Abstract
Candidiasis is a fungal infection caused by yeasts that belong to the genus Candida. There are over 20 species of Candida yeasts that can cause infection in humans, the most common of which is Candida albicans. Candida yeasts normally reside in the intestinal tract and can be found on mucous membranes and skin without causing infection. However, under immunocompromised conditions, Candida can cause significant infections in susceptible patients. Herein, we report a peculiar presentation of a C. albicans cutaneous infection in an immunocompetent young subject. This case widens our knowledge on the C. albicans infections both in terms of host susceptibility and cutaneous manifestations.
Keywords: Candida albicans, cutaneous candidiasis, mycotic infection
Introduction
The skin is a complex and dynamic ecosystem inhabited by bacteria, archaea, viruses, and fungi. Several genera have been reported to be common fungal skin commensals, such as Malassezia, Rhodotorula, Debaromyces, Cryptococcus, and in some sites, Candida.1 In particular, Candida species are frequently colonizers of the skin and mucosal surfaces of our body, including the genitourinary tract, oral cavity, and gastrointestinal tract.2 Over the 200 species of Candida identified, only 15 of them are human pathogens and Candida albicans is the most prevalent.2 Indeed, C. albicans is responsible for a wide range of diseases from more superficial and milder clinical manifestations to life-threatening diseases in immunocompromised patients.3 Its ability of growing in different morphological forms (i.e. unicellular budding yeast, pseudohyphae, and true hyphae) enables this microorganism to become virulent and invade host tissues.4,5 Therefore, variations in the skin environment, antibiotic therapy, underlying diseases, and/or alterations in the immune system can induce the switch of C. albicans from a commensal to a pathogen, thereby causing significant infections in susceptible patients.3,6 Among cutaneous candidiasis, the infection usually affects intertriginous and interdigital areas and its typical clinical presentation is characterized by dry, erosive, erythematous, or scaly skin; flaking collarette; or pustules.7 Herein, we report the case of a 37-year-old otherwise healthy man who had a peculiar and previously unreported cutaneous manifestation of C. albicans infection.
Case report
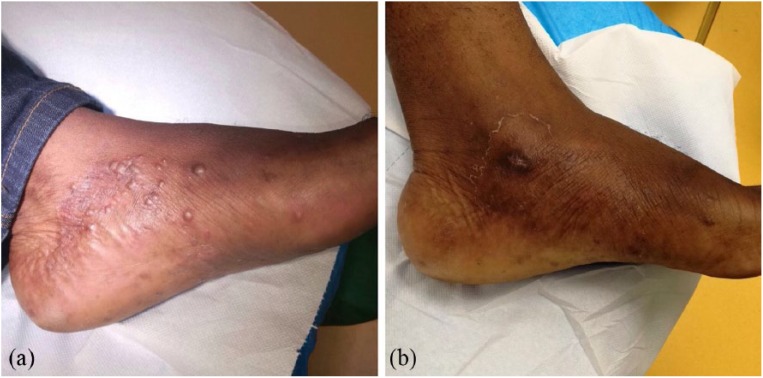
A 37-year-old man referred to our clinic for itchy, painless papules on his left foot (Figure 1(a)). The subject provided a written informed consent for information and images in accordance with the Declaration of Helsinki. The patient reported not to be a smoker, not to suffer from hypertension, and to have a sedentary lifestyle. In addition, he referred that he was not under any antibiotic or corticosteroids therapy nor that he had changed his diet or soaps for personal hygiene. Laboratory tests revealed no sign of diabetes (glycemic values of 86 mg/dL) or immunodeficiency (white blood cell count of 9610 mm−3 and a CD4 lymphocyte count of 825 µL−1).
Figure 1.
Clinical presentation of cutaneous Candida albicans infection. (a) Clinical appearance of the infection showing multiple, hard papules on a scaly, reddish skin. (b) Improvement of the papules after 2 weeks of oral fluconazole treatment.
Clinical examination of patient’s left foot revealed a scaly, reddish skin with multiple, hard papules on the medial surface of the dorsum, with a diameter of 1 mm. No specific distribution pattern was noticed (Figure 1(a)). Their hard consistency ruled out vesicular lesions, whereas unilateral spreading and scaly borders led us to hypothesize a mycotic infection.
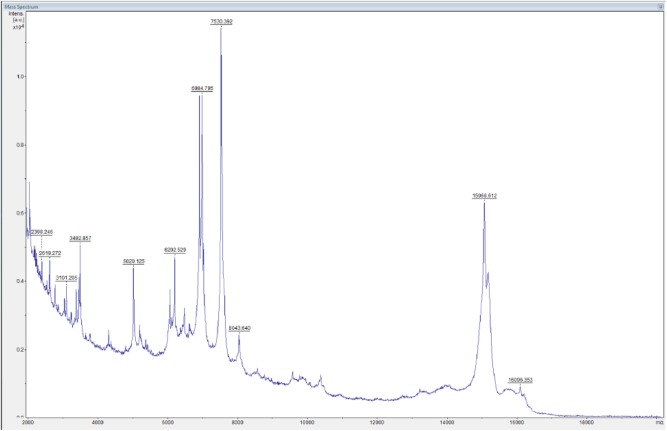
Microbiological culture of cutaneous swabs/skin specimens on Sabouraud plates revealed creamy, rapidly growing, white colonies, confirming a candidiasis. Colony subculturing on chromID® Candida agar (bioMérieux, Inc., France) showed only blue-colored colonies, suggestive for C. albicans species. Species identification was performed by VITEK2 system (bioMérieux, Inc.) and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) analysis. The peptide mass fingerprinting of C. albicans by MALDI-TOF MS is shown in Figure 2. Microscopic examination of methylene blue–stained C. albicans culture highlighted the formation of true hyphae (Figure 3). Histopathological analysis was consistent with a superficial mycosis describing fungal periodic acid–Schiff (PAS)-positive structures in the stratum corneum, orthokeratotic hyperkeratosis, epidermal hyperplasia, spongiosis, vesicles filled with serum and neutrophils, and superficial perivascular lymphocytic infiltrate (data not shown). Antifungal susceptibility tests, carried out by VITEK2 (bioMérieux, Inc.) and Sensititre YeastOne® Systems (Thermo Scientific), following the manufacturer’s instructions (www.mcsdiagnostics.com), showed that the isolate was susceptible to fluconazole. Therefore, the patient received oral fluconazole therapy (100 mg/die) and 2 weeks after treatment showed a clinical improvement characterized by a significant decrease in the number of papules (Figure 1(b)).
Figure 2.
Peptide mass fingerprinting of Candida albicans performed by MALDI-TOF MS.
Figure 3.
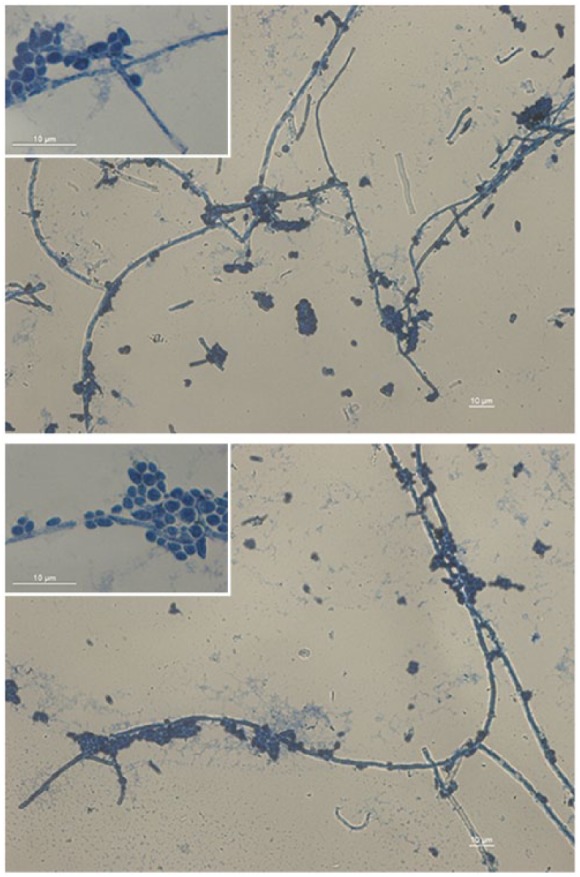
Filamentous stage of development of Candida albicans. Formation of true hyphae in liquid Sabouraud medium of the C. albicans isolated after 72 h at 28°C. The heat-fixed smear was stained with methylene blue. Images were acquired with a magnification of 400× and relative insets at 1000×, using a Leica DM5000B microscope equipped with Digital FireWire Color Camera systems Leica DFX300 and processed using the Leica Application Suite 2.7.0.R1 software (Leica).
Discussion
Skin C. albicans infections may be triggered by physical, chemical and immunological local factors or individual systemic immunodeficiencies.8 Immunological impairments can induce a typical disorder consisting of mucosal, cutaneous and ungual persistent and/or recurrent lesions, known as chronic mucocutaneous candidiasis.9 Immunosuppressed patients are at major risk to develop a C. albicans infections, including premature neonates, diabetics, HIV-positive patients, and those receiving organ transplants or chemotherapy.10 However, also immunocompetent subjects can be susceptible to these infections, but mainly with a healthcare-related acquisition.11 However, the case reported herein involves a patient with a symptomatic cutaneous candidiasis without evidence of local or systemic immunodeficiency condition, as determined by immunologic evaluation. However, the onset and exacerbation of this C. albicans infection could arise from a combination of other factors such as genetic predisposition and environmental factors including skin pH, food, soaps, and house dust mites that might have induced major changes in the local microbiota. In addition, the extensive ability of C. albicans to colonize skin and mucosal surfaces and the expression of infection-associated genes should be taken into account.12 These virulence factors, including the production of different hydrolytic enzymes, adhesins, the ability to form biofilms, and morphological dimorphism, expressed upon slight changes in the environmental condition might enable the onset of the infection.13
In this case report, we describe a presentation of an unusual cutaneous C. albicans infection appearing with unilateral papules in an immunocompetent patient. The uncommon site and the peculiar clinical manifestation of the described infection in this case further broaden the wide spectrum of candidiasis and could aid the diagnosis of unusual C. albicans infections.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mattia Carbotti  https://orcid.org/0000-0001-8484-3205
https://orcid.org/0000-0001-8484-3205
Cecilia Ambrosi  https://orcid.org/0000-0003-2163-1613
https://orcid.org/0000-0003-2163-1613
Valeria Pietropaolo  https://orcid.org/0000-0001-5723-8886
https://orcid.org/0000-0001-5723-8886
References
- 1. Findley K, Oh J, Yang J, et al. (2013) Topographic diversity of fungal and bacterial communities in human skin. Nature 498: 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kashem SW, Kaplan DH. (2016) Skin immunity to Candida albicans. Trends in Immunology 37: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Havlickova B, Czaika VA, Friedrich M. (2008) Epidemiological trends in skin mycoses worldwide. Mycoses 51: 2–15. [DOI] [PubMed] [Google Scholar]
- 4. Sudbery P, Gow N, Berman J. (2004) The distinct morphogenic states of Candida albicans. Trends in Microbiology 12: 317–324. [DOI] [PubMed] [Google Scholar]
- 5. Bineshian F, Yadegari MH, Sharifi Z, et al. (2015) Identification of Candida species using MP65 gene and evaluation of the Candida albicans MP65 gene expression in BALB/C Mice. Jundishapur Journal of Microbiology 8: e18984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coppola R, Zanframundo S, Rinati MV, et al. (2015) Rhodotorula mucilaginosa skin infection in a patient treated with sorafenib. Journal of the European Academy of Dermatology and Venereology 29: 1028–1029. [DOI] [PubMed] [Google Scholar]
- 7. Nenoff P, Krüger C, Schaller J, et al. (2014) Mycology— an update part 2: Dermatomycoses: Clinical picture and diagnostics. Journal of the German Society of Dermatology 12: 749–777. [DOI] [PubMed] [Google Scholar]
- 8. Allen AM, King RD. (1978) Occlusion, carbon dioxide, and fungal skin infections. Lancet 1: 360–362. [DOI] [PubMed] [Google Scholar]
- 9. Kirkpatrick CH. (2001) Chronic mucocutaneous candidiasis. The Pediatric Infectious Disease Journal 20: 197–206. [DOI] [PubMed] [Google Scholar]
- 10. Lanternier F, Cypowyj S, Picard C, et al. (2013) Primary immunodeficiencies underlying fungal infections. Current Opinion in Pediatrics 25: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clancy CJ, Nguyen MH. (1998) Acute community-acquired pneumonia due to Aspergillus in presumably immunocompetent hosts: Clues for recognition of a rare but fatal disease. Chest 114: 629–634. [DOI] [PubMed] [Google Scholar]
- 12. Brown AJ, Odds FC, Gow NA. (2007) Infection-related gene expression in Candida albicans. Current Opinion in Microbiology 10: 307–313. [DOI] [PubMed] [Google Scholar]
- 13. Chaffin WL, López-Ribot JL, Casanova M, et al. (1998) Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiology and Molecular Biology Reviews 62: 130–180. [DOI] [PMC free article] [PubMed] [Google Scholar]