In this study, measuring cotinine levels from maternal serum specimens collected during pregnancy, we investigated the association between nicotine exposure during pregnancy and offspring ADHD.
Abstract
OBJECTIVES:
An association between maternal smoking during pregnancy and offspring attention-deficit/hyperactivity disorder (ADHD) has been shown across several studies based on self-reports. No previous studies have investigated the association of nicotine exposure measured by cotinine levels during pregnancy and offspring ADHD.
METHODS:
In this population-based study, 1079 patients born between 1998 and 1999 and diagnosed with ADHD according to the International Classification of Diseases and 1079 matched controls were identified from Finnish nationwide registers. Maternal cotinine levels were measured by using quantitative immunoassays from maternal serum specimens collected during the first and second trimesters of pregnancy and archived in the national biobank.
RESULTS:
There was a significant association between increasing log-transformed maternal cotinine levels and offspring ADHD. The odds ratio was 1.09 (95% confidence interval [CI] 1.06–1.12) when adjusting for maternal socioeconomic status, maternal age, maternal psychopathology, paternal age, paternal psychopathology, and child’s birth weight for gestational age. In the categorical analyses with cotinine levels in 3 groups, heavy nicotine exposure (cotinine level >50 ng/mL) was associated with offspring ADHD, with an odds ratio of 2.21 (95% CI 1.63–2.99) in the adjusted analyses. Analyses by deciles of cotinine levels revealed that the adjusted odds for offspring ADHD in the highest decile was 3.34 (95% CI 2.02–5.52).
CONCLUSIONS:
The study reveals an association with and a dose-response relationship between nicotine exposure during pregnancy and offspring ADHD. Future studies incorporating maternal smoking and environmental, genetic, and epigenetic factors are warranted.
What’s Known on This Subject:
Exposure to maternal smoking is associated with various adverse perinatal outcomes. Association between maternal smoking and offspring attention-deficit/hyperactivity disorder has been shown across studies. However, the causality of the association has been questioned to be mostly due to familial confounding.
What This Study Adds:
In this first nationwide study, objectively measured nicotine exposure through maternal cotinine levels allows us to overcome underreporting of smoking during pregnancy. We report a strong association as well as a dose-dependent relationship between prenatal nicotine exposure and offspring attention-deficit/hyperactivity disorder.
Despite its proven negative effects on fetal development, smoking during pregnancy remains a significant public health issue. Approximately 7.2% of women who gave birth in the United States smoked cigarettes during pregnancy in 2016.1 The prevalence rate was similar in Finland, with ∼7% of all pregnant women continuing to smoke throughout their pregnancy.2 Exposure to maternal smoking has been associated with various adverse outcomes, such as obstetric complications, low birth weight, preterm birth, sudden infant death syndrome, and increased infections in childhood.3 An association between maternal smoking and offspring attention-deficit/hyperactivity disorder (ADHD) has been shown across numerous studies from different populations.3–7 However, the causality of the association has been questioned to be mostly due to familial confounding.3,8–13 Moreover, to date, the information on maternal smoking is typically based solely on maternal self-report.
In previous studies, maternal self-report of smoking has been shown to underestimate true smoking by 8% to 28%.14–16 Disclosure of smoking is lower among pregnant smokers than women who smoke in general. Cotinine is the most appropriate biomarker indicating nicotine exposure because of the short half-life of nicotine, which is primarily metabolized to cotinine in the liver.17 Although previous studies are based on self-reports of active smoking, cotinine measurements enable quantifying the amount of nicotine exposure and detecting nicotine exposure from other sources, such as nicotine replacement therapy or passive smoking. However, authors of no previous studies have investigated the association of prenatal cotinine levels and the risk of offspring ADHD. In 2 previous studies that have examined the association between maternal cotinine levels in pregnancy and childhood behavioral outcomes on the basis of parental questionnaires, the authors reported no association.18,19
This is the first study to investigate the association between maternal cotinine levels during pregnancy and ADHD diagnosis in offspring. In this population-based case-control study, the use of objectively measured nicotine exposure allows us to overcome underreporting of smoking. On the basis of findings from previous studies revealing an association between self-reported maternal smoking during pregnancy and ADHD diagnosis in offspring, we hypothesized that there is an association between maternal cotinine levels and offspring ADHD. In addition, our study allows us to examine possible dose-response effects between maternal cotinine levels and offspring ADHD.
Methods
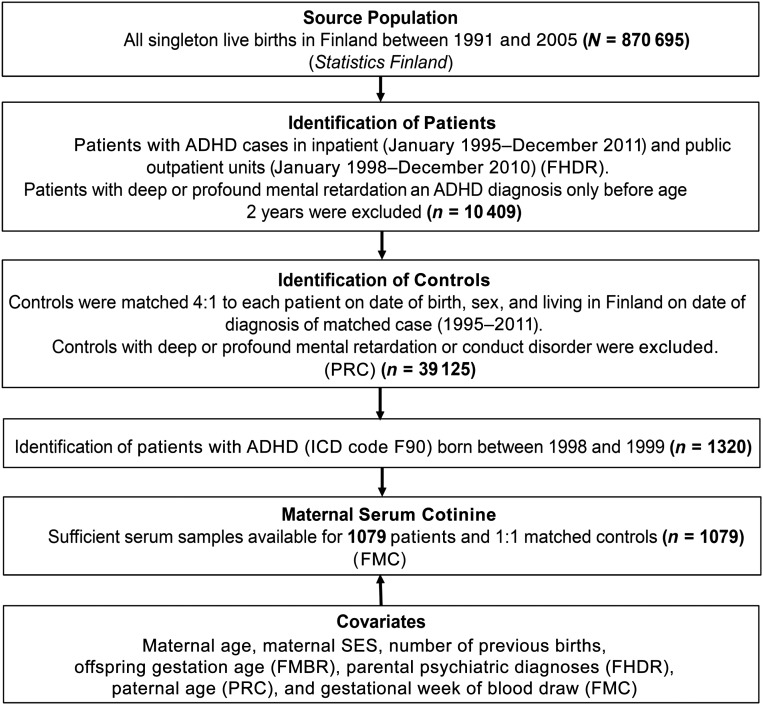
The current study is based on the Finnish prenatal study of ADHD (Fig 1), a nested case-control study derived from all singleton live births in Finland between January 1, 1998, and December 31, 1999, and followed-up for any ADHD diagnosis in the Finnish Hospital Discharge Register (FHDR) by December 31, 2011.
FIGURE 1.
Flowchart of the study design. PRC, Population Register Center.
Finnish Maternity Cohort
All offspring in the Finnish prenatal study of ADHD were derived from the Finnish Maternity Cohort (FMC). The FMC consists of 2 million serum samples collected during the first and early second trimester of pregnancy (fifth to 95th percentile: months 2–4 of pregnancy) from >950 000 women since the beginning of 1983. After informed consent, blood samples were collected at Finnish maternity clinics for the purpose of screening for congenital infections (HIV, hepatitis B, and syphilis). One maternal serum sample was obtained from each pregnancy. The median gestational age of serum collection for subjects in this study was 10 weeks (interquartile range: 8–12 weeks). After the screening, ∼1 to 3 mL of serum from each pregnancy are stored at −25°C in a protected biorepository at Biobank Borealis in Oulu, Finland, and are available for scientific research.20 Linkage between FMC data and the Finnish health registries and other data sources is possible by using the Finnish personal identity code (PIC), which has been assigned to all residents of Finland since 1971.21
Nationwide Registers
The Population Information System was established by the Finnish Population Register Center in 1969 and subsequently computerized in 1971. It is a computerized national register containing basic information about Finnish citizens and foreign permanent residents. The personal data in the system include name, PIC, address, citizenship and native language, family relations, and date of birth and death (if applicable). The Finnish Medical Birth Register (FMBR), established in 1987, includes comprehensive and standardized data on the perinatal period for all live births and stillbirths of fetuses with a birth weight of at least 500 g or a gestational age of at least 22 weeks.
Patients and Controls
The patients with ADHD were identified by linking the information from the FHDR with the FMC using the PIC. The FHDR contains all inpatient diagnoses since 1967 and outpatient diagnoses from specialized services since 1998. The FHDR contains the patient’s PIC, hospital identification, and primary and secondary diagnoses. The diagnostic classification is based on the International Classification of Diseases; the International Classification of Diseases, 10th Revision (ICD-10) has been used since 1996.22 From 1987 to 1995 the diagnoses were coded according to the International Classification of Diseases, Ninth Revision (ICD-9),23 and from 1969 to 1986 according to the International Classification of Diseases, Eighth Revision (ICD-8).24 Finland has a system of regular assessment of children’s physical and psychological development by well-trained health professionals. Almost all children attend free health check-ups at least 15 times during their first 6 years of life followed by annual check-ups at school. ADHD is typically diagnosed on the basis of the assessment of a specialist in psychiatry or neurology in public outpatient services free of charge. A previous study, in which the authors evaluated the validity of the ADHD diagnosis in the FHDR, revealed that 88% of subjects examined met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnostic criteria for ADHD.25
In the current study, the patients with ADHD included singletons born in Finland between 1998 and 1999 and registered in the FHDR with the ICD-10 code for hyperkinetic disorders (F90.0) until 2011. Controls were defined as singleton offspring born in Finland without a diagnosis of ADHD, conduct disorder, or severe or profound intellectual disability in the FHDR. Each participant with ADHD was matched with 1 control on date of birth (±30 days), sex, and place of birth. The controls had to be alive and living in Finland at the date of the ADHD diagnosis of the matched patient. Patients and controls were selected from these years because of the lack of earlier availability of data from the FMBR (established in 1987) and to ensure greater uniformity of the serum specimens with regard to the time of sampling and storage. In the current study, of the 1320 total patients and controls identified, sufficient serum was available in the FMC for 1079 patients and matched controls (n = 1079). The study was approved by the ethical committees of the Hospital District of Southwest Finland and the National Institutes of Health and Welfare. Informed consent was obtained before collection of maternal serum.
Cotinine Measurements
Cotinine measurements from the FMC samples were conducted blind to patient/control status. Serum cotinine levels were measured by using a commercially available quantitative immunoassay kit (OraSure Technologies, Bethlehem, PA) (sensitivity = 96%–97%; specificity = 99%–100%). Intra-assay and interassay variation are 3.5% to 6.2%, and 6.0% to 9.6%, respectively. The results are reported in nanogram per milliliter.
Covariates
The covariates included variables that have been previously shown to be associated with both maternal smoking as well as offspring ADHD: number of previous births, maternal socioeconomic status (SES), maternal psychiatric history, maternal history of ADHD diagnosis, maternal history of substance use disorder, paternal psychiatric history, paternal history of ADHD diagnosis, gestational age of the child, birth weight for gestational age (BWGA; based on national sex-specific growth curves26), maternal age, paternal age, and gestational week of blood draw. Information on number of previous births, maternal SES, maternal age, and gestational age was obtained from the FMBR. Maternal and paternal psychiatric diagnoses were obtained from the FHDR. A parent was defined as having a psychiatric history if he or she had any psychiatric diagnoses registered in the FHDR during his or her lifetime (ICD-10 codes F10–F99 [corresponding to ICD-9 codes 291–316 and ICD-8 codes 291–309]), excluding intellectual disability (ICD-10 codes F70–F79) or a diagnosis of ADHD (ICD-10 code F90.x or ICD-9 code 314.x). For mothers, the association with disorders due to alcohol or substance abuse (ICD-10 codes F10–F19; ICD-9 codes 291–292 and 303–305; and ICD-8 codes 291, 303, and 304) was tested separately from other psychiatric disorders. Paternal age was obtained from the Finnish Population Register Center, and gestational week of blood draw was obtained from the FMC. The covariates were considered for inclusion in the models on the basis of associations with both cotinine exposure and ADHD at P ≤ .1, in accord with standard texts.27
Statistical Analysis
The analysis was based on a nested case-control design, in which the controls for each patient were identified from the population at risk and matched on selected factors (see Patients and Controls). Initially, cotinine was examined as a continuous measure. Because of the skewed distribution of cotinine, the variable was log-transformed before analysis.
To further facilitate data interpretation, we examined maternal cotinine as categorized into deciles and as a 3-class categorical variable: reference (<20 ng/mL); moderate exposure (20–50 ng/mL); and heavy exposure (>50 ng/mL). The deciles for the patient and control groups in the analyses were derived from the cut points of maternal cotinine levels that defined the deciles for this biomarker in the control group. We hypothesized that a significant association would be observed for maternal cotinine classified in the highest decile compared with the reference group, which was defined as the lowest decile. The cutoff points for the 3-class categorical variable were based on the manufacturer’s recommendation and have been used in previous studies on the basis of the FMC serum bank.28,29 These levels also corresponded well with the frequencies of self-reported maternal smoking. Among mothers of patients with ADHS, smoking was reported by 56 of 702 (7.9%) mothers in the reference category of <20 ng/mL, by 62 of 80 (77%) mothers with cotinine levels of 20 to 50 ng/mL, and by 196 of 214 (91.5%) mothers with cotinine levels >50 ng/mL (P < .001). Appropriate to the nested case-control study design, point and interval estimates of odds ratios (ORs) were obtained by fitting conditional logistic regression models for matched pairs. Statistical significance was based on P < .05. All the statistical analyses were performed with SAS software (SAS 9.4; SAS Institute, Inc, Cary, NC).
Results
The mean age of the patients at the time of the ADHD diagnosis was 7.3 years (SD: 1.9; range: 2–13.7 years). As shown in Supplemental Table 7, there was no difference in covariates between 1079 patients included in the analysis and 241 patients not included because of insufficient serum, except for paternal age. The mean paternal age among patients not included was 1.2 years (SD: 6.9) older than that of patients included (P = .02) in the analysis. The mean cotinine level was 27.4 ng/mL (SD: 54.8; range: 0.0–427.7 ng/mL) among patients and 11.3 ng/mL (SD: 34.5; range: 0.0–320.0 ng/mL) among controls. Supplemental Table 4 reveals the distribution of serum cotinine levels and corresponding self-reported smoking status in the sample. As shown in the table, the prevalence of self-reported smoking status increased with increasing serum cotinine levels.
As shown in Supplemental Table 5, maternal SES, psychopathology, substance abuse, ADHD, age, and number of previous births; paternal psychopathology, ADHD, and age; and the child’s gestational age and BWGA were associated (P < .1) with offspring ADHD (see Supplemental Table 5). As shown in Supplemental Table 6, maternal SES, psychopathology, substance use, and age; paternal psychopathology; and the child’s BWGA were associated with maternal serum cotinine levels. Gestational week of blood draw was not associated with these outcomes (see Supplemental Table 6). Maternal SES, psychopathology, substance use, and age; paternal psychopathology; and the child’s BWGA were associated with both offspring ADHD and maternal cotinine levels and therefore fulfilled criteria for confounding. Furthermore, adjustment was made for paternal age to address any potential selection bias because of its difference among included and missing patients with ADHD.
The main findings of the current study are shown in Table 1. The adjusted analyses were performed by using 2 models. Model 1 contained the child’s BWGA, maternal SES, maternal age, maternal psychopathology, maternal substance abuse, paternal age, and paternal psychopathology as confounders. Model 2 contained the same confounders as in model 1 except for paternal psychopathology to specifically assess the effect of maternal factors. There was a significant association between increasing log-transformed maternal cotinine levels and offspring ADHD both in unadjusted analyses (OR: 1.14; 95% confidence interval [CI]: 1.11–1.17) as well as adjusted analyses. In model 1, the OR was 1.09 (95% CI: 1.06–1.12), and in model 2, the OR was 1.10 (95% CI: 1.06–1.13).
TABLE 1.
Association Between Maternal Serum Cotinine (Log-Transformed and 3-Class Categorical Variables) and Offspring ADHD
| Patient | Control | Association With Maternal Serum Cotinine | |||
|---|---|---|---|---|---|
| OR | 95% CI | P | |||
| Maternal cotinine levels (ng/mL), median | 27.4 | 11.3 | — | — | — |
| Log-transformed analysis | |||||
| Unadjusted | — | — | 1.14 | 1.11–1.17 | <.001 |
| Model 1a | — | — | 1.09 | 1.06–1.13 | <.001 |
| Model 2b | — | — | 1.10 | 1.06–1.13 | <.001 |
| Categorical analysis | |||||
| Unadjusted | |||||
| Reference, n (%)c | 777 (72.0) | 936 (86.8) | — | — | — |
| Moderated | 84 (7.8%) | 54 (5.0%) | 1.92 | 1.33–2.77 | <.001 |
| Heavy exposuree | 218 (20.2%) | 89 (8.2%) | 2.95 | 2.25–3.88 | <.001 |
| Model 1a | |||||
| Moderate exposured | — | — | 1.27 | 0.84–1.92 | .25 |
| Heavy exposuree | — | — | 2.21 | 1.64–2.99 | <.001 |
| Model 2b | |||||
| Moderate exposured | — | — | 1.31 | 0.87–1.96 | .20 |
| Heavy exposuree | — | — | 2.27 | 1.69–3.07 | <.001 |
—, not applicable.
Model 1: adjusted for BWGA, maternal SES, maternal age, maternal psychopathology, maternal substance abuse, paternal age, and paternal psychopathology.
Model 2: adjusted for BWGA, maternal SES, maternal age, maternal psychopathology, maternal substance abuse, and paternal age.
Reference: <20 ng/mL.
Moderate exposure: 20–50 ng/mL.
Heavy exposure: >50 ng/mL.
In the categorical analyses, cotinine levels were categorized into 3 groups: heavy ( >50 ng/mL), moderate (20–50 ng/mL), and no or low nicotine exposure (<20 ng/mL). As shown in Table 1, heavy exposure was associated with offspring ADHD in the unadjusted analyses (OR: 2.95; 95% CI: 2.25–3.88) as well as in the adjusted analyses both in model 1 (OR: 2.21; 95% CI: 1.63–2.99) and in model 2 (OR: 2.27; 95% CI: 1.68–3.07). Moderate cotinine levels were associated with offspring ADHD in the unadjusted analyses (OR: 1.92; 95% CI: 1.33–2.77) but did not remain significant in the adjusted models (model 1: OR: 1.27 [95% CI: 0.84–1.92]; model 2: OR: 1.31 [95% CI: 0.87–1.96]).
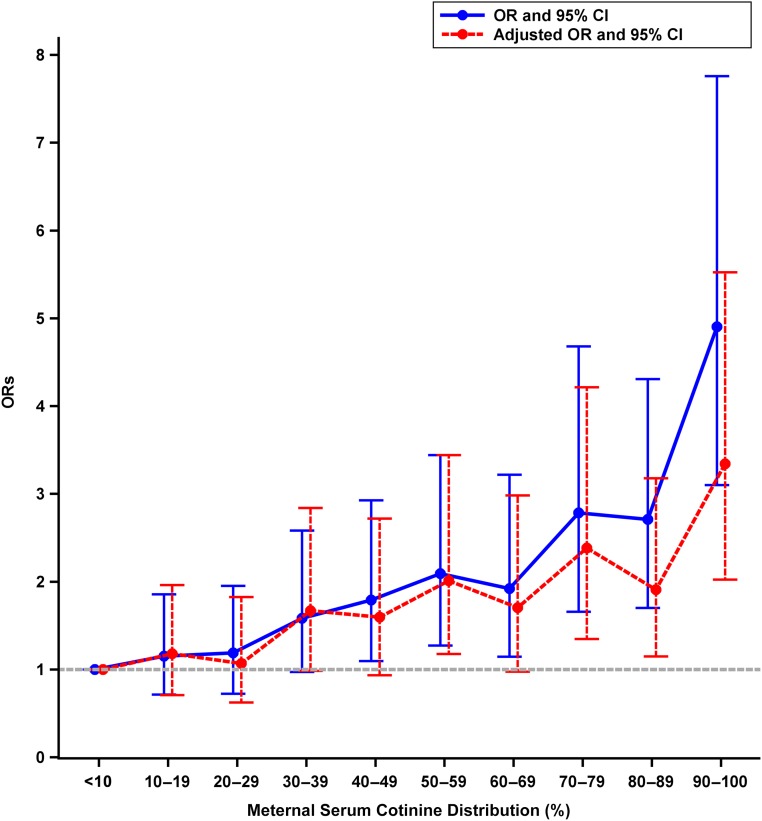
The distribution of cotinine exposure in deciles by case-control status is presented in Table 2. As shown in Fig 2, the strongest association was in the highest decile (90%–100%). The odds for offspring ADHD in the highest decile in the unadjusted analyses was 4.90 (95% CI: 3.10–7.76), whereas in the analyses adjusting for BWGA, maternal SES, maternal age, maternal psychopathology, maternal substance abuse, paternal age, and paternal psychopathology, it was 3.34 (95% CI: 2.02–5.52). For the second highest decile (80%–89%), the association in the unadjusted analysis revealed an OR of 2.71 (95% CI: 1.70–4.31) and in the adjusted analyses, an OR of 1.91 (95% CI: 1.15–3.18; Table 3). The difference between the deciles (80%–89% vs 90%–100%) was significant, revealing that the risk of ADHD was higher with a higher level of nicotine exposure.
TABLE 2.
Maternal Serum Cotinine Levels by Deciles in Patients With ADHD and Matched Controls
| Maternal Cotinine by Deciles,a % | Range, ng/mL | Patients (n = 1079) | Controls (n = 1079) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| <10 | <0.008 | 67 | 6.2 | 107 | 9.9 |
| 10–19 | 0.009–0.011 | 73 | 6.8 | 108 | 10.0 |
| 20–29 | 0.012–0.014 | 66 | 6.1 | 107 | 9.9 |
| 30–39 | 0.015–0.019 | 89 | 8.2 | 109 | 10.1 |
| 40–49 | 0.020–0.024 | 96 | 8.9 | 112 | 10.4 |
| 50–59 | 0.025–0.033 | 99 | 9.2 | 104 | 9.6 |
| 60–69 | 0.034–0.052 | 88 | 8.2 | 108 | 10.0 |
| 70–79 | 0.053–0.165 | 118 | 10.9 | 108 | 10.0 |
| 80–89 | 0.166–41.08 | 139 | 12.9 | 108 | 10.0 |
| ≥90 | ≥41.08 | 244 | 22.6 | 108 | 10.0 |
The cut points for deciles are based on maternal cotinine levels in the control group.
FIGURE 2.
ORs and 95% CIs for maternal serum cotinine (deciles) and offspring ADHD.
TABLE 3.
Association Between Maternal Serum Cotinine Categorized Into Deciles and Offspring ADHD
| Maternal Cotinine by Deciles, % | OR | 95% CI | P | ORa | 95% CI | P |
|---|---|---|---|---|---|---|
| <10 | Reference | — | — | — | Reference | — |
| 10–19 | 1.15 | 0.71–1.85 | .56 | 1.18 | 0.71–1.96 | .53 |
| 20–29 | 1.19 | 0.72–1.95 | .50 | 1.07 | 0.63–1.83 | .81 |
| 30–39 | 1.58 | 0.97–2.58 | .07 | 1.67 | 0.98–2.84 | .06 |
| 40–49 | 1.79 | 1.09–2.93 | .02 | 1.60 | 0.94–2.72 | .09 |
| 50–59 | 2.09 | 1.27–3.44 | .004 | 2.01 | 1.18–3.44 | .01 |
| 60–69 | 1.92 | 1.15–3.2 | .013 | 1.71 | 0.98–2.98 | .06 |
| 70–79 | 2.78 | 1.66–4.68 | <.001 | 2.38 | 1.35–4.21 | .003 |
| 80–89 | 2.71 | 1.70–4.31 | <.001 | 1.91 | 1.15–3.18 | .01 |
| ≥90 | 4.90 | 3.10–7.76 | <.001 | 3.34 | 2.02–5.53 | <.001 |
—, not applicable.
Adjusted for BWGA, maternal SES, maternal age, maternal psychopathology, maternal substance abuse, paternal age, and paternal psychopathology.
Discussion
With this study, we provide evidence for the association between nicotine exposure during pregnancy and increased risk of ADHD in the offspring because previous population-based studies have been based on self-report of smoking during pregnancy. The association between fetal exposure to nicotine, quantified as cotinine during gestation, and ADHD were similar when cotinine was classified as a continuous variable and when it was classified in 3 categories on the basis of cotinine levels. Most importantly, the findings reveal a dose-dependent relationship between fetal exposure to nicotine and risk of ADHD. The findings persisted in all analyses after adjusting for potential confounders, including maternal SES, maternal and paternal psychopathology, maternal and paternal age, and BWGA. To the best of our knowledge, this is the first biomarker-based study to reveal a relationship between fetal nicotine exposure and later offspring ADHD.
The findings add to the evidence of a complex association between exposure to nicotine during pregnancy and later ADHD. A neurobiological mechanism via intrauterine effects seems possible because nicotine crosses the placenta during pregnancy.30 Animal studies have demonstrated an association between in utero nicotine exposure and increased locomotor activity as well as changes in the neurotransmitter systems in the brain.31,32 Human studies have revealed structural and functional changes in the brain associated with prenatal smoking exposure.33 The mechanisms behind these effects are thought to develop through nicotine modulating the maturation of the developing central nervous system. In addition, other components of smoking, such as carbon monoxide, are thought to lead to fetal hypoxia and affect fetal brain development.34
The association between smoking during pregnancy and offspring ADHD may be explained by genetic or social factors.9,35 On the basis of several sibling and family studies, the association between fetal exposure to smoking and ADHD has been suggested to be mostly explained by familial confounding.8–13 These studies, as well as a novel study design on children born with assisted conception,36 suggest that inherited effects and unmeasured household-level confounding seem more likely contributors behind the association than direct intrauterine effects of smoking. There are also contradictory sibling-study findings, including results from a Finnish study with >150 000 sibling pairs, revealing that if the mother stopped smoking between the pregnancies, the second sibling did not have an increased risk of externalizing diagnoses, including ADHD. Correspondingly, if the mother started smoking between the pregnancies, a significantly higher risk was observed in the second sibling.37 A Dutch family study found that maternal compared with paternal smoking during pregnancy was associated with a greater effect, with externalizing problems among offspring. The same study revealed that quitting smoking was associated with less externalizing problems among offspring.38 Furthermore, an American sibling study revealed that familial confounding explained inattentive but not hyperactive and/or impulsive ADHD behaviors, suggesting that the association may vary by phenotype.39
Despite several strengths, sibling comparison studies should also be interpreted with caution given their limitations. It has been suggested that within-sibling estimates will be biased toward the null by measurement error and that they may be either more or less biased than between-family estimates depending on the extent to which siblings share confounders versus the exposure.40 In addition, mothers who vary in their smoking habits during different pregnancies can perhaps not be generalized to all smoking populations.40
It is possible that the association between nicotine exposure during pregnancy and ADHD might partially be explained also by gene-environment interaction. Accumulating data provide evidence that prenatal smoking may act through epigenetic changes via altered DNA methylation and microRNA expression.41,42 The exposure to nicotine may increase the risk of ADHD particularly among children with genetic vulnerability for ADHD. It is also possible that maternal smoking during pregnancy is a proxy risk factor leading to ADHD by independent mechanisms. Smoking during pregnancy is associated with poorer parenting skills, which are associated with child behavioral problems.43
The current study has several strengths, including being based on a large nationwide sample, assessing nicotine exposure with objective biological measurement and including several potential confounders. However, when interpreting the findings, several limitations should be considered. A key question is whether smoking during pregnancy is causally associated with ADHD or is a proxy of another risk factor (eg, familial confounding). Even if most of the effect is due to familial or genetic confounding, the current study reveals that the association between smoking and ADHD has a dose-response effect. The limitation of observational data is that we cannot examine causal processes. We did not have access to biomarkers of sibling pregnancies that would have shed more light on a possible causal link between nicotine exposure during pregnancy and offspring ADHD. However, in the current study, we were able to adjust for several confounders, including BWGA, maternal SES, age, psychopathology, and substance use disorder as well as paternal psychopathology. Information about possible substance use during pregnancy was restricted to register-based substance use diagnoses. In the current study, 5.3% of mothers of children with ADHD had a diagnosis of substance use disorder, which was similar to the estimated prevalence in Finland of 6.4% for the exposure to maternal alcohol and drug dependence.44 The number of parents diagnosed with ADHD in this sample was low, which is a limitation of the study (Supplemental Tables 5 and 6). The underdiagnoses among parents could be primarily because ADHD was not a widely used diagnosis in the parental generation. In addition, it is unlikely that ADHD among parents was treated in inpatient care. Because the FHDR did not cover outpatient diagnoses before 1998, the diagnosis of ADHD among parents is likely underestimated. Finally, the subjects with ADHD included in this study were only those who were referred to specialized services and likely represent the more severe cases of ADHD. However, authors of a previous study reported an 88% validity of the ADHD diagnoses in the FHDR examined against Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for ADHD.7
Conclusions
According to the World Health Organization, smoking is considered 1 of the main public health concerns worldwide.45 The current study reveals a strong association between prenatal nicotine exposure and a dose-dependent relationship with offspring ADHD. This study adds 2 important aspects; first, the use of cotinine as a documented measure of nicotine exposure during pregnancy and second, the finding of a dose-response effect in the association. Given the high prevalence of both smoking during pregnancy and ADHD among children, these findings warrant future studies on the interplay between maternal smoking and environmental, genetic, and epigenetic factors.
Acknowledgments
We thank Mr Jesse Fomin, Ms Jarna Lindroos, and Mr Lauri Sillanmäki for their technical support.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- BWGA
birth weight for gestational age
- CI
confidence interval
- FHDR
Finnish Hospital Discharge Register
- FMBR
Finnish Medical Birth Register
- FMC
Finnish Maternity Cohort
- ICD-8
International Classification of Diseases, Eighth Revision
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, 10th Revision
- OR
odds ratio
- PIC
personal identity code
- SES
socioeconomic status
Footnotes
Dr Sourander conceptualized the study, participated in the study design, drafted the initial manuscript, and contributed to the interpretation of the data and the critical review and revision of the manuscript; Dr Sucksdorff participated in the study design, conducted the literature search, drafted portions of the initial manuscript, and contributed to the interpretation of the data and the critical review and revision of the manuscript; Dr Chudal participated in the study design, drafted portions of the initial manuscript, and contributed to the interpretation of the data and the critical review and revision of the manuscript; Drs Surcell, Gyllenberg, Cheslack-Postava, and Brown conceptualized the study, participated in the study design, and contributed to the interpretation of the data and the critical review and revision of the manuscript; Ms Hinkka-Yli-Salomäki designed the study, conducted the analyses, and contributed to the interpretation of the data and the critical review and revision of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the following funding sources: Academy of Finland (decision 308552), National Institutes of Health, grants 1RO1ES028125-01 and 1(GG012038-01), the Brain and Behavior Research Foundation (Dr Gyllenberg), the Finnish Medical Foundation (Drs Sucksdorff and Gyllenberg), and the University of Turku Graduate School (Drs Sucksdorff and Chudal). This study belongs to the Finnish Psychiatric Birth Cohort Consortium, which is funded by Academy of Finland. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief. 2018;(305):1–8 [PubMed] [Google Scholar]
- 2.Heino A, Vuori E, Gissler M Perinatal statistics - parturients, delivers and newborns 2016 [in Finnish]. Available at: http://urn.fi/URN:NBN:Fi-fe2017103150386. Accessed August 15, 2018
- 3.Huang L, Wang Y, Zhang L, et al. . Maternal smoking and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Pediatrics. 2018;141(1):e20172465. [DOI] [PubMed] [Google Scholar]
- 4.Tiesler CM, Heinrich J. Prenatal nicotine exposure and child behavioural problems. Eur Child Adolesc Psychiatry. 2014;23(10):913–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obel C, Linnet KM, Henriksen TB, et al. . Smoking during pregnancy and hyperactivity-inattention in the offspring–comparing results from three Nordic cohorts. Int J Epidemiol. 2009;38(3):698–705 [DOI] [PubMed] [Google Scholar]
- 6.Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatr. 2005;57(6):359–371 [PubMed] [Google Scholar]
- 7.Joelsson P, Chudal R, Talati A, Suominen A, Brown AS, Sourander A. Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: a finnish nationwide population-based cohort study. BMC Psychiatry. 2016;16:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obel C, Zhu JL, Olsen J, et al. . The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy - a re-examination using a sibling design. J Child Psychol Psychiatry. 2016;57(4):532–537 [DOI] [PubMed] [Google Scholar]
- 9.Skoglund C, Chen Q, D’Onofrio BM, Lichtenstein P, Larsson H. Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry. 2014;55(1):61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavson K, Ystrom E, Stoltenberg C, et al. . Smoking in pregnancy and child ADHD. Pediatrics. 2017;139(2):e20162509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley K, Heron J, Smith GD, Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol. 2012;176(3):261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Onofrio BM, Van Hulle CA, Waldman ID, et al. . Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine Tob Res. 2010;12(4):408–415 [DOI] [PubMed] [Google Scholar]
- 14.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz PM, Homa D, England LJ, et al. . Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173(3):355–359 [DOI] [PubMed] [Google Scholar]
- 16.Tong VT, Althabe F, Alemán A, et al. ; Prenatal Tobacco Cessation Intervention Collaborative . Accuracy of self-reported smoking cessation during pregnancy. Acta Obstet Gynecol Scand. 2015;94(1):106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24 [DOI] [PubMed] [Google Scholar]
- 18.Dürr DW, Høyer BB, Christensen LH, et al. . Tobacco smoking during pregnancy and risk of adverse behaviour in offspring: a follow-up study. Reprod Toxicol. 2015;58:65–72 [DOI] [PubMed] [Google Scholar]
- 19.Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. II. Effects on neurodevelopment at age 5 years. Am J Epidemiol. 1995;142(suppl 9):S19–S29 [DOI] [PubMed] [Google Scholar]
- 20.University of Oulu Biobank Borealis of Northern Finland – from bench to bedside. Available at: www.oulu.fi/university/node/38474. Accessed March 14, 2018
- 21.Gissler M, Surcel H. Combining health register data and biobank data. Stat J IAOS. 2012;28(1–2):53–58 [Google Scholar]
- 22.World Health Organization International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 1992 [Google Scholar]
- 23.World Health Organization International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1977 [Google Scholar]
- 24.World Health Organization International Classification of Diseases, Eighth Revision. Geneva, Switzerland: World Health Organization; 1967 [Google Scholar]
- 25.Joelsson P, Chudal R, Gyllenberg D, et al. . Demographic characteristics and psychiatric comorbidity of children and adolescents diagnosed with ADHD in specialized healthcare. Child Psychiatry Hum Dev. 2016;47(4):574–582 [DOI] [PubMed] [Google Scholar]
- 26.Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45(5–6):446–454 [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1998 [Google Scholar]
- 28.Niemelä S, Sourander A, Surcel HM, et al. . Prenatal nicotine exposure and risk of schizophrenia among offspring in a national birth cohort. Am J Psychiatry. 2016;173(8):799–806 [DOI] [PubMed] [Google Scholar]
- 29.Kapeu AS, Luostarinen T, Jellum E, et al. . Is smoking an independent risk factor for invasive cervical cancer? A nested case-control study within Nordic biobanks. Am J Epidemiol. 2009;169(4):480–488 [DOI] [PubMed] [Google Scholar]
- 30.Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C. Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet Gynecol. 1999;93(1):25–29 [DOI] [PubMed] [Google Scholar]
- 31.Wickström R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol. 2007;5(3):213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bublitz MH, Stroud LR. Maternal smoking during pregnancy and offspring brain structure and function: review and agenda for future research. Nicotine Tob Res. 2012;14(4):388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekblad M, Korkeila J, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L; PIPARI Study Group . Maternal smoking during pregnancy and regional brain volumes in preterm infants. J Pediatr. 2010;156(2):185–190.e1 [DOI] [PubMed] [Google Scholar]
- 34.Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015;104(1):12–18 [DOI] [PubMed] [Google Scholar]
- 35.Obel C, Olsen J, Henriksen TB, et al. . Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder?–Findings from a sibling design. Int J Epidemiol. 2011;40(2):338–345 [DOI] [PubMed] [Google Scholar]
- 36.Thapar A, Rice F, Hay D, et al. . Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66(8):722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekblad M, Lehtonen L, Korkeila J, Gissler M. Maternal smoking during pregnancy and the risk of psychiatric morbidity in singleton sibling pairs. Nicotine Tob Res. 2017;19(5):597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolan CV, Geels L, Vink JM, et al. . Testing causal effects of maternal smoking during pregnancy on offspring’s externalizing and internalizing behavior. Behav Genet. 2016;46(3):378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marceau K, Cinnamon Bidwell L, Karoly HC, et al. . Within-family effects of smoking during pregnancy on ADHD: the importance of phenotype. J Abnorm Child Psychol. 2018;46(4):685–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720 [DOI] [PubMed] [Google Scholar]
- 41.Rzehak P, Saffery R, Reischl E, et al. ; European Childhood Obesity Trial Study group . Maternal smoking during pregnancy and DNA-methylation in children at age 5.5 years: epigenome-wide-analysis in the European Childhood Obesity Project (CHOP)-study. PLoS One. 2016;11(5):e0155554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 2012;24(4):1377–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tandon M, Si X, Belden A, Spitznagel E, Wakschlag LS, Luby J. Parenting practices in pregnancy smokers compared to non smokers. J Clin Med Res. 2013;5(2):84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pajulo M, Savonlahti E, Sourander A, Helenius H, Piha J. Antenatal depression, substance dependency and social support. J Affect Disord. 2001;65(1):9–17 [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization MPOWER: A Policy Package to Reverse the Tobacco Epidemic. Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]