Abstract
Objectives:
To evaluate associations between maternal lifetime traumatic stress and offspring birthweight and examine modifying effects of third trimester cortisol and fetal sex.
Study design:
Analyses included 314 mother-infant dyads from an ethnically mixed pregnancy cohort. Maternal lifetime trauma was reported via the Life Stressor Checklist-Revised (LSC-R). Fenton birthweight for gestational age z-scores (BWGA-z) were calculated. A 3-cm scalp-nearest maternal hair segment collected at birth was assayed to reflect cumulative third trimester cortisol secretion. Multivariable regression was used to investigate associations between maternal lifetime trauma and BWGA-z and examine 2-and 3-way interactions with cortisol and fetal sex. Because subjects with low or high cortisol levels could represent susceptible populations, varying coefficient models that relax the linearity assumption on cortisol level were used to assess modification of maternal lifetime trauma associations with BWGA-z as a function of cortisol.
Results:
Women were primarily minorities (41% Hispanic, 26% black) with ≤12 years education (63%); 63% reported ≥1 traumatic event. Prenatal cortisol modified the association between maternal lifetime trauma and birthweight. Women with higher lifetime trauma and increased cortisol had significantly lower birthweight infants in males; among males exposed to the 90th percentile of cortisol, a 1-unit increase in trauma score was associated with a 0.19-unit decrease in BWGA-z (95% Confidence Interval [CI] -0.34,-0.04). Associations among females were nonsignificant, regardless of cortisol level.
Conclusions:
These findings underscore the need to consider complex interactions among maternal trauma, disrupted in utero cortisol production, and fetal sex to fully elucidate intergenerational effects of maternal lifetime trauma.
Size at birth is a determinant of lifelong function, health and disease1, 2. Minority women and those of disadvantaged socio-economic status (SES) are more likely to have low birthweight infants3, 4. Moreover, birthweight disparities parallel the distribution of associations between disparities in SES and adverse outcomes over the lifecourse5, 6. Identifying modifiable factors influencing birthweight can inform interventions to reduce health disparities over the lifespan.
A variety of maternal stressors have been examined in relation to birthweight with findings varying across studies6–17. The majority of studies considered stress in the prenatal or immediate preconception period with some11–13, 15, 18, 19 but not all8, 9, 20 showing associations with reduced birthweight. Notably, the limited number of studies that consider a woman’s lifetime stress consistently support associations with lower birthweight6, 7, and effects persist when accounting for stress in pregnancy7. Our group21 and others22 demonstrate high rates of trauma exposure in lower income, ethnic minority women of childbearing age. Trauma can result in altered psychophysiological states that persist years after an event which, when carried into pregnancy, can impact the developing fetus23.
Although mechanisms remain unclear, hypothalamic-pituitary-adrenal (HPA) axis functioning and consequent maternal cortisol production play a key role. The placenta modulates fetal effects via 11-β-hydroxysteroid dehydrogenase-type 2 (11β-HSD2) activity24. Chronic stress downregulates 11β-HSD2, increasing placental permeability to cortisol25. Levels of 11β-HSD2 decrease late in pregnancy; thus the fetus can be more vulnerable in the third trimester26. Chronic traumatic stress disrupts other regulatory systems including autonomic nervous system (ANS) and immune functioning, which interact with cortisol in utero to influence fetal development27, 28. Thus, maternal trauma history and altered cortisol in pregnancy may have joint effects on fetal programming. Fetal sex can also modify stress effects29, with evidence of complex associations among stress, cortisol, and fetal sex and increased susceptibility of males to effects of in utero stress 30, 31.
In the current study, we examined associations between maternal lifetime traumatic stress and infant birthweight, modifying effects of third trimester maternal cortisol levels, and differences by fetal sex. We hypothesized that infants born to women with higher lifetime trauma who also had disrupted cortisol production in utero would have lower birthweight and that males would be more susceptible to intrauterine effects of maternal lifetime trauma.
Methods
Pregnant women receiving prenatal care were recruited from Beth Israel Deaconess Medical Center and the East Boston Neighborhood Health Center in Boston, MA from March 2011 - December 2013 and the Mount Sinai Hospital in New York City, NY from April 2013 - July 2014. Eligibility criteria included English- or Spanish-speaking, ≥18 years of age and single gestation pregnancy. Exclusion criteria included maternal intake of ≥7 alcoholic drinks/week prior to or any alcohol after pregnancy recognition, as usage at or above these thresholds have been associated with increased risk of a number of child outcomes32, 33; and congenital abnormalities or chronic child health conditions (eg, severe neurodevelopmental delays) that would impact participation. The relevant institutions’ human studies committees approved procedures and mothers provided written consent in their primary language. Trained research staff approached women in participating prenatal clinics on select clinic days. Of those approached and eligible, N=548 agreed to participate (69.4%); women were enrolled at an average of 25.44 ± 7.0 weeks’ gestation (25 weeks and 3 days ± 7 weeks) and participants and non-participants did not differ on race/ethnicity, education or income. Within 2 weeks of enrollment, mothers completed standardized surveys via in-person interviews. Funding was later obtained to collect hair at delivery; all women who had not delivered (n=359) agreed to hair sampling; 45 were excluded due to insufficient hair length, shift work, exogenous steroid use in the past 6 months, or multiple gestation, leaving a final sample of 314 for the present analysis. Those included in the present analysis did not significantly differ from the sample as a whole based on mean maternal age, race, education level, prenatal smoking, pre-pregnancy body mass index (BMI), or child sex. All questionnaire measures were administered in a face-to-face interview in a private setting by trained research staff.
Main Exposure
Maternal lifetime trauma exposure:
Mothers completed the 30-item Life Stressor Checklist-Revised (LSC-R) to report exposure to potentially traumatic events (e.g., accident or natural disaster, death of someone close, childhood maltreatment, interpersonal violence, sexual assault) over the life course. To classify an event as traumatic, mothers were asked whether there was concern that they or someone else was at risk of death or serious injury in association with the event 34; positive responses were summed to create a lifetime trauma score. The LSC-R was administered by trained research staff via face-to-face interview and has been used in diverse populations with demonstrated test-retest reliability and validity35.
Outcome
Birthweight:
Birthweight was extracted from delivery records. Gestational age was determined by reported last menstrual period (LMP) and compared with obstetrical estimates from the first trimester ultrasound; if the discrepancy was >2 weeks, obstetrical estimates were used36. Sex-specific Fenton birthweight for gestational age z-scores (BWGA-z) were derived37. Z-scores factor in non-linear growth, which reduces bias and residual confounding38.
Effect Modifiers
Cortisol:
Most studies of prenatal HPA axis functioning consider salivary or serum cortisol obtained at discrete time points reflecting cortisol secretion over hours or days39. Hair provides an integrated measure reflecting cortisol levels over weeks to months40. Hair cortisol levels demonstrate expected increases over pregnancy and correlate with salivary cortisol measures and chronic stress41,42. Hair was collected from mothers within 1 week of delivery. A 3 to 9 cm long by 3 mm diameter hair segment (∼50 strands) was collected close to the scalp at the posterior vertex43 and stored at room temperature until shipment for analysis using a published protocol (Kirschbaum laboratory, Dresden, Germany)44. Based on an average monthly hair growth of ∼1 cm, a 3 cm scalp-nearest hair segment was assayed to reflect cumulative maternal third trimester cortisol secretion. Cortisol was quantified using enzyme-linked immunosorbent assay (ELISA) (n=137) (CLIA, IBL-Hamburg, Germany; sensitivity 0.16 ng/ml, inter-assay coefficient of variance 5–7%) or liquid chromatograph-mass spectrometry (LC-MS/MS)(n=177) (sensitivity 0.1 pg/mg, inter-assay variability 3.7–8.8%)44; immunoassay results were converted into standard LC-MS/MS equivalents as previously described45. Cortisol levels were not impacted by chemical treatment in the past year, use of gels, oils or sprays on the day of collection, or mode of delivery; thus these factors were not considered further in analyses.
Fetal Sex:
Sex was determined from birth records.
Covariates
Factors associated with stress and birthweight were considered as confounders including maternal age, education (≤12 years vs >12 years), race/ethnicity (White, black and Hispanic), and body mass index (BMI). BMI was calculated from maternal self-reported pre-pregnancy weight and height as weight (kg) divided by height (m) squared. There is previously demonstrated reliability of self-reported anthropometric measures46.
Statistical analyses
Analyses proceeded in several steps. We first examined the distribution of covariates in the sample overall and by fetal sex. We calculated descriptive statistics and examined differences by sex using the χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Hair cortisol was natural log-transformed to reduce skewness and we calculated z-scores for analyses; we report the raw scale (pg/mg) for comparison with prior studies.
We used linear regression to examine the association between maternal lifetime trauma exposure and BWGA-z, adjusting for confounders. The main model adjusted for fetal sex, third trimester hair cortisol (hereafter referred to as prenatal cortisol), maternal age, race/ethnicity and pre-pregnancy BMI. Subsequent models examined two-way interactions between maternal lifetime trauma and sex and prenatal cortisol. The final model included a three-way interaction between maternal lifetime trauma, prenatal cortisol and fetal sex.
We assessed modification of maternal lifetime trauma associations with BWGA-z by prenatal cortisol using varying coefficient models. These models are similar to a linear regression model, but no longer assume a linear effect of cortisol because stress and disrupted reactivity of the HPA axis can result in either elevated or reduced cortisol levels47 and both elevated and blunted cortisol production have been associated with low birthweight48, 49. Thus, cortisol is entered into the model as an unspecified, potentially nonlinear, smooth function that is estimated from the data both in the main effect of cortisol and the cortisol x trauma interaction. The resulting model allows the association of trauma and birthweight to vary as a potentially nonlinear function of cortisol. The varying coefficient model takes the form BWGA-zi = β0 + β1LSCRi + β2(ci) + β3(ci) LSCRi + β4xi + εi, where β2(ci) is the main, potentially nonlinear smooth association between birthweight z-score and prenatal cortisol level when LSCRi=0; β3(ci) is the association between maternal lifetime trauma exposure and BWGA-z for a subject having cortisol level ci; and xi is a vector containing the additional covariates. Figures were created to present estimates and corresponding confidence intervals for β1 + β3(c) the association of maternal lifetime trauma with BWGA-z, as a function of prenatal cortisol level c. To examine effect modification by fetal sex, we stratified the above model by fetal sex.
Results
Descriptive Data
Table 1 shows sample characteristics overall and by fetal sex. Women were primarily minorities (41.1% Hispanic, 26.4% Black) and the majority reported ≤12 years education (63.1%); 197 (62.7%) reported one or more traumatic events in their lifetime. There were no significant differences by sex for maternal age, race/ethnicity, education, pre-pregnancy BMI, or maternal lifetime trauma score. Median prenatal hair cortisol was 6.65 pg/mg (Intraquartile Range (IQR) 2.8–22.9) with higher levels in mothers of male vs. female infants.
Table 1.
PRISM participant characteristics
| All children | Male | Female | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| (N=314) | (n=153) | (n=161) | |||||
| Maternal age at enrollment (year; mean, SD b) | 30.0 | 5.9 | 29.7 | 5.7 | 30.4 | 6.1 | 0.28 |
| Race/Ethnicity (n, %) | |||||||
| White | 102 | 32.5 | 48 | 31.4 | 54 | 33.5 | 0.89 |
| Black | 83 | 26.4 | 42 | 27.5 | 41 | 25.5 | |
| Hispanic | 129 | 41.1 | 63 | 41.2 | 66 | 41.0 | |
| Maternal education status (n, %) | |||||||
| ≤12 years | 198 | 63.1 | 103 | 67.3 | 95 | 59.0 | 0.13 |
| >12 years | 116 | 36.9 | 50 | 32.7 | 66 | 41.0 | |
| Maternal pre-pregnancy BMI (kg/m2; median, IQR) | 24.4 | 21.9–29.3 | 25.1 | 22.5–30.7 | 24.0 | 21.9–28.3 | 0.09 |
| Maternal lifetime trauma exposure: LSC-R (mean, SD) c | 1.79 | 2.33 | 1.76 | 1.97 | 1.81 | 2.63 | 0.07 |
| Prenatal cortisol d | |||||||
| Original scale (pg/mg; median, IQR) | 6.65 | 2.8–22.9 | 9.13 | 3.8–25.4 | 5.14 | 2.1–17.0 | <0.01 |
| z-score; ln-transformed (mean, SD) c | −0.05 | 0.97 | 0.08 | 0.95 | −0.16 | 0.97 | 0.02 |
| Fenton birthweight for gestational age z-score (mean, SD) | −0.11 | 0.90 | −0.11 | 0.99 | −0.10 | 0.80 | 0.82 |
| Gestational age at birth (weeks; median, IQR) | 39.1 | 38.1–40.1 | 39.2 | 38–40.1 | 39.0 | 38.4–40.0 | 0.99 |
p-value comparing boys and girls (χ2 test for categorical variables; Wilcoxon rank-sum test for continuous variables)
Standard Deviation (SD)
Maternal lifetime trauma exposures assessed as number of endorsed events meeting DSM-5 Criterion A via the Life Stressor ChecklistRevised (LSC-R).
Maternal third trimester hair cortisol
Maternal lifetime trauma and BWGA-z scores: Main effects
In multivariable regression models adjusted for child's sex and maternal age, race/ethnicity, education, BMI and hair cortisol, there was no significant main effect of maternal lifetime trauma on BWGA-z; sex and prenatal cortisol were not independently associated with BWGA-z.
Effect modification by third trimester hair cortisol and child’s sex
To investigate whether the association between maternal lifetime trauma and BWGA-z varied by sex or prenatal cortisol level, we examined two-way interactions for maternal lifetime trauma with 1) prenatal cortisol and 2) fetal sex. The interaction term for maternal lifetime trauma x prenatal cortisol was marginally significant (P = .058), and the interaction term for maternal lifetime trauma x sex was not significant (P=0.91). In the model including a three-way interaction for maternal lifetime trauma x prenatal cortisol x sex, the main effect of trauma was essentially unchanged; however, we observed a statistically significant three-way interaction (P=0.02).
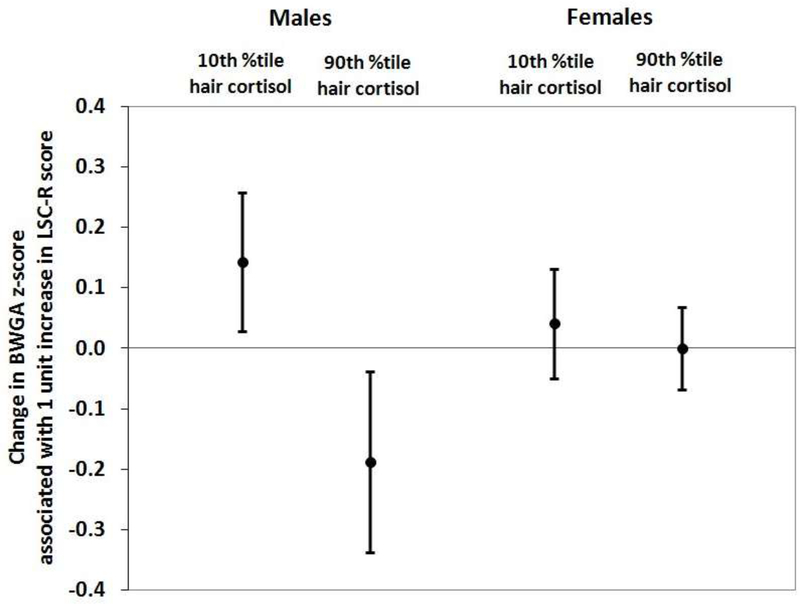
To demonstrate variations in the association between maternal lifetime trauma and BWGA-z across prenatal cortisol levels and by sex, we calculated predicted effect estimates of trauma on BWGA-z based on results of the linear regression models incorporating interactions between maternal lifetime trauma, prenatal cortisol, and sex. We considered the following four conditions to highlight associations at the upper and lower levels of prenatal cortisol level: 1) boys exposed to prenatal cortisol at the 10th percentile, 2) boys exposed to prenatal cortisol at the 90th percentile, 3) girls exposed to prenatal cortisol at the 10th percentile, and 4) girls exposed to prenatal cortisol at the 90th percentile. We emphasize that this model was fit using the original continuous cortisol measures, and these percentiles are chosen for interpreting the magnitudes of the trauma x cortisol interaction terms. These results are presented in Figure 1. Among males exposed to prenatal cortisol at the 90th percentile (i.e., 74.03 pg/mg), a 1-unit increase in maternal trauma score was associated with a 0.19-unit decrease (95% CI -0.34,-0.04) in BWGA-z, and among those exposed to prenatal cortisol at the 10th percentile (i.e., 1.14 pg/mg), a 1-unit increase in trauma score was associated with a 0.14-unit increase (95% CI 0.03,0.26) in BWGA-z. We found no association between maternal trauma score and BWGA-z in females regardless of prenatal cortisol levels.
Figure 1. Predicted effect estimates for maternal lifetime trauma exposure on infant birthweight for gestational age z-score (BWGA-z) by prenatal cortisol level and fetal sex.
Estimated change in BWGA z-scores (with 95% CI) associated with a one-unit increase in maternal trauma exposure (continuous ratings from the LSC-R). Estimates from multivariableadjusted regression model under the following four conditions: 1) males born to mothers with prenatal hair cortisol level at 10th percentile, 2) males born to mothers with prenatal hair cortisol level at 90th percentile, 3) females born to mothers with prenatal hair cortisol level at 10th percentile, and 4) females born to mothers with prenatal hair cortisol level at 90th percentile. Prenatal cortisol (natural log-transformed z-score) was assessed from maternal hair representing the third trimester of pregnancy. Analyses adjusted for maternal age at enrollment, race/ethnicity, maternal education status, and pre-pregnancy BMI.
Effect modification by hair cortisol: Varying coefficient models
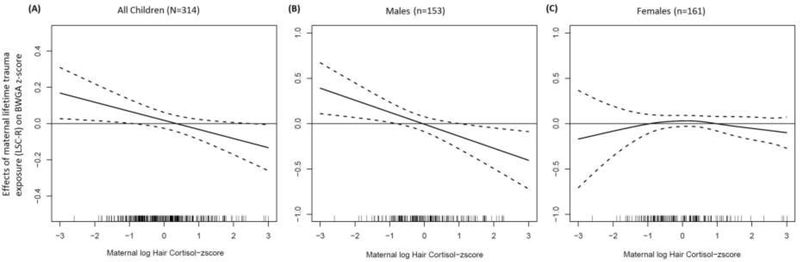
We further examined the trauma x cortisol interactions on birthweight using varying coefficient models to allow for nonlinear trends in the BWGA – trauma associations across the range of cortisol levels. To present the findings, we use graphical displays to present the estimated associations between maternal lifetime trauma and infant birthweight along a continuum of cortisol levels. Figure 2, A presents estimates of the change in BWGA-z associated with a one-unit increase in maternal trauma exposure at each value of prenatal cortisol exposure. Models were adjusted for fetal sex and maternal age, education, race/ethnicity, and pre-pregnancy BMI. The association between maternal lifetime trauma and infant BWGA-z varied by prenatal cortisol level. Among infants exposed to the highest levels of prenatal cortisol, maternal trauma exposure was inversely associated with BWGA-z. In contrast, among infants exposed to lower prenatal cortisol, maternal trauma was associated with higher BWGA-z. Results were statistically significant at the upper and lower levels of prenatal cortisol as evidenced by the 95% confidence intervals (CI) (represented by dotted bands) not containing zero. In the overall sample, the interaction term for the multivariable-adjusted regression model was marginally significant (P=0.058, Table 2).
Figure 2. Association between maternal lifetime trauma exposure and infant birthweight for gestational age z-score (BWGA-z): Effect modification by prenatal cortisol overall and by fetal sex.
Change in BWGA-z for a one-unit increase in maternal trauma at each cortisol value estimated as a smooth function of cortisol (solid line) and 95% pointwise confidence intervals (CIs, dashed lines). Areas in which 95% CIs fall above or below zero indicate intervals of cortisol z-score in which there is a significant association between trauma and BWGA-z. (A) overall sample, (B) Male fetal sex, and (C) female fetal sex. Analyses adjusted for (A) fetal sex, maternal age, race/ethnicity, education, and BMI and (B, C) maternal age, race/ethnicity, education, and BMI
Table 2.
Multivariable-adjusted regression modelsa examining maternal lifetime trauma exposure (LSC-R) in relation to birthweight for gestational age zscore
| Interaction Models | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Model | Maternal Lifetime Traumab x Fetal Sex Model |
Maternal Lifetime Traumab x Cortisolc Model |
Maternal Lifetime Traumab x Cortisolc x Fetal Sex Model |
|||||||||
| beta | s.e. d | p | beta | s.e. d | p | beta | s.e. d | p | beta | s.e. d | p | |
| Maternal Lifetime Trauma b | 0.01 | 0.02 | 0.59 | 0.01 | 0.03 | 0.62 | 0.02 | 0.02 | 0.39 | 0.02 | 0.03 | 0.46 |
| Fetal sex (male as referent) | −0.03 | 0.10 | 0.75 | −0.02 | 0.13 | 0.86 | −0.05 | 0.10 | 0.64 | 0.04 | 0.13 | 0.79 |
| Prenatal cortisol c | 0.06 | 0.06 | 0.25 | 0.06 | 0.06 | 0.26 | 0.14 | 0.07 | 0.04 | 0.08 | 0.09 | 0.41 |
| Interaction terms: | ||||||||||||
| Maternal Lifetime Trauma x Fetal Sex | −0.01 | 0.05 | 0.91 | −0.04 | 0.05 | 0.43 | ||||||
| Maternal Lifetime Trauma x Prenatal Cortisol | −0.04 | 0.02 | 0.0 58 | −0.02 | 0.02 | 0.48 | ||||||
| Prenatal Cortisol x Fetal Sex | 0.24 | 0.14 | 0.09 | |||||||||
| Maternal Lifetime Trauma x Prenatal Cortisol x | −0.12 | 0.05 | 0.02 | |||||||||
| Fetal Sex | ||||||||||||
Covariates include child's sex, prenatal hair cortisol, maternal age at enrollment, race/ethnicity, maternal educational status, pre-pregnancy BMI
Maternal lifetime trauma exposure assessed as number of endorsed events meeting DSM-5 Criterion A via the Life Stressor Checklist-Revised (LSC-R).
Third-trimester hair cortisol z-score, ln-transformed
Figure 2, B and C present sex-stratified estimates of associations between maternal trauma and BWGA-z as a smooth function of prenatal cortisol, adjusted for maternal age, race/ethnicity, and pre-pregnancy BMI. Findings among males were similar to findings in the overall sample: in males exposed to higher prenatal cortisol, maternal lifetime trauma was inversely associated with BWGA-z, and among those with lower cortisol exposure, maternal trauma was positively associated with BWGA-z. The dotted lines (representing 95% CI) indicate that associations at the extremes of prenatal cortisol are statistically significant. In females, the association between maternal trauma and BWGA-z was not statistically significant regardless of prenatal cortisol (Figure 2, C).
Discussion
These analyses did not demonstrate an independent relationship between increased maternal lifetime trauma and birth weight adjusted for gestational age. Rather, higher maternal lifetime trauma exposure was associated with offspring birthweight adjusted for gestational age only after considering modifying effects of in utero cortisol levels. Specifically, children born to mothers with higher lifetime trauma exposure and higher third trimester cortisol levels had significantly lower birthweight, and children born to mothers with higher lifetime trauma exposure and lower third trimester cortisol had significantly higher birthweight. Finally, only males were vulnerable to the joint effects of maternal trauma exposure and higher cortisol late in pregnancy.
The present study builds on a growing body of literature demonstrating that trauma occurring over a mother’s lifetime can contribute to adverse pregnancy outcomes50. Such findings are of public health significance given epidemiological data showing high rates of trauma exposure among pregnant women and women of childbearing age, particularly for lower-income, racial/ethnic minority samples21, 51. A majority of women in this racially/ethnically mixed, lower income pregnancy cohort experienced one or more traumatic events in their lifetime; this prevalence is consistent with other reports in samples of similar sociodemographics21, 51.
Several lines of prior evidence can inform why those women with a history of trauma exposure who secreted higher levels of cortisol in late pregnancy were most likely to have lower birthweight infants. Trauma exposure can result in altered psychophysiological states, including abnormal reactivity of the HPA axis with alterations persisting years after an event. Therefore, women exposed to trauma can enter pregnancy with disrupted physiological states (e.g., aberrant HPA axis reactivity, enhanced inflammation)52 that in turn influence fetal programming. Another recent study found that maternal trauma history was only associated with lower birthweight and preterm delivery when prenatal maternal psychological dysfunction was considered23. Specifically, associations between increased anxiety late in pregnancy and lower birthweight were significantly stronger in women with a trauma history. Although the study did not examine potential underlying mechanisms, mood disorders in pregnancy can be associated with disrupted stress responsivity and reactivity (e.g., HPA, ANS disruption) and increased fetal exposure to cortisol53.
Elevated maternal prenatal cortisol production has been associated with reduced birthweight in prior studies. Bolten et al reported that higher prenatal cortisol levels were associated with smaller size at birth, independent of maternal perceived stress54. Valladares et al linked partner violence during pregnancy to high salivary cortisol levels, which in turn were associated with reduced birthweight55.
We observed sex-specific effects, as associations between maternal lifetime trauma exposure, elevated prenatal cortisol and birthweight were evident in males but not females. For example, in terms of birthweight in grams among males who were born at gestational age 39 weeks (the mean and median of the sample included in the analysis), among those exposed to prenatal cortisol at the 90th percentile, a 1-unit increase in maternal trauma score corresponds to an 88 gram decrease in birthweight and among those exposed to prenatal cortisol at the 10th percentile, a 1-unit increase in maternal trauma score corresponds to a 65 gram increase in birthweight. Fetal sex may impact the association between maternal psychosocial stress and birthweight given reported differences in fetal growth as well as maternal HPA axis activity by fetal sex56. Our results are consistent with literature showing that males are more susceptible to effects of in utero stress and stress correlates on fetal growth31, 57. For example, Thayer et al found that elevated maternal cortisol levels assessed prior to conception disproportionately impacted fetal growth in boys.31 Bublitz et al showed that lower maternal SES was associated with altered maternal cortisol production and lower birthweight in male infants.58
Future studies are needed to elucidate mechanisms underlying these associations. Recently, epigenetic programming has been receiving increasing attention in this context. Maternal stress influences placental permeability to cortisol through altered production of 11β-HDS2 with evidence that chronic stress and prenatal mood disorders are associated with downregulation of 11β-HDS2 and increased placental permeability to cortisol49. Changes in epigenetic regulation of both 11β-HDS259 and the glucocorticoid receptor60 may play a role and also show sex-specific effects61, 62.
This study has a number of strengths, including the racially/ethnically mixed, lower income, higher risk sample, the consideration of effects of maternal trauma on birthweight using a lifecourse framework, and use of hair cortisol as an integrated measure of maternal cortisol secretion in the third trimester to consider interactions between trauma and HPA axis disruption in pregnancy. Our measure of hair cortisol demonstrated expected increases across pregnancy, which is consistent with the literature to date40, 41, and correlations between trimester-specific cortisol levels were high and ranged from 0.89–0.96. First and second trimester cortisol levels were available for a smaller sample of the study population (given differences in hair length at delivery); we replicated analyses using first and second trimester cortisol levels and the overall inferences were unchanged. Our analyses focused on third trimester maternal hair cortisol levels given that the developing fetus may be particularly vulnerable late in pregnancy to elevated cortisol63. Birthweight was considered as a continuous measure, rather than low/extremely low birthweight, which is important as lower birthweight in the normal range has been associated with adverse health outcomes49.
There are also some limitations which will be important to consider in future studies. Maternal lifetime trauma exposure was obtained by self-report via the LSC-R. However, the LSC-R is a reliable measure of lifetime trauma exposure, with data suggesting possible under-reporting of trauma exposure which would lead to an underestimate of association64. The LSC-R was collected prior to birth and is thus unlikely to have been reported differentially by birthweight. In addition, we obtained funding to measure hair cortisol 6 months after study initiation and had to exclude women recruited prior to cortisol assessment.
This study underscores the need to consider complex interactions among maternal trauma, biomarkers of disrupted stress reactivity in utero, and fetal sex in order to more fully elucidate the intergenerational effects of maternal trauma on offspring health and development. These findings also highlight the importance of taking a lifecourse approach which hypothesizes that a woman’s life experiences prior to pregnancy can shape the development of the fetus and pregnancy outcomes as well as elucidating the etiology of perinatal health disparities65, 66. Early identification of women at elevated risk of having lower birthweight babies can help reduce adverse maternal-child outcomes. Identifying a prior history of trauma and providing interventions, for example treatment for associated mood disturbances67, could lead to improved perinatal outcomes that have lifelong implications for health.
Acknowledgments
Data collection was supported by the National Institutes of Health (NIH) (R01HL095606 [to R.W. and M.E.] and R21HD00359 [to R.W.]). Phenotyping and biostatistical support was funded by the NIH (P30 ES023515 and P30 ES000002). K.B. received research support from the NIH (K99ES024116 and R00ES024116). The other authors declare no conflicts of interest.
Abbreviations:
- SES
Socio-economic status
- 11β-HSD2
11-β-hydroxysteroid dehydrogenase-type 2
- ANS
Autonomic nervous system
- LSC-R
Life Stressor Checklist-Revised
- LMP
Last menstrual period
- BWGA-z
Birthweight for gestational age z-scores
- ELISA
Enzyme-linked immunosorbent assay
- LC-MS/MS
Liquid chromatograph-mass spectrometry
- BMI
Body mass index
- IQR 95%
Intraquartile Range
- CI 95%
Confidence Interval
- s.e.
Standard error
- SD
Standard Deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. International journal of epidemiology. 2011;40:647–61. [DOI] [PubMed] [Google Scholar]
- [2].Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Seminars in reproductive medicine. 2011;29:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–72. [DOI] [PubMed] [Google Scholar]
- [4].Aizer A, Currie J. The intergenerational transmission of inequality: maternal disadvantage and health at birth. Science. 2014;344:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Maternal and child health journal. 2003;7:13–30. [DOI] [PubMed] [Google Scholar]
- [6].Strutz KL, Hogan VK, Siega-Riz AM, Suchindran CM, Halpern CT, Hussey JM. Preconception stress, birth weight, and birth weight disparities among US women. American journal of public health. 2014;104:e125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Witt WP, Cheng ER, Wisk LE, Litzelman K, Chatterjee D, Mandell K, et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. American journal of public health. 2014;104 Suppl 1:S81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial adversity during pregnancy is associated with length of gestation and offspring size at birth: evidence from a populationbased cohort study. Psychosomatic medicine. 2010;72:419–26. [DOI] [PubMed] [Google Scholar]
- [9].Wing DA, Ortega-Villa AM, Grobman WA, Hediger ML, Grewal J, Pugh SJ, et al. Maternal stress and neonatal anthropometry: the NICHD Fetal Growth Studies. American journal of obstetrics and gynecology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rondo PH, Ferreira RF, Nogueira F, Ribeiro MC, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. European journal of clinical nutrition. 2003;57:266–72. [DOI] [PubMed] [Google Scholar]
- [11].Su Q, Zhang H, Zhang Y, Zhang H, Ding D, Zeng J, et al. Maternal Stress in Gestation: Birth Outcomes and Stress-Related Hormone Response of the Neonates. Pediatrics and neonatology. 2015;56:376–81. [DOI] [PubMed] [Google Scholar]
- [12].Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, et al. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic medicine. 2008;70:688–94. [DOI] [PubMed] [Google Scholar]
- [13].Littleton HL, Bye K, Buck K, Amacker A. Psychosocial stress during pregnancy and perinatal outcomes: a meta-analytic review. J Psychosom Obstet Gynaecol. 2010;31:219–28. [DOI] [PubMed] [Google Scholar]
- [14].Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: A systematic review. J Affect Disord. 2017;225:18–31. [DOI] [PubMed] [Google Scholar]
- [15].Nkansah-Amankra S, Luchok KJ, Hussey JR, Watkins K, Liu X. Effects of maternal stress on low birth weight and preterm birth outcomes across neighborhoods of South Carolina, 2000–2003. Maternal and child health journal. 2010;14:215–26. [DOI] [PubMed] [Google Scholar]
- [16].Harville EW, Boynton-Jarrett R, Power C, Hypponen E. Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study. Arch Pediatr Adolesc Med. 2010;164:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Witt WP, Litzelman K, Cheng ER, Wakeel F, Barker ES. Measuring stress before and during pregnancy: a review of population-based studies of obstetric outcomes. Maternal and child health journal. 2014;18:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Borders AE, Grobman WA, Amsden LB, Holl JL. Chronic stress and low birth weight neonates in a low-income population of women. Obstet Gynecol. 2007;109:331–8. [DOI] [PubMed] [Google Scholar]
- [19].Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. American journal of obstetrics and gynecology. 2010;203:34 e1–8. [DOI] [PubMed] [Google Scholar]
- [20].Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. The relationship between psychological distress during pregnancy and birth weight for gestational age. Acta Obstet Gynecol Scand. 1996;75:32–9. [DOI] [PubMed] [Google Scholar]
- [21].Rich-Edwards JW, James-Todd T, Mohllajee A, Kleinman K, Burke A, Gillman MW, et al. Lifetime maternal experiences of abuse and risk of pre-natal depression in two demographically distinct populations in Boston. International journal of epidemiology. 2011;40:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gelaye B, Zhong QY, Basu A, Levey EJ, Rondon MB, Sanchez S, et al. Trauma and traumatic stress in a sample of pregnant women. Psychiatry Res. 2017;257:506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blackmore ER, Putnam FW, Pressman EK, Rubinow DR, Putnam KT, Matthieu MM, et al. The Effects of Trauma History and Prenatal Affective Symptoms on Obstetric Outcomes. J Trauma Stress. 2016;29:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond). 2007;113:1–13. [DOI] [PubMed] [Google Scholar]
- [25].Welberg LA, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol. 2005;186:R7–R12. [DOI] [PubMed] [Google Scholar]
- [26].Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–44. [DOI] [PubMed] [Google Scholar]
- [27].Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2017. [DOI] [PubMed] [Google Scholar]
- [28].Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23:943–56. [DOI] [PubMed] [Google Scholar]
- [29].Lee A, Mathilda Chiu YH, Rosa MJ, Jara C, Wright RO, Coull BA, et al. Prenatal and postnatal stress and asthma in children: Temporal- and sex-specific associations. J Allergy Clin Immunol. 2016;138:740–7 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Enlow MB, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ. Maternal Lifetime Trauma Exposure, Prenatal Cortisol, and Infant Negative Affectivity. Infancy. 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thayer ZM, Feranil AB, Kuzawa CW. Maternal cortisol disproportionately impacts fetal growth in male offspring: evidence from the Philippines. Am J Hum Biol. 2012;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118:1411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: a meta-analytical review. Alcohol Alcohol. 2003;38:295–304. [DOI] [PubMed] [Google Scholar]
- [34].American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- [35].Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder In: Keane JWTM, editor. Assessing psychological trauma and PTSD. New York: Guilford1997; p. 192–238. [Google Scholar]
- [36].Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008;22:587–96. [DOI] [PubMed] [Google Scholar]
- [37].Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36. [DOI] [PubMed] [Google Scholar]
- [40].Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–7. [DOI] [PubMed] [Google Scholar]
- [41].D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology & behavior. 2011;104:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schreier HM, Enlow MB, Ritz T, Coull BA, Gennings C, Wright RO, et al. Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress. 2016;19:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stalder T, Kirschbaum C. Analysis of cortisol in hair--state of the art and future directions. Brain Behav Immun. 2012;26:1019–29. [DOI] [PubMed] [Google Scholar]
- [44].Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J Epidemiol Community Health. 2015;69:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Russell E, Kirschbaum C, Laudenslager ML, Stalder T, de Rijke Y, van Rossum EF, et al. Toward standardization of hair cortisol measurement: results of the first international interlaboratory round robin. Ther Drug Monit. 2015;37:71–5. [DOI] [PubMed] [Google Scholar]
- [46].Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wells S, Tremblay PF, Flynn A, Russell E, Kennedy J, Rehm J, et al. Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress. 2014;17:334–42. [DOI] [PubMed] [Google Scholar]
- [48].D'Anna-Hernandez KL, Hoffman MC, Zerbe GO, Coussons-Read M, Ross RG, Laudenslager ML. Acculturation, maternal cortisol, and birth outcomes in women of Mexican descent. Psychosomatic medicine. 2012;74:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis--2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. [DOI] [PubMed] [Google Scholar]
- [50].Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Social science & medicine. 1996;42:589–97. [DOI] [PubMed] [Google Scholar]
- [51].Mezey G, Bacchus L, Bewley S, White S. Domestic violence, lifetime trauma and psychological health of childbearing women. BJOG. 2005;112:197–204. [DOI] [PubMed] [Google Scholar]
- [52].Suzuki A, Poon L, Papadopoulos AS, Kumari V, Cleare AJ. Long term effects of childhood trauma on cortisol stress reactivity in adulthood and relationship to the occurrence of depression. Psychoneuroendocrinology. 2014;50:289–99. [DOI] [PubMed] [Google Scholar]
- [53].O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O'Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;37:818–26. [DOI] [PubMed] [Google Scholar]
- [54].Bolten MI, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Archives of women's mental health. 2011;14:33–41. [DOI] [PubMed] [Google Scholar]
- [55].Valladares E, Pena R, Ellsberg M, Persson LA, Hogberg U. Neuroendocrine response to violence during pregnancy--impact on duration of pregnancy and fetal growth. Acta Obstet Gynecol Scand. 2009;88:818–23. [DOI] [PubMed] [Google Scholar]
- [56].Giesbrecht GF, Campbell T, Letourneau N, Team APS. Sexually dimorphic adaptations in basal maternal stress physiology during pregnancy and implications for fetal development. Psychoneuroendocrinology. 2015;56:168–78. [DOI] [PubMed] [Google Scholar]
- [57].Bale TL. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin Neurosci. 2016;18:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bublitz MH, Vergara-Lopez C, O'Reilly Treter M, Stroud LR. Association of Lower Socioeconomic Position in Pregnancy with Lower Diurnal Cortisol Production and Lower Birthweight in Male Infants. Clin Ther. 2016;38:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jensen Pena C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7:e39791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Palma-Gudiel H, Cordova-Palomera A, Eixarch E, Deuschle M, Fananas L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: a meta-analysis. Epigenetics. 2015;10:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ostlund BD, Conradt E, Crowell SE, Tyrka AR, Marsit CJ, Lester BM. Prenatal Stress, Fearfulness, and the Epigenome: Exploratory Analysis of Sex Differences in DNA Methylation of the Glucocorticoid Receptor Gene. Front Behav Neurosci. 2016;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. [DOI] [PubMed] [Google Scholar]
- [63].Cherak SJ, Giesbrecht GF, Metcalfe A, Ronksley PE, Malebranche ME. The effect of gestational period on the association between maternal prenatal salivary cortisol and birth weight: A systematic review and meta-analysis. Psychoneuroendocrinology. 2018;94:49–62. [DOI] [PubMed] [Google Scholar]
- [64].McHugo GJ, Caspi Y, Kammerer N, Mazelis R, Jackson EW, Russell L, et al. The assessment of trauma history in women with co-occurring substance abuse and mental disorders and a history of interpersonal violence. J Behav Health Serv Res. 2005;32:113–27. [DOI] [PubMed] [Google Scholar]
- [65].Malat J, Jacquez F, Slavich GM. Measuring lifetime stress exposure and protective factors in life course research on racial inequality and birth outcomes. Stress. 2017;20:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rohan AM, Onheiber PM, Hale LJ, Kruse TL, Jones MJ, Gillespie KH, et al. Turning the ship: making the shift to a life-course framework. Maternal and child health journal. 2014;18:423–30. [DOI] [PubMed] [Google Scholar]
- [67].Grote NK, Spieker SJ, Lohr MJ, Geibel SL, Swartz HA, Frank E, et al. Impact of childhood trauma on the outcomes of a perinatal depression trial. Depress Anxiety. 2012;29:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]