ABSTRACT
Background
Postprandial lipemia is a risk factor for cardiovascular disease. Dairy products differ in nutrient content and food matrix, and little is known about how different dairy products affect postprandial triglyceride (TG) concentrations.
Objective
We investigated the effect of meals with similar amounts of fat from different dairy products on postprandial TG concentrations over 6 h in healthy adults.
Methods
A randomized controlled cross-over study was performed on 47 subjects (30% men), with median (25th–75th percentile) age of 32 (25–46) y and body mass index of 23.6 (21.0–25.8) kg/m2. Meals included 1 of butter, cheese, whipped cream, or sour cream, corresponding to 45 g of fat (approximately 60 energy%). Serum concentrations of TGs (primary outcome), and total cholesterol (TC), low density lipoprotein cholesterol (LDL cholesterol), high density lipoprotein cholesterol (HDL cholesterol), insulin, glucose, non-esterified fatty acids, and plasma glucose-dependent insulinotropic polypeptide (secondary outcomes) were measured before the meal and 2, 4, and 6 h postprandially. Incremental AUC (iAUC) was calculated for the responses, and data were analyzed using a linear mixed model.
Results
Sour cream induced a 61% larger TG-iAUC0–6 h compared to whipped cream (P < 0.001), a 53% larger TG-iAUC0–6 h compared to butter (P < 0.001), and a 23% larger TG-iAUC0–6 h compared to cheese (P = 0.05). No differences in TG-iAUC0–6 h between the other meals were observed. Intake of sour cream induced a larger HDL cholesterol-iAUC0–6 h compared to cheese (P = 0.01). Intake of cheese induced a 124% larger insulin iAUC0–6 h compared to butter (P = 0.006). No other meal effects were observed.
Conclusions
High-fat meals containing similar amount of fat from different dairy products induce different postprandial effects on serum TGs, HDL cholesterol, and insulin in healthy adults. The potential mechanisms and clinical impact of our findings remain to be further elucidated. The study was registered at www.clinicaltrials.gov as NCT02836106.
Keywords: cheese, butter, cream, sour cream, fermentation, triglycerides, HDL cholesterol, glucose-dependent insulinotropic polypeptide, insulin, non-esterified fatty acids
Introduction
Triglyceride-rich lipoproteins (TRLs) and their remnants are strongly associated with cardiovascular disease (CVD) and all-cause mortality (1–6). Although measurement of fasting triglyceride (TG) concentrations is more common in clinical practice, nonfasting TG concentrations have been shown to be a stronger and more independent predictor of CVD (7–9). It is well documented that high-fat meals lead to a transient increase in TRLs such as chylomicrons and very low density lipoproteins. A recent review investigated the postprandial TG responses of high-fat meals (40–95 g fatty acids) with different fat qualities (10). The main finding was that meals rich in saturated fat (mostly from butter) induced larger TG responses than meals with polyunsaturated fat. These different acute effects on lipids are in agreement with cohort studies and randomized controlled trials showing reduced CVD risk and improved lipid profile by replacing saturated fat with unsaturated fat (11–13). In addition, overweight subjects and subjects with components of the metabolic syndrome have been shown to respond differently to a high-fat meal than healthy normal weight subjects (14, 15), with a TG response lasting for up to 12 h in overweight subjects with elevated fasting TG levels (16). As most people eat several times per day, most of the day is spent in a postprandial state with TG concentrations above fasting levels (17, 18).
Dairy fat consists of approximately 70% saturated fatty acids, of which about 11% is myristic acid (14:0) and 29% is palmitic acid (16:0) (19). These 2 fatty acids are known to increase the level of cholesterol-rich low density lipoproteins (LDLs) in plasma, which can lead to excessive subendothelial retention and subsequently atherosclerosis (20, 21). Human dietary intervention studies have shown that chronic intakes of butter increase LDL cholesterol in plasma, whereas cheese appears to have less pronounced effects (22–25). However, evidence from epidemiological studies on dairy products and risk of CVD or mortality is inconclusive. Three recent cohorts report an association between high intake of milk and increased risk of mortality (26, 27), but most studies indicate a decreased or neutral CVD or mortality risk from intake of dairy products, especially fermented dairy products such as cheese and yoghurt (26, 28–33). An explanation for these contradictory results could be that dairy products are a heterogeneous food group with major differences in nutrient composition. For example, calcium content varies greatly among different dairy products and increased calcium intake has been shown to dampen postprandial lipemia (34). Also, different food structures and industrial processes impact the nutrients in dairy products, and may thereby affect metabolic responses (35). One study has shown that whole milk and fermented milk induce different postprandial TG responses (36), and a recent study found a larger increase in postprandial TG concentrations in the first 2 h after intake of cream cheese compared to butter and cheddar cheese (37).
How intake of different dairy products affects postprandial TG concentrations in subjects with various body compositions has, to the best of our knowledge, not been reported. Thus, the aim of this study was to investigate how high-fat meals containing similar amounts of fat from different dairy products affect the 6 h postprandial TG response, measured as the incremental AUC (iAUC), in both lean and overweight or obese subjects. The hypothesis was that there would be different postprandial TG responses after intake of meals containing similar fat content from different dairy products, and that obesity would increase the postprandial response.
Subjects and Methods
Subjects
Subjects aged 18–70 y were recruited between June 2016 and January 2017 through advertisements in social media and local newspapers as well as by posters at the University of Oslo, Oslo and Akershus University College of Applied Sciences, and Oslo University Hospital, Norway. Subjects were distributed to 1 of 2 study groups based on their BMI and waist circumference. Inclusion criteria for the lean group was BMI 18.5–25 kg/m2, waist circumference <80 cm for women and <94 cm for men, and fasting TG concentration <1.3 mmol/L. Inclusion criteria for the overweight and obese group was BMI ≥25 kg/m2 and waist circumference ≥80 cm for women and ≥94 cm for men. Common inclusion criteria for both groups was less than 5% weight change in the previous 3 mo, and willingness to eat a high-fat meal with 3 slices of bread and a dairy product for breakfast 4 times. Exclusion criteria for both groups were as follows: severe chronic disease (including type 1 and type 2 diabetes mellitus), CVD or cancer in the previous 6 mo, fasting blood glucose ≥7 mmol/L, C-reactive protein >10 mg/L, total cholesterol (TC) >6.1 mmol/L (aged 18–29 y), >6.9 mmol/L (aged 30–49 y), >7.8 mmol/L (≥50 y), blood pressure >160/100 mm Hg, thyroid stimulating hormone >4 mU/L, hemoglobin <120 gram/L, blood donation 2 mo before the first test day or during the study period, pregnant or lactating, allergy or intolerance against gluten, milk protein, or lactose, smoking, excessive alcohol consumption (>40 g daily), use of lipid-lowering or anti-inflammatory medications, hormonal treatment (except contraceptives or stable dose of thyroxine) or unwillingness to stop use of ω-3 fatty acid supplements 4 wk before the screening visit and during the study period. Subjects were contacted by telephone, and those who met the inclusion criteria were invited to a final screening visit at the Department of Nutrition at the University of Oslo for blood sampling and anthropometric measurements.
Study design
The randomized controlled cross-over study was conducted at the Centre for Clinical Nutrition at the Department of Nutrition, University of Oslo, Norway. The subjects were served 4 high-fat meals with different dairy products but containing similar amounts of fat. Each meal consisted of 3 toasted slices of white bread (Pågen Rosta), raspberry jam (Nora Bringebærsyltetøy), and 1 of butter (B) (TINE Smør), full-fat milk- and cream based medium-hard cheese (C) (TINE Gräddost), whipped cream (WC) (TINE Kremfløte), or sour cream (SC) (TINE Seterrømme), corresponding to 45 g of fat and approximately 60 energy % (E%) from fat. Each slice of bread was decorated with a small basil leaf to improve the participants’ visual impression of the meals. The subjects were blinded to the allocation because they did not know in which order they were to eat the 4 test meals. The meals were not blinded, although all meals were prepared so that they would look similar (Supplemental Figure 1). The principal investigator randomly allocated the subjects to 1 of 4 fixed test meal orders (Order 1: B_C_WC_SC, Order 2: C_WC_SC_B, Order 3: WC_SC_B_C, Order 4: SC_B_C_WC; B = butter, C = cheese, WC = whipped cream, SC = sour cream) by block randomization using Microsoft Excel's random generator. Allocation ratio was 1:1:1:1. Meal compositions and nutritional data were calculated using the software Dietist Net (Kost och näringsdata AB) and are shown in Table 1. The fatty acid composition for each of the dairy products is shown in Supplemental Table 1. There was a 3–5 wk long wash-out period between each test day for premenopausal women not taking contraceptives, and a minimum wash-out period of 2 wk for other participants. Subjects were encouraged to retain their habitual dietary and physical activity patterns during the whole study period. Before each test day, participants were given written guidelines to fast for 12 h, not perform any strenuous physical activity or drink alcohol in the previous 24 h, and not eat any fatty food after 1800 the night before. Subjects were instructed to eat 1 of 2 low-fat meals with the intention to standardize the last meal before the fast. Each test day was started with weight and waist circumference measurement and a fasting blood sample collection via venous puncture by a trained bioengineer. Subjects were given 20 min to eat the test meal (together with water if wanted, with a maximum 1 L of water in total during the test day), and blood samples were drawn at 2, 4, and 6 h after eating the meal. The participants stayed at the study center during the postprandial period where they could sit and work or study. Subjects were encouraged to be as physically inactive as possible during the 6 h of blood sampling. After the last blood sample, all subjects were offered a meal.
TABLE 1.
Meal compositions and nutritional data1
| B | C | WC | SC | |
|---|---|---|---|---|
| Meal composition | ||||
| White toast bread, g | 84 | 84 | 84 | 84 |
| Raspberry jam, g | 20 | 20 | 20 | 20 |
| Butter (82% fat), g | 52 | — | — | — |
| Cheese (38% fat), g | — | 113 | — | — |
| Whipped cream (38% fat), g | — | — | 113 | — |
| Sour cream (35% fat), g | — | — | — | 123 |
| Nutritional data | ||||
| Energy, kJ | 2614 | 2981 | 2712 | 2726 |
| Energy, kcal | 629 | 715 | 652 | 655 |
| Carbohydrates, g | 45.3 | 45.1 | 48.4 | 48.6 |
| Carbohydrates, E% | 30.5 | 26.6 | 31.3 | 31.3 |
| Protein, g | 8.8 | 30.0 | 10.9 | 11.2 |
| Protein, E% | 5.7 | 17.2 | 6.9 | 7.0 |
| Fat, g | 44.8 | 45.1 | 45.1 | 45.2 |
| Fat, E% | 63.7 | 56.2 | 61.8 | 61.6 |
| Calcium, mg | 9.0 | 678.0 | 85.0 | 94.0 |
1B, meal rich in fat from butter; C, meal rich in fat from medium-hard cheese; E%, energy %; kcal, kilo calories; kJ, kilo Joule; SC, meal rich in fat from sour cream; WC, meal rich in fat from whipped cream.
Clinical measurements
Weight was measured with use of The Medical Body Composition Analyzer Seca 515/514 (Seca, software version 1.1), and waist circumference was measured according to WHO guidelines (38). Body composition was determined by Dual Energy X-ray Absorptiometry (GE Lunar iDXA, Software: EnCore v16), using a valid method for abdominal visceral fat estimation, on the first and last test day to ensure that there were no changes in fat mass during the study period that could influence the TG response (39). The abdominal fat region was measured, as previously described (40), from the caudal limit at the top of the iliac crest to the cephalic limit at the base of the skull. The visceral fat was then calculated by subtracting subcutaneous fat from the total fat mass. Blood pressure was measured 3 consecutive times in the subjects’ non-dominant arm in a sitting position with use of a Dinamap Carescape v100 (GE Medical System) at the screening visit.
Blood sampling and standard biochemical measurements
Serum was collected in silica gel tubes (Becton Dickenson Vacutainer Systems) and kept at room temperature for 30–60 min to ensure complete blood coagulation. The samples were centrifuged for 15 min at 1500 × g (2700 rpm) (Thermo Fischer Scientific) and stored in a refrigerator or at −80°C until analysis. Plasma was obtained from EDTA tubes (Becton Dickenson Vacutainer Systems) and kept on ice for <15 min before being centrifuged at 2000 × g for 15 min at 4°C (Thermo Fischer Scientific). Aliquots of the samples were taken, then frozen at −80°C for glucose-dependent insulinotropic polypeptide (GIP) analysis.
Serum TGs were measured with use of an enzymatic colorimetric assay using photometry (ADVIA Chemistry XPT system) (41). Serum TC, LDL cholesterol, and HDL cholesterol were measured with use of enzymatic assays (ADVIA Chemistry XPT system) (42, 43). Apolipoprotein A1 (apo A1) and apo B were measured through use of polyethylene glycol fortified immunoturbidimetry (ADVIA Chemistry XPT system), and serum glucose was measured through use of an enzymatic assay with hexokinase and glucose-6-phosphate dehydrogenase enzymes (ADVIA Chemistry XPT system) (44), while serum high sensitivity C-reactive protein was measured with use of immunoturbidimetry (ADVIA Chemistry XPT system) and serum insulin was measured with use of a 2-site sandwich immunoassay with direct chemiluminescent technology (ADVIA Centaur system). All measurements were performed according to routine methods at an accredited medical laboratory (Fürst Medical Laboratory, Oslo, Norway), and were analyzed after each study visit. The analytical CVs for the biochemical measurements were 1.9% for TGs and TC, 2.0% for LDL cholesterol, and 2.1% for HDL cholesterol. The analytical CVs for apo A, apo B, glucose, and high sensitivity C-reactive protein were 2.4%, 3.7%, 1.3%, and 3.1%, respectively (Fürst Medical Laboratory).
GIP analysis
Plasma concentrations of GIP were analyzed with a Milliplex Map Kit for human metabolic hormone magnetic bead panel (Cat. # HMHEMAG-34K, EMD Millipore Corporation). All the samples were measured in duplicate along with controls with a Bio-Plex 200 system, based on Luminex xMAP technology (Bio-Rad Laboratories Inc.) (45).
Non-esterified fatty acids (NEFAs) analysis
Serum levels of NEFAs were determined by a contract laboratory (Vitas AS, Oslo, Norway) with a commercial NEFA-HR in vitro enzymatic colorimetric method (Wako Diagnostics). Samples were thawed overnight in a refrigerator and kept on ice during sample preparation and analysis.
Fatty acid analysis of the test products
Because the fatty acid composition of dairy fat may vary as a result of seasonal variations, samples from each dairy product were frozen at −20°C from 3 different time points during the study period, and analyzed by Vitas (Vitas AS). Dairy product samples (butter, cheese, whipped cream, and sour cream) were centrifuged at 17562 × g (14000 rpm) for 20 min, and the upper phase was dissolved in hexane, and methylated with 0.5 M sodium methoxide (NaOCH3). After mixing and centrifuging, the hexane phase was injected into the gas chromatography-flame ionization detector. The analysis was performed on a 7890A GC with a split/splitless injector, a 7683B automatic liquid sampler, and flame ionization detection (Agilent Technologies). Separations were performed on a Varian CP7421 (200 mm × 0.25 mm i.d.) column (Varian Inc). Identification of peaks was done by a combination of FAME 37 mixture (CRM47885) from Supelco Inc and USP FAME mixture Catalog#1,269,119. A total of 12 samples was analyzed with 3 samples of each dairy product, and the analyses were all performed together.
Dietary intake assessment
Habitual dietary pattern from the last year was assessed using a retrospective FFQ developed at the Department of Nutrition, University of Oslo (46). The FFQ was filled out by the participants before the screening visit, and a registered dietitian checked that this had been completed correctly at the screening visit. The FFQs were analyzed using the food database AE-14 and KBS software system (KBS version 7.3, 2017). Regular consumption of the dairy products included in the study was calculated based on the FFQ data. Milk intake included milk products with various fat contents, butter included mainly bread spread, cheese included products such as hard cheese, brown cheese, feta cheese, cream cheese, and cottage cheese, and sour cream intake included both high- and low-fat variants.
Ethics
The study was approved by the Regional Committees for Medical and Health Research Ethics (2016/418/REK sør-øst B) and conducted according to the principles of the Declaration of Helsinki. All subjects gave their written informed consent to participate. The study was registered at www.clinicaltrials.gov as NCT02836106.
Statistics
The iAUC 0–6 h was calculated based on the trapezoid method (47). Sample size was based on number of participants from previous intervention studies with high-fat meals or dairy products using postprandial TG-iAUC as outcome (18, 34, 48). The number of participants ranged from 7 to 18, and we calculated a drop-out rate of 20%. We therefore needed to recruit 22 subjects in each group. Because we did not manage to include the expected sample size of the overweight group, and had a larger drop-out rate than expected in the lean group, we performed a post hoc calculation of the sample size based on the change in TG-iAUC0–6 h between B and SC in the overweight group. Our post hoc analysis showed that with 80% power and a significance level of α = 0.05, we would have needed 16 subjects to be able to find a significant TG δ = 0.68 with SD = 0.9. Because there was no significant meal*group effect in the initial TG-iAUC analysis (data not shown), we decided to combine the 2 groups in the final analyses, and adjust the statistical model for BMI. Data from the postprandial measurements were analyzed with an intention-to-treat analysis by a linear mixed model using Stata Special Edition 14.2 (StataCorp LLC). All subjects that completed ≥1 test day were included in the analyses. The linear mixed model included the variables id (random effect), meal, visit number, age, sex, and BMI (fixed effects) for TG-iAUC (primary outcome), LDL cholesterol-iAUC, HDL cholesterol-iAUC, TC-iAUC, glucose-iAUC, insulin-iAUC, NEFA-iAUC, and GIP-iAUC (secondary outcomes). The meal*BMI interaction was tested for TG-iAUC but excluded from the final model as it was shown to be nonsignificant. Significance level was set to α = 0.05. To adjust for multiple testing when comparing single meals, Bonferroni correction was applied (i.e., all P values were multiplied by 6). Pairwise meal comparisons were performed by combining the appropriate regression coefficients from the linear mixed model. Baseline characteristic data is presented as median (25th–75th percentile), and postprandial data as mean ± SEM, unless otherwise indicated.
Results
Baseline characteristics
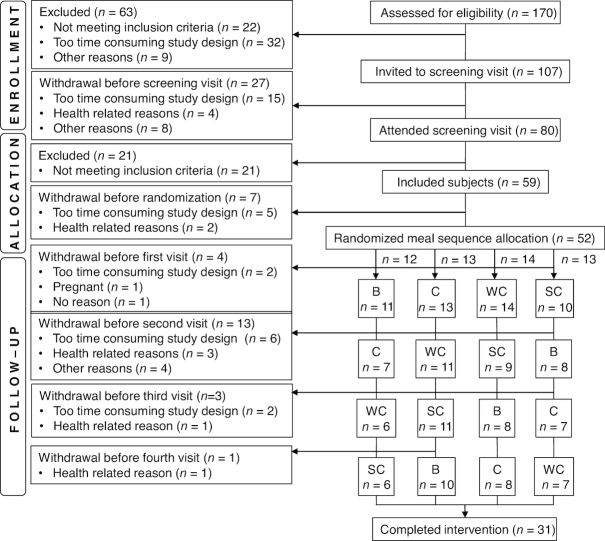
In total 170 subjects were assessed for eligibility, of whom 59 subjects were included and 52 were randomly assigned to a meal order. Forty-seven subjects completed the first visit and were included in the final analyses. In total, 31 subjects completed all 4 test meals (flowchart shown in Figure 1). Baseline characteristics from the participants’ first visit are shown in Table 2. The median age (25th–75th percentile) of the subjects was 32 (25–46) y and the median BMI (25th–75th percentile) was 23.6 (21.0–25.8) kg/m2. More women than men participated, and 12% of the women were postmenopausal. The habitual dietary intake is shown in Table 3, and includes the habitual intake of milk and the study-related dairy products before the subjects entered the study. The participants remained weight stable, and no changes in body composition occurred during the study period (data not shown).
FIGURE 1.
Flowchart of the study participants. B, meal rich in fat from butter; C, meal rich in fat from medium-hard cheese; SC, meal rich in fat from sour cream; WC, meal rich in fat from whipped cream.
TABLE 2.
Baseline characteristics of the study subjects1
| All subjects (n = 47) | |
|---|---|
| Descriptives | |
| Age, y | 32.0 (25.0–46.0) |
| Gender, n (%) | |
| Male | 14 (30) |
| Female2 | 33 (70) |
| Postmenopausal women, n (%) | 4 (12) |
| Blood pressure, mmHg | |
| Systolic | 113 (108–119) |
| Diastolic | 66 (62–71) |
| Anthropometrics | |
| BMI, kg/m2 | 23.6 (21.0–25.8) |
| Waist circumference, cm | 78.0 (71.0–90.0) |
| Females | 74.0 (67.5–79.5) |
| Males | 88.5 (82.8–102.5) |
| Biochemical measurements in serum | |
| Triglycerides, mmol/L | 0.87 (0.70–1.08) |
| Total cholesterol, mmol/L | 4.85 (4.30–5.50) |
| LDL cholesterol, mmol/L | 2.85 (2.50–3.60) |
| HDL cholesterol, mmol/L | 1.60 (1.38–2.03) |
| Apolipoprotein B, g/L | 0.90 (0.80–1.10) |
| Apolipoprotein A-1, g/L | 1.60 (1.48–1.70) |
| Glucose, mmol/L | 4.70 (4.50–5.05) |
| Insulin, pmol/L | 42 (26–67) |
| hsCRP, mg/L | 0.7 (0.4–1.5) |
1Data are presented as median (25th–75th percentile) or percentage, n = 47. All biochemical measurements are analyzed in serum. HDL, high density lipoprotein; hsCRP, high sensitivity C-reactive protein; LDL, low density lipoprotein.
2Six women used contraceptives.
TABLE 3.
Habitual dietary intake of the 47 healthy subjects1
| All subjects (n = 47) | |
|---|---|
| Energy, MJ/d | 9.3 (7.9–10.9) |
| Total fat, E% | 35.0 (30.2–39.8) |
| SFA, E% | 12.6 (10.1–15.1) |
| MUFA, E% | 13.1 (11.4–15.6) |
| PUFA, E% | 6.1 (4.8–7.3) |
| Protein, E% | 17.7 (16.4–19.2) |
| Carbohydrates, E% | 41.5 (37.4–44.1) |
| Fiber, E% | 2.4 (2.0–3.0) |
| Fiber, g/d | 27.6 (20.9–38.2) |
| Sugars, E% | 4.4 (2.7–6.7) |
| Alcohol, E% | 1.9 (0.4–3.8) |
| Milk, g/d | 204.6 (100.0–408.0) |
| Butter, g/d | 4.1 (0.1–10.4) |
| Cheese, g/d | 31.0 (21.0–49.5) |
| Whipped cream, g/d | 0.0 (0.0–0.1) |
| Sour cream, g/d | 5.2 (2.2–9.8) |
1Data are presented as median (25th–75th percentile). Milk intake includes milk of various fat contents but not milk in tea or coffee. Butter intake is mainly as bread spread. Cheese intake is mainly as various yellow hard cheeses but also includes brown cheese, feta cheese, cottage cheese, cream cheese, and similar products. Sour cream intake includes both high- and low-fat variants. E%, energy %.
Differences between test meals
TG serum concentrations
Fasting TG concentrations did not significantly differ between visits (data not shown). There was a significant meal effect on the postprandial TG-iAUC0–6 h (P < 0.001) (Figure 2, Supplemental Table 2). Intake of SC induced a significantly larger serum TG-iAUC0–6 h than all other dairy products (P < 0.001 for SC compared with WC, and SC compared with B; P = 0.05 for SC compared with C) (Figure 3,Supplemental Table 3). The mean TG-iAUC0–6 h for SC was 61%, 53%, and 23% larger compared with intake of WC, B, and C, respectively (Figure 3). Age, sex, and BMI did not have a significant impact on the TG-iAUC0–6 h, but there was a borderline effect of sex (P = 0.051) (Supplemental Table 2). In addition, visit number (the order in which the meals were consumed) did not have a significant impact (data not shown). BMI was also exchanged with abdominal visceral adipose tissue, lean mass, fat %, and fat mass (g), but none of these factors influenced the TG-iAUC0–6 h in the mixed model (data not shown).
FIGURE 2.
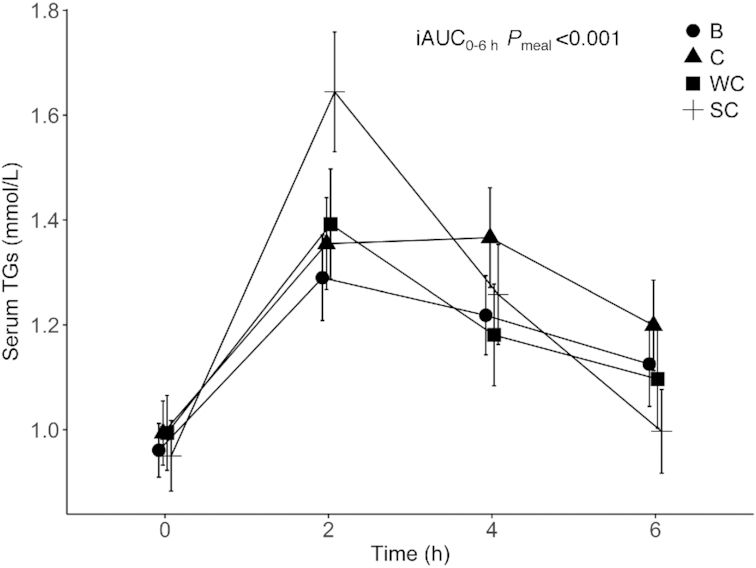
Changes in serum TG concentrations after intake of meals containing butter, cheese, whipped cream, and sour cream in healthy adults. Values are means ± SEMs. n = 36 (B), n = 35 (C), n = 38 (WC), n = 36 (SC). B, meal rich in fat from butter; C, meal rich in fat from medium-hard cheese; iAUC, incremental AUC; SC, meal rich in fat from sour cream; TG, triglyceride; WC, meal rich in fat from whipped cream.
FIGURE 3.
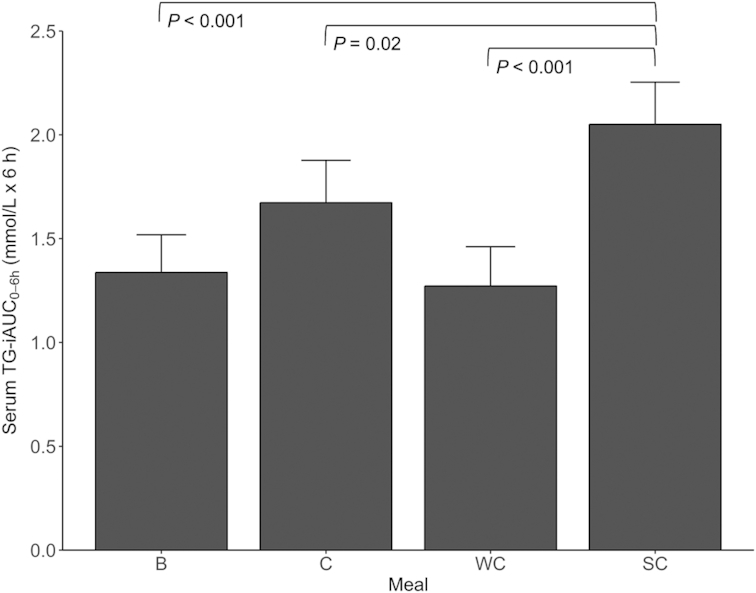
Serum TG-iAUC0–6 h concentrations after intake of meals containing butter, cheese, whipped cream, and sour cream in healthy adults. Values are means ± SEMs. Pairwise meal comparisons were performed with all meal combinations. Only statistically significant Bonferroni corrected P values are shown in the figure. n = 36 (B), n = 35 (C), n = 38 (WC), n = 36 (SC). B, meal rich in fat from butter; C, meal rich in fat from medium-hard cheese; iAUC, incremental AUC; SC, meal rich in fat from sour cream; TG, triglyceride; WC, meal rich in fat from whipped cream.
TC, LDL cholesterol, and HDL cholesterol serum concentrations
Fasting TC, LDL cholesterol, and HDL cholesterol concentrations did not significantly differ between visits (data not shown). There was a significant meal effect on the postprandial HDL cholesterol-iAUC0–6 h (P = 0.02) (Supplemental Table 2). Intake of SC induced a larger serum HDL cholesterol-iAUC0–6 h compared to C (P = 0.01) (Figure 4, Supplemental Table 3). There was no significant meal effect on the postprandial TC-iAUC0–6 h (P = 0.37) or LDL cholesterol-iAUC0–6 h (P = 0.21) (Supplemental Table 2). The mixed model analysis showed that BMI influenced the iAUC0–6 h for TC (P = 0.03), LDL cholesterol (P = 0.007), and HDL cholesterol (P = 0.03). In addition, sex (P = 0.03) and age (P = 0.01) influenced the LDL cholesterol-iAUC0–6 h (Supplemental Table 2). There was no significant meal effect on serum apo B-iAUC0–6 h (P = 0.30), and the concentrations of apo B were relatively constant during the postprandial period (data not shown).
FIGURE 4.
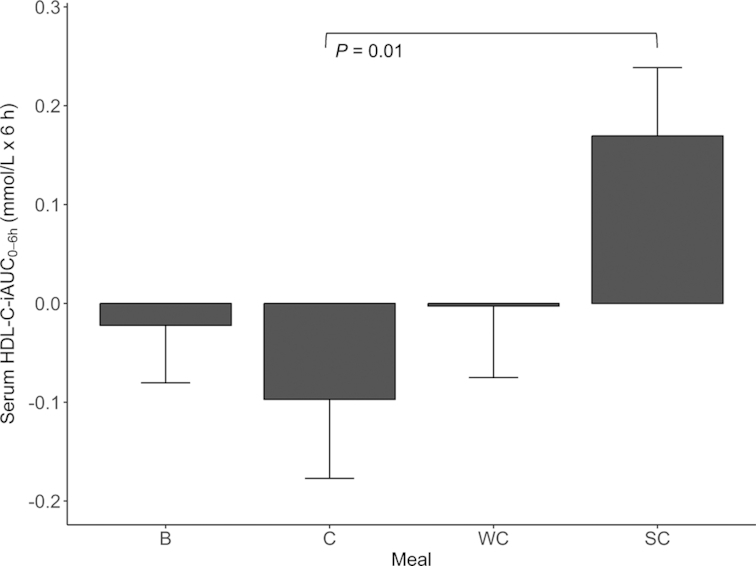
Serum HDL cholesterol-iAUC0–6 h concentrations after intake of meals containing butter, cheese, whipped cream, and sour cream in healthy adults. Values are means ± SEMs. Pairwise meal comparisons were performed with all meal combinations. Only statistically significant Bonferroni corrected P values are shown in the figure. n = 36 (B), n = 35 (C), n = 38 (WC), n = 36 (SC). B, meal rich in fat from butter; C, meal rich in fat from medium-hard cheese; HDL, high density lipoprotein; iAUC, incremental AUC; SC, meal rich in fat from sour cream; WC, meal rich in fat from whipped cream.
Insulin, glucose, and NEFA serum concentrations
Fasting glucose and NEFA concentrations did not significantly differ between visits, but there was a significant difference between visit 1 and visit 3 for fasting insulin concentration (data not shown). There was no significant meal effect on the postprandial serum glucose-iAUC0–6 h (P = 0.26) (Figure 5A, Supplemental Table 2) or serum NEFA-iAUC0–6 h (P = 0.06) (Figure 5C, Supplemental Table 2). There was a significant meal effect on the postprandial serum insulin-iAUC0–6 h (P = 0.006) (Figure 5B, Supplemental Table 2). Intake of C induced a 124% larger serum insulin-iAUC0–6 h compared to B (P = 0.006) (Supplemental Table 3). Sex had a significant impact on insulin-iAUC0–6 h (P = 0.007) and glucose-iAUC0–6 h (P = 0.04), and age on glucose-iAUC0–6 h (P < 0.001), but not on insulin-iAUC0–6 h (P = 0.95). BMI had a significant impact on insulin-iAUC0–6 h (P = 0.001), but not on glucose-iAUC0–6 h (P = 0.19) (Supplemental Table 2).
FIGURE 5.
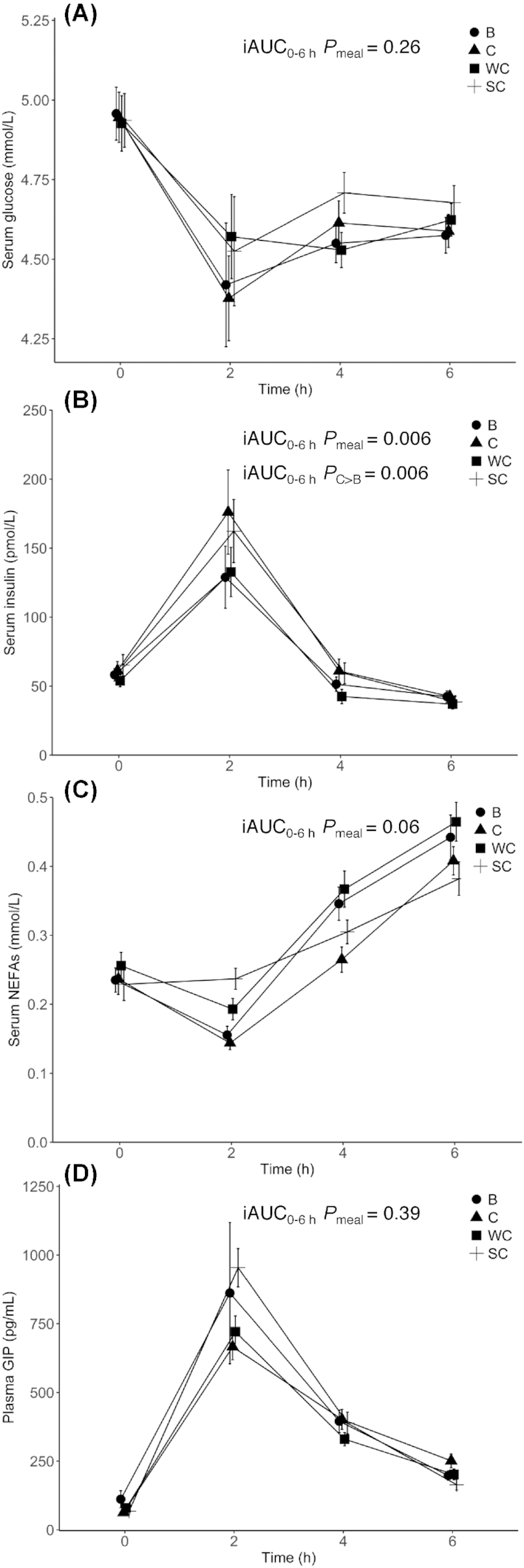
Changes in serum glucose (Panel A), insulin (Panel B), NEFA (Panel C), and plasma GIP (Panel D) concentrations after intake of meals containing butter, cheese, whipped cream, and sour cream in healthy adults. Values are means ± SEMs. Pairwise meal comparisons were performed with all meal combinations. Only statistically significant Bonferroni corrected P values are shown in the figure. Panel A: n = 35 (B), n = 35 (C), n = 38 (WC), n = 35 (SC). Panel B: n = 35 (B), n = 34 (C), n = 38 (WC), n = 35 (SC). Panel C: n = 36 (B), n = 35 (C), n = 38 (WC), n = 36 (SC). Panel D: n = 35 (B), n = 35 (C), n = 38 (WC), n = 34 (SC). B, meal rich in fat from butter; C, meal rich in fat from medium-hard cheese; GIP, glucose-dependent insulinotropic polypeptide; iAUC, incremental AUC; NEFA, non-esterified fatty acid; SC, meal rich in fat from sour cream; WC, meal rich in fat from whipped cream.
GIP plasma concentrations
Fasting GIP concentrations did not significantly differ between visits (data not shown). There was no significant meal effect on the postprandial plasma GIP-iAUC0–6 h (P = 0.39) (Figure 5D, Supplemental Table 2). Sex and age had significant impacts on GIP-iAUC0–6 h (P = 0.03 for both), whereas BMI had no significant impact on GIP-iAUC0–6 h (P = 0.85) (Supplemental Table 2).
Discussion
Our study showed divergent postprandial effects on the serum TG-iAUC0–6 h, the HDL cholesterol-iAUC0–6 h, and the insulin-iAUC0–6 h after high-fat meals with different dairy products containing similar fat and fatty acid composition. To our knowledge, this is the first study to investigate postprandial TG effects of standardized meals with equal amounts of fat from different dairy products in healthy adults.
Dairy products are a heterogeneous food group with substantial variations in nutrient content as well as food matrix (35, 49). Thus, it seems appropriate to view dairy products as separate product categories, but there are no custom classifications used in epidemiologic research (35). During the postprandial period there is an accumulation of TRLs in the circulation caused by the competition between intestinal- and hepatic-derived TRLs for the lipolytic activity of lipoprotein lipase (LPL), and receptor-mediated uptake (50). Accumulated TRLs may interact with leukocytes and vascular cells, and thereby influence the arterial wall, and contribute to formation of foam cells and thus increase the CVD risk (51). Sour cream is a fermented product, and fermented products have been associated with neutral or beneficial effects on cardiovascular health (28–33). We showed that intake of SC induced a significantly larger serum TG-iAUC0–6 h than all the other dairy meals, which may indicate that sour cream exerts a more atherogenic response compared to the other products. However, it remains to be determined why the fat particles in SC induce a larger serum TG-iAUC0–6 h compared to the other dairy product meals with similar fat content. However, as can be seen in Figure 2, the TG concentration peaked at 2 h after SC, and it has been shown in a large cohort study that postprandial TG concentration within 2 h is not associated with CVD risk, whereas TG concentration at 2–4 h is associated with CVD risk (7).
To further understand potential mechanisms behind the observed differences in TG-iAUC responses between the meals, we analyzed postprandial responses of insulin, glucose, NEFAs, and GIP. Insulin is known to stimulate the activity of LPL, and GIP is known to increase insulin secretion (52) and LPL activity (53). Both GIP and insulin increased 2 h after intake of all meals, but there were no significant meal effects that could explain why SC induced a larger serum TG-iAUC0–6 h compared to the other dairy products. At the same time point, serum glucose and NEFA concentrations were lower compared to fasting levels, as expected from the insulin response. However, the lack of meal effects for these variables indicates that these variables cannot explain the different SC serum TG-iAUC0–6 h response. The GIP concentrations were highest 2 h after intake of SC, which might affect LPL activity. This might partly explain the rapid TG decrease 2 h after intake of SC. However, this speculation needs to be further elucidated to understand whether GIP has a role in the different TG responses. The lack of significant meal effects for glucose and GIP may be a result of the time points used for blood sampling. Blood samples were taken at 0, 2, 4, and 6 h postprandially, and a more frequent blood sampling in the early postprandial phase could possibly have given different results. For example, in a previous study, an increase in plasma GIP was reported 30 min after intake of milk (36).
As sour cream is fermented homogenized cream, its nutrient composition is the same as for whipped cream but the fat droplets differ. Milk fat consists of fat droplets covered by a milk fat globule membrane. When going through homogenization, the droplets become smaller and increase in number (49). Smaller fat droplets generate more lipolysis than bigger ones (54, 55), and it has been shown that homogenized milk resulted in initially quicker digestion of the fat droplets compared with raw milk under simulated gastrointestinal tract conditions (56). This homogenization hypothesis is further supported by Drouin-Chartier et al. (37), who recently found that intake of homogenized cream cheese induced a larger postprandial TG increase compared with butter and cheddar cheese in the first 2 h in healthy subjects, which mirrors the SC response in our study, even though we did not perform statistical analysis at this time point.
The total fat content and the fatty acid composition was similar in the 4 dairy product meals, but the protein content of the meals differed, with cheese having the highest protein and energy contents. It has been demonstrated that postprandial lipid response is affected by the protein quantity and quality in high-fat meals (57–59). Cheese contains a large amount of casein, and casein has been shown to cause a more pronounced postprandial lipemia than whey protein, when given as part of a high-fat meal to postmenopausal women (58), whereas the opposite has been shown in obese men (60). In the present study, 70% of the subjects were women but only 4 were postmenopausal. Calcium from dairy products has been shown to dampen postprandial lipemia, most probably because of reduced fat absorption (34), and to attenuate increases in TC and LDL cholesterol induced by long-term intake of saturated fat (61). However, as there was no significant difference in TG-iAUC0–6 h after intake of C (highest content of protein and calcium) and B (lowest content of protein and calcium), our results are not in line with these studies. Nevertheless, the larger insulin-iAUC0–6 h seen from C compared to B could be explained by the higher protein content in cheese as milk proteins are known to stimulate insulin secretion (62, 63). On the contrary, we did not observe any meal effect on GIP, which is also known to increase after protein intake (64). Interestingly, sex and age had significant impacts on GIP-iAUC0–6 h, and a sex effect has recently been shown by others (65). The sex effect on GIP is also in accordance with the observed higher LPL activity in women compared to men (66, 67).
There was no significant meal effect on TC-iAUC0–6 h, which is in accordance with previous observations (68, 69), and neither did we find a significant meal effect on LDL cholesterol-iAUC0–6 h. However, we observed that intake of SC increased HDL cholesterol and induced a significantly larger HDL cholesterol-iAUC0–6 h compared to C, which is an interesting finding as previous studies have shown neutral or decreasing HDL cholesterol concentrations postprandially after an oral fat load (68–72). As HDL cholesterol is inversely associated with future cardiovascular events (7), the clinical relevance of this postprandial increase in HDL cholesterol after intake of SC with no effect on TC and LDL cholesterol should be further investigated.
Our study has several strengths including a controlled cross-over design with 4 meals containing both fermented and unfermented dairy products, and a relatively large study population with a broad range of BMI, compared with previous postprandial studies. The meals were equal in fat content and only differed by the type of dairy product. All routine measurements were performed in an accredited Norwegian medical laboratory (ISO 15,189), thereby strengthening the results. One limitation of the study is that we cannot be sure that all participants followed the guidelines to fast for 12 h, not perform any strenuous physical activity or drink alcohol in the previous 24 h, and not eat any fatty food after 1800 before each test day. Furthermore, because we were interested in investigating the effect of the dairy products as whole foods, the meals were not adjusted for differing nutrient and calorie contents. Another limitation is that we did not measure the postprandial response for longer than 6 h, and therefore did not allow for the circulating TG concentrations to return to baseline and information about the circulating concentrations in the late postprandial period was thus missed. TG concentrations were analyzed continuously after each study visit, and not in a batch after the study was finished, which may have influenced the results. However, the analyses from all study visits contained blood samples from subjects who consumed different meals, therefore this effect would have influenced the TG response for all meals.
Lastly, we did not measure postprandial apo B-48 concentrations, which could have provided information about the origins of the TRLs.
In conclusion, our results demonstrate that high-fat meals with different dairy products induce different postprandial effects on serum TG-iAUC0–6 h. The intake of SC induced a significantly larger TG-iAUC0–6 h compared to all the other dairy products, and a significantly larger HDL cholesterol-iAUC0–6 h compared to the intake of C. The potential mechanisms and the clinical impact of our findings will require additional studies.
Supplementary Material
Acknowledgments
We would like to thank Navida Akhter Sheikh for help with blood sampling and all logistics, Anne Randi Enget for help with blood sampling, Anne Lene Nordengen for preparing meals and blood samples, Geir Florholmen for dual energy X-ray absorptiometry scanning, Anne Marte Wetting Johansen for analyzing the FFQs, and Professor Jason Matthews for reading and editing the manuscript. The authors’ responsibilities were as follows—PH, KBH, LKLØ, HKB, MT, and SMU: designed the study; PH, KBH, and SMU: conducted the study; PH, LKLØ, and MT: performed statistical analyses; GOG and ASB: provided essential material; GSR and K-HH: performed laboratory analysis, PH, KBH, LKLØ, HKB, GOG, GSR, K-HH, MT, and SMU: wrote the manuscript; PH, KBH, and SMU: primary responsibility for the final content of the manuscript; and all authors: read and approved the final paper.
Notes
Supported by the Research Council of Norway (IPN244633), University of Oslo, and the Throne-Holst Foundation for Nutrition Research, Oslo, Norway.
Author disclosures: PH, LKLØ, HKB, GSR, K-HH, and MT, no conflicts of interest. SMU has received research grants from Mills DA, and Olympic Seafood, none of which are related to the content of this manuscript; KBH has received research grants and/or personal fees from Mills DA, Olympic Seafood, Kaneka, Amgen, Sanofi, and Pronova, none of which are related to the content of this manuscript. TINE partially funded the study via the Research Council of Norway, and ASB and GOG are employed at TINE. None of them own any stocks in the company.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Apo, apolipoprotein; B, meal rich in fat from butter; C, meal rich in fat from cheese; CVD, cardiovascular disease; E%, energy %; GIP, glucose-dependent insulinotropic polypeptide; HDL, high density lipoprotein; iAUC, incremental AUC; LDL, low density lipoprotein; LPL, lipoprotein lipase; NEFA; non-esterified fatty acid; SC, meal rich in fat from sour cream; TC, total cholesterol; TG, triglyceride; TRL, triglyceride-rich lipoprotein; WC, meal rich in fat from whipped cream.
References
- 1. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–63. [DOI] [PubMed] [Google Scholar]
- 2. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. [DOI] [PubMed] [Google Scholar]
- 3. Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med. 2011;270(1):65–75. [DOI] [PubMed] [Google Scholar]
- 4. Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118(20):2047–56. [DOI] [PubMed] [Google Scholar]
- 5. Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9(3):258–70. [DOI] [PubMed] [Google Scholar]
- 6. Toth PP. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16. [DOI] [PubMed] [Google Scholar]
- 8. Iso H, Imano H, Yamagishi K, Ohira T, Cui R, Noda H, Sato S, Kiyama M, Okada T, Hitsumoto S et al.. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis. 2014;237(1):361–8. [DOI] [PubMed] [Google Scholar]
- 9. Lindman AS, Veierod MB, Tverdal A, Pedersen JI, Selmer R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian Counties Study. Eur J Epidemiol. 2010;25(11):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monfort-Pires M, Delgado-Lista J, Gomez-Delgado F, Lopez-Miranda J, Perez-Martinez P, Ferreira SR. Impact of the content of fatty acids of oral fat tolerance tests on postprandial triglyceridemia: systematic review and meta-analysis. Nutrients. 2016;8(9):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S et al.. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89(5):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ulven SM, Leder L, Elind E, Ottestad I, Christensen JJ, Telle-Hansen VH, Skjetne AJ, Raael E, Sheikh NA, Holck M et al.. Exchanging a few commercial, regularly consumed food items with improved fat quality reduces total cholesterol and LDL-cholesterol: a double-blind, randomised controlled trial. Br J Nutr. 2016;116(8):1383–93. [DOI] [PubMed] [Google Scholar]
- 13. Vafeiadou K, Weech M, Altowaijri H, Todd S, Yaqoob P, Jackson KG, Lovegrove JA. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr. 2015;102(1):40–8. [DOI] [PubMed] [Google Scholar]
- 14. Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220(1):22–33. [DOI] [PubMed] [Google Scholar]
- 15. Jackson KG, Walden CM, Murray P, Smith AM, Lovegrove JA, Minihane AM, Williams CM. A sequential two meal challenge reveals abnormalities in postprandial TAG but not glucose in men with increasing numbers of metabolic syndrome components. Atherosclerosis. 2012;220(1):237–43. [DOI] [PubMed] [Google Scholar]
- 16. Cohn JS. Postprandial lipemia and remnant lipoproteins. Clin Lab Med. 2006;26(4):773–86. [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98(3):458–73. [DOI] [PubMed] [Google Scholar]
- 18. Clemente G, Mancini M, Nazzaro F, Lasorella G, Rivieccio A, Palumbo AM, Rivellese AA, Ferrara L, Giacco R. Effects of different dairy products on postprandial lipemia. Nutr Metab Cardiovasc Dis. 2003;13(6):377–83. [DOI] [PubMed] [Google Scholar]
- 19. Devle H, Vetti I, Naess‐Andresen CF, Rukke EO, Vegarud G, Ekeberg D. A comparative study of fatty acid profiles in ruminant and non‐ruminant milk. Eur J Lipid Sci Technol. 2012;114(9):1036–43. [Google Scholar]
- 20. Muller H, Kirkhus B, Pedersen JI. Serum cholesterol predictive equations with special emphasis on trans and saturated fatty acids. an analysis from designed controlled studies. Lipids. 2001;36(8):783–91. [DOI] [PubMed] [Google Scholar]
- 21. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H et al.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Goede J, Geleijnse JM, Ding EL, Soedamah-Muthu SS. Effect of cheese consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73(5):259–75. [DOI] [PubMed] [Google Scholar]
- 23. Biong AS, Muller H, Seljeflot I, Veierod MB, Pedersen JI. A comparison of the effects of cheese and butter on serum lipids, haemostatic variables and homocysteine. Br J Nutr. 2004;92(5):791–7. [DOI] [PubMed] [Google Scholar]
- 24. Hjerpsted J, Leedo E, Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr. 2011;94(6):1479–84. [DOI] [PubMed] [Google Scholar]
- 25. Nestel PJ, Chronopulos A, Cehun M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur J Clin Nutr. 2005;59(9):1059–63. [DOI] [PubMed] [Google Scholar]
- 26. Tognon G, Nilsson LM, Shungin D, Lissner L, Jansson JH, Renstrom F, Wennberg M, Winkvist A, Johansson I. Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr. 2017;105(6):1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michaelsson K, Wolk A, Langenskiold S, Basu S, Warensjo Lemming E, Melhus H, Byberg L. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32(4):269–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drouin-Chartier JP, Brassard D, Tessier-Grenier M, Cote JA, Labonte ME, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drouin-Chartier JP, Cote JA, Labonte ME, Brassard D, Tessier-Grenier M, Desroches S, Couture P, Lamarche B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7(6):1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hjerpsted J, Tholstrup T. Cheese and cardiovascular disease risk: a review of the evidence and discussion of possible mechanisms. Crit Rev Food Sci Nutr. 2016;56(8):1389–403. [DOI] [PubMed] [Google Scholar]
- 32. Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr. 2014;99(5 Suppl):1235s–42s. [DOI] [PubMed] [Google Scholar]
- 33. Tapsell LC. Fermented dairy food and CVD risk. Br J Nutr. 2015;113(Suppl 2):S131–5. [DOI] [PubMed] [Google Scholar]
- 34. Lorenzen JK, Nielsen S, Holst JJ, Tetens I, Rehfeld JF, Astrup A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am J Clin Nutr. 2007;85(3):678–87. [DOI] [PubMed] [Google Scholar]
- 35. Thorning TK, Bertram HC, Bonjour JP, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC et al.. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105(5):1033–45. [DOI] [PubMed] [Google Scholar]
- 36. Sanggaard KM, Holst JJ, Rehfeld JF, Sandstrom B, Raben A, Tholstrup T. Different effects of whole milk and a fermented milk with the same fat and lactose content on gastric emptying and postprandial lipaemia, but not on glycaemic response and appetite. Br J Nutr. 2004;92(3):447–59. [DOI] [PubMed] [Google Scholar]
- 37. Drouin-Chartier JP, Tremblay AJ, Maltais-Giguere J, Charest A, Guinot L, Rioux LE, Labrie S, Britten M, Lamarche B, Turgeon SL et al.. Differential impact of the cheese matrix on the postprandial lipid response: a randomized, crossover, controlled trial. Am J Clin Nutr. 2017;106(6):1358–65. [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. 2011.
- 39. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). 2012;20(6):1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olarescu NC, Jorgensen AP, Godang K, Jurik AG, Froslie KF, Bollerslev J. Dual-energy X-ray absorptiometry is a valid method to estimate visceral adipose tissue in adult patients with Prader-Willi syndrome during treatment with growth hormone. J Clin Endocrinol Metab. 2014;99(9):E1727–31. [DOI] [PubMed] [Google Scholar]
- 41. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077–80. [PubMed] [Google Scholar]
- 42. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–5. [PubMed] [Google Scholar]
- 43. Okada M, Matsui H, Ito Y, Fujiwara A, Inano K. Low-density lipoprotein cholesterol can be chemically measured: a new superior method. J Lab Clin Med. 1998;132(3):195–201. [DOI] [PubMed] [Google Scholar]
- 44. Bergmeyer HU, Gawehn K, Lund P. Methods of Enzymatic Analysis: Vol. 2: Weinheim: Verlag Chemie; New York: Academic Press, 1974. [Google Scholar]
- 45. Ingerslev AK, Mutt SJ, Laerke HN, Hedemann MS, Theil PK, Nielsen KL, Jorgensen H, Herzig KH, Bach Knudsen KE. Postprandial PYY increase by resistant starch supplementation is independent of net portal appearance of short-chain fatty acids in pigs. PLoS One. 2017;12(10):e0185927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carlsen MH, Lillegaard IT, Karlsen A, Blomhoff R, Drevon CA, Andersen LF. Evaluation of energy and dietary intake estimates from a food frequency questionnaire using independent energy expenditure measurement and weighed food records. Nutr J. 2010;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carstensen M, Thomsen C, Hermansen K. Incremental area under response curve more accurately describes the triglyceride response to an oral fat load in both healthy and type 2 diabetic subjects. Metabolism. 2003;52(8):1034–7. [DOI] [PubMed] [Google Scholar]
- 48. Lewis GF, O'Meara NM, Soltys PA, Blackman JD, Iverius PH, Druetzler AF, Getz GS, Polonsky KS. Postprandial lipoprotein metabolism in normal and obese subjects: comparison after the vitamin A fat-loading test. J Clin Endocrinol Metab. 1990;71(4):1041–50. [DOI] [PubMed] [Google Scholar]
- 49. Michalski M-C, Januel C. Does homogenization affect the human health properties of cow's milk?. Trends Food Sci Technol. 2006;17(8):423–37. [Google Scholar]
- 50. Bjorkegren J, Packard CJ, Hamsten A, Bedford D, Caslake M, Foster L, Shepherd J, Stewart P, Karpe F. Accumulation of large very low density lipoprotein in plasma during intravenous infusion of a chylomicron-like triglyceride emulsion reflects competition for a common lipolytic pathway. J Lipid Res. 1996;37(1):76–86. [PubMed] [Google Scholar]
- 51. Botham KM, Wheeler-Jones CP. Postprandial lipoproteins and the molecular regulation of vascular homeostasis. Prog Lipid Res. 2013;52(4):446–64. [DOI] [PubMed] [Google Scholar]
- 52. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. [DOI] [PubMed] [Google Scholar]
- 53. Eckel RH, Fujimoto WY, Brunzell JD. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 1979;28(12):1141–2. [DOI] [PubMed] [Google Scholar]
- 54. Armand M, Pasquier B, Andre M, Borel P, Senft M, Peyrot J, Salducci J, Portugal H, Jaussan V, Lairon D. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am J Clin Nutr. 1999;70(6):1096–106. [DOI] [PubMed] [Google Scholar]
- 55. Fave G, Coste TC, Armand M. Physicochemical properties of lipids: new strategies to manage fatty acid bioavailability. Cell Mol Biol (Noisy-le-grand). 2004;50(7):815–31. [PubMed] [Google Scholar]
- 56. Liang L, Qi C, Wang X, Jin Q, McClements DJ. Influence of homogenization and thermal processing on the gastrointestinal fate of bovine milk fat: in vitro digestion study. J Agric Food Chem. 2017;65(50):11109–17. [DOI] [PubMed] [Google Scholar]
- 57. O'Reilly EM, Holub BJ, Laidlaw M, Garrioch C, Wlodek MG. Development of a standardized clinical protocol for ranking foods and meals based on postprandial triglyceride responses: The Lipemic Index. ISRN Vasc Med. 2011;2011:6. [Google Scholar]
- 58. Pal S, Ellis V, Ho S. Acute effects of whey protein isolate on cardiovascular risk factors in overweight, post-menopausal women. Atherosclerosis. 2010;212(1):339–44. [DOI] [PubMed] [Google Scholar]
- 59. Westphal S, Taneva E, Kastner S, Martens-Lobenhoffer J, Bode-Boger S, Kropf S, Dierkes J, Luley C. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis. 2006;185(2):313–9. [DOI] [PubMed] [Google Scholar]
- 60. Mariotti F, Valette M, Lopez C, Fouillet H, Famelart MH, Mathe V, Airinei G, Benamouzig R, Gaudichon C, Tome D et al.. Casein compared with whey proteins affects the organization of dietary fat during digestion and attenuates the postprandial triglyceride response to a mixed high-fat meal in healthy, overweight men. J Nutr. 2015;145(12):2657–64. [DOI] [PubMed] [Google Scholar]
- 61. Soerensen KV, Thorning TK, Astrup A, Kristensen M, Lorenzen JK. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am J Clin Nutr. 2014;99(5):984–91. [DOI] [PubMed] [Google Scholar]
- 62. Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80(5):1246–53. [DOI] [PubMed] [Google Scholar]
- 63. van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr. 2000;72(1):96–105. [DOI] [PubMed] [Google Scholar]
- 64. Giezenaar C, Luscombe-Marsh ND, Hutchison AT, Standfield S, Feinle-Bisset C, Horowitz M, Chapman I, Soenen S. Dose-dependent effects of randomized intraduodenal whey-protein loads on glucose, gut hormone, and amino acid concentrations in healthy older and younger Men. Nutrients. 2018;10(1):pii: E78. doi:10.3390/nu10010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giezenaar C, Luscombe-Marsh ND, Hutchison AT, Lange K, Hausken T, Jones KL, Horowitz M, Chapman I, Soenen S. Effect of gender on the acute effects of whey protein ingestion on energy intake, appetite, gastric emptying and gut hormone responses in healthy young adults. Nutr Diabetes. 2018;8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61(10):1145–61. [DOI] [PubMed] [Google Scholar]
- 67. Despres JP, Gagnon J, Bergeron J, Couillard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Plasma post-heparin lipase activities in the HERITAGE Family Study: the reproducibility, gender differences, and associations with lipoprotein levels. HEalth, RIsk factors, exercise Training And GEnetics. Clin Biochem. 1999;32(3):157–65. [DOI] [PubMed] [Google Scholar]
- 68. Nikkila M, Solakivi T, Lehtimaki T, Koivula T, Laippala P, Astrom B. Postprandial plasma lipoprotein changes in relation to apolipoprotein E phenotypes and low density lipoprotein size in men with and without coronary artery disease. Atherosclerosis. 1994;106(2):149–57. [DOI] [PubMed] [Google Scholar]
- 69. Lupattelli G, Pasqualini L, Siepi D, Marchesi S, Pirro M, Vaudo G, Ciuffetti G, Mannarino E. Increased postprandial lipemia in patients with normolipemic peripheral arterial disease. Am Heart J. 2002;143(4):733–8. [DOI] [PubMed] [Google Scholar]
- 70. Rathnayake KM, Weech M, Jackson KG, Lovegrove JA. Impact of meal fatty acid composition on postprandial lipaemia, vascular function and blood pressure in postmenopausal women. Nutr Res Rev. 2018:1–11. [DOI] [PubMed] [Google Scholar]
- 71. Westerveld HT, Meijer E, Erkelens DW, de Bruin TW. Postprandial reduction in high-density lipoprotein cholesterol concentrations in postmenopausal women: improvement by 17β-estradiol. Metabolism. 1996;45(7):827–32. [DOI] [PubMed] [Google Scholar]
- 72. Pirro M, Lupattelli G, Siepi D, Palumbo B, Roscini AR, Marchesi S, Schillaci G, Mannarino E. Postprandial lipemia and associated metabolic disturbances in healthy and hyperlipemic postmenopausal women. Metabolism. 2001;50(3):330–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.