ABSTRACT
Background
Menaquinone-4 (MK4), a vitamin K metabolite, is converted from phylloquinone through a process that requires intermediates of endogenous cholesterol production. Recent evidence suggests that MK4 is involved in kidney function.
Objective
The purpose of this study was to determine the effect of atorvastatin treatment on MK4 formation in young and old male mice.
Methods
C57BL/6 male mice (4-mo-old and 20-mo-old) were randomly assigned to either a diet containing 300 mg atorvastatin/kg with 3 mg phylloquinone/kg or a control diet containing 3 mg phylloquinone/kg for 8 wk. During week 8, all mice received deuterium-labeled phylloquinone in the diet. Labeled and unlabeled phylloquinone and MK4 in liver, kidney, brain, and intestine were measured by atmospheric pressure chemical ionization LC/MS. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase gene expression was quantified by reverse transcriptase-PCR. Tissue MK4 and phylloquinone concentrations were compared between atorvastatin treatment groups with use of general linear models.
Results
There was no age-treatment interaction on MK4 tissue concentrations. In atorvastatin-treated mice, total MK4 and percentage of deuterium-labeled MK4 in kidney were both approximately 45% lower compared to values in mice not given atorvastatin (all P < 0.05). MK4 concentrations did not differ between groups in any other tissue measured.
Conclusion
In male mice, atorvastatin reduced endogenous MK4 formation in the kidney, but not other organs. These observations are consistent with our hypothesis that cholesterol metabolism is involved in the generation of MK4. Further research is needed to understand potential regulatory mechanisms and the unique functions of MK4 in the kidney.
Keywords: atorvastatin, cholesterol intermediates, geranylgeranyl pyrophosphate, menaquinones, phylloquinone, statins, vitamin K
Introduction
Vitamin K represents a group of vitamers essential for carboxylation of vitamin K-dependent proteins involved in various physiological processes, including blood coagulation and regulation of calcification (1). These vitamers include phylloquinone and menaquinones (MKn), where the “n” indicates the number of isoprenoid units in the side chain. All vitamin K forms are bioactive and provide the reducing agent for carboxylation of vitamin K-dependent proteins; however, they vary in their degree of physiological bioactivity (2, 3). A unique characteristic of vitamin K metabolism is the tissue-specific difference in conversion of dietary phylloquinone to menaquinone-4 (MK4) (4). Furthermore, dietary phylloquinone intake can influence tissue concentrations of MK4 (5). Identifying factors that influence conversion of phylloquinone to MK4 will provide insight into the potential function of MK4, including regulation of pro-inflammatory cytokines, cell growth, and protection against oxidative stress (6–9).
Vitamin K metabolism and cellular cholesterol metabolism are linked because endogenous MK4 production requires an isoprenoid side chain and geranylgeranyl pyrophosphate (GGPP). GGPP is synthesized from isopentenyl pyrophosphate and farnesyl pyrophosphate, intermediate metabolites in biosynthesis of cholesterol (10). The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, also known as statins, are potent inhibitors of endogenous cholesterol synthesis and thus inhibit biosynthesis of isoprenoid intermediates, including GGPP (11). In vitro studies have demonstrated that statins reduce the conversion of dietary phylloquinone to MK4, by limiting GGPP availability (12). In addition, supplementary GGPP rescues MK4 production, illustrating an interaction between cholesterol synthesis and MK4, and potential regulation of MK4 formation (12). The contribution of cholesterol precursors to tissue concentrations of MK4 or the potential regulatory mechanisms involving enzymatic activity in both the vitamin K metabolic pathway and cholesterol synthesis pathway are not well understood. The objective of this study was to determine the effect of atorvastatin on endogenous production of MK4 in C57BL/6 male mice provided a diet that exceeded recommended intakes of phylloquinone.
Methods
Animals and diets
Expanding on the previous C57BL/6 mouse model used for dietary manipulation studies (13), we chose to optimize an aging mouse model for comparison to young adult mice (14). All mice were acclimated on AIN-93G diet (TD.94045, Harlan Teklad) for 2 wk, and transitioned to a control diet (TD.120060, Harlan Teklad with 5% tocopherol stripped corn oil, containing 3 mg phylloquinone/kg). The experimental diet was a modification of TD.97053 (Supplemental Table 1). Eight-mo-old retired male breeder C57BL/6 mice were obtained from Charles River Laboratory and aged to 20 mo on the control diet, at which time statin intervention was initiated. In addition, younger 4-mo-old male mice were obtained from Charles River Laboratory 2 wk before initiation of the statin intervention. A 2 × 2 factorial design was used to evaluate age and diet effects (Supplemental Figure 1). Male mice were weight-matched and randomly assigned to a diet containing 300 mg atorvastatin/kg with 3 mg phylloquinone/kg or a control diet containing 3 mg phylloquinone/kg (TD.120060, Harlan Teklad with 5% tocopherol stripped corn oil) for 8 wk ad libitum, resulting in 4 groups of 8 mice each. Atorvastatin dosing was targeted to <3 mg/kg body weight/d, to minimize risk of liver toxicity during chronic exposure (15) and to account for extensive first-pass metabolism in the gut and liver (16). During week 8, all mice received deuterium-labeled phylloquinone in the diet (0.02 mg/mouse/d). Deuterium-labeled collard greens were hydroponically grown in a controlled environment at the USDA-Agricultural Research Service Children's Nutrition Research Center in Houston, Texas, as previously described (17).
Body weights were measured weekly. Mice were maintained in AAALAC-accredited facilities with an environmentally controlled atmosphere (22°C, 45% relative humidity, 15 air changes of 100% fresh hepa-filtered air per h and a 12/12-h light/dark cycle). Animals were observed daily for clinical signs of distress or disease. At the end of the experiment, mice were killed with carbon dioxide and subsequent cervical dislocation, followed by tissue collection. Tissues of interest (brain, liver, intestine—cleaned of luminal contents—and kidney) were harvested, frozen immediately in liquid nitrogen, and stored at −80°C until time of analysis. All protocols were approved by the HNRCA Tufts University Animal Care and Use Committee.
Mass spectrometry
Tissues (0.10–0.20 g wet weight) were homogenized in PBS through use of a Powergen homogenizer (Fisher Scientific). Concentrations of unlabeled phylloquinone, deuterium-labeled phylloquinone, unlabeled MK4, and deuterium-labeled MK4 were measured in tissue homogenates by atmospheric pressure chemical ionization LC-MS (Agilent Technologies) (Supplemental Table 2, Supplemental Table 3, and Supplemental Figure 2), as described elsewhere (18) and are presented as pmol/g tissue. Data were collected with use of Agilent Chemistation software (Version C.01.05). Efforts to measure isoprenoid intermediates including isopentenyl pyrophosphate, geranyl pyrophosphate, farnesyl pyrophosphate, and GGPP by UPLC-MS/MS (Agilent Technologies) were attempted with a modified method of Henneman et al. 2011 (19), but we were unable to detect any isoprenoid intermediates in the tissue.
RT-quantitative PCR
We profiled expression of the following genes encoding enzymes involved in vitamin K and cholesterol metabolism: 1) vitamin K epoxide reductase complex subunit 1 (vkorc1); 2) vitamin K epoxide reductase complex subunit 1 like 1 (vkorc1l1); 3) gamma glutamyl carboxylase (ggcx); 4) UbiA prenyl transferase domain-containing 1 (ubiad); HMG-CoA reductase (hmgcr); and LDL receptor (ldlr). All primer/probe sets for real-time PCR were TaqMan gene expression assays (Applied Biosystems). Total RNA was isolated from tissues (kidney, liver, brain, and intestine) with Trizol reagent and the PureLink RNA Mini Kit (Ambion Life Technologies) following the manufacturer's instructions. The cDNA was synthesized with use of the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Real-time PCR was performed with TaqMan Universal Master Mix II, no UNG and a QuantStudio 6 Flex System (Applied Biosystems). Relative expression was calculated through use of the 2-ΔΔCt method and statistical analyses were performed on ΔCt values. Gapdh was used as the control gene. All primer information is provided in Supplemental Table 4.
Plasma and tissue lipid concentrations
Plasma triglycerides were quantified with a kit from Wako Pure Chemical Industries, Ltd. Plasma total cholesterol was quantified with the Cholesterol/Cholesterol Ester Quantitation Kit (Abcam). Both assays were carried out per manufacturers’ instructions. Tissue concentrations of total cholesterol were quantified through use of the colorimetric Total Cholesterol Assay Kit (Cell Biolabs), per the manufacturer's instructions.
Statistical analyses
Sample size calculations were based on previously reported data of deuterium-labeled phylloquinone tissue concentrations, with parameters of α = 0.05; power = 80%; effect size = 209 pmol/g and standard deviation = 113.2 pmol/g for liver concentrations of phylloquinone. Accounting for multiple group comparisons, 8 animals were required per group. We initially evaluated the effects of age, atorvastatin treatment, and tissue and their interactions on total concentrations and % labeled phylloquinone and MK4 with a 3-factor ANOVA model. In the analyses of tissue phylloquinone concentrations, the atorvastatin treatment groups were combined because the 3-factor interaction was not significant (age*atorvastatin treatment*tissue P = 0.13) and there was no effect of atorvastatin on tissue total or % labeled phylloquinone (all atorvastatin treatment*tissue P = 0.49). However, we detected a significant interaction between age and tissue (age*tissue P < 0.01), so we analyzed the effect of age on phylloquinone in each tissue separately. In contrast, for the MK4 analyses, the age groups were combined because there was no effect of age on tissue MK4 (all P values > 0.44). We did analyze the effect of atorvastatin on MK4 in each tissue separately because we detected an interaction between tissue and atorvastatin treatment with respect to MK4 (P < 0.04). The effect of age, atorvastatin, and their interaction on tissue cholesterol concentrations were analyzed through use of a 2-factor ANOVA. All models were assessed with use of diagnostics for assumptions of homogeneity of variance. No outliers were found that influenced significance in the full model. Data were tested for normality with the Shapiro-Wilk test. Significance was set at P < 0.05, and all analyses were carried out with SAS v 9.4. Data are reported as means ± SEM.
Results
Body weight
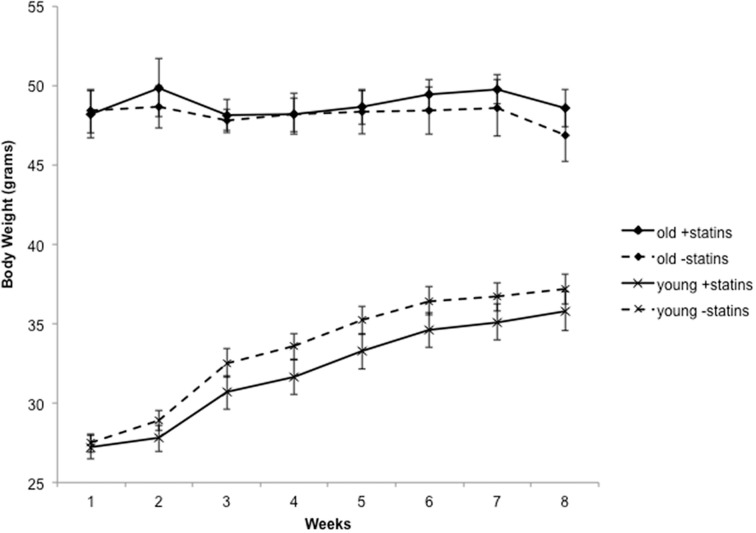
Following 8 wk of atorvastatin treatment, there was no difference in body weight between atorvastatin treatment groups and the control groups (P = 0.50). The only differences in body weight were observed between the young and older mice, with older mice having significantly higher body weight (Figure 1, P < 0.01).
FIGURE 1.
Effect of atorvastatin on weekly body weight in male C57BL/6 mice aged 4 mo and 20 mo, analyzed by repeated measures ANOVA. Data presented as means ± SEM. N = 8 animals per group. Significant difference between ages (P < 0.01) but no significant difference by statin treatment.
Tissue concentrations of phylloquinone
Older mice had significantly higher total phylloquinone concentration in liver, but there was no significant difference in total phylloquinone concentrations between age groups in kidney and intestine (Table 1). Although older mice had an approximately 40% higher concentration of hepatic total phylloquinone, they had a 50% lower percentage of labeled phylloquinone in liver, and an approximately 30% lower percentage of labeled phylloquinone in intestine when compared to young mice (P < 0.01). There were no differences observed in percentage of labeled phylloquinone in the kidney between young and old mice.
TABLE 1.
Effect of age on tissue concentrations of total (pmol/g) and labeled phylloquinone (%) in young (4 mo) and old (20 mo) C57BL/6 male mice1
| Total phylloquinone, pmol/g | Labeled phylloquinone, % | |||||
|---|---|---|---|---|---|---|
| Tissue | Young | Old | P 2 | Young | Old | P 2 |
| Kidney | 14.1 ± 2.33 | 17.4 ± 1.97 | 0.30 | 78.4 ± 6.48 | 70.5 ± 3.76 | 0.31 |
| Liver | 16.4 ± 1.44 | 25.0 ± 2.42 | 0.005 | 48.1 ± 5.00 | 21.3 ± 4.83 | <0.001 |
| Intestine | 18.1 ± 4.76 | 9.78 ± 2.11 | 0.11 | 85.9 ± 6.37 | 61.4 ± 6.23 | 0.01 |
| Brain3 | ND | ND | ND | ND | ||
1Data presented as mean ± SEM, n = 16 per group.
2Significances at P < 0.05.
3ND, not detectable; concentration was below lower limit of detection for PK and MK4 (Lower Limit of Detection = 0.01 pmol/g).
Tissue concentrations of menaquinone-4
Total MK4 and percentage of deuterium-labeled MK4 were reduced by approximately 40% in the kidney of atorvastatin-treated mice (Table 2). There was no effect of atorvastatin treatment on total MK4 (all P values > 0.31), or percentage of deuterium-labeled MK4 (all P values > 0.78) in brain or intestine. There was no MK4 detected in the liver in any group. The phylloquinone and MK4 composition of each tissue by experimental group is presented in Supplemental Figure 3.
TABLE 2.
Effect of atorvastatin on tissue concentrations of total (pmol/g) and labeled MK4 (%) in C57BL/6 male mice1
| Total MK4, pmol/g | Labeled MK4, % | |||||
|---|---|---|---|---|---|---|
| Tissue | Statin + | Statin − | P 2 | Statin + | Statin − | P 2 |
| Kidney | 13.8 ± 2.65 | 23.4 ± 3.95 | 0.05 | 20.7 ± 3.8 | 35.7 ± 3.2 | 0.008 |
| Liver3 | ND | ND | ND | ND | ||
| Intestine | 2.88 ± 0.81 | 3.62 ± 0.74 | 0.51 | 22.9 ± 5.6 | 24.7 ± 3.5 | 0.78 |
| Brain | 13.9 ± 1.49 | 19.7 ± 1.75 | 0.31 | 19.8 ± 3.5 | 19.7 ± 1.8 | 0.96 |
1Data presented as mean ± SEM, n = 16 per group.
2Significances at P < 0.05.
3ND, not detectable; concentration was below lower limit of detection for PK and MK4 (Lower Limit of Detection = 0.01 pmol/g).
Circulating cholesterol and triglycerides
After 8 wk, plasma total cholesterol was not significantly different in the atorvastatin-treated groups (Supplemental Figure 4, all P values > 0.52). Plasma triglycerides were reduced by approximately 20% in young mice treated with atorvastatin (P = 0.004). There was no effect of atorvastatin treatment on circulating triglycerides in older mice (Supplemental Figure 5, P = 0.67).
Tissue concentrations of cholesterol
Liver, kidney, brain, and intestine total cholesterol concentrations were not significantly different in the atorvastatin-treated groups (Supplemental Table 5). Older mice had lower liver cholesterol concentrations (Supplemental Table 5, P = 0.01) but higher intestinal cholesterol concentrations compared to young mice (Supplemental Table 5, P = 0.003).
Gene expression
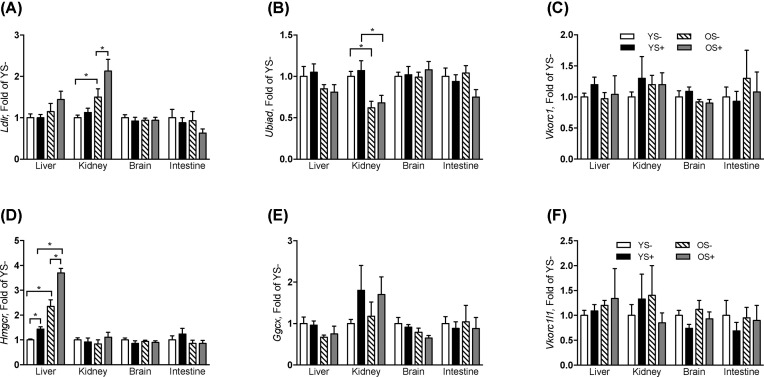
Because statins exert their pharmacological effect by inhibiting HMG-CoA reductase in the liver, we evaluated the effect of atorvastatin treatment on gene expression of hmgcr and ldlr, genes directly involved in cholesterol synthesis and recycling. In all tissues, there was no effect of age on hmgcr expression, except in the liver where hmgcr was significantly (P < 0.05) increased by ∼2-fold in older mice. Atorvastatin increased expression of hmgcr in liver compared to those without atorvastatin treatment (Figure 2). There was no effect of atorvastatin on hmgcr expression in any other tissue.
FIGURE 2.
Gene expression of ldlr (A), ubiad(B), vkorc1 (C), hmgcr (D), ggcx (E), and vkorc1l1 (F) in tissues from C57BL/6 male mice aged 4 mo (Young, Y) and 20 mo (Old, O) fed a phylloquinone control diet with or without atorvastatin (Statin, S) for 8 wk. Data are displayed as expression relative to the young control diet group and are presented as mean ± SEM, n = 8. Bars represent 2-group comparisons of interest with endpoints of each bar indicating the 2 groups being compared. Asterisk above a bar denotes significant difference at P < 0.05. vkorc1, vitamin K epoxide reductase complex 1; vkorc1l1, vitamin K epoxide reductase complex 1 like 1; ggcx, gamma glutamyl carboxylase; ubiad, ubiA domain containing protein 1; hmgcr: HMG-CoA reductase; ldlr: low density lipoprotein receptor.
Ldlr expression in kidney was higher in older mice receiving atorvastatin compared to older mice not receiving atorvastatin. Older mice not treated with atorvastatin had higher ldlr expression in kidney compared to young mice not treated with atorvastatin. There was no effect of age or atorvastatin treatment on ldlr expression in any other tissue. There was no effect of statin treatment or age on expression of vkorc1, vkorc1l1, or ggcx, in any tissue (liver, kidney, brain, or intestine). Older mice had lower expression of ubiad in kidney compared to younger mice. However, there was no effect of statin treatment on expression of ubiad in kidney.
Discussion
We evaluated the effect of atorvastatin treatment on MK4 production in young and old male mice to test the hypothesis that tissue-specific MK4 synthesis and cholesterol metabolism are linked. The conversion of phylloquinone to MK4 is proposed to be a multistep process involving side chain cleavage from dietary phylloquinone, and prenylation of the naphthoquinone ring to produce MK4 by the suggested prenyltransferase UBIAD (12, 20–22). In this study, atorvastatin significantly reduced both total and deuterium-labeled concentrations of MK4 in kidneys of both young and old mice. In other tissues that are capable of converting phylloquinone to MK4, including brain and intestine, we saw no effect of atorvastatin treatment on MK4. Our finding that atorvastatin reduced MK4 formation in the kidney may be clinically relevant as recent evidence proposes an interaction between calcification associated with chronic kidney disease and MK4 formation (23, 24). Furthermore, there is growing evidence that low vitamin K is implicated in renal disease (25, 26). The results of our study emphasize the need for future experiments designed to evaluate the role of MK4 in the kidney and/or in rodent models of chronic kidney disease.
In contrast to our findings in the kidney, there was no effect of atorvastatin treatment in the brain, where the sole form of vitamin K is MK4. Lipophilic statins including atorvastatin do not readily cross the blood-brain barrier, unless it undergoes a chemical modification of lactonization increasing its permeability (27). Therefore, the lack of an effect on MK4 is consistent with the inability of atorvastatin to enter the brain. Because the liver lacks the ability to convert dietary phylloquinone to MK4 (5, 13), it was not unexpected that MK4 was undetected in this tissue. The lack of effect of atorvastatin on the intestinal production of MK4 is less clear, as deuterium-labeled phylloquinone provided in the diet would be the primary substrate for labeled MK4. Okano et al. (28) proposed that the side chain can be removed from phylloquinone within intestinal cells and the naphthoquinone ring is then transported to a destination tissue for MK4 formation. However, our data do not support this hypothesis.
The isoprenoid intermediate in the cholesterol synthesis pathway, GGPP, is required for endogenous synthesis of MK4, as demonstrated with in vitro models (12). Current hypotheses suggest the link between cholesterol synthesis and MK4 formation to be antagonistic such that as MK4 formation increases, GGPP is depleted, which results in greater conversion of farnesyl pyrophosphate to GGPP and ultimately reduces cholesterol synthesis. In contrast, a reduction in MK4 formation may shunt more GGPP to cholesterol synthesis and potentially lead to cholesterol accumulation. It is plausible the differences in MK4 concentrations observed are attributed to decreased concentrations of GGPP as a direct result of atorvastatin, and further exacerbated by an increase in shunting of GGPP towards cholesterol synthesis to combat the atorvastatin interference. Tissue concentrations of cholesterol were quantified, and there was no difference between atorvastatin treatment groups. Cholesterol intermediates/metabolites including GGPP were not measurable, thus limiting our interpretation that GGPP shunting occurs.
The suggested role of UBIAD in the conversion of phylloquinone to MK4 is through isoprenoid side chain cleavage and/or attachment to the napthoquinone ring of phylloquinone (12). UBIAD is also implicated in cholesterol metabolism. Recently, Schumacher et al. demonstrated, in a fibroblast cell line, that UBIAD can regulate degradation of HMG-CoA reductase, but this regulation is such that non-steroid isoprenoid intracellular concentrations remain stable in a cholesterol-replete state (29). This mechanism has been implicated in the condition termed Schnyder corneal dystrophy, which is caused by a mutation in the ubiad gene resulting in abnormal serum lipids and corneal cholesterol deposition (30–32). In comparison, we found that inhibiting HMG-CoA reductase with atorvastatin decreased the non-steroid isoprenoid MK4 in the kidney, in the absence of an effect on ubiad gene expression. Schumacher et al. did not measure MK4 and we did not measure other non-steroid isoprenoid metabolites. However, our findings when combined with those of Schumacher et al. (29) suggest that future studies are needed to clarify the role of UBIAD in cholesterol and vitamin K metabolism in specific tissues and the potentially related clinical conditions.
The isolated effect of atorvastatin on kidney MK4 concentrations may be related to the low dose of statin provided in the diet, the tissue-specific rate of conversion from phylloquinone to MK4 in the different tissues, or a tissue-specific response to statins. It was recently reported that specific lipophilic statins, such as that used in this study, were more likely to bind directly to UBIAD, altering enzyme functionality, compared to aqueous statins (12). However, the magnitude of these effects in vivo and potential regulatory mechanisms are unknown. Additional information regarding UBIAD enzyme activity and isoprenoid substrate availability is necessary to determine the contribution of cholesterol synthesis in the conversion of dietary phylloquinone to MK4.
Strengths of this study include the use of a mouse model that has previously been optimized for dietary phylloquinone interventions and well-characterized methodology with deuterium-labeled phylloquinone. In addition, we studied older and younger mice, consistent with current recommendations of the National Institutes of Health to use aging animal models. Finally, we were able to induce changes in MK4 formation without deleterious effects of statin treatment, such as hepatic toxicity (33). However, we were unable to directly measure isoprenoid precursors in tissues, which is a limitation. The effect of atorvastatin treatment on plasma and tissue cholesterol and on liver gene expression of hmgcr was minimal, and may be related to the low dose of atorvastatin in the diet. Statins manipulate LDL cholesterol and have little to no effect on HDL cholesterol, the major component of circulating plasma lipoproteins in mice, which may explain our observations and also explain why statins are less effective in reducing total cholesterol in rodent models compared to humans (34, 35). In addition, plasma lipid levels were in the normal range and thus likely to be unaffected by statin treatment. The animals were not deprived of food so it is plausible that the higher intestinal cholesterol concentrations reflected differences in this non-food-deprived state. We measured gene expression, which may not correlate with protein levels or enzyme activity. Because we limited our study to males, generalizability to females is uncertain. Sex-specific differences in mice have been identified previously (13). Because this was the first study to evaluate the effect of statin treatment on vitamin K metabolism in vivo, we selected genes known to be involved in cholesterol metabolism. Future studies are needed to better characterize the role of MK4 in kidney by evaluating transcriptional and translational mechanisms in kidney metabolism, as well as newly emerging pathways in cholesterol metabolism (29).
In summary, we found that atorvastatin can interfere with MK4 formation in the kidney. The dynamic regulation of endogenous cholesterol synthesis and lack of a demonstrated effect on cholesterol metabolism through the use of atorvastatin suggests that isoprenoid intermediates may be shunted away from MK4 formation to maintain cellular cholesterol concentrations when in a deplete state. However, the effect on tissue physiology and function remains unclear as the role of MK4 in kidney has yet to be determined. The clinical relevance of vitamin K forms, their function, and the conversion of phylloquinone to MK4 in different tissues is also not yet determined. Therefore, future studies are necessary to advance our understanding of vitamin K metabolism in the kidney and other tissues.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SGH, MKS, SLB, and DS: designed the research; MKS, SGH, XF, and DS: conducted the research; SGH: analyzed data; SGH, MKS, and SLB: wrote the paper; MAG, SL-F, AK, and ASG: reviewed the data, aided in interpretation of results, and reviewed the manuscript; SLB: primary responsibility for final content; and all authors: read and approved the final paper.
Notes
Supported by the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, NIH/NIDDK grant T32 DK062032, NIH/NIAMS grant K01 AR063176, and the USDA Human Nutrition Research Center on Aging Gerald Cassidy Student Innovation Award.
Author disclosures: SGH, MKS, XF, MAG, DS, SL-F, AK, AG, and SLB, no conflicts of interest.
Supplemental Tables 1–5 and Supplemental Figures 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: GGCX, gamma glutamyl carboxylase (ggcx); GGPP, geranylgeranyl pyrophosphate; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; LDLr, LDL receptor (ldlr); MK4, menaquinone-4; UBIAD, UbiA prenyl transferase domain-containing 1 (ubiad); VKORC1, vitamin K epoxide reductase complex subunit 1 (vkorc1); VKORC1L1, vitamin K epoxide reductase complex subunit 1 like 1 (vkorc1l1).
References
- 1. Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55:345–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suttie JW, Booth SL. Vitamin K. Adv Nutr. 2011;2:440–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013;4:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thijssen HHW, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996;75:121. [DOI] [PubMed] [Google Scholar]
- 5. Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr. 2012;142:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshida T, Miyazawa K, Kasuga I, Yokoyama T, Minemura K, Ustumi K, Aoshima M, Ohyashiki K. Apoptosis induction of vitamin K2 in lung carcinoma cell lines: the possibility of vitamin K2 therapy for lung cancer. Int J Oncol. 2003;23:627–32. [PubMed] [Google Scholar]
- 8. Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M et al.. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278:43919–27. [DOI] [PubMed] [Google Scholar]
- 9. Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Vitamin K2 induces phosphorylation of protein kinase A and expression of novel target genes in osteoblastic cells. J Mol Endocrinol. 2007;39:239–47. [DOI] [PubMed] [Google Scholar]
- 10. Nickerson ML, Bosley AD, Weiss JS, Kostiha BN, Hirota Y, Brandt W, Esposito D, Kinoshita S, Wessjohann L, Morham SG et al.. The UBIAD1 prenyltransferase links menaquinone-4 synthesis to cholesterol metabolic enzymes. Hum Mutat. 2013;34:317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuipers HF, van den Elsen PJ. Immunomodulation by statins: inhibition of cholesterol vs. isoprenoid biosynthesis. Biomed Pharmacother. 2007;61:400–7. [DOI] [PubMed] [Google Scholar]
- 12. Hirota Y, Nakagawa K, Sawada N, Okuda N, Suhara Y, Uchino Y, Kimoto T, Funahashi N, Kamao M, Tsugawa N et al.. Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. PLoS One. 2015;10:e0125737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harshman SG, Fu X, Karl JP, Barger K, Lamon-Fava S, Kuliopulos A, Greenberg AS, Smith D, Shen X, Booth SL. Tissue concentrations of vitamin K and expression of key enzymes of vitamin K metabolism are influenced by sex and diet but not housing in C57BL/6 mice. J Nutr. 2016;146:1521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE et al.. Commentary geroscience : linking aging to chronic disease. Cell. 2014;159:709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706.LP-1721. [DOI] [PubMed] [Google Scholar]
- 16. Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–60. [DOI] [PubMed] [Google Scholar]
- 17. Dolnikowski GG, Sun Z, Grusak MA, Peterson JW, Booth SL. HPLC and GC/MS determination of deuterated vitamin K (phylloquinone) in human serum after ingestion of deuterium-labeled broccoli. J Nutr Biochem. 2002;13:168–74. [DOI] [PubMed] [Google Scholar]
- 18. Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem. 2009;81:5421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henneman L, van Cruchten AG, Kulik W, Waterham HR. Inhibition of the isoprenoid biosynthesis pathway: detection of intermediates by UPLC-MS/MS. Biochim Biophys Acta. 2011;1811:227–33. [DOI] [PubMed] [Google Scholar]
- 20. Cheng W, Li W. Structural insights into ubiquinone biosynthesis in membranes. Science. 2014;343:878–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suvarna K, Stevenson D, Meganathan R, Hudspeth ME. Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA gene from Escherichia coli. J Bacteriol. 1998;180:2782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakagawa K, Sawada N, Hirota Y, Uchino Y, Suhara Y, Hasegawa T, Amizuka N, Okamoto T, Tsugawa N, Kamao M et al.. Vitamin K2 biosynthetic enzyme, UBIAD1 is essential for embryonic development of mice. PLoS One. 2014;9:e104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Z, Qureshi AR, Parini P, Hurt-Camejo E, Ripsweden J, Brismar TB, Barany P, Jaminon AM, Schurgers LJ, Heimbürger O et al.. Does statins promote vascular calcification in chronic kidney disease?. Eur J Clin Invest. 2017;47:137–48. [DOI] [PubMed] [Google Scholar]
- 24. McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, Holden RM. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835–44. [DOI] [PubMed] [Google Scholar]
- 25. McCabe KM, Adams MA, Holden RM. Vitamin K status in chronic kidney disease. Nutrients. 2013;5:4390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCabe KM, Booth SL, Fu X, Ward E, Adams MA, Holden RM. Vitamin K metabolism in a rat model of chronic kidney disease. Am J Nephrol. 2017;45:4–13. [DOI] [PubMed] [Google Scholar]
- 27. Wood WG, Eckert GP, Igbavboa U, Müller WE. Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci. 2010;1199:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okano T, Nakagawa K, Kamao M. In vivo metabolism of vitamin K: in relation to the conversion of vitamin K1 to MK-4. Clin Calcium. 2009;19:1779–87. [PubMed] [Google Scholar]
- 29. Schumacher MM, Jun D-J, Johnson BM, DeBose-Boyd RA. UbiA prenyltransferase domain-containing protein-1 modulates HMG-CoA reductase degradation to coordinate synthesis of sterol and nonsterol isoprenoids. J Biol Chem. 2018;293:312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin BR, Frausto RF, Vo RC, Chiu SY, Chen JL, Aldave AJ. Identification of the first de novo UBIAD1 gene mutation associated with Schnyder corneal dystrophy. J Ophthalmol. 2016;2016:1968493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orr A, Dubé M-P, Marcadier J, Jiang H, Federico A, George S, Seamone C, Andrews D, Dubord P, Holland S et al.. Mutations in the UBIAD1 gene, encoding a potential prenyltransferase, are causal for Schnyder crystalline corneal dystrophy. PLoS One. 2007;2:e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss JS, Kruth HS, Kuivaniemi H, Tromp G, White PS, Winters RS, Lisch W, Henn W, Denninger E, Krause M et al.. Mutations in the UBIAD1 gene on chromosome short arm 1, region 36, cause Schnyder crystalline corneal dystrophy. Investig Opthalmology Vis Sci. 2007;48:5007. [DOI] [PubMed] [Google Scholar]
- 33. Parker RA, Garcia R, Ryan CS, Liu X, Shipkova P, Livanov V, Patel P, Ho SP. Bile acid and sterol metabolism with combined HMG-CoA reductase and PCSK9 suppression. J Lipid Res. 2013;54:2400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roglans N, Verd JC, Peris C, Alegret M, Vázquez M, Adzet T, Diaz C, Hernández G, Laguna JC, Sánchez RM. High doses of atorvastatin and simvastatin induce key enzymes involved in VLDL production. Lipids. 2002;37:445–54. [DOI] [PubMed] [Google Scholar]
- 35. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J et al.. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.