Abstract
Purpose of review
The aim of this study was to describe the newest development in cystic fibrosis (CF) care, CF transmembrane conductance regulator (CFTR) modulator therapies.
Recent findings
Phase II results showing CFTR modulator triple therapies are more effective than current CFTR modulators.
Summary
CFTR modulator therapy targets the protein defective in CF and boosts its function, but the drug must match mutation pathobiology. Ivacaftor, a CFTR potentiator, was the first modulator approved in 2012, with impressive improvement in lung function and other measures of disease in patients with gating and other residual function mutations (∼10% of CF patients). In 2015, the combination of lumacaftor, a CFTR corrector, and ivacaftor was approved for patients homozygous for the F508del mutation (∼40–50% of the CF population) with positive but less impressive clinical response and 10–20% incidence of intolerance. A next-generation CFTR corrector, tezacaftor, with ivacaftor equally effective and better tolerated than lumacaftor, has also received US Food and Drug Administration approval. Novel CFTR correctors, entering Phase 3 trials in triple modulator combination with tezacaftor-ivacaftor, appear substantially more effective for patients who are homozygous for the F508del mutation and can provide benefit for patients with a single F508del mutation. This offers promise of effective CFTR modulator therapy for nearly 90% of CF patients.
Keywords: cystic fibrosis, cystic fibrosis transmembrane conductance regulator modulator, personalized medicine
INTRODUCTION
Cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators take a fundamental targeted treatment approach to CF by improving function of the defective CFTR protein that causes CF. Modulators have efficacy specific to the functional type or class of CFTR mutation that the patient carries (see Mutation Classes below). Currently targeted to groups of patients based on CFTR mutation class, truly personalized medicine would entail predictive individual testing and treatment, a process dubbed theratyping [1]. In the 2016 Cystic Fibrosis Foundation (CFF) Patient Registry report, median predicted survival for an individual born with CF in 2016 is 47.7 years [2]. Most therapies that have increased survival are based on minimizing end-organ symptomatology and disease complications. CFTR modulators represent a different and more fundamental approach.
CFTR is a cell membrane anion channel affecting fluid and ion content of exocrine tissues [3,4]. It also interacts with a respiratory epithelial sodium channel (ENaC) and functionally affects many other cell proteins [5,6]. Inheritance of two functionally defective CF gene alleles from both parents results in CF. Dysfunction of CFTR in the sweat gland causes an increased salt in the sweat; in the airways, deficient chloride and bicarbonate secretion causes thick secretions leading to a vicious cycle of mucus obstruction, chronic infection and inflammation. Varying disease severity not only results from CFTR genotype but also other genetic and environmental factors. Organs with functionally important cells expressing CFTR (e.g. respiratory tract, sweat gland, pancreas, hepatobiliary tract, vas deferens) are affected, resulting in a multisystem, heterogeneous disease.
MUTATION CLASSES
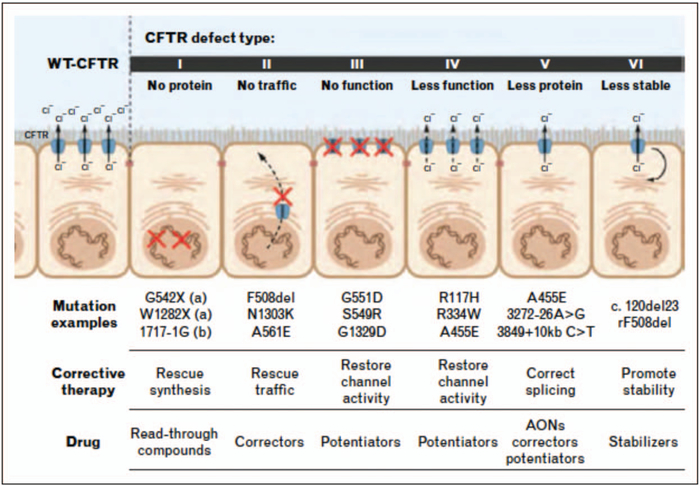
There are six functional CFTR mutation classes (Fig. 1) [7]. Class I results in deficient CFTR protein synthesis and is usually caused by a stop codon in a nonsense mutation. Class II mutations include F508del and result in protein folding and trafficking defects, causing nascent CFTR protein to be marked for degradation before it can reach its functional site in the cell membrane. Nearly half of all CF patients are homozygous for the F508del mutation and another 35–40% are heterozygous for F508del. Class III mutations result in CFTR protein that reaches the cell membrane but has defective regulation and does not sufficiently allow movement of anions; these are known as gating mutations. Class IV mutations result in CFTR proteins that have decreased anion conductance due to pore defects. There is reduced synthesis of the CFTR protein with class V mutations, often from a splicing defect. Class VI results in decreased CFTR membrane stability resulting in increased turnover [3,4]. It is important to note that a single mutation can result in multiple class defects; for example, Class II F508del also results in Class III and Class V defects [8].
FIGURE 1.
Functional classification of cystic fibrosis transmembrane conductance regulator mutations causing cystic fibrosis with major examples in each class, associated approach to targeted CFTR modulator therapies and drug type nomenclature. Current approved drugs include the potentiator ivacaftor, the corrector lumacaftor and the corrector tezacaftor. AONs, antisense oligonucleotides; WT, wild type. Reproduced with permission from [7].
DRUG DEVELOPMENT
Cells collected from patients with CF can be grown in cell culture and have been utilized to understand CF pathophysiology and screen drug candidates. Bronchial epithelial cell cultures (BECs) grown from patients with CF maintain morphological and functional characteristics of the CF airway and can be used to examine effects of candidate compounds on ion transport, airway surface fluid, mucus and ciliary movement [9].
As the CFTR gene was cloned in 1989, researchers have been working towards correcting the dysfunction. Substantial effort was initially put into gene therapy to replace the mutant gene in respiratory epithelia with wild-type DNA, but this approach, while generally shown to be possible and well tolerated, has not to date yielded beneficial clinical results [10]. Research efforts then moved towards rescuing endogenous CFTR protein.
In this approach, the CFF provided substantial financial support to Vertex, a biopharmaceutical company, to incentivize this endeavour. Highthroughput screening is a method of drug discovery utilizing robotics, data processing and sensitive plate readers, which allows for rapid testing of numerous potentially bioactive molecules. Multiple small molecules that could improve the function of the mutant CFTR protein in vitro were identified by high throughput screening using cell-based chloride transport assays, validated using BECs from patients with defined CFTR mutations, and further optimized and tested in animal toxicity models before entering clinical trials. This approach obviates reliance on genetically engineered CF animal models, which now exist in several mammalian species but have limitations in modelling clinical CF.
Promising drug candidates enter phased clinical trials. Phase I trials evaluate safety and pharmacokinetics. In Phase 1, a small number of healthy volunteers receive drug, drug metabolism is measured and safety assessed. Phase 2 trials examine safety with longer exposures and early evidence (often biomarkers) of efficacy in patients with the disease of interest, typically enrolling several dozen to at least 100 patients. Phase 3 trials are pivotal for approval, determining yet longer exposure safety and clinically relevant efficacy measures in a large patient population. These are typically randomized double-blinded and placebo-controlled. After US Food and Drug Administration (FDA) approval, Phase 4 studies, also called postmarketing surveillance, are carried out (sometimes mandated) to evaluate long-term safety.
CURRENT DRUGS
In January 2012, ivacaftor (Kalydeco, Vertex Pharmaceuticals, Boston, Massachusetts, USA) was the first CFTR modulator approved by the FDA. It is a potentiator, that is increasing activity of CFTR in the cell membrane by increasing open channel probability. Clinical trials in the most common class III mutation, G551D (present in 4–5% of patients), showed impressive results. Improved lung function with an average 10% absolute improvement in forced expiratory volume in 1 s (FEV1) percentage predicted occurred within the first 2 weeks and was sustained over 48 weeks, with a 55% decrease in pulmonary exacerbations, improved quality of life and improved nutritional status [11]. Sweat chloride dramatically decreased into the normal range in most patients [11]. Notably, these clinical effects correspond to a 30–50% in-vitro restoration of CFTR activity in G551D-containing BECs. Follow-up studies have shown these clinical benefits are sustained long-term [12–14]. With further clinical trial data, FDA approval has been extended down to age 2 years [15,16■]. Over the last 5 years, clinical trial based FDA approval was expanded to include nine more gating mutations [17,18]. In 2017, the FDA approved use of ivacaftor for an additional 23 mutations with residual function. This extended approval was based on in-vitro data utilizing in-vitro cell-based assays wherein individual mutations could be tested for their response to ivacaftor [19]. Approval based on predictive in-vitro cell-based functional data was unprecedented. As these mutations are very rare, clinical trials with sufficient patient numbers for adequate statistical power are not possible.
Although ivacaftor provided promising therapy for CF, it was only available to nearly 5% of the population of patients with CF after initial approval (and still only ∼10% after additional approvals). Finding a medication to improve the function of the F508del mutation would be of greater import, as nearly 90% of patients with CF have at least one F508del allele. Unfortunately, ivacaftor alone did not show clinical improvement in patients homozygous for F508del [20]. The CFTR corrector VX-809 (lumacaftor) which reduces the protein folding error was studied. By itself lumacaftor was also ineffective in clinical trial [21]. But as more CFTR with the F508del mutation does reach the cell membrane with lumacaftor, if given together with ivacaftor, it can help improve F08del CFTR function. Homozygous F508del BECs show nearly 25% restoration of CFTR activity in vitro with combination lumacaftor-ivacaftor treatment. Clinical trials of lumacaftor-ivacaftor in patients with homozygous F508del were successful [22]. The pivotal Phase 3 trials did not improve lung function as much as the earlier studies with ivacaftor in G551D patients (i.e. ∼3% absolute increase in FEV1 percentage predicted, resulting from patients distributing into a typical bell-shaped drug response curve, while almost all gating mutation patients respond to ivacaftor). However, exacerbations were decreased by nearly 40% during the 24-week study period [22]. In July 2015, the FDA approved lumacaftor-ivacaftor (Orkambi, Vertex Pharmaceuticals, Boston, Massachusetts, USA) for patients at least 12 years of age homozygous for F508del. However, patients with only one copy of F508del did not show clinical improvement, presumably due to less drug substrate [23]. The durability of the clinical benefit of lumacaftor-ivacaftor in homozygous F508del patients has been demonstrated in epidemiologic follow-up studies, in which nearly 40% slowing of the rate of lung function decline over several years is similar to that achieved by ivacaftor in G551D patients [12–14,24■■]. In September 2016, FDA approval was extended to patients homozygous for F508del as young as age 6 years based on pivotal trials showing safety and efficacy in this age group [25,26].
Although a therapy for nearly 50% of CF patients homozygous for F508del is exciting, Orkambi has tolerability issues and drug-drug interactions may be troublesome. There are substantial rates of intolerance and side effects, most notably in those with low lung function [27,28].
Tezacaftor is a second-generation CFTR corrector, which has been shown in Phase 3 trials to have improved tolerability compared with lumacaftor when used in combination with ivacaftor while having similar clinical benefit to Orkambi in homozygous F508del patients [22,29■■], or those with one F508del allele and a residual function second CFTR mutation [30■]. Tezacaftor-ivacaftor (Symdeko, Vertex Pharmaceuticals, Boston, Massachusetts, USA) received FDA approval in February of 2018 [31] and is expected to replace Orkambi for homozygous F508del patients intolerant to or unwilling to use Orkambi. Perhaps more significantly, tezacaftor-ivacaftor provides a backbone for a triple combination (two correctors and a potentiator) CFTR modulator approach to patients with either one or two copies of F508del, as two-site biosynthetic correction and in-vitro functional testing in appropriate cell models predict robust (>50%) restoration of CFTR activity [8,32]. It is hoped that this ‘triple combo’ approach will thus be able to provide effective therapy for 90% of CF patients.
THE FUTURE
Phase 1–2 trials adding different next-generation correctors to tezacaftor-ivacaftor to make a triple therapy are ongoing. Early results have been reported with improvements in lung function (absolute increase in FEV1 of 7–12%) incremental to tezacaftor-ivacaftor [33]. On 1 February 2018, Vertex announced it has chosen VX-445 and VX-659 as corrector drugs that will move forward into Phase 3 testing in triple combination therapies [34]. Phase 3 trials for VX-445 are anticipated to start in early 2018 and VX-659 in late 2018.
For the nearly 10% of CF patients unresponsive to F508del functional restoration due to disease arising from severe non-F508del CFTR mutations, efforts are proceeding in a variety of distinct research areas, including read-through agents for stop codons in nonsense mutations, DNA or RNA replacement (gene therapy or RNA therapy), antisense oligonucleotides for certain splice mutations and gene-editing of stem cells for regenerative medicine [35,36]. Although these efforts are mostly occurring in laboratory research or at best early phase clinical trials, we can expect eventual surmounting of technological hurdles in each of these approaches. Ultimately, the need for daily small molecule therapy is likely to be complemented or superseded by treatments provided in one or few administrations. In a genetic disease such as CF, such an ultimate treatment would likely need to occur or start in early infancy before onset of irreversible structural organ damage and self-perpetuating positive feedback loops of infection, inflammation and metabolic derangements characteristic of established CF disease.
THE BIG PICTURE
Although modulator therapy is a new and exciting form of treatment with a great potential, there is a large cost associated inherent in the idea of personalized medicine for rare diseases. Small molecule CFTR modulator therapy, for example, will be lifelong and current drug prices will make providing therapy difficult to sustain. Currently, Kalydeco costs $310000, Orkambi $259 000 and Symdeko $292000 annually. The next generation of modulators will not likely decrease in price. If the goal of providing modulator therapy for all F508del mutation-bearing patients is met, there could be more than 50 000 patients worldwide requiring these therapies. Gene or stem cell therapy will likely be even more expensive. Currently, in the United States, payers are generally covering these costs with modulators prescribed on label. In Europe, ivacaftor has been accessible through health insurance; however, lumacaftor-ivacaftor has not been made universally available. Some health systems initially rejected modulators based on insufficient cost-effectiveness data. Longer-term studies may help address this, as disease modification (reduction in rate of lung function decline) appears independent of early lung function response or magnitude [24■■,37,38]. Paying for these therapies out of pocket is prohibitive for most. Since USA approval of Orkambi Vertex has made reimbursement agreements with some European countries, such as Germany, Austria, Ireland, Italy and Luxemburg, but patients in the UK and France may not yet have access [39]. Although Vertex is currently the only company with CFTR modulators on the market, there are other companies with compounds in clinical testing. Galapagos, a partner of AbbVie, has multiple modulator compounds in development, some of which are currently in Phase 1–2 testing [40,41]. Compounds in an interesting new class of CFTR modulator, termed ‘amplifiers’, appear to specifically boost CFTR RNA and protein production in a mutation-agnostic manner, and may, by boosting CFTR substrate levels for correctors and potentiators, play a role in new CFTR modulator combinations [42,43]. As more CFTR modulators progress in development and hopefully reach approval, market competition will offer hope in making personalized CF medicine more affordable and accessible.
CONCLUSION
Since the introduction of ivacaftor, CF therapy has entered a new era with small molecule CFTR modulators that are tailored to a patient’s specific CFTR mutations. Although the first generation of drugs are imperfect, ongoing research and drug development will likely improve their tolerability and efficacy. In addition, there is hope that once these medications have been tested and approved in younger children, we may be able to prevent the phenotype of CF from developing. Although this is promising, as noted above, there is a significant minority of patients who currently do not have CFTR modulator therapy available, most notably patients with only one copy of F508del and a second minimal function mutation, up to 40% of the CF population. Triple modulator combinations will hopefully fill this heterozygote F508del patient gap. Those patients with class I mutations caused by stop codons also have no therapies available. Class I mutations, which result in a shortened transcript and no functional CFTR protein, prove a different and more difficult problem to solve with modulators. One promising drug for class I mutations was halted after Phase 3 trials did not reach the primary endpoint [44]. In addition, patients with other rare mutations likely will need mutationagnostic approaches or very specifically targeted molecular fixes. Resources for drug development to make drugs available to all CFTR mutations as well as the cost to sustain treatment for CF patients treated with modulator therapy will be significant. As it has taken the collaboration of industry, the CF Foundation and academia to bring these therapies to patients, it will take further collaboration and broader evolution of our healthcare system to address cost and access issues and fulfil the ultimate dream of researchers, care givers, patients and loved ones: the end of CF.
KEY POINTS.
CFTR modulators are a new therapy that can correct the protein responsible for causing cystic fibrosis.
Ivacaftor is a very effective medication but is only available to a minority of patients with specific CFTR mutations. Lumacaftor-ivacaftor is modestly effective and applicable in nearly 50% of CF patients homozygous for the F508del mutation, and tezacaftor-ivacaftor, a successor combination, with improved tolerability and pharmacokinetics, is now available.
There are next-generation modulators in triple combination configuration in clinical trials expected to have improved results for patients with either one or two copies of F508del, that is nearly 90% of CF patients. Other rare non-F508del CF mutations will require novel approaches.
Acknowledgments
Financial support and sponsorship
EB is funded by the Marion and Jack Euphrat Pediatric Translational Medicine Fellowship under the Stanford Child Health Research Institute under the NIH-NCATS-CTSA grant #UL1 TR001085 and the Stanford Training Program in Pulmonary via the National Heart, Lun, and Blood Institute of the National Institute of Health under the award T32HL129970.
Footnotes
Conflicts of interest
EB has no relationships to disclose. RM is a consultant to Vertex, Abbvie and Proteostasis.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 2015; 16:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2016-patient-registry-annual-data-report.pdf [Internet]. Cystic Fibrosis Foundation, Bethesda, Maryland: Available at: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf. [Accessed 3 January 2018] [Google Scholar]
- 3.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet Lond Engl 2009; 373:1891–1904. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005; 352:1992–2001. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein RC, Lockwood SR, Lide E, et al. Regulation ofendogenous ENaC functional expression by CFTR and (F508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011; 300:L88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pankow S, Bamberger C, Calzolari D, et al. ΔF508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature 2015; 528:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaral MD. Novel personalized therapies for cystic fibrosis: treating the basic defect in all patients. J Intern Med 2015; 277:155–166. [DOI] [PubMed] [Google Scholar]
- 8.Veit G, Avramescu RG, Chiang AN, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 2016; 27:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuberger T, Burton B, Clark H, Goor FV. Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the preclinical testing of CFTR modulators Cystic fibrosis [Internet]. Humana Press; 2011. p. 39–54. (Methods in Molecular Biology). Available at: https://link.springer.com/protocol/10.1007/978-1-61779-117-8_4. [Accessed 22 January 2018] [DOI] [PubMed] [Google Scholar]
- 10.Hart SL, Harrison PT. Genetic therapies for cystic fibrosis lung disease. Curr Opin Pharmacol 2017; 34:119–124. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKone EF, Borowitz D, Drevinek P, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med 2014; 2:902–910. [DOI] [PubMed] [Google Scholar]
- 14.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015; 192:836–842. [DOI] [PubMed] [Google Scholar]
- 15.Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013; 187:1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies JC, Cunningham S, Harris WT, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med 2016; 4:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■This was the first study to explore ivacaftor’s effects on pancreatic function. There was an increase in faecal elastase seen with ivacaftor treatment, even increasing beyond the cut-off for pancreatic sufficiency in 26% of the individuals. Pancreatic insufficiency was previously thought to be irreversible.
- 17.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551 D gating mutation. J Cyst Fibros 2014; 13:674–680. [DOI] [PubMed] [Google Scholar]
- 18.Moss RB, Flume PA, Elborn JS, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med 2015; 3:524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durmowicz AG, Lim R, Rogers H, et al. Food and Drug Administration’s experience with ivacaftor in cystic fibrosis. establishing efficacy using in vitro data in lieu of a clinical trial. Ann Am Thorac Soc 2018; 15:1–2. [DOI] [PubMed] [Google Scholar]
- 20.Flume PA, Liou TG, Borowitz DS, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest 2012; 142: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 2012; 67:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373:220–231. [DOI] [PubMed] [Google Scholar]
- 23.Rowe SM, McColley SA, Rietschel E, et al. Lumacaftor/ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del-CFTR. Ann Am Thorac Soc 2016; 14:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konstan MW, McKone EF, Moss RB, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017; 5:107–118. [DOI] [PubMed] [Google Scholar]; ■■This study describes the use of the CFF Patient Registry comparing patients homozygous for F508del allele on lumacaftor-ivacaftor into propensity-matched comparators to show that lumacaftor-ivacaftor decreases the rate of decline in FEV1.
- 25.Milla CE, Ratjen F, Marigowda G, et al. Lumacaftor/Ivacaftor in patients aged 6–11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med 2016; 195:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017; 5:557–567. [DOI] [PubMed] [Google Scholar]
- 27.Hubert D, Chiron R, Camara B, et al. Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J Cyst Fibros 2017; 16:388–391. [DOI] [PubMed] [Google Scholar]
- 28.Jennings MT, Dezube R, Paranjape S, et al. An observational study of outcomes and tolerances in patients with cystic fibrosis initiated on lumacaftor/ivacaftor. Ann Am Thorac Soc 2017; 14:1662–1666. [DOI] [PubMed] [Google Scholar]
- 29.Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017; 377:2013–2023. [DOI] [PubMed] [Google Scholar]; ■■This study describes the results of the clinical trials using tezacaftor-ivacaftor in patients homozygous for F508del and shows that it is comparable in efficacy to lumacaftor-ivacaftor without the intolerance or drug-drug interactions seen in lumacaftor-ivacaftor use.
- 30.Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017; 377: 2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■This study describes the trial showing that tezacaftor-ivacftor provides incremental benefit to ivacaftor in patients who are heterozygous for a residual function CFTR mutation.
- 31.FDA approves SYMDEKOTM (tezacaftor/ivacaftor and ivacaftor) to treat the underlying cause of cystic fibrosis in people ages 12 and older with certain mutations in the CFTR gene [Internet]. Vertex. Available at: http://investors.vrtx.com//releasedetail.cfm?ReleaseID=1057241. [Accessed 13 February 2018] [Google Scholar]
- 32.Okiyoneda T, Veit G, Dekkers JF, et al. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol 2013; 9:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vertex announces positive phase 1 & phase 2 data from three different triple combination regimens in people with cystic fibrosis who have one F508del mutation and one minimal function mutation (F508del/Min) [Internet]. Vertex. Available at: http://investors.vrtx.com//releasedetail.cfm?ReleaseID=1033559. [Accessed 3 January 2018] [Google Scholar]
- 34.Vertex selects two next-generation correctors, VX-659 and VX-445, to advance into phase 3 development as part of two different triple combination regimens for people with cystic fibrosis [Internet]. Available at: http://investors.vrtx.com//releasedetail.cfm?ReleaseID=1055958. [Accessed 13 February 2018]
- 35.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med 2016; 4:662–674. [DOI] [PubMed] [Google Scholar]
- 36.Elborn JS, Davies J. Clinical trial research in focus: ensuring new cystic fibrosis drugs fulfil their potential. Lancet Respir Med 2017; 5:681–683. [DOI] [PubMed] [Google Scholar]
- 37.Konstan M, McKone E, Moss R, et al. Relationship between rate of percentage predsuted FEV1 decline and baseline and acute change in percentage predicted FEV1 in patients with cystic fibrosis treated with lumacaftor/ ivacaftor. Poster Presentation at European Cystic Fibrosis Society Annual Meeting; June 7–10, 2018, Spain. [Google Scholar]
- 38.Heltshe SL, Rowe SM, Skalland M, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Ivacaftor-treated CF patients derive long-term benefit despite no short-term clinical improvement. Am J Respir Crit Care Med 2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stendahl M Vertex inks another reimbursement deal in Europe for top-selling drug Orkambi [Internet]. Boston Business Journal. Available at: https://www.bizjournals.com/boston/news/2017/07/13/vertex-inks-another-reimbursement-deal-in-europe.html. [Accessed 3 January 2018] [Google Scholar]
- 40.Update on progress in cystic fibrosis programs [Internet]. Galapagos. Available at: http://www.glpg.com/press-releases. [Accessed 3 January 2018] [Google Scholar]
- 41.Galapagos goes for broke in cystic fibrosis [Internet]. Galapagos. Available at: http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=748910&isEPVantage=yes&utm_source=STAT%20Newsletters&utm_campaign=76794b4cc0-Readout&utm_medium=E-Mail&utm_term=0_8cab1d7961-76794b4cc0-150448013. [Accessed 3 January 2018] [Google Scholar]
- 42.Molinski SV, Ahmadi S, Ip W, et al. Orkambi® and amplifier co-therapy improves function from a rare CFTR mutation in gene-edited cells and patient tissue. EMBO Mol Med 2017; 9:1224–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proteostasis therapeutics announces positive clinical results from studies of PTI-428, PTI-801 and PTI-808 in healthy volunteers and patients with cystic fibrosis [Internet]. Proteostasis Therapeutics, Inc; Available at: /news-releases/news-release-details/proteostasis-therapeutics-announces-positive-clinical-results. [Accessed 22 January 2018] [Google Scholar]
- 44.PTC therapeutics announces results from pivotal phase 3 clinical trial of ataluren in patients living with nonsense mutation cystic fibrosis (NASDAQ:PTCT) [Internet]. PTC Therapeutics, Inc; Available at: http://ir.ptcbio.com/releasedetail.cfm?ReleaseID=1015471. [Accessed 5 January 2018] [Google Scholar]