Abstract
Background:
Positive association between resting heart rate (RHR) and risk of type 2 diabetes (T2D) has been documented in several studies. However, whether RHR is an independent predictor of T2D and its potential interaction with other risk factors of T2D remain unclear.
Methods:
We conducted a prospective cohort study of 31,156 men from the Health Professionals Follow-up Study (1992–2012). Cox proportional hazard model was used to examine the association between RHR and T2D risk. We further examined whether this association is modified by known risk factors. Lastly, we conducted a meta-analysis of prospective cohort studies.
Results:
During 505,380 person-years of follow-up, we identified 2,338 incident T2D cases. The multivariable-adjusted hazard ratio (HR) comparing the highest vs. lowest categories of RHR was 1.69 (95% confidence interval (CI), 1.43–2.01). Increase in 10 bpm of RHR was associated with 19% increased risk of T2D in the fully adjusted model (HR, 1.19; 95% CI, 1.14–1.24). The HRs of T2D associated with RHR was stronger among those with normal weight or without hypertension (P-interaction<.001). Moreover, RHR with other known risk factors cumulatively increased T2D risk. A meta-analysis consistently showed a positive association between RHR and T2D risk (The summary relative risk (RR) for highest vs. lowest RHR, 1.44; 95% CI, 1.20–1.74, n=12, the summary RR per 10 bpm increase, 1.17; 95% CI, 1.09–1.26, n=13).
Conclusions:
High RHR was independently associated with increased risk of T2D. Our findings suggest that RHR, with other known risk factors, could be a useful tool to predict T2D risk.
Keywords: Resting heart rate, Type 2 diabetes, Risk factor, Interaction, Prospective cohort, Meta-analysis
Introduction
Globally, diabetes mellitus mortality rate has increased by 62% between 1990 and 2016.1 As the eighth leading cause of death worldwide, diabetes mellitus results in about 1.5 million deaths every year.1,2 In the United States (US) alone, there were 71.5 thousand deaths from diabetes mellitus in 2016 and 30 million people (9.4% of the US population) living with it.1,3 Type 2 diabetes (T2D), which accounts for 90% of all diagnosed cases of diabetes, could be largely prevented through changes in physical activity, healthy diet and weight control.2,4
Resting heart rate (RHR) has been commonly used as a simple and useful diagnostic and predictive tool for cardiovascular disease in clinical settings.5 More recently, epidemiological studies have found an association between elevated RHR and increased risk of T2D, after adjusting for potential confounders.6–16 In these studies, elevated RHR was hypothesized as a maker of an imbalanced autonomic nervous system, favoring sympathetic over-activity, which is linked to insulin sensitivity, impaired glucose uptake and hyperglycemia.17 However, the majority of these studies were small and had short follow-up, which can contribute to publication bias and lead to reverse causation. Residual confounding was also a major limitation in some studies that did not adjust for obesity, physical activity, diet, smoking hypertension and medications.17 In addition, none of these studies comprehensively investigated whether the association between elevated RHR and T2D is modified by other known risk factors for diabetes. Interaction analysis can be useful for searching new risk factors in low-risk groups and can provide further insight on mechanisms causing T2D.18
Therefore, we investigated the association between RHR and T2D using data from the Health Professionals Follow-up Study, a large prospective US cohort of 31,156 men followed-up over 15 years on average. We additionally assessed whether this association was modified by age, body mass index (BMI), physical activity, diet quality, smoking, family history of diabetes, and hypertension. Lastly, we conducted a meta-analysis of existing prospective cohort studies, including the current study, on RHR and risk of T2D.
Methods
Study population
The Health Professionals Follow-up Study was initiated in 1986 when 51,529 male health professionals aged 40 to 75 years were enrolled. The cohort included 29,683 dentists, 4,185 pharmacists, 3,745 optometrists, 2,218 osteopath physicians, 1,600 podiatrists, and 10,098 veterinarians. Participants completed a detailed questionnaire on their lifestyle and medical information at baseline enrollment and every two years thereafter. The follow-up rate for the Health Professionals Follow-up Study was 95.9%. This investigation was approved by the Institutional Review Board of the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital.
Resting heart rate and covariates assessments
In 1992, participants were asked to report their RHR (beat per minute (bpm)) after sitting for 10 to 15 minutes. Detailed information on covariates, including age, height, weight, family history of disease, medication use, smoking status and physical activity, were collected at enrollment in 1986 and updated through biennial questionnaires. Diet was assessed using validated food frequency questionnaires every four years.
Case ascertainment
From the biennial questionnaire, participants self-reported a newly diagnosed diabetes, and a supplementary questionnaire was sent to confirm the diabetes cases. We confirmed the diagnosis of diabetes if the participants met ≥1 of following criteria: 1) ≥1 classic symptoms (i.e., excessive thirst, polyuria, weight loss, or hunger) plus fasting blood glucose ≥140 mg/dL (7.8 mmol/L) or random blood glucose ≥200 mg/dL (11.1 mmol/L); 2) elevated blood glucose on two different occasions (i.e., fasting blood glucose ≥140 mg/dL (7.8 mmol/L) or random blood glucose ≥200 mg/dL (11.1 mmol/L), or blood glucose ≥200 mg/dL after 2-hour oral-glucose tolerance testing) with no symptoms; 3) treatment with hypoglycemic drugs (i.e., insulin or oral hypoglycemic agent). Our criteria were consistent with those proposed by the National Diabetes Data Group.19 Of note, the threshold for fasting blood glucose was changed to ≥126 mg/dl (7.0 mmol/l) in 1998 and HbA1c ≥6.5% was additionally included in the criteria from 2010.20,21
Statistical analysis
Among participants who provided data for RHR at baseline in 1992, we excluded those who had been previously diagnosed with cardiovascular disease, cancer (excluding non-melanoma skin cancer) or T2D (n=5,936) as these medical conditions can affect RHR.22 We also excluded those who reported extreme RHR values below 30 or over 150 bpm (n=42).
Person-years of follow-up accrued from the baseline when RHR was available until the time of diagnosis of T2D, death or the end of study (January 2012), whichever came first. We used Cox proportional hazard models to compute hazard ratio (HR)s and 95% confidence interval (CI)s of T2D risk associated with RHR. RHR was categorized into 6 groups based on the predefined cut points (<60, 60–64, 65–69, 70–74, 75–79, ≥80 bpm). RHR was also used as a continuous variable (10 bpm increment). Age in month and calendar year were jointly stratified in the model to finely control for confounding by age and calendar time. In a multivariable analysis, we adjusted for race, family history of diabetes, alcohol consumption, total calorie intake, smoking status, intake of dietary factors, including trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, whole grain, and glycemic load, body mass index, physical activity, high blood pressure at baseline, and medication use at baseline (i.e., betablocker, thiazide diuretic, furosemide-like diuretic, calcium channel blocker, other antihypertensive drug and antiarrhythmic drug). Since antihypertensive drugs and hypertension may affect RHR and T2D, we further conducted sensitivity analyses excluding men with antihypertensive drug use and hypertension. Additionally, we ran a sensitivity analysis adjusting for vigorous activity instead of total physical activity because vigorous activity may better adjust for physical fitness.
To explore whether the association between RHR and risk of T2D differed by potential effect modifiers, we conducted stratified analyses by known risk factors for T2D (i.e., age, BMI, physical activity, diet quality, smoking status, family history of diabetes, and hypertension) and by different antihypertensive drugs (i.e., betablocker, thiazide diuretic, furosemide-like diuretic, calcium channel blocker, and other antihypertensive drug). We tested for interaction by including cross-product terms of exposure and stratification variables in the models. We also examined a joint association between RHR and aforementioned risk factors in relation to risk of T2D. For joint analyses, RHR was categorized into tertiles and known risk factors were analyzed separately and as a combined score. The score was calculated for each individual by adding the number of risk factors. The combined score ranged from 0 to 8 and higher scores indicated having more risk factors of T2D. Since BMI is a strong risk factor for T2D, participants received 0 to 2 scores (0=normal weight, 1=overweight, 2=obese).
We updated a previous meta-analysis23 by searching Medline, Embase, Web of Science and Cochrane library databases for articles published from January 2015 through September 2018. Two authors independently extracted the relavent studies using the following terms: (“resting heart rate” OR “heart rate” OR “resting pulse”) AND (“type 2 diabetes” OR “diabetes”). To be eligible in our assessment, a study had to have evaluated the association between RHR and the incidence of T2D in a cohort study design. We also searched the reference lists of relevant publications to identify more studies. A total of 14 studies, including the current study, were included for the meta-analysis. Random effects models were used to calculate summary relative risk (RR)s and 95% CIs for the highest vs. lowest categories of RHR and for 10 bpm increase of RHR (linear dose-response analysis). Subgroup and meta-regression analyses were conducted to explore sources of heterogeneity using a priori selected variables (i.e., geographic location, exposure and outcome measures, follow-up period, number of cases, exclusion of unhealthy participants and confounding adjustment). Cochran Q test and I2 were used to assess heterogeneity between studies. Potential publication bias was assessed using the Begg and Egger tests.
All statistical tests were two-sided and P<0.05 was considered significant. We used SAS 9.4 (SAS institute Inc., Cary, NC, USA) for the cohort analysis and STATA version 14.0 software (StataCorp, College Station, TX, USA) for the meta-analysis.
Results
Baseline characteristics
During 505,380 person-years of follow-up of 31,156 men, we identified 2,338 incident cases of T2D. Baseline characteristics of the participants are shown in Table 1. The mean age and BMI of participants were 58.3±9.3 years and 25.5±3.1 kg/m2, respectively. Participants with higher RHR engaged in less physical activities and had lower diet quality and higher BMI. The percentage of current smokers was higher, while the percentage of beta-blocker use was lower, among participants in higher categories of RHR.
Table 1.
Age-standardized baseline characteristics of participants according to resting heart rate in men (Health Professionals Follow-up Study, 1992–2012)
| Resting heart rate (beat per minute) |
||||||
|---|---|---|---|---|---|---|
| <60 | 60–64 | 65–69 | 70–74 | 75–79 | ≥80 | |
| Participants, N | 4295 | 8430 | 5239 | 6412 | 2905 | 3875 |
| Resting heart rate (beat per minute) | 53.6 (4.4) | 61.4 (1.7) | 66.9 (1.3) | 71.6 (1.2) | 76.5 (1.2) | 84.4 (7.0)a |
| Age (yrs)† | 57.9 (9.6) | 58.6 (9.6) | 58.3 (9.2) | 58.6 (9.4) | 58.2 (9.0) | 57.8 (8.9)a |
| BMI (kg/m2) | 24.9 (2.8) | 25.3 (2.9) | 25.5 (3.0) | 25.7 (3.3) | 25.9 (3.3) | 26.1 (3.5)a |
| Physical activity (MET-h/week) | 42.4 (35.5) | 33.4 (29.9) | 31.3 (29.3) | 28.0 (27.8) | 25.2 (25.1) | 23.3 (24.9)a |
| Total calorie intake (kcal/day) | 1994 (556) | 1959 (547) | 1965 (555) | 1960 (559) | 1961 (557) | 1989 (575) |
| Alcohol consumption (g/day) | 9.81 (13.4) | 9.94 (13.6) | 10.19 (13.8) | 10.51 (14.7) | 10.95 (15.3) | 11.86 (16.5)a |
| P:S ratio | 0.63 (0.21) | 0.60 (0.19) | 0.59 (0.18) | 0.58 (0.18) | 0.58 (0.18) | 0.56 (0.17)a |
| Trans fat (% of total energy) | 1.29 (0.50) | 1.35 (0.50) | 1.37 (0.50) | 1.40 (0.51) | 1.41 (0.49) | 1.46 (0.51)a |
| Cereal fiber (g/day) | 7.01 (4.30) | 6.35 (3.97) | 6.21 (3.64) | 6.00 (3.54) | 5.87 (3.33) | 5.53 (3.19)a |
| Whole grain (g/day) | 28.2 (20.8) | 24.5 (18.2) | 23.6 (17.6) | 22.5 (18.1) | 21.5 (16.6) | 19.9 (15.9)a |
| Glycemic load | 131.6 (44.5) | 125.8 (42.4) | 125.2 (42.7) | 123.6 (42.6) | 122.8 (42.5) | 122.1 (42.9)a |
| Diet z-score (SD)‡ | 0.68 (2.91) | 0.18 (2.67) | 0.01 (2.55) | −0.18 (2.56) | −0.28 (2.42) | −0.63 (2.40)a |
| white, % | 96.6 | 95.8 | 96.1 | 95.5 | 95.0 | 94.8a |
| Ever smoker, % | 43.0 | 48.1 | 48.8 | 49.4 | 51.8 | 56.9a |
| Family history of diabetes, % | 14.5 | 15.1 | 14.7 | 14.9 | 14.8 | 15.7a |
| Hypertension, % | 17.7 | 17.2 | 17.4 | 18.5 | 16.9 | 21.6a |
| Betablocker use, % | 11.3 | 8.2 | 7.3 | 5.2 | 4.2 | 4.1a |
| Thiazide diuretic use, % | 3.3 | 3.6 | 3.4 | 4.1 | 3.7 | 4.8a |
| Furosemide-like diuretic use, % | 0.8 | 0.9 | 1.0 | 1.0 | 1.0 | 1.5a |
| Calcium channel blocker use, % | 5.1 | 5.4 | 5.2 | 5.9 | 4.6 | 5.9a |
| Other antihypertensive drug use, % | 3.7 | 4.3 | 4.3 | 5.3 | 5.1 | 7.0a |
| Antiarrhythmic drug use, % | 1.2 | 1.0 | 0.8 | 0.7 | 0.8 | 0.4a |
Data are mean (SD) for continuous variables and percentage for categorical variables.
Value is not age adjusted
Diet z-score was calculated by standardizing and summarizing continuously scaled dietary variables (polyunsaturated fat to saturated fat ratio, trans fat, cereal fiber, whole grain, and glycemic load) P for trend across categories of resting heart rate.
P<0.001
P<0.05
RHR and T2D risk
Table 2 shows the association between RHR and risk of T2D in men. Overall, elevated RHR was positively associated with increased risk of T2D. The age-adjusted HR of T2D comparing the highest to lowest categories of RHR was 2.53 (95% CI, 2.15–2.98). Adjustment for potential confounders substantially attenuated this association (multivariable-adjusted HR of 1.82 (95% CI, 1.54–2.15). When we further adjusted for physical activity in the model, we observed weakened but nevertheless strong positive association that remained significant (HR, 1.69; 95% CI, 1.43–2.01). Increase in 10 bpm of RHR was associated with 19% increased risk of T2D in the fully adjusted model (HR, 1.19; 95% CI, 1.14–1.24). The results did not change after excluding those with antihypertensive use and hypertension. Adjustment of vigorous activity instead of total physical activity yielded consistent results (data not shown).
Table 2.
Hazard ratio (95% CI) of type 2 diabetes associated with resting heart rate in men (1992–2012)
| Resting heart rate (beat per minute) |
|||||||
|---|---|---|---|---|---|---|---|
| <60 | 60–64 | 65–69 | 70–74 | 75–79 | ≥80 | Per 10 bpm | |
| All participants | |||||||
| Cases | 210 | 511 | 381 | 504 | 284 | 448 | |
| Person-year | 72173 | 138413 | 86184 | 102209 | 46568 | 59833 | |
| Age-adjusted | 1 (ref) | 1.25 (1.07–1.47) | 1.49 (1.26–1.77) | 1.66 (1.42–1.95) | 2.07 (1.73–2.47) | 2.53 (2.15–2.98) | 1.31 (1.26–1.36) |
| Mutivariable-adjusted† | 1 (ref) | 1.14 (0.97–1.35) | 1.30 (1.09–1.54) | 1.39 (1.18–1.64) | 1.66 (1.38–1.99) | 1.82 (1.54–2.15) | 1.21 (1.16–1.26) |
| Mutivariable-adjusted‡ | 1 (ref) | 1.11 (0.95–1.31) | 1.25 (1.06–1.49) | 1.33 (1.13–1.56) | 1.55 (1.29–1.86) | 1.69 (1.43–2.01) | 1.19 (1.14–1.24) |
| Excluding those with any antihypertensive drug use | |||||||
| Cases | 146 | 380 | 275 | 356 | 219 | 343 | |
| Person-year | 60783 | 116946 | 73680 | 87144 | 40376 | 50262 | |
| Age-adjusted | 1 (ref) | 1.34 (1.10–1.62) | 1.54 (1.26–1.88) | 1.68 (1.38–2.03) | 2.24 (1.82–2.77) | 2.81 (2.32–3.42) | 1.35 (1.29–1.40) |
| Mutivariable-adjusted† | 1 (ref) | 1.15 (0.95–1.39) | 1.26 (1.03–1.54) | 1.28 (1.05–1.55) | 1.66 (1.35–2.06) | 1.88 (1.54–2.29) | 1.23 (1.17–1.28) |
| Mutivariable-adjusted‡ | 1 (ref) | 1.11 (0.91–1.34) | 1.21 (0.98–1.48) | 1.21 (0.99–1.47) | 1.53 (1.24–1.89) | 1.72 (1.41–2.10) | 1.20 (1.14–1.26) |
| Excluding those with any antihypertensive drug use or hypertension | |||||||
| Cases | 128 | 339 | 241 | 331 | 190 | 304 | |
| Person-year | 57380 | 110838 | 69621 | 81497 | 37846 | 46272 | |
| Age-adjusted | 1 (ref) | 1.35 (1.10–1.66) | 1.53 (1.24–1.90) | 1.80 (1.47–2.20) | 2.23 (1.78–2.80) | 2.91 (2.37–3.58) | 1.35 (1.29–1.42) |
| Mutivariable-adjusted† | 1 (ref) | 1.15 (0.94–1.41) | 1.23 (0.99–1.53) | 1.36 (1.11–1.67) | 1.65 (1.31–2.06) | 1.93 (1.56–2.38) | 1.23 (1.17–1.29) |
| Mutivariable-adjusted‡ | 1 (ref) | 1.11 (0.90–1.36) | 1.18 (0.95–1.47) | 1.28 (1.04–1.58) | 1.51 (1.20–1.90) | 1.77 (1.43–2.18) | 1.20 (1.15–1.27) |
Adjusted for age, race (White or non-White), family history of diabetes (yes or no), alcohol consumption (0, 0.1–4.9, 5–9.9, 10–14.9, ≥15 g/day), total calorie intake (quintiles), smoking status (never, quit≥10yrs, quit<10 yrs, or current smokers), intake of dietary factors including trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, whole grain, and glycemic load (quintiles), body mass index (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), high blood pressure at baseline (yes or no), and medication use at baseline (i.e., betablocker, thiazide diuretic, furosemide-like diuretic, calcium channel blocker, other antihypertensive drug and antiarrhythmic drug) (yes or no)
Additionally adjusted for physical activity (<3, 3–8.9, 9–17.9, 18–26.9, ≥27 MET-h/week)
Figure 1 presents the results of the updated meta-analysis including the current study. The summary RR for the highest vs. lowest RHR and T2D was 1.44 (95% CI: 1.20–1.74, I2: 92.9%, 12 studies) and the summary RR per 10 bpm increase in RHR was 1.17 (95% CI: 1.09–1.26, I2: 91.8%, 13 studies), but there was a large heterogeneity across studies. Subgroup and meta-regression analyses showed no significant heterogeneity between subgroups (Supplementary table 2). The Begg and Egger tests showed no evidence of publication bias (data not shown).
Figure 1.
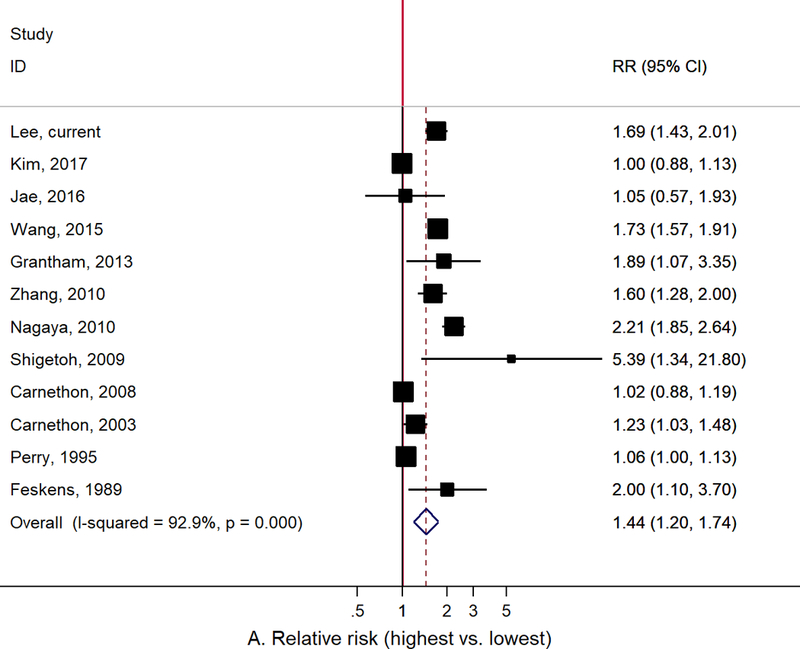
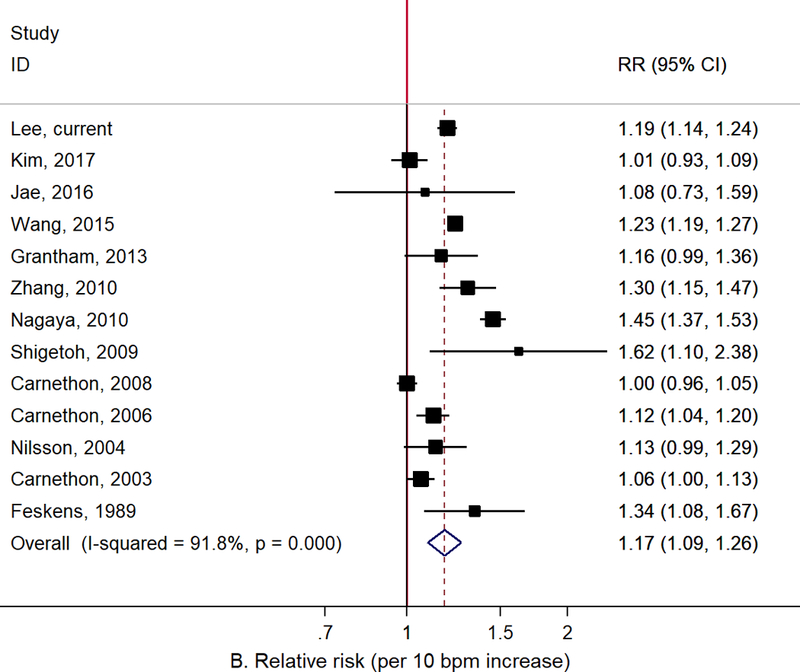
Meta-analysis of the association between resting heart rate and type 2 diabetes: A. Resting heart rate for the highest vs. lowest categories, B. Resting heart rate for 10 bpm increase.
Interaction between RHR and known risk factors for T2D
The association between RHR and risk of T2D significantly differed by BMI (P for interaction<.001) and high blood pressure (P for interaction<.001) (Table 3). The positive association between RHR and T2D (on the multiplicative scale) was stronger among those with normal weight or without hypertension. However, there were no significant differences by age, physical activity, diet quality, smoking status and family history of diabetes. Moreover, we did not find a significant effect modification by different types of antihypertensive drugs although the association between RHR and risk of T2D tended to be stronger among non-antihypertensive drug users in general (Supplementary table 3).
Table 3.
Hazard ratio (95% CI) of type 2 diabetes associated with resting heart rate stratified by known risk factors† in men (1992–2012)
| Resting heart rate (beat per minute) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <60 | 60–64 | 65–69 | 70–74 | 75–79 | ≥80 | Per 10 bpm | P interaction | ||
| Age | |||||||||
| <60 yrs | 1 (ref) | 1.18 (0.91–1.52) | 1.19 (0.91–1.56) | 1.38 (1.07–1.78) | 1.48 (1.12–1.97) | 1.74 (1.34–2.25) | 1.19 (1.12–1.26) | 0.19 | |
| ≥60 yrs | 1 (ref) | 1.06 (0.86–1.31) | 1.30 (1.04–1.61) | 1.28 (1.03–1.58) | 1.57 (1.24–2.00) | 1.61 (1.29–2.02) | 1.18 (1.12–1.25) | ||
| Body mass index | |||||||||
| <25 kg/m2 | 1 (ref) | 1.34 (0.93–1.93) | 1.52 (1.03–2.25) | 1.70 (1.17–2.47) | 2.40 (1.58–3.64) | 2.63 (1.77–3.90) | 1.34 (1.23–1.46) | <.001 | |
| 25–29.9 kg/m2 | 1 (ref) | 1.05 (0.84–1.30) | 1.28 (1.02–1.60) | 1.29 (1.04–1.60) | 1.44 (1.13–1.85) | 1.68 (1.34–2.11) | 1.18 (1.12–1.25) | ||
| ≥30 kg/m2 | 1 (ref) | 1.02 (0.73–1.43) | 0.95 (0.66–1.36) | 1.05 (0.75–1.48) | 1.27 (0.88–1.83) | 1.23 (0.87–1.73) | 1.09 (1.00–1.18) | ||
| Physical activity | |||||||||
| <25 MET-h/week | 1 (ref) | 1.03 (0.82–1.29) | 1.13 (0.90–1.43) | 1.26 (1.01–1.58) | 1.48 (1.17–1.88) | 1.59 (1.27–1.99) | 1.18 (1.12–1.24) | 0.51 | |
| ≥25 MET-h/week | 1 (ref) | 1.23 (0.97–1.56) | 1.42 (1.10–1.83) | 1.41 (1.10–1.81) | 1.63 (1.21–2.21) | 1.88 (1.44–2.47) | 1.21 (1.13–1.30) | ||
| Diet quality | |||||||||
| <median | 1 (ref) | 1.05 (0.84–1.31) | 1.19 (0.94–1.49) | 1.25 (1.00–1.55) | 1.42 (1.11–1.81) | 1.57 (1.26–1.97) | 1.17 (1.11–1.24) | 0.41 | |
| ≥median | 1 (ref) | 1.21 (0.95–1.53) | 1.35 (1.04–1.74) | 1.45 (1.14–1.86) | 1.76 (1.34–2.33) | 1.94 (1.49–2.53) | 1.22 (1.14–1.30) | ||
| Ever smoking | |||||||||
| No | 1 (ref) | 1.16 (0.93–1.46) | 1.32 (1.04–1.68) | 1.30 (1.03–1.64) | 1.66 (1.28–2.14) | 1.59 (1.24–2.02) | 1.16 (1.09–1.23) | 0.48 | |
| Yes | 1 (ref) | 1.06 (0.84–1.34) | 1.19 (0.93–1.52) | 1.36 (1.07–1.71) | 1.48 (1.14–1.92) | 1.79 (1.41–2.26) | 1.22 (1.15–1.29) | ||
| Family history of diabetes | |||||||||
| No | 1 (ref) | 1.11 (0.92–1.34) | 1.31 (1.08–1.60) | 1.40 (1.16–1.70) | 1.65 (1.34–2.04) | 1.71 (1.40–2.08) | 1.19 (1.14–1.25) | 0.59 | |
| Yes | 1 (ref) | 1.18 (0.86–1.62) | 1.15 (0.82–1.61) | 1.12 (0.80–1.56) | 1.35 (0.93–1.96) | 1.75 (1.25–2.44) | 1.18 (1.08–1.28) | ||
| Hypertension | |||||||||
| No | 1 (ref) | 1.13 (0.93–1.38) | 1.24 (1.01–1.52) | 1.32 (1.08–1.61) | 1.54 (1.24–1.91) | 1.80 (1.47–2.21) | 1.20 (1.14–1.26) | <.001 | |
| Yes | 1 (ref) | 1.09 (0.81–1.46) | 1.34 (0.98–1.82) | 1.37 (1.01–1.84) | 1.58 (1.13–2.22) | 1.47 (1.08–2.01) | 1.14 (1.05–1.24) | ||
All models were adjusted for age, race (White or non-White), family history of diabetes (yes or no), alcohol consumption (0, 0.1–4.9, 5–9.9, 10–14.9, ≥15 g/day), total calorie intake (quintiles), smoking status (never, quit≥10yrs, quit<10 yrs, or current smokers), intake of dietary factors including trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, whole grain, and glycemic load (quintiles), body mass index (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), high blood pressure at baseline (yes or no), medication use at baseline (i.e., betablocker, thiazide diuretic, furosemide-like diuretic, calcium channel blocker, other antihypertensive drug and antiarrhythmic drug) (yes or no) and physical activity (<3, 3–8.9, 9–17.9, 18–26.9, ≥27 MET-h/week)
Number of cases: age (<60, n=1033; ≥60, n=1305), body mass index (<25, n=441; 25–29.9, n=1277; ≥30, n=620), physical activity (<25, n=1512; ≥25, n=826), diet quality (<median, n=1384; ≥median, n=954), ever smoking (no, n=1137; yes, n=1201), family history of diabetes (no, n=1761; yes, n=577), hypertension (no, n=1028; yes, n=1310)
When we examined the joint association of RHR and known risk factors in relation to T2D risk, there was a significant multiplicative interaction between higher RHR and greater number of risk factors (Table 4). Compared to those with low RHR and ≤1 risk factor, men with high RHR and 3 and 4+ risk factors had 7.0 and 14.8 times increased risk of T2D, respectively. Similarly, we found a strong multiplicative interaction of RHR and each individual risk factor (i.e., age, BMI, physical activity, diet quality, smoking, family history of diabetes and hypertension) with T2D risk.
Table 4.
Joint association of resting heart rate and known risk factors in relation to type 2 diabetes in men (1992–2012)
| Resting heart rate (beat per minute) |
|||
|---|---|---|---|
| Tertile 1 (<63) | Tertile 2 (63–71) | Tertile 3 (≥72) | |
| Age | |||
| <60 yrs | 1 (ref) | 1.19 (1.00–1.41) | 1.50 (1.28–1.75) |
| ≥60 yrs | 1.24 (1.05–1.48) | 1.54 (1.31–1.81) | 1.73 (1.47–2.02) |
| BMI | |||
| <25 kg/m2 | 1 (ref) | 1.18 (0.92–1.52) | 1.84 (1.47–2.32) |
| 25–29.9 kg/m2 | 2.71 (2.19–3.36) | 3.71 (3.02–4.55) | 3.94 (3.22–4.83) |
| ≥30 kg/m2 | 8.16 (6.37–10.5) | 7.28 (5.72–9.28) | 9.19 (7.39–11.4) |
| Physical activity | |||
| <25 MET-h/week | 1 (ref) | 1.31 (1.10–1.56) | 1.54 (1.30–1.82) |
| ≥25 MET-h/week | 1.37 (1.15–1.62) | 1.57 (1.34–1.84) | 1.89 (1.63–2.20) |
| Diet quality | |||
| <median | 1 (ref) | 1.29 (1.09–1.52) | 1.57 (1.33–1.84) |
| ≥median | 1.25 (1.06–1.48) | 1.45 (1.23–1.70) | 1.68 (1.44–1.96) |
| Ever smoking | |||
| No | 1 (ref) | 1.23 (1.06–1.44) | 1.40 (1.21–1.63) |
| Yes | 1.13 (0.96–1.34) | 1.34 (1.15–1.57) | 1.65 (1.43–1.91) |
| Family history of diabetes | |||
| No | 1 (ref) | 1.23 (1.08–1.40) | 1.48 (1.31–1.67) |
| Yes | 1.95 (1.61–2.35) | 2.26 (1.89–2.69) | 2.47 (2.11–2.90) |
| Hypertension | |||
| No | 1 (ref) | 1.22 (1.03–1.45) | 1.57 (1.34–1.83) |
| Yes | 1.73 (1.45–2.06) | 2.04 (1.73–2.42) | 2.21 (1.89–2.59) |
| Number of risk factors† | |||
| 0–1 | 1 (ref) | 1.83 (1.05–3.17) | 2.13 (1.20–3.78) |
| 2 | 2.32 (1.44–3.74) | 2.83 (1.76–4.53) | 3.38 (2.12–5.39) |
| 3 | 4.52 (2.90–7.03) | 5.72 (3.71–8.83) | 6.98 (4.56–10.7) |
| 4+ | 10.5 (6.91–15.9) | 12.2 (8.10–18.5) | 14.8 (9.83–22.3) |
All models were adjusted for age, race (White or non-White), family history of diabetes (yes or no), alcohol consumption (0, 0.1–4.9, 5–9.9, 10–14.9, ≥15 g/day), total calorie intake (quintiles), smoking status (never, quit≥10yrs, quit<10 yrs, or current smokers), intake of dietary factors including trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, whole grain, and glycemic load (quintiles), body mass index (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), high blood pressure at baseline (yes or no), medication use at baseline (i.e., betablocker, thiazide diuretic, furosemide-like diuretic, calcium channel blocker, other antihypertensive drug and antiarrhythmic drug) (yes or no) and physical activity (<3, 3–8.9, 9–17.9, 18–26.9, ≥27 MET-h/week)
Risk factors included age, body mass index, physical activity, diet quality, smoking, family history of diabetes, and hypertension
Discussion
In this large prospective cohort study of US men, we found a strong positive association between RHR and T2D risk. RHR was a stronger predictor of T2D among those with normal weight or with no hypertension. Moreover, RHR had a multiplicative interaction with known risk factors in relation to T2D risk.
Our findings were consistent with previous studies that have suggested an association between elevated RHR and increased risk of T2D. In the meta-analysis published in 2015, the summary RR for the highest vs. lowest RHR was 1.83 (95% CI: 1.28–2.60, I2: 88%, 7 studies) and the summary RR per 10 bpm increase in RHR was 1.20 (95% CI: 1.07–1.34, I2: 93%, 9 studies).17 We updated this meta-analysis with all published papers since then (a total of 14 prospective cohort studies including the current study) and found consistently strong positive association, albeit studies were heterogeneous with regards to study population, design, and data analysis (Figure 1 and Supplementary table 1). The observed findings should be interpreted with careful consideration of the following potential biases.
First, RHR and T2D relationship is particularly susceptible to confounding. For instance, age, BMI, lifestyle and medical conditions are known to be associated with T2D and they can also affect RHR.24–26 While previous studies have adjusted for some of the potential confounders (e.g., demographics, lifestyle factors, disease status, medications, and biomarkers), no study has fully adjusted for a comprehensive set of known confounders to the extent we did in our study. That being said, we could not adjust for baseline glucose and insulin sensitivity, which were controlled for in several other studies. Similar to our results, prior studies have also reported substantial attenuation in the magnitude of the association between RHR and T2D after adjusting for lifestyle factors, medical conditions and/or biomarkers. Most of them found the positive association between RHR and T2D to be significant in their final models.
Second, RHR is prone to reverse causation because RHR may reflect subclinical chronic diseases.27,28 Thus, it is important to exclude participants with preexisting diseases or medication use in the analysis. Reverse causation could be a greater concern in studies with short follow-up period because undiagnosed health condition may change RHR while increasing T2D risk. More than half of the published studies had shorter than 10 years of follow-up period (mostly <5 years). Additionally, only a few studies had conducted sensitivity analyses to test for robustness of their findings. With 15 years of follow up, our primary and sensitivity analyses showed a robust positive association between RHR and T2D after excluding participants with cardiovascular disease, cancer, hypertension or antihypertensive use. These results strengthen the evidence that RHR may have an independent association with T2D risk.
Third, measurement error in RHR is inevitable as various factors may have acute and/or chronic effect on RHR. The Health Professional Follow-up Study collected self-reported RHR and thus our study is prone to more measurement error than studies that have used objective measures (i.e., electrocardiogram). However, measurement error is likely to be random given the nature of our prospective study design. Also, because our study included health professionals with expert medical knowledge and they measured their own RHR, such measurement error is likely to be minimal and less likely to be affected by acute stress that could be caused in a medical setting (white coat syndrome). The concern for measurement error in RHR is further reduced given that our results were highly consistent with previous studies that had used objective RHR measure.
Lastly, in our updated review of all the relevant publications, we found heterogeneous study populations across the studies. Studies were mostly done in Asia (6 studies), followed by Europe (3 studies), USA (3 studies), and Australia (1 study). While these studies had different participant characteristics (e.g., race/ethnicity, urban vs. rural, general population vs. employee, etc.), the positive association between RHR and T2D was persistently found.
Several biological mechanisms may explain the observed association between RHR and T2D. First, RHR is an indicator of autonomic activity.29 Parasympathetic nerve fibers stimulate pancreatic B-cells to release insulin while sympathetic activation inhibits the insulin secretion from the pancreas. More importantly, sympathetic overactivity can impair glucose uptake in skeletal muscle by inducing vasoconstriction and reducing skeletal muscle blood flow.30 Sympathetic overactivity is also associated with stimulation of the renin-angiotensin-aldosterone system and result in insulin resistance.31 Second, RHR is a marker of physical fitness with faster RHR potentially reflecting lower cardiorespiratory fitness.32 It is known that low cardiorespiratory fitness is associated with increased risk of chronic diseases, including T2D.33 While we found significant and positive association between RHR and T2D even after adjusting for total physical activity or vigorous activity, it is worth noting that physical activity is not a perfect measure of physical fitness and hence residual confounding may be present. To our knowledge, only one study has adjusted for physical fitness using an exercise test to measure peak oxygen consumption, which did not alter the association between RHR and T2D risk.34 Another recent study suggested that elevated RHR was associated with mortality, independent of physical fitness and physical activity.35 Thus, it is unlikely that residual confounding by physical fitness would substantially alter the conclusion of our study. For an unmeasured confounder to fully explain the observed association between RHR and T2D, it should have a minimum RR of 1.64 in relation to both RHR and T2D in the multivariable-adjusted models.36,37
Interestingly, we found a stronger HR of T2D associated with RHR among those with normal weight or without hypertension. Two other studies, including the Australian Diabetes Obesity and Lifestyle study and the Chicago Heart Association Detection Project in industry, have reported a significant positive association between RHR and T2D among non-obese individuals, but not among obese individuals.8,10 Our finding on stronger association between RHR and T2D among those who were younger, active, or without family history of diabetes (although not statistically significant) suggests that RHR may be a stronger predictor of T2D among low risk group for T2D. A large Chinese prospective study showed a significant interaction between age and RHR in relation to T2D risk, with the association being stronger among younger individuals (<50 years).14 Another interesting finding from the current study was that RHR and other known risk factors had a significant joint association with T2D risk. Only a few studies have examined joint association of RHR with BMI, waist-to-hip ratio, and blood pressure with T2D.11,15,16 Our study suggests that RHR in combination with other known risk factors (i.e., age, BMI, physical activity, diet, smoking status, family history of diabetes and hypertension) may provide further evidence to predict future T2D risk.
The present study has several strengths. To our knowledge, this study is the largest prospective cohort study of US men with long follow-up period. Moreover, this is the first study to conduct stratified and joint analyses using all established risk factors of T2D. Lastly, we had detailed information on important confounders such as lifestyle factors, medical history and medications. There are several data limitations as well. First, measurement error in self-reported RHR is inevitable. Yet, measurement error is likely to be non-differential and hence bias the estimates towards the null. Second, RHR was measured only once at the baseline, so changes in RHR over the follow-up are unknown. A recent study, with two RHR measurements, reported that increase in RHR over 2 years predicts T2D risk, although this study did not find a significant association between baseline RHR and T2D.38 Futher studies are needed to examine whether change in RHR is predictive of type 2 dibetese and how the magnitude of association between RHR and T2D changes over time. Third, although we thoroughly adjusted for possible confounders, residual confounding by unmeasured confounders (e.g., physical fitness and baseline glucose/insulin sensitivity) cannot be entirely ruled out in observational studies. Lastly, our cohort included predominantly White male health professionals which may limit the generalizability of our findings, but homogeneity of the study population enhances the internal validity.
Although it is difficult to draw a definitive causal relationship from observational study design, we demonstrated a fairly robust positive association between RHR and T2D risk in US men. The association was stronger in low-risk groups and significant joint association was found between RHR and other known risk factors in respect to T2D risk. Taken together with accumulating evidence suggesting the usefulness of RHR as a diagnostic tool for several chronic diseases and a prognostic tool for patients,5,39–45 our findings suggest that RHR has a potential to be used independently, and in addition to other known risk factors, to predict future T2D in the clinical settings.
Supplementary Material
Funding
This work was supported by the National Institutes of Health (UM1 CA167552 and R01 HL35464). Leandro Fórnias Machado de Rezende receives a doctoral fellowship from Sao Paulo Research Foundation (FAPESP), grants #2014/25614–4 and #2016/21390–0.
Footnotes
Conflict of interest
The authors declared no conflicts of interest.
References
- 1.Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1084–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. Global report on diabetes World Health Organization; 2016. [Google Scholar]
- 3.Prevention. CfDCa. National Diabetes Statistics Report 2017.
- 4.Group DPPR. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine 2002;2002(346):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aune D, Sen A, ó’Hartaigh B, et al. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality–A systematic review and dose–response meta-analysis of prospective studies. Nutrition, Metabolism and Cardiovascular Diseases 2017. [DOI] [PubMed]
- 6.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes. Circulation 2003;107(17):2190–2195. [DOI] [PubMed] [Google Scholar]
- 7.Carnethon MR, Prineas RJ, Temprosa M, Zhang Z-M, Uwaifo G, Molitch ME. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes care 2006;29(4):914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnethon MR, Yan L, Greenland P, et al. Resting heart rate in middle age and diabetes development in older age. Diabetes care 2008;31(2):335–339. [DOI] [PubMed] [Google Scholar]
- 9.Feskens EJ, Kromhout D. Cardiovascular risk factors and the 25-year incidence of diabetes mellitus in middle-aged men. American journal of epidemiology 1989;130(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 10.Grantham N, Magliano D, Tanamas S, Söderberg S, Schlaich M, Shaw J. Higher heart rate increases risk of diabetes among men: The Australian Diabetes Obesity and Lifestyle (AusDiab) Study. Diabetic medicine 2013;30(4):421–427. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya T, Yoshida H, Takahashi H, Kawai M. Resting heart rate and blood pressure, independent of each other, proportionally raise the risk for type-2 diabetes mellitus. International journal of epidemiology 2010;39(1):215–222. [DOI] [PubMed] [Google Scholar]
- 12.Perry IJ, Wannamethee SG, Walker MK, Thomson A, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. Bmj 1995;310(6979):560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigetoh Y, Adachi H, Yamagishi S-i, et al. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. American journal of hypertension 2008;22(2):151–155. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Cui L, Wang Y, et al. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. International journal of epidemiology 2015;44(2):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang SY, Wu JH, Zhou JW, et al. Overweight, resting heart rate, and prediabetes/diabetes: A population-based prospective cohort study among Inner Mongolians in China. Scientific reports 2016;6:23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Shu XO, Xiang YB, et al. Resting heart rate and risk of type 2 diabetes in women. International journal of epidemiology 2010;39(3):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aune D, B OH, Vatten LJ. Resting heart rate and the risk of type 2 diabetes: A systematic review and dose--response meta-analysis of cohort studies. Nutrition, metabolism, and cardiovascular diseases : NMCD 2015;25(6):526–534. [DOI] [PubMed] [Google Scholar]
- 18.Szklo M, Nieto F. Epidemiology: beyond the basics, 3rd edition, Jones & Bartlett Learning; Burlington, VT: 2014. [Google Scholar]
- 19.Group NDD. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28(12):1039–1057. [DOI] [PubMed] [Google Scholar]
- 20.Standards of medical care in diabetes−−2010. Diabetes care 2010;33 Suppl 1:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavin JR III, Alberti K, Davidson MB, DeFronzo RA. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care 1997;20(7):1183. [DOI] [PubMed] [Google Scholar]
- 22.Palatini P, Julius S. The physiological determinants and risk correlations of elevated heart rate. American journal of hypertension 1999;12(S1):3S–8S. [PubMed] [Google Scholar]
- 23.Aune D, ó Hartaigh B, Vatten L. Resting heart rate and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Nutrition, Metabolism and Cardiovascular Diseases 2015;25(6):526–534. [DOI] [PubMed] [Google Scholar]
- 24.Korat AVA, Willett WC, Hu FB. Diet, lifestyle, and genetic risk factors for type 2 diabetes: a review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-up Study. Current nutrition reports 2014;3(4):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi J, Hozawa A, Ohkubo T, et al. Factors Affecting Home-Measured Resting Heart Rate in the General Population* The Ohasama Study. American journal of hypertension 2005;18(9):1218–1225. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Kesteloot H. Anthropometric, lifestyle and metabolic determinants of resting heart rate. A population study. European heart journal 1999;20(2):103–110. [DOI] [PubMed] [Google Scholar]
- 27.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. European Heart Journal 2004;25(5):363–370. [DOI] [PubMed] [Google Scholar]
- 28.Rogowski O, Shapira I, Shirom A, Melamed S, Toker S, Berliner S. Heart rate and microinflammation in men: a relevant atherothrombotic link. Heart (British Cardiac Society) 2007;93(8):940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease. Journal of the American College of Cardiology 2008;51(18):1725–1733. [DOI] [PubMed] [Google Scholar]
- 30.Julius S, Gudbrandsson T, Jamerson K, Andersson O. The interconnection between sympathetics, microcirculation, and insulin resistance in hypertension. Blood pressure 1992;1(1):9–19. [DOI] [PubMed] [Google Scholar]
- 31.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clinical and experimental hypertension 2001;23(1–2):45–55. [DOI] [PubMed] [Google Scholar]
- 32.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. New England Journal of Medicine 1993;328(8):533–537. [DOI] [PubMed] [Google Scholar]
- 33.Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. Jama 1989;262(17):2395–2401. [DOI] [PubMed] [Google Scholar]
- 34.Jae SY, Kurl S, Laukkanen JA, et al. Exercise heart rate reserve and recovery as predictors of incident type 2 diabetes. The American journal of medicine 2016;129(5):536 e537–536. e512. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart (British Cardiac Society) 2013;99(12):882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Annals of internal medicine 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 37.Mathur MBDP, Riddell CA, VanderWeele TJ. Website and R package for computing E-values. Epidemiology 2018;in press. [DOI] [PMC free article] [PubMed]
- 38.Kim G, Lee YH, Jeon JY, et al. Increase in resting heart rate over 2 years predicts incidence of diabetes: A 10-year prospective study. Diabetes & metabolism 2017;43(1):25–32. [DOI] [PubMed] [Google Scholar]
- 39.Böhm M, Reil J-C, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. The American journal of medicine 2015;128(3):219–228. [DOI] [PubMed] [Google Scholar]
- 40.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. The Lancet 2008;372(9641):817–821. [DOI] [PubMed] [Google Scholar]
- 41.Lee DH, Park S, Lim SM, et al. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast cancer research and treatment 2016;159(2):375–384. [DOI] [PubMed] [Google Scholar]
- 42.Palatini P, Benetos A, Julius S. Impact of increased heart rate on clinical outcomes in hypertension. Drugs 2006;66(2):133–144. [DOI] [PubMed] [Google Scholar]
- 43.Yang HI, Kim HC, Jeon JY. The association of resting heart rate with diabetes, hypertension, and metabolic syndrome in the Korean adult population: The fifth Korea National Health and Nutrition Examination Survey (1873–3492 (Electronic)). [DOI] [PubMed]
- 44.Park J, Kim JH, Park Y, et al. Resting heart rate is an independent predictor of advanced colorectal adenoma recurrence (1932–6203 (Electronic)). [DOI] [PMC free article] [PubMed]
- 45.Lee MK, Lee DH, Park S, Kim SI, Jeon JY. Relationship between resting heart rate and metabolic risk factors in breast cancer patients. Clinica Chimica Acta 2018. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


