included for qualitative analysis [Figure 1].
Figure 1.
Flowchart for study selection process
Data abstraction
Characteristics of included studies
Nine of the 10 included studies were designed as parallel group studies. Only one study was a crossover study.[21] Placebo was used in only one trial.[15]
Regarding the country of origin of the 10 studies, three[13,19,21] were conducted in Iran, two[14,20] in Thailand, one study in each of the following: Italy,[16] Croatia,[17] Japan,[18] and Germany.[22] In one study, the trial site is not clearly mentioned.[15] Four studies received funding.[14,18,19,20,21,22] In one study, no financial support was received.[13] No information is available regarding any financial support in five studies.
Sample size calculation was carried out in four studies.[14,17,19,20] It was not required in one study as it was an exploratory study.[22]
Most of the studies were conducted for 4 weeks.[13,14,19] A study conducted by Montaldo et al.[16] was of the longest duration among selected studies and was conducted for 6 months. Two studies were conducted for 2 weeks.[17,21] The duration of one study was 1 week.[18]
Study population
Six hundred and forty-five was the number of total participants with xerostomia, both drug induced and radiation induced. Among included studies, minimum sample size was of 24[22] and maximum was of 134.[16]
Reasons of xerostomia
In four studies, the reason of xerostomia was radiation induced,[13,19,20,21] whereas in four studies, it was drug induced.[14,17,22] One study was conducted on patients with diabetes to determine the effect of diabetes on SF.[16] In one study, patients with neutropenia were included in the study.[18]
Types of interventions provided
Various products used in included studies are listed below:
Oral moisturizing jelly (OMJ): A novel edible saliva substitute compared with commercial artificial saliva gel.[14]
Herbal powder of Alcea digitata and Malva sylvestris compared with Hypozalix (artificial saliva).[13,19]
Immunologically active saliva substitutes (IASS): Immunologically active salivary substitute containing lactoperoxidase, lysozyme, glucose oxidase, and lactoferrin.[16]
(Buccotherm®, Laboratoire Odost, Castera-Verduzan, France), (oral spray based on thermal spring water), Xeros (Xeros®, Dentaid, Barcelona, Spain), (commercially available mouthwash containing betaine, sodium fluoride, and hydroxyethyl cellulose), and marshmallow root (Zagreb City Farmacy, Zagreb, Croatia) (marshmallow root is traditionally used in Croatia to alleviate dry mouth symptoms).[17]
Commercially available carboxymethylcellulose (CCMC): CMC is a polymer derived from natural cellulose. It is used in saliva substitute formulation as a thickening agent. King Chulalongkorn Memorial Hospital (KCMH) artificial saliva is a liquid CMC-based preparation that has been compounded and dispensed at KCMH.[20]
Oral balance: Commercially available artificial saliva substitute.[18]
bioXtra gel (Laclede, Inc., Rancho Dominguez, CA, USA), (commercial synthetic saliva substitute, combination of lactoferrin, lysozyme, and lactoperoxidase).[21]
DC161-DP0292 aqueous solution (Pierre Fabre Medical Devices) (glycerol, povidone K30, copovidone, xanthan gum, potassium chloride, xylitol, marshmallow concentrated hydroglycerined extract, anhydrous disodium hydrogen phosphate, potassium dihydrogen phosphate, macrogolglycerol 40 hydroxystearate, potassium sorbate, benzylic alcohol, and pure water) and Aequasyal oily solution (Carilène Laboratory, Montesson, France) (94.4% tri-esters of glycerol oxidized fatty acids of vegetal origin [corn oil]; silicium dioxide; food aromas: orange, grapefruit, and mint; and aspartame).[22]
Artificial saliva, 3% citric acid, and distilled water (placebo).[15]
Primary and secondary outcome measures used in included studies
In most of the trials, primary outcome was the subjective assessment of relief from dry mouth or effectiveness of treatment.13,14,15,20,21,22] In one trial, it was the secondary outcome.[17] In three trials, the primary outcome was QOL.[17,19,20]
Other primary outcomes were patient satisfaction,[14] flow rate of unstimulated whole saliva (UWS),[15] assessment of oral health status in patients with type 2 diabetes mellitus (DM),[16] and total bacterial count on buccal mucosa.[18]
Secondary outcome measures were objective dry mouth score, salivary pH, buffering capacity of saliva, SF rate,[14] and any observed adverse events.[19] In a study conducted by Donath et al.,[22] the secondary outcome was evaluation of dry mouth over 2 h after second application of product. Parameters used for evaluation were the time of onset of action, time of maximum effect, and duration of action.[22]
Risk of bias assessment
Randomization: Only two of 10 trials were not randomized.[14,18] In one study, randomized list was generated using Microsoft Excel Inc. USA, version 15, with block randomization method (non-stratified, with the same block lengths).[19]
Allocation concealment: It was carried out in two studies.[15,19] In one of these two studies, only statistician was kept blinded.[19]
Blinding: Blinding was assessed for both patients and outcome assessors. Patients were blinded in only two trials.[15,20] Outcome assessors were blinded in two trials.[15,16]
Incomplete outcome: Three studies were found to have unclear risk of attrition bias.[17,18,21]
Selective reporting: All the studies were found to be at low risk of selective reporting bias.
Other sources of bias: The study sponsored by a pharmaceutical company and the trial report seemed focused on the product of the same companies.[18] In one trial group, similarity was questionable.[18]
Overall risk of bias: Only one study was found to be at low risk of bias.[15] Five studies were judged to be at high risk[14,18,19,20,22] and four studies were judged to be at unclear risk of bias.[13,14,17,21] Risk of bias for individual studies is discussed in Table 2.
Table 2.
Risk of bias in included studies
| S. no. | Author | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| 1 | Ameri et al.[13] | Done but the method of randomization not stated | Unclear data | Unclear data | Unclear data | In the experimental group, 75 subjects initially participated and 62 completed the study | Low risk of bias | Unclear risk of selection bias, performance bias, detection bias. High risk for attrition bias |
| 2 | Dalodom et al.[14] | Randomization not done | Not done | Not done | Not done | Of 126, 118 patients completed the study | Low risk of bias | High risk of selection, attrition, and performance bias |
| 3 | Femiano et al.[15] | Using random number generator computer software | Done | Done | Done | Complete outcome data, all 54 subjects completed the study | Low risk of bias | Low risk of bias |
| 4 | Montaldo et al.[16] | Randomized but method of randomization not stated | Insufficient information | Not done | Done | Complete outcome data, all 134 patients completed the study | Low risk of bias | Unclear risk of selection bias. Although blinding was not done, it is unlikely to cause bias as the outcomes were assessed by blinded investigators |
| 5 | Skrinjar et al.[17] | Randomization was performed with a random number generator | Insufficient information | Open-label study | Open-label study | Insufficient information. Not mentioned the number of participants completed the study | Low risk | Unclear risk for selection and attrition bias. High risk for performance and detection bias |
| 6 | Sugiura et al.[18] | Not done | Not done | Not done | Not done | It was not mentioned that how many participants completed the study | Low risk of bias | High risk of bias. Although blinding was not done, it would not affect the result as the outcome assessment was based on laboratory tests. Received financial support from the corporation who is exclusive agent of product used in the study |
| 7 | Heydarirad et al.[19] | Randomized list was generated using Microsoft Excel, version 15, with block randomization method (non-stratified, with the same block lengths) | Done for statistician only | Not done | Not done | Complete outcome data provided. In control group 4 and in herbal group 3 subjects discontinued the study. | Low risk of bias | High risk for performance and detection bias |
| 10 | Vadcharavivad and Boonroung[20] | Randomization done but method of randomization not stated | Not done | Patients blinded | Not blinded | Complete outcome data provided. All patients completed the questionnaires before randomization and at the end of 14-day treatment period | Low risk of bias | High risk for selection bias, detection bias |
| 11 | Gookizadeh et al.[21] | Randomization done but method of randomization not stated | Unclear | Unclear | Unclear | Not mentioned number of participants that completed the study | Low risk of bias | Unclear risk of bias |
| 12 | Donath et al.[22] | Computer-generated randomization | Unclear | Open-label study | Open-label study | Complete data provided. All 24 subjects completed the study | Low risk of bias | High risk of performance and detection bias. Unclear risk of selection bias. Pierre Fabre Medical Devices provided funding for the study. Few authors were employees of Pierre Fabre Laboratories |
Effectiveness of the interventions
1. Herbal versus Hypozalix: The study was a randomized controlled trial conducted in Iran. This study compared the efficacy of herbal products (M. sylvestris and A. digitata) and Hypozalix (Biocodex, France) (artificial saliva) in reducing radiation-induced xerostomia in 62 subjects. The study duration was of 4 weeks and the outcome was measured at the end of 2 weeks and 4 weeks using visual analog scale (VAS). At the end of 4 weeks, significant decrease in VAS score was noticed in herbal group as compared to artificial saliva group.[13]
The second study that compared same interventions was also conducted in Iran on 60 subjects with radiotherapy completed 2 months before this study. Sample size calculation was carried out. The outcome measure was QOL, measured using questionnaire. Group was similar at baseline (Heydarirad et al.[19]). The study concluded that study group taking traditional persian medicine preparations experienced improvement in more areas of their xerostomia-associated symptoms as well as QOL as compared to the group using Hypozalix spray.[19]
2. OMJ versus commercial artificial saliva gel: This study was designed as pre-post design and conducted in Thailand. Subjects enrolled were having DM and/or hypertension and received medical therapy for at least 1 year. Initially, 126 patients were recruited and 118 patients completed the study. Primary outcome was patient satisfaction and was measured by subjective dry mouth score. Secondary outcome measures were SF rate, salivary pH, buffering capacities, and objective dry mouth score. Objective dry mouth score was examined for their signs of dry mouth, including loss of pooled saliva, mouth mirror stickiness, stringy or foamy appearance, labial dehydration, and irresponsiveness to parotid stimulation.
Approximately 65% of patients selected OMJ and rest 35% preferred commercial artificial saliva gel. The highest preference of OMJ was correlated with easily swallowing, extra deliciousness, and more hydrating. Subjective dry mouth scores reduced 73% at the end of 2 weeks and 88% at the end of 1 month from their baseline data, after using OMJ.
In case of objective dry mouth scores, results were not changed significantly at the end of 2 weeks for OMJ, but at the end of 4 weeks, 16% reduction was observed. Increase in buffering capacity and slight increase in SF were noticed after the use of OMJ.[14]
3. IASS: The study was conducted in Italy, which included 134 patients with diabetes. Subjects were randomly divided into two groups of 67 people each. Immunologically active salivary substitutes containing lactoperoxidase, lysozyme, glucose oxidase, and lactoferrin were provided to one group for 6 months. The set provided to trial subjects included toothpaste, mouthwash, and a moistening gel. Instructions were given to brush teeth thrice daily with the set toothpaste and to rinse with the set mouthwash. Subjects were told to apply the supplied moistening gel to their tongue and gums at night and whenever they feel dry. The second group was not provided anything. Patients in both groups were told not to change their eating, drinking, or smoking habits. Patients who were not given salivary substitutes showed a higher risk of having both gingivitis, positive yeast counts, and a higher amount of plaque.[16]
4. Buccotherm, Xeros, and marshmallow root: Randomized, open-label study was conducted on 60 patients with mean age of 64 years, having drug-induced hyposalivation. Patients with unstimulated SF rate <0.2 mL/min were randomized into three groups. In the first group, 30 patients were using Buccotherm; in the second group, 15 patients were using Xeros; and in the third group, 15 patients were using marshmallow root. Duration of study was 2 weeks. Patients were instructed to use one of the products four times a day. Primary outcome measure was QOL, assessed by oral health impact profile questionnaire. The second outcome measure was feeling of dryness, assessed by VAS. The study found that all three saliva substitutes showed significant improvement but Buccotherm was superior to Xeros and marshmallow root.[17]
5. CCMC and KCMH: This questionnaire-based study conducted in Thailand included 50 subjects with 50.6 years of mean age. Subjects completed radiotherapy for at least 1 month before enrollment in the trial. Outcome measures were severity level of xerostomia and xerostomia-related QOL. It was found that commercially available CMC-based saliva substitute showed better outcomes in improving the severity of xerostomia, speech difficulty, taste alteration, and frequency of sipping water compared with KCMH after 14 days.[20]
6. Oral balance: The study included 18 patients with neutropenia undergoing hematopoietic cell transplantation and 10 healthy subjects in control group. Outcome measure was total bacterial counts on buccal mucosa assessed by quantitative polymerase chain reaction amplification of 16s rDNA. It concluded that no significant increase in bacterial counts was observed, associated with the use of Oral balance. The study may be subject to high risk of bias as it received financial support from corporation, which was exclusive agent of product used in this study.[18]
7. bioXtra gel: The study was conducted to evaluate the efficacy of bioXtra gel in improving radiation-induced xerostomia, lactobacillus colony count, C. albicans in the saliva cultures, and oral cavity PH. A total of 55 patients with radiation-induced xerostomia were included in the study. Patients received two bioXtra gel for 2 weeks (one spoon, three times a day). Assessment was carried out of xerostomia, swallowing, and oral cavity examined for infections and teeth condition initially and after 2 weeks. Oral cavity pH, lactobacillus colony count, and C. albicans were checked in laboratory. The study indicated a significant superiority of bioXtra gel for xerostomia during the day and speech impairment, but it did not indicate significant effect in swallowing xerostomia. The bioXtra gel increased the pH.[21]
8. DC161-DP0292: This was an exploratory, randomized, open‐label, active‐controlled, four‐period, and crossover study conducted at a single center in Germany. Three formulations (two aqueous and one oily) of DC161 oral spray, a new saliva substitute, and an active comparator, Aequasyal oral spray (oily), were assessed for clinical efficacy in terms of their relief on drug‐induced xerostomia and other associated symptoms. The subjective intensity of various symptoms was self‐assessed by the subject using a 100-mm VAS, a validated and well‐described assessment tool. The four periods of 1-day study were separated by a washout of 3 days maximum. A total of 40 subjects were screened, of which 24 were selected for the study. All subjects completed the study. Of the three DC161 formulations tested, only DC161‐DP0292 showed a reduction in mouth dryness. Subjects’ overall evaluation of the product was higher for DC161‐DP0292 than comparator, following both applications were not blinded, both the investigator and the subjects were aware of the formulations being investigated and could bias the subjective assessments.[22]
9. Artificial saliva, 3% citric acid, and distilled water: A total of 78 patients complaining of a sensation of dry mouth were clinically evaluated, of these, 54 were selected for the study. At the end of the study (30 days), all patients were evaluated by investigator, who was blinded to the form of therapy given to each group. All patients completed the study. After 15 min. of solution intake, 12 patients (67%) belonging to the artificial saliva group, 9 (50%) from the citric acid group, and 2 (11%) from the water group reported significant symptomatological improvement. After 1 h of solution intake, 7 patients (39%) from the artificial saliva group, 10 (56%) from the citric acid group, and 0 from the water group noted significant symptomatological improvement. Both salivary substitutes and citric acid proved to be effective against dry mouth, but 3% citric acid provided a longer lasting beneficial effect in relief of the dry mouth feeling compared with salivary substitutes 1 h after use. Neither the 3% citric acid nor the saliva substitutes affected the UWS flow rate.[15]
DISCUSSION
The studies included in this review evaluated a range of different interventions and were mostly found to be at high or unclear risk of bias. Therefore, due to lack of evidences, it was difficult to conclude the most effective product or intervention. However, the findings of all the studies included in this review were summarized.
The selected studies were categorized under following categories:
Studies conducted on subjects with drug-induced xerostomia
Studies conducted on subjects with radiation-induced xerostomia
Others
Drug-induced xerostomia
Four studies were conducted on subjects with drug-induced xerostomia. In a study conducted by Skrinjar et al.,[17] all tested agents Buccotherm, Xeros, and marshmallow root showed beneficial effect in alleviating hyposalivation symptoms and improvement in QOL, but Buccotherm was found to be superior to other two.[17]
Results of one study showed that the use of OMJ for 2 weeks significantly reduced the symptoms of dry mouth, whereas the use for 1 month reduced the signs of xerostomia, prevented the decline of salivary pH, and improved buffering capacities. OMJ was equally effective in patients taking 1–2 and 3–7 medications. Furthermore, 65% of patients preferred OMJ over a commercial product.[14]
An aqueous formulation—DC161‐DP0292—reduced the intensity of dryness of mouth at least as well as the comparator, Aequasyal oral spray; DC161‐DP0292 provided a fast relief and a long‐lasting effect on mouth dryness. Both products improved other symptoms such as swallowing and speaking, even when applied just before a meal.[22]
A study conducted by Femiano et al.[15] showed that both artificial saliva and citric acid provided immediate relief from oral dryness. Citric acid also provided a longer lasting feeling of oral moistness at 1 h after use owing to its protracted activity on salivary gland function. None of the drugs tested affected UWS flow.[15]
Radiation-induced xerostomia
Four studies were conducted on subjects with radiation-induced xerostomia. Of these four, two studies compared M. sylvestris and A. digitata (herbal) with artificial saliva substitutes. Both studies concluded that herbal compounds showed more improvement in xerostomia-associated symptoms as well as QOL as compared to the group using Hypozalix spray at the end of 4 weeks.[13,19]
bioXtra gel relieved xerostomia during the day and night and speech impairment, but xerostomia, while eating and drinking water during a meal, indicated no significant difference after 2 weeks. In addition, bioXtra gel increased the PH and the candida counts,[21] commercially available CMC-based saliva substitute was found effective in improving the severity of xerostomia, speech difficulty, taste alteration, and frequency of sipping water after 14 days of treatment.[20] Results suggest that relief from radiation-induced xerostomia during swallowing was provided by CMC-based saliva substitutes used by Vadcharavivad and Boonroung[20] and not by bioXtra.
All four studies used questionnaire or VAS to measure outcomes; therefore, lack of objective outcome measures for assessment may introduce bias. More clinical trials with large sample size addressing this issue are required.
Others
In this category, two studies were included that do not fall into aforementioned two categories; one study included patients with neutropenia undergoing hematopoietic cell transplantation[18] and the other was conducted on patients with type 2 DM.[16] A study conducted by Montaldo et al.[16] concluded that a therapy with IASS can be useful in the reduction of the amount of plaque, gingivitis, and positive yeast counts. The use of Oral balance may reduce symptoms caused by hyposalivation without promoting infection.[18]
A similar review carried out in 2009 by Hahnel et al.[12] suggested that significant differences are present in various saliva substitutes concerning the review parameters and indicated that further studies need to be conducted on the properties of saliva substitutes. After 10 years from the latter review, there is a lack of strong evidence available. However, all the tested products reduced the dryness of mouth; the effect lasted for short duration. There is no effective treatment for xerostomia till now. Therefore, there is a need to find and test newer products for their efficacy.
A recent study concluded that artificial salivary substitutes with its biological, rheological properties can be a great adjunct influencing the success of prosthodontic treatment by restoring the health of dehydrated mucosa.[25] Available saliva substitutes for xerostomia management should be used according to the individual patient’s concerns, preferences, and oral health needs. Patient’s current oral health status, hygiene regime, and compliance should be considered while making a treatment plan for patients with xerostomia.[26]
Limitations
This review was limited to the last 10 years and to English language studies, which may preclude some information about the topic to be retrieved. Gray literature was not considered. Full texts of two studies were not found after contacting the authors as they did not reply.[23,24] Therefore, those studies could not be included in this review, which may cause bias. This review may be subject to publication bias as unpublished literature was not included in the study.
Recommendation for further research
Well-designed randomized controlled trials for dry mouth are required to provide evidence to improve clinical care. Patient satisfaction is an important consideration. Therefore, crossover trials, a design that allows more efficiency in determining patient preferences, would be more appropriate.[27]
CONCLUSION
The studies included in this review were carried out on different artificial saliva products; therefore, it is challenging to reach a definite conclusion. Nevertheless, published studies suggest that all tested products reduced signs and symptoms of xerostomia. Herbal products were reported to show more improvement than artificial saliva in case of radiation-induced xerostomia. Oral spray presentation and 3% citric acid provide a long‐lasting effect on drug-induced xerostomia. Products that improved dryness symptoms during swallowing and speaking were OMJ, oral spray (Aequasyal), and DC161‐DP0292 in case of drug-induced xerostomia. Management of xerostomia may be performed in combination of available products according to the individual patient’s concerns, preferences, and oral health requirements. It was not possible to compare efficacy to reduce signs and symptoms of products because of heterogenicity among studies in terms of study product, site, and duration. In addition, most existing studies had high risk of bias. Therefore, there is a need of more randomized controlled trials on effects of various products conducted according to Consolidated Standards of Reporting Trials (CONSORT) statements with large sample size to reach an unbiased conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tenovuo J, Lagerlöf F. Saliva. In: Thylstrup A, Fejerskov O, editors. Textbook of clinical cardiology. 2nd ed. Copenhagen, Denmark: Munksgaard; 1994. [Google Scholar]
- 2.Edgar M, Dawes C, O’Mullane D. 3rd ed. London, UK: BDJ Books; 2004. Saliva and oral health. [Google Scholar]
- 3.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66:648–53. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 4.Axelsson P. Vol. 2. Batavia, IL: Quintessence Books; 2000. Diagnosis and risk prediction of dental caries. [Google Scholar]
- 5.Douglas CR. 5th ed. São Paulo, Brazil: Robe Editorial; 2002. Tratado de fisiologia aplicada à saúde. [Google Scholar]
- 6.Thelin WR, Brennan MT, Lockhart PB, Singh ML, Fox PC, Papas AS, et al. The oral mucosa as a therapeutic target for xerostomia. Oral Dis. 2008;14:683–9. doi: 10.1111/j.1601-0825.2008.01486.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–6. [PubMed] [Google Scholar]
- 8.Mouly SJ, Orler JB, Tillet Y, Coudert AC, Oberli F, Preshaw P, et al. Efficacy of a new oral lubricant solution in the management of psychotropic drug-induced xerostomia: a randomized controlled trial. J Clin Psychopharmacol. 2007;27:437–43. doi: 10.1097/jcp.0b013e31814db434. [DOI] [PubMed] [Google Scholar]
- 9.Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68:419–27. doi: 10.1016/0030-4220(89)90140-0. [DOI] [PubMed] [Google Scholar]
- 10.Löfgren CD, Wickström C, Sonesson M, Lagunas PT, Christersson C. A systematic review of methods to diagnose oral dryness and salivary gland function. BMC Oral Health. 2012;12:29. doi: 10.1186/1472-6831-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil-Montoya JA, Silvestre FJ, Barrios R, Silvestre-Rangil J. Treatment of xerostomia and hyposalivation in the elderly: a systematic review. Med Oral Patol Oral Cir Bucal. 2016;21:e355–66. doi: 10.4317/medoral.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahnel S, Behr M, Handel G, Bürgers R. Saliva substitutes for the treatment of radiation-induced xerostomia—a review. Support Care Cancer. 2009;17:1331–43. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- 13.Ameri A, Heydarirad G, Rezaeizadeh H, Choopani R, Ghobadi A, Gachkar L. Evaluation of efficacy of an herbal compound on dry mouth in patients with head and neck cancers: a randomized clinical trial. J Evid Based Complementary Altern Med. 2016;21:30–3. doi: 10.1177/2156587215590232. [DOI] [PubMed] [Google Scholar]
- 14.Dalodom S, Lam-Ubol A, Jeanmaneechotechai S, Takamfoo L, Intachai W, Duangchada K, et al. Influence of oral moisturizing jelly as a saliva substitute for the relief of xerostomia in elderly patients with hypertension and diabetes mellitus. Geriatr Nurs. 2016;37:101–9. doi: 10.1016/j.gerinurse.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Femiano F, Rullo R, di Spirito F, Lanza A, Festa VM, Cirillo N. A comparison of salivary substitutes versus a natural sialagogue (citric acid) in patients complaining of dry mouth as an adverse drug reaction: a clinical, randomized controlled study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e15–20. doi: 10.1016/j.tripleo.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Montaldo L, Montaldo P, Papa A, Caramico N, Toro G. Effects of saliva substitutes on oral status in patients with type 2 diabetes. Diabet Med. 2010;27:1280–3. doi: 10.1111/j.1464-5491.2010.03063.x. [DOI] [PubMed] [Google Scholar]
- 17.Skrinjar I, Vucicevic Boras V, Bakale I, Andabak Rogulj A, Brailo V, Vidovic Juras D, et al. Comparison between three different saliva substitutes in patients with hyposalivation. Clin Oral Investig. 2015;19:753–7. doi: 10.1007/s00784-015-1405-8. [DOI] [PubMed] [Google Scholar]
- 18.Sugiura Y, Soga Y, Yamabe K, Tsutani S, Tanimoto I, Maeda H, et al. Total bacterial counts on oral mucosa after using a commercial saliva substitute in patients undergoing hematopoietic cell transplantation. Support Care Cancer. 2010;18:395–8. doi: 10.1007/s00520-009-0789-x. [DOI] [PubMed] [Google Scholar]
- 19.Heydarirad G, Rezaeizadeh H, Choopani R, Mosavat SH, Ameri A. Efficacy of a traditional Persian medicine preparation for radiation-induced xerostomia: a randomized, open-label, active-controlled trial. J Integr Med. 2017;15:201–8. doi: 10.1016/S2095-4964(17)60333-9. [DOI] [PubMed] [Google Scholar]
- 20.Vadcharavivad S, Boonroung T. Effects of two carboxymethylcellulose-containing saliva substitutes on post-radiation xerostomia in head and neck cancer patients related to quality of life. Asian Biomed. 2013;7:93–202. [Google Scholar]
- 21.Gookizadeh A, Emami H, Najafizadeh N, Roayaei M. Clinical evaluation of BIOXTRA in relieving signs and symptoms of dry mouth after head and neck radiotherapy of cancer patients at Seyed-Al-Shohada Hospital, Isfahan, Iran. Adv Biomed Res. 2012;1:72. doi: 10.4103/2277-9175.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donath F, Tonner F, Chavda R, Gatignol JP, Bouyrie J. Randomized trial of the efficacy and safety of a new oral spray for drug-induced xerostomia. Clin Exp Dent Res. 2016;2:112–20. doi: 10.1002/cre2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salom M, Hachulla E, Bertolus C, Deschaumes C, Simoneau G, Mouly S. Efficacy and safety of a new oral saliva equivalent in the management of xerostomia: a national, multicenter, randomized study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:301–9. doi: 10.1016/j.oooo.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Milleman JL, Milleman KR, Santos SL, Proskin HM, Battershell KK, DiMarino JC. Subjective assessment of Enamelon® preventive treatment gel in a self-reported dry-mouth population. Compend Contin Educ Dent. 2016;37:e5–8. [PubMed] [Google Scholar]
- 25.Saraswathy B, Ganapathy D. Salivary substitutes in prosthodontics. Int J Recent Adv Multidisciplinary Res. 2016;3:1350–2. [Google Scholar]
- 26.Dost F, Farah CS. Stimulating the discussion on saliva substitutes: a clinical perspective. Aust Dent J. 2013;58:11–7. doi: 10.1111/adj.12023. [DOI] [PubMed] [Google Scholar]
- 27.Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials. 2009;10:27. doi: 10.1186/1745-6215-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]