In a propensity score–matched cohort of 772 children with tracheostomy hospitalized with influenza, those receiving antiviral medications after admission have 15% shorter LOS.
Abstract
BACKGROUND:
Early administration of anti-influenza medications is recommended for all children hospitalized with influenza. We investigated whether early use of anti-influenza medications is associated with improved outcomes in children with tracheostomy hospitalized with influenza.
METHODS:
We performed a multicenter retrospective cohort study through the Pediatric Health Information System database for patients aged 30 days to 19 years who were discharged between October 1, 2007, and September 30, 2015 with diagnostic codes for both influenza and tracheostomy. Our primary predictor was receipt of anti-influenza medications on hospital day 0 or 1. We used propensity score matching to adjust for confounding by indication. Primary outcomes were length of stay (LOS) and 30-day all-cause revisit rate (emergency department visit or hospital admission).
RESULTS:
Of 1436 discharges screened, 899 met inclusion criteria. The median admission age was 5 years (interquartile range: 2–10). The majority had multiple complex chronic conditions (median 3; interquartile range: 3–4) and technology dependence, such as gastrostomy tube (73.6%). After matching 772 unique admissions by propensity score, LOS was shorter for the cohort receiving early anti-influenza medications (6.4 vs 7.5 days; P = .01) without increase in revisit rate (27.5% vs 24.1%; P = .28). More than 80% in both cohorts received empirical antibiotics, and the duration of antibiotic therapy was similar (5.0 vs 5.6 days; P = .11).
CONCLUSIONS:
Early use of anti-influenza medications in children with tracheostomy hospitalized with influenza is associated with shorter LOS, but these children continue to receive antibiotics despite identification and treatment of their viral infections.
What’s Known on This Subject:
Early administration of anti-influenza medications is recommended for all children hospitalized with influenza, but its use remains inconsistent. These medications shorten duration of illness for outpatients and length of hospital stay for those requiring intensive care or experiencing influenza complication.
What This Study Adds:
Early administration of anti-influenza medications in children with tracheostomy is associated with 1-day shorter (15%) length of stay. Early use of these medications is not associated with difference in hospital readmission rates or days on antibiotics.
Influenza is a respiratory tract infection that accounts for 7% to 10% of respiratory hospitalizations in children worldwide.1,2 In the Unites States, influenza contributes to 11 000 to 45 000 hospitalizations and 200 to 1300 excess deaths each season.3 Children with chronic medical conditions, such as pulmonary and neurologic diseases, are particularly at high risk for influenza complications,4,5 and they comprise more than half of influenza-related deaths among children in the United States.6–8 They are at high risk of hospitalizations from influenza,4,6–8 which are prolonged5 and costly.9 Therefore, strategies to improve the care of influenza in children with medical complexity (CMC) are needed.
Because influenza prevention can be challenging in CMC because of suboptimal coverage10,11 and effectiveness8,12 of influenza vaccine, it is important for clinicians in emergency departments (EDs) and inpatient settings to optimize management of influenza in this population. Early administration of anti-influenza medication has been shown to reduce the risk of influenza-related complications in the outpatient setting,13,14 and timely administration for all children hospitalized with influenza has been recommended.15,16 Most children hospitalized with influenza, however, do not receive anti-influenza medications in a timely fashion.17,18 Furthermore, evidence regarding efficacy of anti-influenza medications among CMC is limited.
Children with tracheostomy represent a unique population with medical complexity19 who experience recurrent admissions related to respiratory tract infection.19–21 Despite their elevated risk of viral infections,22,23 efficacy of anti-influenza medications remains unknown in this population. We investigated whether early use of anti-influenza medications was associated with improved outcomes in children with tracheostomy hospitalized with influenza.
Methods
Data Source
We conducted a multicenter, retrospective cohort study using the Pediatric Health Information System (PHIS) database. The PHIS database contains administrative and billing data from inpatient and observation units, ED, and ambulatory surgery encounters at 49 freestanding pediatric hospitals in the United States. Data are deidentified at the time of submission and subjected to rigorous quality checks before inclusion in the database.24 The database allows longitudinal patient tracking by using encrypted patient medical record numbers. The Children’s Hospital Los Angeles Institutional Review Board reviewed the study and granted an exemption according to 45 Code of Federal Regulations 46.101(b)(4).
Patient Selection
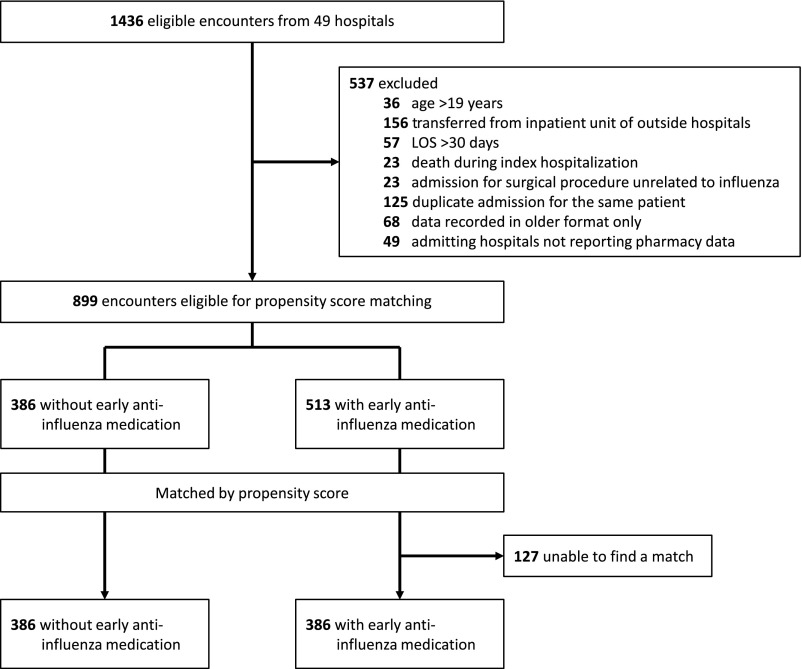
We included patients with admission age 30 days to 19 years discharged between October 1, 2007, and September 30, 2015, with (1) International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes representing presence of tracheostomy (V44.0, V55.0, 519.00, 519.00, 519.01, 519.02, and 519.09) and (2) influenza infection (487.xx and 488.xx; Fig 1). These ICD-9-CM codes have been previously used to identify hospitalized pediatric patients with the presence of tracheostomy19,21,25 and laboratory-confirmed influenza with high specificity and positive predictive value.26–29
FIGURE 1.
CONSORT diagram for patient selection.
We excluded patients with any of the following characteristics: (1) age 20 years or older to exclude adult patients; (2) transfer from an outside hospital with inpatient status, given interest in initial treatment of influenza and length of stay (LOS); (3) extreme outliers in LOS defined as median plus 3 times the interquartile range (IQR); (4) death during the index hospitalization because the patients could not have a revisit; (5) presence of surgical procedures unrelated to influenza within first 2 days of hospitalization to minimize the possibility of including patients who acquired or manifested with influenza infection during hospitalizations for other reasons; or (6) missing variables in the database. To prevent overrepresentation by patients with multiple eligible encounters, we first assigned a computer-generated random whole number to each admission episode and subsequently selected 1 admission episode for each patient for analysis.
Primary Exposure
Our primary exposure was early receipt of anti-influenza medications, defined as prescription of amantadine, rimantadine, oseltamivir, zanamivir, or peramivir on day 0 or 1 of hospitalization. We used this definition to be inclusive and minimize bias, considering our patient recruitment period. Given the robust data quality management and encryption of medical record numbers and other identifying patient information, we did not review clinical charts to validate whether data around anti-influenza medication administration reported in PHIS were accurate.
Outcomes
Our primary outcomes were LOS in days and 30-day all-cause revisit, defined as any repeat presentation to an inpatient unit, observation unit, or ED. Previous studies have used LOS28,30 and 30-day all-cause revisit29 to measure the impact of anti-influenza therapy in children who were hospitalized. We chose to evaluate all-cause revisits rather than those related to influenza or other respiratory tract infections, given the wide variety of comorbidities of patients with tracheostomy and difficulty assessing causality of revisit based on administrative data alone. Our secondary outcome was the number of days the patient received antibiotics that treat bacterial tracheostomy-associated respiratory tract infections.31
Covariates
Demographic covariates included age at admission in years, sex, race and/or ethnicity, and insurance status. Race and/or ethnicity was categorized as non-Hispanic white, non-Hispanic African American, Hispanic, and other. Insurance status was categorized as private, government, and other. Medical covariates were complex chronic conditions (CCCs), presence of gastrostomy, baseline dependence of mechanical ventilation, receipt of care at ICU, and antibiotics on day 0 or 1 of hospitalization. CCCs have previously been defined on the basis of specific ICD-9-CM codes.32,33 Gastrostomy status was defined as having any of the ICD-9-CM codes at discharge: v44.1, v55.1, or 536.4x.34 Baseline ventilator dependence was defined as having any of the ICD-9-CM codes falling under v46.1x at discharge.21
Statistical Analysis
For all discharge episodes that met inclusion and exclusion criteria, descriptive statistics (eg, mean, SD, and frequencies) were calculated for the entire sample and for both exposure groups. Next, bivariate analyses were conducted to determine if receipt of early anti-influenza medications was associated with any of the covariates and outcomes of interest. We used χ2 tests for categorical covariates and Student’s t test for continuous covariates.
To assess the effect of receipt of early anti-influenza medication on our outcomes, we subsequently used propensity score matching to adjust for confounding by indication.35 We included the following covariates (chosen a priori) to generate propensity score on the basis of multivariate logistic regression: patient age, sex, race and/or ethnicity, presence of neurologic and neuromuscular CCCs, premature birth, total number of CCCs, presence of gastrostomy tube, baseline ventilator dependence, and receipt of systemic antibiotics and ICU care on day 0 or 1 of hospitalization. Propensity score and matched samples were generated in R (version 3.5.0) by using the package MatchIt.36,37 We performed 1-to-1 matching without replacement using optimal matching strategy, aiming to minimize total within-pair difference of the propensity score (optimal matching). Patients without matches were removed from analysis. We used Student’s t test to compare LOS and days on antibiotics. χ2 test was used for comparison of 30-day all-cause revisit rate.
Results
A total of 1436 discharge episodes met inclusion criteria of which 899 discharge episodes were eligible for propensity score matching (Fig 1). Characteristics of the cohort eligible for propensity score matching are described in Table 1. The median age of the cohort was 5 years (IQR: 2–10 years). The cohort was predominately male (56.5%; n = 508), non-Hispanic white (40.4%; n = 363), and on public insurance (75.5%; n = 679). The median number of CCCs was 3 (IQR: 3–4), and all patients had respiratory CCCs. Gastrointestinal (82.9%; n = 745), neurologic and neuromuscular (50.6%; n = 466), and other congenital CCCs (33.5%; n = 314) were also commonly identified. Most patients had technology dependence, such as gastrostomytube (73.6%; n = 676) and baseline mechanical ventilator dependence (25.5%; n = 229). Nearly 40% received care at ICU after admission. Systemic antibiotics were prescribed to the majority of the patients after admission (n = 739; 82.2%) and for most days of hospitalization (average 5.3 days; 95% confidence interval [CI] 5.0–5.6).
TABLE 1.
Unadjusted Characteristics of Patients With Tracheostomy Hospitalized With Influenza Categorized According to Administration of Anti-influenza Medications on Hospital Day 0 or 1
| All Patients (N = 899) | Received Anti-influenza Medications on d 0 or 1 (n = 513) | No Anti-influenza Medications on d 0 or 1 (n = 386) | P | |
|---|---|---|---|---|
| Age, median (IQR), y | 5 (2–10) | 5 (2–11) | 4 (2–9) | .004 |
| Sex, n (%) | .77 | |||
| Male | 508 (56.5) | 292 (56.9) | 216 (56.0) | |
| Female | 391 (43.5) | 221 (43.1) | 170 (44.0) | |
| Race and ethnicity, n (%) | .43 | |||
| White | 363 (40.4) | 209 (40.7) | 154 (39.9) | |
| African American | 218 (24.2) | 126 (24.6) | 92 (23.8) | |
| Hispanic or Latino | 236 (26.3) | 126 (24.6) | 110 (28.5) | |
| Multiracial, other, and/or unknown | 82 (9.1) | 52 (10.1) | 30 (7.8) | |
| Insurance, n (%) | .29 | |||
| Private | 186 (20.7) | 106 (20.7) | 80 (20.7) | |
| Public | 679 (75.5) | 392 (76.4) | 287 (74.4) | |
| Other or unknown | 34 (3.8) | 15 (2.9) | 19 (4.9) | |
| CCCs, n (%) | ||||
| Cardiovascular | 182 (20.2) | 101 (19.7) | 81 (21.0) | .63 |
| Gastrointestinal | 745 (82.9) | 437 (85.2) | 308 (79.8) | .03 |
| Hematologic | 37 (4.1) | 23 (4.5) | 14 (3.6) | .52 |
| Malignancy | 17 (1.9) | 12 (2.3) | 5 (1.3) | .26 |
| Metabolic | 55 (6.1) | 31 (6.0) | 24 (6.2) | .91 |
| Neurologic | 466 (51.8) | 271 (52.8) | 195 (50.5) | .49 |
| Renal and urologic | 66 (7.3) | 38 (7.4) | 28 (7.3) | .93 |
| Respiratory | 899 (100) | 513 (100) | 386 (100) | — |
| Other congenital | 314 (34.9) | 189 (36.8) | 125 (32.4) | .17 |
| Premature and neonatal | 133 (14.8) | 69 (13.5) | 64 (16.6) | .19 |
| Transplant | 10 (1.1) | 6 (1.2) | 4 (1.0) | .85 |
| Total CCCs, median (IQR) | 3 (3–4) | 3 (3–4) | 3 (3–4) | .16 |
| Technology dependence, n (%) | ||||
| Gastrostomy tube | 676 (75.2) | 398 (77.6) | 278 (72.0) | .06 |
| Baseline mechanical ventilator dependence | 229 (25.5) | 136 (26.5) | 93 (24.1) | .41 |
| Care after admission, n (%) | ||||
| ICU stay on d 0 or 1 | 334 (37.2) | 195 (38.0) | 139 (36.0) | .54 |
| Antibiotics on d 0 or 1 | 739 (82.2) | 421 (82.1) | 318 (82.4) | .90 |
—, not applicable.
Of this non–propensity-matched cohort, 513 patients (57.1%) received anti-influenza medications on hospital day 0 or 1. In this group, 499 received oseltamivir only, 10 received both oseltamivir and calcium channel blockers (amantadine or rimantadine), and only 2 received calcium channel blockers. No patient in our study cohort received peramivir or zanamivir. Those who received anti-influenza medications were older (median age of 5 vs 4 years; P = .004) and more likely to have gastrointestinal CCCs (85.2% vs 79.8%; P = .03). Those receiving anti-influenza medications had statistically shorter LOS (average 6.6 vs 7.5 days; P = .02) but no differences in 30-day revisit rates (26.1% vs 24.1%; P = .49) or days of antibiotics (5.2 vs 5.6 days; P = .19). Variations at the hospital level accounted for between 0% and 2.3% of variance in the outcomes of interest.
We successfully matched 386 patients who received early anti-influenza medications to those who did not receive early anti-influenza medications. Characteristics of this population are detailed in Table 2. After matching, the differences in age and gastrointestinal CCCs were not statistically significant. Of the 386 that received early anti-influenza medications, 379 (98.2%) received oseltamivir as the sole anti-influenza medication on day 0 or 1. As noted in Table 3, the LOS was shorter for the cohort that received early anti-influenza medications (6.4 vs 7.5 days; P = .01), representing an absolute change in LOS of 1.1 days (95% CI 0.3–2.0). No statistically significant differences were detected in 30-day all-cause revisit rate (27.5% for early anti-influenza medications versus 24.1% for no early anti-influenza medications; P = .28). After a closer look at revisit rates, rates of hospital readmission and of ED visits within 30 days of discharges were similar in the 2 cohorts. Systemic antibiotics were prescribed on the majority of hospital days in both cohorts with no statistically significant difference (5.0 vs 5.6 days; P = .11).
TABLE 2.
Propensity Score–Matched Characteristics of Patients With Tracheostomy Hospitalized With Influenza Categorized According to Early Administration of Anti-influenza Medications
| All Patients (N = 772) | Received Anti-influenza Medications on d 0 or 1 (n = 386) | No Anti-influenza Medications on d 0 or 1 (n = 386) | P | |
|---|---|---|---|---|
| Age, median (IQR), y | 4 (2–9) | 4 (2–9) | 4 (2–9) | .74 |
| Sex, n (%) | .83 | |||
| Male | 435 (56.3) | 219 (56.7) | 216 (56.0) | |
| Female | 337 (43.7) | 167 (43.3) | 170 (44.0) | |
| Race and ethnicity, n (%) | .47 | |||
| White | 309 (40.0) | 155 (40.2) | 154 (39.9) | |
| African American | 185 (24.0) | 93 (24.1) | 92 (23.8) | |
| Hispanic or Latino | 207 (26.8) | 97 (25.1) | 110 (28.5) | |
| Multiracial, other, and/or unknown | 71 (9.2) | 41 (10.6) | 30 (7.8) | |
| Insurance, n (%) | .55 | |||
| Private | 159 (20.6) | 79 (20.5) | 80 (20.7) | |
| Public | 581 (75.3) | 294 (76.2) | 287 (74.4) | |
| Other or unknown | 32 (4.1) | 13 (3.4) | 19 (4.9) | |
| CCCs, n (%) | ||||
| Cardiovascular | 152 (19.7) | 71 (18.4) | 81 (21.0) | .37 |
| Gastrointestinal | 629 (81.5) | 321 (83.2) | 308 (79.8) | .23 |
| Hematologic | 28 (3.6) | 14 (3.6) | 14 (3.6) | — |
| Malignancy | 14 (1.8) | 9 (2.3) | 5 (1.3) | .28 |
| Metabolic | 42 (5.4) | 18 (4.7) | 24 (6.2) | .34 |
| Neurologic | 393 (50.9) | 198 (51.3) | 195 (50.5) | .83 |
| Renal and urologic | 55 (7.1) | 27 (7.0) | 28 (7.3) | .89 |
| Respiratory | 772 (100) | 386 (100) | 386 (100) | — |
| Other congenital | 255 (33.0) | 130 (33.7) | 125 (32.4) | .70 |
| Premature and neonatal | 127 (16.5) | 63 (16.3) | 64 (16.6) | .92 |
| Transplant | 8 (1.0) | 4 (1.0) | 4 (1.0) | — |
| Total CCCs, median (IQR) | 3 (3–4) | 3 (3–4) | 3 (3–4) | .81 |
| Technology dependence, n (%) | ||||
| Gastrostomy tube | 563 (72.9) | 285 (73.8) | 278 (72.0) | .57 |
| Baseline mechanical ventilator dependence | 190 (24.6) | 97 (25.1) | 93 (24.1) | .74 |
| Care after admission, n (%) | ||||
| ICU stay on d 0 or 1 | 286 (37.0) | 147 (38.1) | 139 (36.0) | .55 |
| Antibiotics on d 0 or 1 | 635 (82.3) | 317 (82.1) | 318 (82.4) | .93 |
—, not applicable.
TABLE 3.
Propensity Score–Matched Outcomes in Patients With Tracheostomy Hospitalized With Influenza Categorized According to Early Administration of Anti-influenza Medications
| All Patients (N = 772) | Received Anti-influenza Medications on d 0 or 1 (n = 386) | No Anti-influenza Medications on d 0 or 1 (n = 386) | P | |
|---|---|---|---|---|
| LOS, mean (95% CI), d | 6.9 (6.5–7.4) | 6.4 (5.8–7.0) | 7.5 (6.9–8.1) | .01 |
| 30-d revisit, n (%) | 199 (25.8) | 106 (27.5) | 93 (24.1) | .29 |
| 30-d hospital readmission | 151 (19.6) | 76 (19.7) | 75 (19.4) | .93 |
| 30-d ED revisit | 62 (8.0) | 35 (9.1) | 27 (7.0) | .29 |
| Days on antibiotics, mean (95% CI) | 5.3 (5.0–5.6) | 5.0 (4.6–5.5) | 5.6 (5.1–6.1) | .11 |
Because we excluded 127 patients in the matched propensity analysis, we conducted a post hoc sensitivity analysis to assess if the outcomes and effect sizes seen in the matched cohort remained stable when those who were unmatched were included. To perform the sensitivity analyses, we conducted traditional multivariable regression (linear for LOS and logistic for 30-day revisit) of all 899 eligible patients. We included all demographic and clinical characteristics in Table 1 as covariates. We obtained results like those seen in the propensity score–matched cohort for which receipt of early anti-influenza medications was independently associated with shorter LOS (absolute difference −0.98 days; P = .01) and similar 30-day revisit rates (adjusted odds ratio 1.12; P = .48).
Discussion
In our retrospective, propensity score–matched cohort study of nearly 800 children who were hospitalized with tracheostomy and influenza, we have demonstrated that early administration of anti-influenza medications is associated with shorter LOS by >1 day and no increase in 30-day all-cause revisit rate. Our study population was notable for a high degree of medical complexity with multiple CCCs and technology dependence. More than 80% of our study population received systemic antibiotics after admission. Regardless of receipt of early anti-influenza medications, systemic antibiotics were given for >75% of hospital days in our population with documented viral infection.
Although early administration of anti-influenza medication decreases influenza-related complications for CMC in the outpatient setting,13,14 this study is the first to reveal that early anti-influenza medication administration may improve clinical outcomes in a subpopulation of CMC. The results of our study are comparable to previous studies that revealed association with anti-influenza medications and shorter LOS without increase in readmission rate among children who experienced ICU admissions28 or complications from influenza.29 The changes in LOS in the current study are similar to the 1-day reduction in duration of illness for children who receive anti-influenza medications in the outpatient setting.14,38 Compared with the population in these studies, our population was notable for high medical complexity with multiple chronic care conditions. Our study captured a unique population that was not previously studied, and early use of anti-influenza medications was again associated with similar improvements in LOS.
The rate of timely administration of anti-influenza medications was <60% in our study population. This indicates that, despite recommendations, clinicians have difficulty following the clinical guidelines for early anti-influenza medication administration16,39 in this medically complex patient population that has increased morbidity from influenza and its complications. With our results, we affirm the recommendation for timely administration of antiviral medications in all children hospitalized with influenza39 by demonstrating its impact in a population of CMC. The LOS reductions seen in the current study may decrease family and caregiver burden and costs to the health care system. Previous quality improvement efforts to ensure the provision of early anti-influenza medications in children who were hospitalized uncovered barriers for providers to adhere to the guideline recommendations.40 Given the impact revealed in our study, future quality improvement efforts might specifically target CMC to ensure the provision of early anti-influenza medication administration.
We found widespread and prolonged use of systemic antibiotics in our patient cohort despite identification of a viral infection. There are several possible reasons for high rates of prolonged antibiotic use in our study. First, there are no evidence-based guidelines for differentiating and diagnosing bacterial and viral tracheostomy-associated respiratory tract infections. With high rates of positive bacterial respiratory culture results in children with tracheostomy,31 some providers may continue to treat positive respiratory culture results in the setting of a known viral infection. Second, bacterial coinfection is a known complication of influenza6,41,42; therefore, some providers may choose to continue antibiotics because of concerns for bacterial coinfection or secondary complications. Third, some patients may have had concomitant bacterial infections (eg, urinary tract infections) for which they appropriately continued to receive antibiotics. Fourth, concomitant infections with other respiratory viruses may have contributed to delayed response to anti-influenza medications, and clinicians may have chosen to continue antibiotics. Finally, some clinicians may be reluctant to discontinue antibiotics on a patient who is clinically improving on antibiotics, even with a viral source. The role of continued antibiotics in viral tracheostomy-related respiratory tract infections may merit future research.
Our study has some limitations because of the retrospective design using an administrative database. First, because the PHIS database only includes freestanding children’s hospitals, it is possible that exclusion of patients transferred from inpatient units of other hospitals resulted in exclusion of children with higher acuity; thus, our results may not be generalizable to all patients with tracheostomy hospitalized with influenza. Second, the PHIS database has no laboratory test results; therefore, we may have missed some patients with influenza or inappropriately included patients who did not have documented influenza. However, in previous studies, ICD-9-CM codes have been shown to have high specificity and positive predictive value for identifying children hospitalized with laboratory-confirmed influenza.26,27 Third, although we attempted to adjust for confounding by indication by using propensity score matching, additional important patient and hospital characteristics, such as influenza vaccination status and duration of illness before admission, are not available from the database. These patient-level and hospital-level factors could not be included in our propensity score but may have influenced anti-influenza medication prescribing patterns and study outcomes. The impact of these factors on propensity for timely receipt of anti-influenza medications and outcomes in children who are hospitalized is incompletely understood. A previous study of >30 000 admissions due to influenza (including >6000 children) revealed similar rates of timely administration of anti-influenza medications regardless of duration of illness preceding hospitalization.17 Differences in care pathways between individual hospitals were unavailable from the database and may have accounted for differences in our outcomes. However, we found that differences in treating hospitals accounted for 0% to 2.3% of variance in all outcomes, which suggests any variations due to hospital-level factors, such as care pathways, did not have significant influence on the outcomes. Lastly, because mortality is rare among children hospitalized with influenza,6–8 our cohort lacked sufficient patient deaths to examine this as an outcome.
The strengths of this study are its large sample size and use of propensity score matching. Propensity score matching is a statistical method used to adjust for confounding by indication,35 and it has been used in studies in which authors use PHIS data to assess the effect of various interventions.28,34,43 We chose this method rather than multivariate regression because of its potential to handle a larger number of covariates with the sample size of our study44 and to simulate randomized controlled trials by distinguishing covariates, exposure, and outcomes a priori.35 Although excluding those without matches could introduce bias, our sensitivity analysis using multivariate regression analyses with all eligible subjects found similar results. Because of ethical challenges conducting randomized, placebo-controlled prospective trials with anti-influenza medications,45 quasi-experimental design such as this study may provide the strongest evidence for early use of anti-influenza medication.
Conclusions
Early administration of anti-influenza medications is associated with reduction of LOS by 15% in children who are hospitalized with tracheostomy. With our results, we support the recommendation for timely administration of these medications for all children hospitalized with influenza.16,39 With ongoing difficulties in providing effective influenza prevention11,12 and increased risk of hospitalization,4,6–8 adherence to current influenza treatment guidelines in the inpatient setting remains especially important for the care of CMC. With this study, we also offer insight into antibiotic prescribing behavior regarding children with tracheostomy hospitalized with viral respiratory tract infections. Authors of future studies should investigate the role of quality improvement efforts in optimizing the care of CMC with influenza and the effect of antibiotics in children with tracheostomy during viral illness.
Glossary
- CCC
complex chronic condition
- CI
confidence interval
- CMC
children with medical complexity
- ED
emergency department
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- LOS
length of stay
- PHIS
Pediatric Health Information System
Footnotes
Dr Miyakawa conceptualized and designed the study, drafted the analytic plan and the initial manuscript, and reviewed and revised the manuscript; Mr Barreto conducted the statistical analyses and reviewed the manuscript; Drs Kato and Neely critically reviewed and revised the manuscript; Dr Russell conceptualized and designed the study, created the database, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Russell is a KL2 Scholar awarded under the KL2 Mentoring Research Career Development Award through Southern California Clinical and Translational Science Institute at Keck School of Medicine, University of Southern California. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health through grant award KL2TR000131. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Lafond KE, Nair H, Rasooly MH, et al. ; Global Respiratory Hospitalizations—Influenza Proportion Positive (GRIPP) Working Group . Global role and burden of influenza in pediatric respiratory hospitalizations, 1982-2012: a systematic analysis. PLoS Med. 2016;13(3):e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keren R, Zaoutis TE, Bridges CB, et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA. 2005;294(17):2188–2194 [DOI] [PubMed] [Google Scholar]
- 5.Coffin SE, Zaoutis TE, Rosenquist AB, et al. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics. 2007;119(4):740–748 [DOI] [PubMed] [Google Scholar]
- 6.Bhat N, Wright JG, Broder KR, et al. ; Influenza Special Investigations Team . Influenza-associated deaths among children in the United States, 2003-2004. N Engl J Med. 2005;353(24):2559–2567 [DOI] [PubMed] [Google Scholar]
- 7.Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States, 2004-2012. Pediatrics. 2013;132(5):796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics. 2018;141(4):e20172918. [DOI] [PubMed] [Google Scholar]
- 9.Keren R, Zaoutis TE, Saddlemire S, Luan XQ, Coffin SE. Direct medical cost of influenza-related hospitalizations in children. Pediatrics. 2006;118(5). Available at: www.pediatrics.org/cgi/content/full/118/5/e1321 [DOI] [PubMed] [Google Scholar]
- 10.Santibanez TA, Grohskopf LA, Zhai Y, Kahn KE. Complete influenza vaccination trends for children six to twenty-three months. Pediatrics. 2016;137(3):e20153280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian C, Wang H, Wang W, Luo X. Influenza vaccination coverage among US children from 2004/2005 to 2015/2016 [published online ahead of print May 15, 2018]. J Public Health (Oxf). doi: 10.1093/pubmed/fdy081 [DOI] [PubMed] [Google Scholar]
- 12.Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139(5):e20164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics. 2009;124(1):170–178 [DOI] [PubMed] [Google Scholar]
- 14.Venkatesan S, Myles PR, Leonardi-Bee J, et al. Impact of outpatient neuraminidase inhibitor treatment in patients infected with influenza A(H1N1)pdm09 at high risk of hospitalization: an individual participant data metaanalysis. Clin Infect Dis. 2017;64(10):1328–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics; Committee on Infectious Diseases . Policy statement–recommendations for prevention and control of influenza in children, 2010-2011. Pediatrics. 2010;126(4):816–826 [DOI] [PubMed] [Google Scholar]
- 16.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM; Centers for Disease Control and Prevention (CDC) . Antiviral agents for the treatment and chemoprophylaxis of influenza — recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(1):1–24 [PubMed] [Google Scholar]
- 17.Appiah GD, Chaves SS, Kirley PD, et al. Increased antiviral treatment among hospitalized children and adults with laboratory-confirmed influenza, 2010-2015. Clin Infect Dis. 2017;64(3):364–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumental S, Huisman E, Cornet MC, et al. Pandemic A/H1N1v influenza 2009 in hospitalized children: a multicenter Belgian survey. BMC Infect Dis. 2011;11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Das P, Roberson DW, et al. Hospitalizations in children with preexisting tracheostomy: a national perspective. Laryngoscope. 2015;125(2):462–468 [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Mamey MR, Russell CJ. Factors associated with 30-day all-cause hospital readmission after tracheotomy in pediatric patients. Int J Pediatr Otorhinolaryngol. 2017;103:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell CJ, Thurm C, Hall M, Simon TD, Neely MN, Berry JG. Risk factors for hospitalizations due to bacterial respiratory tract infections after tracheotomy. Pediatr Pulmonol. 2018;53(3):349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber SI, Erdman DD, Pur SL, et al. Outbreak of adenovirus genome type 7d2 infection in a pediatric chronic-care facility and tertiary-care hospital. Clin Infect Dis. 2001;32(5):694–700 [DOI] [PubMed] [Google Scholar]
- 23.Neu N, Plaskett T, Hutcheon G, Murray M, Southwick KL, Saiman L. Epidemiology of human metapneumovirus in a pediatric long-term care facility. Infect Control Hosp Epidemiol. 2012;33(6):545–550 [DOI] [PubMed] [Google Scholar]
- 24.Children’s Hospital Association PHIS. Available at: https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system. Accessed March 5, 2018
- 25.Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keren R, Wheeler A, Coffin SE, Zaoutis T, Hodinka R, Heydon K. ICD-9 codes for identifying influenza hospitalizations in children. Emerg Infect Dis. 2006;12(10):1603–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feemster KA, Leckerman KH, Middleton M, et al. Use of administrative data for the identification of laboratory-confirmed influenza infection: the validity of influenza-specific ICD-9 codes. J Pediatric Infect Dis Soc. 2013;2(1):63–66 [DOI] [PubMed] [Google Scholar]
- 28.Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30(11):962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brogan TV, Hall M, Sills MR, et al. Hospital readmissions among children with H1N1 influenza infection. Hosp Pediatr. 2014;4(6):348–358 [DOI] [PubMed] [Google Scholar]
- 30.Bueno M, Calvo C, Méndez-Echevarría A, et al. Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr Infect Dis J. 2013;32(10):1066–1069 [DOI] [PubMed] [Google Scholar]
- 31.McCaleb R, Warren RH, Willis D, Maples HD, Bai S, O’Brien CE. Description of respiratory microbiology of children with long-term tracheostomies. Respir Care. 2016;61(4):447–452 [DOI] [PubMed] [Google Scholar]
- 32.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e99 [DOI] [PubMed] [Google Scholar]
- 33.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnhart DC, Hall M, Mahant S, et al. Effectiveness of fundoplication at the time of gastrostomy in infants with neurological impairment. JAMA Pediatr. 2013;167(10):911–918 [DOI] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):28 [Google Scholar]
- 37.Keller B, Tipton E. Propensity score analysis in R: a software review. J Educ Behav Stat. 2016;41(3):326–348 [Google Scholar]
- 38.Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis. 2018;66(10):1492–1500 [DOI] [PubMed] [Google Scholar]
- 39.Committee on Infectious Diseases Recommendations for prevention and control of influenza in children, 2017 - 2018 [published correction appears in Pediatrics. 2018;141(1):e20173535]. Pediatrics. 2017;140(4):e20172550. [DOI] [PubMed] [Google Scholar]
- 40.Murphy A, Lindegren ML, Schaffner W, et al. Improving influenza testing and treatment in hospitalized children. Hosp Pediatr. 2018;8(9):570–577 [DOI] [PubMed] [Google Scholar]
- 41.Reed C, Kallen AJ, Patton M, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. 2009;28(7):572–576 [DOI] [PubMed] [Google Scholar]
- 42.Randolph AG, Vaughn F, Sullivan R, et al. ; Pediatric Acute Lung Injury and Sepsis Investigator’s Network and the National Heart, Lung, and Blood Institute ARDS Clinical Trials Network . Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics. 2011;128(6). Available at: www.pediatrics.org/cgi/content/full/128/6/ee1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keren R, Shah SS, Srivastava R, et al. ; Pediatric Research in Inpatient Settings Network . Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128 [DOI] [PubMed] [Google Scholar]
- 44.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69(3):345–357 [DOI] [PubMed] [Google Scholar]
- 45.Dawood FS, Jara J, Gonzalez R, et al. A randomized, double-blind, placebo-controlled trial evaluating the safety of early oseltamivir treatment among children 0-9 years of age hospitalized with influenza in El Salvador and Panama. Antiviral Res. 2016;133:85–94 [DOI] [PubMed] [Google Scholar]