ABSTRACT
Background
Novel oils high in monounsaturated fatty acids (MUFAs) and low in saturated fatty acids (SFAs) are an alternative to partially hydrogenated oils high in trans-unsaturated fatty acids. There is widespread use of high-MUFA oils across the food industry; however, limited knowledge of their cardiovascular impact exists.
Objectives
We investigated the effects of diets containing canola oil, high-oleic acid canola oil (HOCO), and a control oil blend (diet formulated to emulate a Western fat profile) on lipids, lipoproteins, and apolipoproteins (apos), as secondary outcomes of the trial.
Methods
In a multi-center, double-blind, randomized, 3-period crossover, controlled feeding trial, men (n = 44) and women (n = 75) with a mean age of 44 y, mean body mass index (BMI; in kg/m2) of 31.7, and an increased waist circumference plus ≥1 metabolic syndrome criteria consumed prepared, weight-maintenance diets containing canola oil [17.5% MUFAs, 9.2% polyunsaturated fatty acids (PUFAs), 6.6% SFAs], HOCO (19.1% MUFAs, 7.0% PUFAs, 6.4% SFAs), or control oil (10.5% MUFAs, 10.0% PUFAs, 12.3% SFAs) for 6 wk with ≥4-wk washouts. Fasting serum lipids were assessed at baseline and 6 wk. Diet effects were examined using a repeated measures mixed model.
Results
Compared with the control, canola and HOCO diets resulted in lower endpoint total cholesterol (TC; −4.2% and −3.4%; P < 0.0001), LDL cholesterol (−6.6% and −5.6%; P < 0.0001), apoB (−3.7% and −3.4%; P = 0.002), and non-HDL cholesterol (−4.5% and −4.0%; P = 0.001), with no differences between canola diets. The TC:HDL cholesterol and apoB:apoA1 ratios were lower after the HOCO diet than after the control diet (−3.7% and −3.4%, respectively). There were no diet effects on triglyceride, HDL cholesterol, or apoA1 concentrations.
Conclusions
HOCO, with increased MUFAs at the expense of decreased PUFAs, elicited beneficial effects on lipids and lipoproteins comparable to conventional canola oil and consistent with reduced cardiovascular disease risk in adults with central adiposity. This trial was registered at www.clinicaltrials.gov as NCT02029833.
Keywords: apolipoproteins, canola oil, cardiovascular disease risk, dietary fatty acids, dietary intervention, high-oleic acid canola oil, lipids, lipoproteins, metabolic syndrome, Western diet
Introduction
Reduction of dietary SFAs and replacement with unsaturated fatty acids in the context of a healthy diet represents a cornerstone of nutritional recommendations for the prevention and treatment of cardiovascular disease (CVD) (1–4). Canola oil is a commonly consumed vegetable oil that is low in SFAs, moderate in PUFAs, and rich in MUFAs (62% oleic acid) (5), with numerous cardioprotective benefits (6, 7). Canola oil is also available in a high-oleic acid variety (HOCO; 71% oleic acid) that is equivalent in SFAs and proportionally lower in PUFAs (8).
The development of HOCO and incorporation into the food supply was spurred, in part, by the recognition of the adverse cardiovascular health effects of industrially produced trans-unsaturated fatty acids (TFAs) from partially hydrogenated vegetable oils (PHVOs) (9). High-MUFA oils are a reasonable substitute for TFA-containing fats and oils given their favorable fatty acid profiles that are consistent with dietary guidance and their ability to achieve or exceed the functional characteristics of PHVOs (i.e., oxidative stability, shelf life, fry life, neutral flavor) (10–12). Food applications for HOCO include replacing margarine and shortening in commercial baked goods and frying oil for restaurant deep-frying and commercial frying of packaged snacks and chips (10, 12). These foods are primary energy sources among US adults (13). Given the FDA's required removal of added TFAs (14), high-MUFA oils are becoming the “new standard” of oil across the food industry and, thus, intake is likely to become pervasive.
Research involving the cardiovascular health impact of HOCO on atherogenic biomarkers is scarce (15, 16). Of particular concern is that widespread consumption of higher-MUFA, lower-PUFA oils will decrease the intake of total PUFAs, the preferred class of unsaturated fatty acids to replace SFAs in the context of a healthy diet for cardioprotection (17). Although conventional canola oil has beneficial effects on CVD lipid and lipoprotein biomarkers (6), we cannot assume that increased MUFAs at the expense of decreased PUFAs in HOCO will elicit identical impacts. We previously investigated the effects of consuming oils with differing unsaturated fat profiles, including HOCO and regular canola oil, in individuals with metabolic syndrome (MetS) criteria in the Canola Oil Multi-center Intervention Trial I (COMIT I), the trial preceding the project herein. The canola and HOCO treatments did not differ in endpoint lipids, lipoproteins, or apos following 4 wk of feeding (16). COMIT II was conducted to address additional knowledge gaps of the effects of HOCO on novel and established CVD risk markers.
The objective of the present study was to examine the effects of diets containing conventional canola oil and HOCO on lipids, lipoproteins, and apos compared to a control diet with a fatty acid composition characteristic of a Western diet in individuals with MetS risk factors. We hypothesized the lipid, lipoprotein, and apo response would be similar between the two canola diets, with greater benefit relative to the Western diet. This article presents the first systematic assessment of the shift in fatty acids in reformulated canola oil compared with conventional canola oil, as well as a Western diet fat profile.
Methods
Participants
Males and females (aged 20–65 y) with MetS risk factors were eligible for the study. Risk for MetS was defined as an increased waist circumference (International Diabetes Federation cut points: men ≥94 cm, women ≥80 cm) plus at least one of the following secondary inclusion criteria: elevated fasting blood glucose (≥5.6 mmol/L), TG (≥1.7 mmol/L), systolic blood pressure (≥130 mmHg), diastolic blood pressure (≥85 mmHg); and/or decreased high-density lipoprotein cholesterol (HDL cholesterol; men <1 mmol/L, women <1.3 mmol/L). Exclusion criteria included: smokers; consumption of >14 alcoholic beverages per week; use of prescription lipid-modifying medications in the last 3 mo or chronic anti-inflammatory medications; kidney disease, liver disease, diabetes, or uncontrolled thyroid disease; and pregnant or lactating women.
Study design
COMIT II was a double-blind, randomized, controlled feeding, crossover, clinical trial that consisted of three, 6-wk feeding periods separated by ≥4-wk washout periods. The trial was conducted from 2014–2016 at 4 research centers in North America [Richardson Center for Functional Foods and Nutraceuticals, University of Manitoba (RCFFN); Institute of Nutrition and Functional Foods, Laval University (INAF); Canadian Center for Agri-Food Research in Health and Medicine, St. Boniface Hospital Albrechtsen Research Center (SBRC); Departments of Nutritional Sciences and Biobehavioral Health, The Pennsylvania State University]. The respective centers’ ethics review boards approved the COMIT II protocol and related documents, and the procedures followed were in accordance with the Declaration of Helsinki as revised in 1983. All participants provided written informed consent at screening prior to enrollment. Randomization.com was used to generate the random allocation sequence, with 6 possible sequences and an allocation ratio of 1:1:1:1:1:1. The sequences were assigned to each participant in the prespecified order as he or she was enrolled in the trial by the study coordinators. This trial was registered at www.clinicaltrials.gov as NCT02029833.
In COMIT I, the feeding periods were 4 wk in length (18), however, a 6-wk feeding period was chosen for COMIT II to allow assessment of the effects of prolonged intervention on CVD risk markers. Although lipids are responsive to dietary intervention by 14 d, 6 wk allowed the participants to reach a steady state of lipid concentrations (19) and also accommodated assessment of other outcomes that require longer duration for measurable change (i.e., body composition, vascular measures). A break of a minimum of 4 wk between diet periods was selected for compliance purposes and to reduce participant scheduling burden; this also ensured sufficient washout of the prior diet effects.
Controlled diets and oil interventions
During the feeding periods, participants were provided with an isocaloric, healthy, weight-maintenance base diet with one of the following oils: canola oil (Canola Harvest 100% Canola Oil, Richardson International), HOCO (Canola Harvest High Oleic Low Linolenic Canola Oil, Richardson International, Canada), or control oil [blend of ghee (Verka), safflower oil (eSutras), coconut oil (eSutras), and flaxseed oil (Shape Foods)]. The conventional canola oil and HOCO contained approximately 60% and 70% oleic acid, respectively. HOCO is a specialty canola cultivar that was developed through traditional plant breeding (11) to selectively reduce the total PUFA content, namely linoleic and α-linolenic acids, resulting in a higher oleic acid and proportionately lower PUFA content compared with conventional canola. The oil blend in the control diet was approximately 49% ghee, 29% safflower oil, 14% flaxseed oil, and 8% coconut oil, and was designed so that when it was added to the base diet the overall fat profile approximated the average fatty acid profile of a contemporary Western-style diet. The most recent estimate of average intake among US adults (NHANES 2015–2016) for SFAs, MUFAs, and PUFAs is 12%, 12%, and 8% of total energy, respectively (20).
The 3 experimental diets were identical in percentage of energy from macronutrients, but differed in fatty acid composition due to the presence of the intervention oils (Table 1). The kitchen staff at each site prepared breakfast, lunch, dinner, and snacks for the participants, adhering to a 7-d rotating menu. The diets were calorie controlled for weight maintenance, calculated using the Harris Benedict Formula, and monitored by daily weighing at each participating center prior to food pick-up. If a participant exhibited weight change during the first 2 wk of diet period 1, the caloric content was adjusted appropriately by switching to a higher or a lower calorie menu (menus were available in 300 kcal increments). The canola experimental diets were higher in MUFAs and lower in SFAs compared with the control diet.
TABLE 1.
Macronutrient composition of the 3 experimental diets containing the oils1
| Canola oil diet | HOCO diet | Control oil diet | |
|---|---|---|---|
| Protein | 15.87 | 15.87 | 15.71 |
| Carbohydrate | 50.79 | 50.79 | 50.75 |
| Fat | 35.26 | 35.26 | 35.21 |
| MUFA | 17.45 | 19.11 | 10.50 |
| Oleic acid | 15.55 | 17.86 | 5.92 |
| PUFA | 9.21 | 7.02 | 9.96 |
| α-Linolenic acid | 2.10 | 0.76 | 1.73 |
| Linoleic acid | 6.42 | 5.56 | 7.28 |
| SFA | 6.56 | 6.43 | 12.26 |
1The average macronutrient composition from the 7-d rotating menu, estimated at 3000 kcal, using Food Processor Nutrition Analysis Software (ESHA Research). Nutrients are presented as percentage of total energy. HOCO, high-oleic acid canola oil.
The oils were incorporated into a smoothie containing frozen strawberries, orange sherbet, and skim milk, which was divided into 2 portions and consumed daily in the morning and evening to avoid gastrointestinal distress from the fat load. The total volume of the smoothie and relative proportion of the ingredients (non-oil ingredients 1:1:1) was adjusted to participants’ caloric needs. For example, for a 3,000 kcal/d diet, the smoothie contained 60 g oil, 200 g skim milk, 200 g strawberries, and 200 g orange sherbet. The intervention oils provided approximately 18% of total energy for all levels of caloric intake.
Participants were instructed to consume all foods provided and to avoid consumption of extraneous food items and calorie-containing beverages. Measures to optimize compliance have been described previously (16). All study personnel and participants were blinded to the diets, with the exception of the kitchen staff responsible for smoothie preparation.
Sample collection and analyses
Participants underwent various clinical tests on 2 consecutive days at baseline (days 1 and 2) and endpoint (days 41 and 42) of each diet period, and the mean values were calculated for all parameters. Anthropometric measures included weight, height, and waist circumference, and clinical procedures included seated blood pressure, DXA scans, and fasting blood draw. All blood draws followed 12 h without food or drink except water and 48 h without alcohol. Blood was allowed to clot, separated by centrifugation, aliquoted into microtubes, and stored at −80°C. Frozen serum samples were shipped on dry ice to St. Michael's Hospital (Toronto, ON, Canada), the central laboratory for multi-site analyses of lipids, lipoproteins, and apos.
The endpoints of interest were total cholesterol (TC), TG, LDL cholesterol, HDL cholesterol, non-HDL cholesterol, apolipoprotein A1 (apoA1), apolipoprotein B (apoB), and the TC:HDL cholesterol and apoB:apoA1 ratios. TC, TG, and HDL cholesterol were quantitatively determined by an enzymatic, colorimetric method on a Roche/Hitachi cobas c 501 analyzer (Roche Diagnostics). LDL cholesterol was estimated according to the Friedewald equation (21). However, for 4 time point samples, due to high serum TG concentrations (>4.52 mmol/L), LDL cholesterol was not calculated and recorded as a missing value. Non-HDL cholesterol was calculated as TC – HDL cholesterol. ApoA1 and apoB were quantitatively determined by endpoint nephelometry on a BN ProSpec nephelometer (Siemens). The TC: HDL cholesterol and apoB: apoA1 ratios were calculated from original values.
Statistical methods
The primary outcome of COMIT II was body composition with supplementary measurement of visceral adiposity measured by DXA (www.clinicaltrials.gov NCT02029833). Outcomes reported herein are secondary outcomes. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc.). The primary analysis was endpoint-to-endpoint comparison (mean of days 41 and 42) of lipids, lipoproteins, and apos across the 3 diets. A secondary analysis was performed to assess absolute change from baseline within each diet. Data were analyzed per protocol to assess the efficacy of the diet response and missing data were not imputed. Participants with a weight change of >5% during any diet were removed from the analyses to eliminate the confounding effects of substantial weight change on the outcomes. All values for the primary and secondary analyses are presented as least squares mean ± SEMs and P ≤ 0.05 was considered significant.
The effects of the diets on the lipid, lipoprotein, and apo outcomes were assessed using a repeated measures mixed model (proc mixed), with subject, diet sequence, and study center as random effects and time as the repeated effect. Factors assessed in the model include diet (canola oil diet, HOCO diet, control oil diet), time (diet period 1, 2, 3), sex (male, female), center (RCFFN, INAF, SBRC, The Pennsylvania State University), and diet sequence, and the following interactions: diet-by-time, diet-by-sex, diet-by-center, and diet-by-sequence. The diet-specific baseline value of the dependent variable was used as a covariate. Final models included diet and only significant terms. Tukey–Kramer adjusted P values were used for multiple pairwise comparisons between diets, only when there was a significant main effect of diet. Normality of the residuals from the final models was assessed and nonnormal dependent variables were log transformed. Within-diet changes from baseline were assessed by the least squares means P values from the final mixed model output. The effect of the diets on DXA-measured weight (both endpoint and change from baseline) was assessed as described above.
The COMIT II sample size was calculated according to the primary outcome, body composition, and a sample size of 140 was determined to detect a 55 g change in android fat mass using the variance parameter in android fat mass from the COMIT I trial (22), and assuming a 20% dropout rate. For analysis of the secondary outcomes, a sample size of 119 offered 97% power to detect a difference of 10% in LDL cholesterol between diets, with α = 0.017.
Results
Baseline characteristics
The flow of participants through COMIT II and inclusion for the lipid and lipoprotein analyses are depicted in Figure 1. One hundred and twenty-five participants completed the study, with a dropout rate of ∼28%. Participants who had a weight change of >5% during any diet period were removed (n = 6). Table 2 presents the baseline characteristics (diet period 1, days 1 and 2) of the remaining COMIT II participants included in the analyses (n = 119). Participants were predominately female and middle-aged with class I obesity (BMI 30.0–34.9 kg/m2). Approximately 38% of the participants met the clinical criteria for a MetS diagnosis at baseline (i.e., at least 3 risk factors). The individual MetS criteria of TG, HDL cholesterol, glucose, and blood pressure were on average within healthy ranges at baseline, suggesting one individual criterion did not drive study enrollment. Among the 119 participants enrolled who finished the trial, the percentage with elevated blood glucose, hypertriglyceridemia, reduced HDL cholesterol, or hypertension was 23%, 35%, 41%, and 33%, respectively.
FIGURE 1.
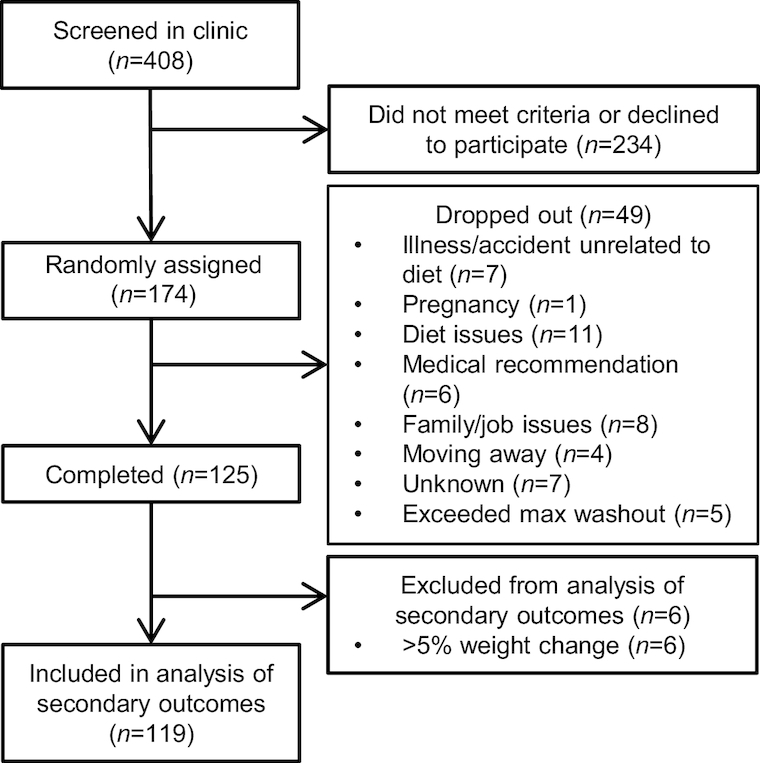
Flow diagram of the COMIT II participants for inclusion in the lipid and lipoprotein outcome analyses. COMIT, canola oil multicenter intervention trial; max, maximum.
TABLE 2.
Baseline characteristics of the COMIT II participants1
| Variable | Value2 |
|---|---|
| Sex | |
| Female | 75 (63%) |
| Male | 44 (37%) |
| Anthropometric measures | |
| Age, y | 44 ± 13 (22–65) |
| Weight, kg3 | 91.3 ± 18.7 (60.4–146.4) |
| BMI, kg/m2 | 31.7 ± 5.3 (22.6–52.6) |
| MetS criteria | |
| Waist circumference, cm | 105 ± 13 (80–151) |
| Female | 103 ± 12 (80–131) |
| Male | 109 ± 13 (94–151) |
| TGs, mmol/L4 | 1.60 ± 0.73 (0.33–3.67) |
| HDL-C, mmol/L4 | 1.33 ± 0.35 (0.67–2.49) |
| Female (n = 75) | 1.41 ± 0.35 (0.87–2.49) |
| Male (n = 43) | 1.20 ± 0.31 (0.67–1.97) |
| Glucose, mmol/L4 | 5.30 ± 0.59 (4.16–8.00) |
| Blood pressure, mmHg | |
| Systolic blood pressure | 120 ± 14 (88–164) |
| Diastolic blood pressure4 | 79 ± 11 (54–100) |
| Number of MetS criteria5 | |
| 0 | 1, 0.85% |
| 1 | 29, 24.79% |
| 2 | 43, 36.75% |
| 3 | 27, 23.08% |
| 4 | 12, 10.26% |
| 5 | 5, 4.27% |
| Additional cardiovascular disease risk biomarkers | |
| Total cholesterol, mmol/L4 | 5.17 ± 0.90 (3.38–7.36) |
| LDL-C, mmol/L4 | 3.11 ± 0.75 (1.04–5.33) |
1Values are means ± SDs (minimum–maximum) or frequency (%), n = 119. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MetS, metabolic syndrome.
2Data collected on days 1 and 2 of diet period 1. Fasting lipids, lipoproteins, and glucose were assessed in serum.
3Weight was measured using a scale at each participating center (i.e., not DXA weight).
4 n = 118 due to missing values.
5 n = 117 due to missing values. Enrolled participants met the requirements of an increased waist circumference plus one additional factor at the screening visit; values present here are from the baseline visits of diet period 1.
Weight stability
Table 3 shows mean DXA-measured body weight at baseline (days 1 or 2) and endpoint (days 41 or 42), and the absolute weight change for the COMIT II participants used in the lipid and lipoprotein analyses (n = 119). All diets modestly reduced body weight from baseline (<1 kg; P < 0.0001 for all). No differences between the 3 diets in endpoint weight or weight change were observed (P = 0.19).
TABLE 3.
DXA-measured weights at baseline, endpoint, and the changes from baseline after consumption of diets containing canola oil, HOCO, or control oil for 6 wk in adults with central adiposity plus at least one additional MetS factor1
| Diet | Baseline (kg) | Endpoint (kg) | Change (kg/6 wk) |
|---|---|---|---|
| Canola Oil | 90.03 ± 1.71 | 89.12 ± 1.68 | −0.65 ± 0.16 * |
| HOCO | 90.37 ± 1.69 | 89.46 ± 1.68 | −0.92 ± 0.15 * |
| Control Oil | 90.28 ± 1.69 | 89.41 ± 1.69 | −0.87 ± 0.16 * |
1Values are means ± SEMs, n = 119. *Different from 0, P ≤ 0.05. HOCO, high-oleic acid canola oil; MetS, metabolic syndrome.
Endpoint-to-endpoint mean comparisons
The primary analysis of endpoint-to-endpoint comparisons (mean of days 41 and 42) between the 3 diets is presented in Table 4. Compared with the control oil diet, consumption of both regular canola oil and HOCO diets resulted in lower endpoint means for TC (canola compared with control: P = < 0.0001; HOCO compared with control: P = 0.002), LDL cholesterol (canola compared with control: P = < 0.0001; HOCO compared with control: P = 0.0002), apoB (canola compared with control: P = 0.005; HOCO compared with control: P = 0.01), and non-HDL cholesterol (canola compared with control: P = 0.002; HOCO compared with control: P = 0.008). There were no significant differences between canola oil and HOCO diets for these parameters. The TC: HDL cholesterol ratio was lower following the HOCO diet compared with the control (HOCO compared with the control: P = 0.01), as well as the apoB: apoA1 ratio (HOCO compared with the control: P = 0.02; canola compared with the control: P = 0.06). There was a trend toward a diet effect on HDL cholesterol (P = 0.09); no diet effects on TG or apoA1 were observed. An effect of time was observed on TC, HDL cholesterol, LDL cholesterol, apoA1, and apoB, with no significant diet-by-time interaction for any parameters (data not shown). There was a significant diet-by-center interaction for apoB, with a higher endpoint value after HOCO at RCFFN compared with SBRC (data not shown; differences of LSM estimate = 0.09 g/L; P for interaction = 0.04).
TABLE 4.
Endpoint-to-endpoint comparisons of fasting serum lipids, lipoproteins, and apos following the consumption of diets containing canola oil, HOCO, or control oil for 6 wk in adults with central adiposity plus at least one additional MetS factor1
| Canola oil diet | HOCO diet | Control oil diet | P for diet effect | P for time effect | |
|---|---|---|---|---|---|
| TC, mmol/L | 4.54 ± 0.04 a | 4.58 ± 0.04 a | 4.74 ± 0.04 b | <0.0001 | 0.01 |
| TGs, mmol/L | 1.45 ± 0.04 | 1.44 ± 0.04 | 1.40 ± 0.04 | NS | NS |
| HDL-C, mmol/L | 1.25 ± 0.01 | 1.28 ± 0.01 | 1.26 ± 0.01 | NS | 0.005 |
| LDL-C, mmol/L | 2.64 ± 0.04 a | 2.67 ± 0.04 a | 2.83 ± 0.04 b | <0.0001 | 0.02 |
| TC:HDL-C ratio | 3.82 ± 0.04 a,b | 3.77 ± 0.04 a | 3.92 ± 0.04 b | 0.02 | NS |
| apoA1, g/L | 1.44 ± 0.01 | 1.46 ± 0.01 | 1.45 ± 0.01 | NS | 0.003 |
| apoB, g/L | 0.87 ± 0.01 a | 0.88 ± 0.01 a | 0.91 ± 0.01 b | 0.002 | 0.04 |
| apoB:apoA1 ratio | 0.619 ± 0.01 a,b | 0.616 ± 0.01 a | 0.64 ± 0.01 b | 0.01 | NS |
| Non-HDL-C, mmol/L | 3.30 ± 0.05 a | 3.31 ± 0.05 a | 3.45 ± 0.05 b | 0.001 | NS |
1Values are least squares means ± SEMs, n = 119. Labeled means in a row without a common superscript letter differ, P ≤ 0.05. A repeated measures mixed model was used to assess the effects of diet, time, sex, center, and sequence, and the interactions diet-by-time, diet-by-sex, diet-by-center, and diet-by-sequence. The diet-specific baseline value was used as a covariate. Final models included diet and only significant terms. Pairwise comparisons were assessed using the Tukey–Kramer method when there was a significant effect of diet. HOCO, high-oleic acid canola oil; MetS, metabolic syndrome; NS, P > 0.05; TC, total cholesterol.
Absolute change from baseline
The secondary analysis of change from baseline for all lipid and lipoprotein parameters within each diet is shown in Figure 2. All diets reduced TC, LDL cholesterol, non-HDL cholesterol, HDL cholesterol, apoB, and apoA1 from baseline (P < 0.0001 for all). TG (canola: P = 0.0182, HOCO: P = 0.0053, control: P = 0.0002), the TC: HDL cholesterol ratio (canola and HOCO: P < 0.0001, control: P = 0.0002), and the apoB: apoA1 ratio (canola and HOCO: P < 0.0001, control: P = 0.006) were also reduced from baseline. Differences between diets in change from baseline were similar to the endpoint comparisons, with the exception of apoB.
FIGURE 2.
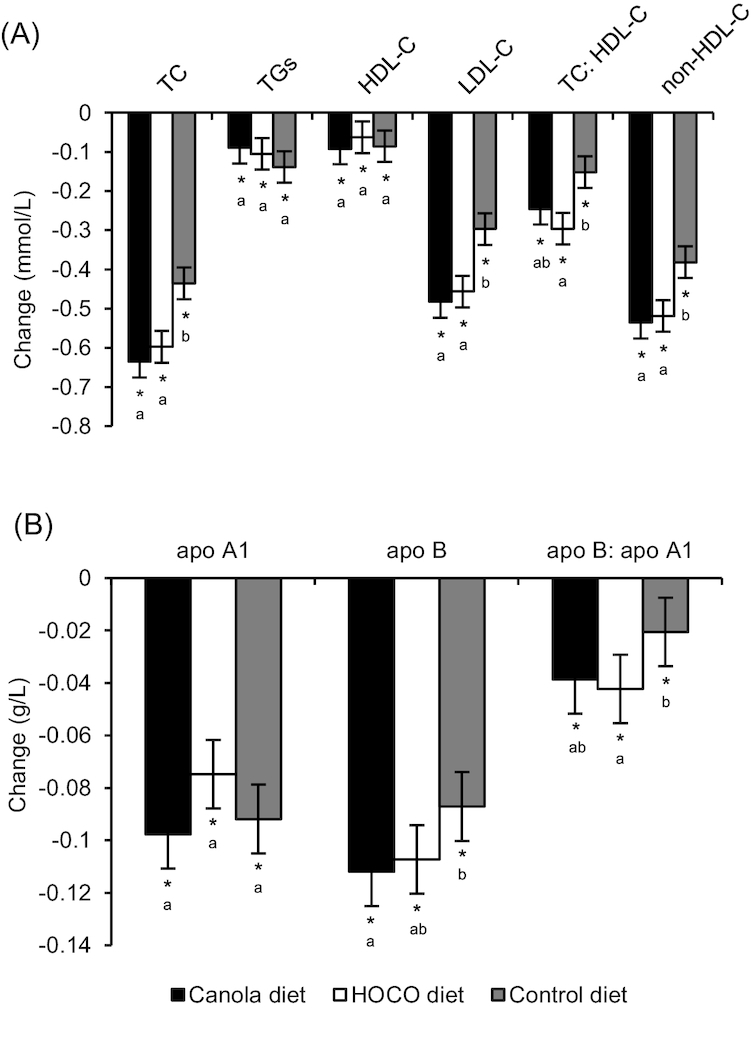
Absolute change (endpoint–baseline) in (A) lipids and lipoproteins and (B) apos following the consumption of diets containing canola oil, HOCO, and control oil for 6 wk in adults with central adiposity plus at least one additional MetS factor. Values are least squares mean ± SEM, n = 119. *Different from 0, P ≤ 0.05. Labeled means in a group without a common letter differ, P ≤ 0.05. HOCO, high-oleic acid canola oil; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MetS, metabolic syndrome; TC, total cholesterol.
Discussion
COMIT II is the first double-blind, randomized, controlled feeding, crossover study to compare the effects of diets containing conventional canola oil and HOCO against a control diet with a fatty acid composition consistent with Western intakes. Herein, we report the lipid, lipoprotein, and apo response, secondary outcomes of the COMIT II study, in participants with MetS risk factors. The principal finding is that 42 d of canola oil and HOCO consumption similarly lowered endpoint TC, LDL cholesterol, apoB, and non-HDL cholesterol, and to a greater magnitude than the Western diet control oil. Further, the TC: HDL cholesterol and apoB: apoA1 ratios were reduced after HOCO compared with the control. These data indicate that HOCO, with increased MUFAs at the expense of decreased PUFAs, elicited beneficial effects on atherogenic lipids and lipoproteins comparable to canola oil and consistent with CVD risk reduction.
High-oleic oils are being increasingly incorporated into the food supply to replace PHVOs high in TFAs (10, 12), although the health effects of the widespread consumption of high-oleic oils remain unclear. Investigation into the clinical cardiovascular impact of HOCO is necessary to identify any unfavorable effects of this novel oil on cardiovascular biomarkers. Only 2 clinical trials to date have assessed the effects of HOCO on lipid and lipoprotein endpoints (15, 16), the primary biomarker targets for atherosclerotic CVD risk reduction (23). A previous study from our group, COMIT I, assessed the effects of controlled feeding of 5 dietary oils that varied in unsaturated fatty acid compositions, including canola oil and HOCO, on lipids and lipoproteins in individuals at risk of or with MetS (n = 130) (16). Endpoint values following 28 d of canola oil and HOCO in COMIT I were 4.81 ± 0.14 and 4.77 ± 0.14 mmol/L for TC, and 2.91 ± 0.08 and 2.86 ± 0.08 mmol/L for LDL cholesterol, respectively. Herein, we report numerically lower TC and LDL cholesterol endpoint values after 42 d of canola oil and HOCO. Analogous to the current report, the 2 COMIT I canola diets did not differ in endpoint values for any parameters. Gillingham et al. was the first to investigate the effects of a high-oleic acid rapeseed oil diet compared with a Western control diet on lipids and lipoproteins (15). Following 28 d of controlled feeding in hypercholesterolemic participants (n = 36), endpoint TC, LDL cholesterol, and non-HDL cholesterol were lower after the high-oleic phase (5.27 ± 0.14, 3.10 ± 0.12, and 3.94 ± 0.14 mmol/L) compared with the Western diet control phase (5.65 ± 0.16, 3.53 ± 0.14, and 4.28 ± 0.17 mmol/L). These findings are consistent with the COMIT II study results, with differences in endpoint values likely due to the variation in populations studied. While these trials provide important insights into the effects of HOCO on CVD biomarkers compared with canola oil (16) and a Western diet (15), COMIT II is the first study to simultaneously examine diets containing conventional canola oil or HOCO and compared to a diet with a contemporary Western fatty acid profile.
We were not surprised to report no differences in the 2 diets containing the canola oil and HOCO on lipid outcomes. We utilized the Katan Calculator for a post hoc predicted differences in blood lipids and lipoproteins following replacement of the COMIT II control diet with the canola oil and HOCO diets, and found that the predicted changes were very similar (data not shown). Although HOCO and canola oil have unique fatty acid profiles when analyzed as independent oils, the COMIT II study design diluted assessment of the proportional fatty acid difference. The intervention oils provided approximately 50% of the total daily fat; thus, the remaining 50% was provided by other fat sources (i.e., mayonnaise, salad dressing, dairy fat) in equivalent amounts across diets, resulting in very modest fatty acid differences between the canola oil diet and HOCO diet. Therefore, the conclusion of a lipid and lipoprotein benefit of HOCO similar to canola oil and relative to control is in the context of 6 wk of intake when incorporated as approximately 18% of total energy (60 g per 3,000 kcal). Diet effects on lipids and lipoproteins following higher intakes of HOCO and canola oil (i.e., >18% of total energy) are unknown. A higher oil dosage is not recommended in the Dietary Guidelines for Americans healthy US-style eating pattern (i.e., 2,000 kcal, 27 g oil; 3,000 kcal, 44 g oil) (2) and modeling exercises suggest risk of essential fatty acid deficiency following elevated intake of high-oleic acid oils (24). Thus, we cannot conclude the longer-term implications of high-oleic oil consumption or the effects of higher dosages, and future research should consider the potential adverse effects of overconsumption for pertinent dietary recommendations.
MetS is defined as a cluster of 3 or more co-occurring interrelated conditions, including abdominal obesity, dysglycemia, dyslipidemia, and/or hypertension, and is associated with increased risk of cardiometabolic disease (25). The COMIT II study participants were required to have at least 2 MetS criteria at the screening visit, one of which was required to be an elevated waist circumference. In contrast to the NCEP ATP III waist circumference criteria (men ≥102 cm, women ≥88 cm), the International Diabetes Federation cut points were used (men ≥94 cm, women ≥80 cm) to identify individuals who may benefit from dietary intervention in the earlier stages of cardiometabolic disease risk. These inclusion criteria were also consistent with those of COMIT I (18). Further, the inclusion criteria of 2 rather than the syndrome definition of 3 factors (25) were selected to increase the generalizability of our findings to a sample that is highly representative of the North American population. An analysis of 2003–2012 NHANES data reported MetS prevalence among adults as 33%, with higher rates among women and Hispanics (26); the COMIT II sample had slightly higher rates at baseline (38%), likely due to the predominance of women (63%). MetS prevalence increases markedly with age [approximately 18% among 18–39 y and 50% among 60+ y in the US (26)], underscoring the relevance of this syndrome as the proportion of the older population rapidly grows. Further, MetS prevalence is ∼21% among Canadian adults, with substantially higher estimates of individuals having components of the syndrome (i.e., 67% have ≥1 and 44% have ≥2 criteria) (27). These rates are concerning since MetS is associated with a 5-fold greater risk of incident diabetes (28) and a 2-fold greater risk of incident CVD events and mortality (29). Thus, selecting a sample of metabolically compromised adults is relevant to a considerable portion of the population and is appropriate for lifestyle intervention trials that aim to identify dietary strategies for chronic disease prevention and risk reduction among North Americans, with important implications for dietary counseling and nutrition policy recommendations. Previous investigations of the effects of canola oil on lipid and lipoprotein parameters in individuals at risk for or with MetS have been limited to 3 trials, including COMIT I, all of which reported lipid-lowering benefits of canola oil (16, 30, 31).
Although there is a substantial evidence base to support the cardioprotective benefits of canola oil, very few trials have directly compared a canola oil-based diet to a control diet with the fatty acid composition of the average, contemporary Western diet (6). This was a noted limitation of COMIT I (16) and prevents the determination of how diets enriched in canola oil fare as a replacement for a diet with the fat profile typical of Western intakes, as well as confirmation of the absence of adverse lipid effects. According to the latest NHANES food consumption data (2015–2016), the average intake of SFAs, MUFAs, and PUFAs among US adults is 12%, 12%, and 8% of total energy, respectively (20), percentages that the COMIT II control diet aimed to emulate (i.e., 12% SFAs, 11% MUFAs, and 10% PUFAs). The SFA content of the control diet was roughly 2-fold that of the canola oil and HOCO diets, and the MUFA content was appreciably lower than the 2 canola diets, although still aligned with average intakes. Ghee, coconut oil, safflower oil, and flaxseed oil were included in the control blend to generate the targeted fatty acid profile, which was based on an exhaustive evaluation of oil combinations during the COMIT II study design. Some of the individual fatty acids in the control diet were not directly congruent with Western intake. However, approximately 50% of the control oil blend was butter based (i.e., butter oil/ghee), a major source of animal fat in the Western diet, and only 8% was from coconut oil. Future research should incorporate fats and oils more representative of Western sources (i.e., corn oil, animal fats) when designing a control arm with a Western diet fatty acid profile, or provide a single fat source for the control for a comparative test of culinary oils.
COMIT II had numerous strengths, including a tightly regulated, controlled feeding, double-blind, multi-center, randomized, crossover design with a large sample size and inclusion of a commonly consumed oil. The crossover design allowed subjects to act as their own controls during each diet period, and the controlled feeding aspect reduced confounding variables characteristic of free-living designs. Moreover, blood was sampled on 2 consecutive days at the baseline and 2 consecutive days at the endpoint of each experimental period, allowing calculation of mean values and possible attenuation of intra-individual variability of lipid parameters. Further, collection of diet-specific blood samples on days 1 and 2 ensured attainment of precise baseline values (data not shown), in contrast to assumed return to initial baseline value post-washout, for inclusion as a covariate in the primary analysis. A limitation of COMIT II is small reductions in body weight (<1 kg) that were observed across all diets; however, it is not uncommon to see some degree of weight loss in controlled feeding trials, and is likely attributable to shifts from the habitual diet to a generally healthier controlled feeding eating pattern (i.e., lower sodium, lower SFAs, higher fiber, among others). This may explain the differences in all measured outcomes compared with baseline, particularly in the control condition. Importantly, the magnitude of weight reduction did not differ across diets and, thus, it is unlikely that the weight loss meaningfully mediated the lipid and lipoprotein diet response. Future assessments of individuals with MetS criteria should utilize the NCEP ATP III abdominal obesity cut points with a higher waist circumference threshold for inclusion (25).
In summary, canola oil and HOCO improved the lipid and lipoprotein profile compared to a control oil with a fatty acid composition characteristic of Western intakes in individuals with at least 2 MetS symptoms. Incorporating high-oleic acid and/or conventional canola oils into the diet by replacing dietary sources higher in SFA is an effective strategy to improve lipid and lipoprotein parameters and thus, reduce atherosclerotic CVD risk.
Acknowledgments
The authors’ contributions were as follows—PJHJ, PMK-E, SGW, PWC, BL, PC, DJAJ, CGT, and PZ: designed research; KJB, JAF, SSH, JS, XC, VG, JM-G, DP, AW, SCSJ, and JR: conducted the research; PWC: performed biochemical analyses; KJB: analyzed data; KJB: wrote the paper; PJHJ, PMK-E, SGW, PWC, BL, DJAJ, and CGT: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
This research is part of the Canola Agri-Science Cluster, with funding provided by Agriculture and Agri-Food Canada, the Canola Council of Canada, Alberta Canola, SaskCanola, Dow Agro Sciences, and the Manitoba Canola Growers. The research was also supported by the National Center for Research Resources, Grant UL1 RR033184, which is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127. In addition, this research was supported by NIH Grant 2T32DK007703. The funders did not play a role in the data analysis, interpretation, or manuscript preparation.
Author disclosures: KJB, JAF, PC, SSH, JS, XC, VG, JM-G, DP, AW, and SCSJ, no conflicts of interest. PMK-E has received research funding from: California Walnut Commission, Ag Canada and Canola Oil Council, California Strawberry Commission, Ocean Spray Cranberries, National Cattlemen's Beef Association, McCormick Science Institute, International Nut & Dried Fruit Council, Hass Avocado Board. PMK-E has served on the following advisory boards: HumanN, Avocado Nutrition Science Advisors, Seafood Nutrition Partnership. SGW has received consulting funds, travel funds, and research funding from the Canola Council of Canada and the McCormick Science Institute. SGW has received research and travel funds from Flax Canada, the California Walnut Commission, American Pistachio Growers, and Hershey's. SGW has received research funding from the Almond Board of California, the Hass Avocado Board, the National Fisheries Institute, Dairy Management Incorporated, General Mills, Reliant Pharmaceuticals, Unilever, and the National Cattleman's Beef Fund. PWC has received funding from the Canadian Institutes of Health Research and the Canadian Diabetes Association. BL is Chair of Nutrition at Université Laval, which is supported by private endowments from Pfizer, La Banque Royale du Canada, and Provigo-Loblaws. BL has received funding in the last 5 years from the Canadian Institutes for Health Research, the Natural Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada (Growing Forward program supported by the Dairy Farmers of Canada (DFC), Canola Council of Canada, Flax Council of Canada, Dow Agrosciences), Dairy Research Institute, Dairy Australia, Danone Institute, Merck Frosst, Pfizer, and Atrium Innovations for which Douglas Laboratories manufacture and market omega-3 supplements. BL served as the Chair of the peer-review Expert Scientific Advisory Council of DFC. BL is also an Advisory Board member of the Canadian Nutrition Society, the Conseil pour les initiatives de progrès en alimentation and has served as an Advisory Expert for the Saturated Fat panel of the Heart and Stroke Foundation of Canada. BL has also received honoraria from the International Chair on Cardiometabolic risk, DFC, and the World Dairy Platform as an invited speaker in various conferences. DJAJ has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Center Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). DJAJ has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. DJAJ has been on the speaker's panel, served on the scientific advisory board, and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and the Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism, and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. DJAJ received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. DJAJ received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). DJAJ is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, ALJ, is a director and partner of Glycemic Index Laboratories, Inc., and his sister, CB, received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies. CGT has received funding in the past 5 years from CIHR, NSERC, Agriculture and Agri-Food Canada (Growing Forward and the Canola/Flax Agri-Science Cluster, Pulse Agri-Science Cluster, Canada-Manitoba Agri-Food Research Development Initiative), Research Manitoba, Manitoba Energy Science and Technology, Manitoba Agri-Health Research Network, Alberta Innovates, Alberta Crop Industry Development Fund, Alberta Canola Producers Commission, Alberta Pulse, Saskatchewan Pulse Growers, Canadian Diabetes Association, and MITACS. PZ has received funding in the past 5 years from CIHR, NSERC, Agriculture and Agri-Food Canada (Growing Forward and the Canola/Flax Agri-Science Cluster, Pulse Agri-Science Cluster, Canada-Manitoba Agri-Food Research Development Initiative), Research Manitoba, Manitoba Energy Science and Technology, Manitoba Agri-Health Research Network, Alberta Innovates, Alberta Crop Industry Development Fund, Alberta Canola Producers Commission, Alberta Pulse, Saskatchewan Pulse Growers, Canadian Diabetes Association, and MITACS. PJHJ's research related to a variety of oils and fats has been supported by grants and contracts from both industry and nonindustry sources, including the Canola Council of Canada, Dairy Farmers of Canada, Canadian Institutes for Health Research, Natural Sciences and Engineering Research Council of Canada, Heart and Stroke Foundation of Canada, and National Institutes of Health Rare Diseases Network. PJHJ also serves as a committee member for the Soy Nutrition Institute, and the North American International Life Sciences Institute's Lipids Committee. PJHJ is President of Nutritional Fundamentals for Health Inc., which markets functional foods and nutraceuticals.
Abbreviations used: COMIT II, canola oil multi-center intervention trial II; HOCO, high-oleic acid canola oil; INAF, Institute of Nutrition and Functional Foods; MetS, metabolic syndrome; PHVO, partially hydrogenated vegetable oils; PSU, The Pennsylvania State University; RCFFN, Richardson Center for Functional Foods and Nutraceuticals; SBRC, St. Boniface Hospital Albrechtsen Research Center; TC, total cholesterol; TFA, trans-unsaturated fatty acids.
References
- 1. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE et al.. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960–84. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2015–2020. 8th ed Washington, DC: U.S. Government Printing Office; 2015. [Google Scholar]
- 3. Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris-Etherton P, Sikand G, La Forge R, Daniels SR, Wilson DP, Morris PB et al.. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9(6 Suppl):S1–122. e1. [DOI] [PubMed] [Google Scholar]
- 4. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 5. U.S. Department of Agriculture Agricultural Research Service. National nutrient database for standard reference legacy release April, 2018: 04582, oil, canola. v.3.9.4.1 2018-06-11 ed, 2018.
- 6. Lin L, Allemekinders H, Dansby A, Campbell L, Durance-Tod S, Berger A, Jones PJ. Evidence of health benefits of canola oil. Nutr Rev. 2013;71(6):370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. Qualified health claims: letter of enforcement discretion - unsaturated fatty acids from canola oil and reduced risk of coronary heart disease (docket no. 2006Q-0091); 2006.
- 8. U.S. Department of Agriculture Agricultural Research Service. National nutrient database for standard reference legacy release April, 2018: 04698, oil, industrial, canola, high oleic. v.3.9.4.1 2018-06-11 ed, 2018.
- 9. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–13. [DOI] [PubMed] [Google Scholar]
- 10. Tarrago-Trani MT, Phillips KM, Lemar LE, Holden JM. New and existing oils and fats used in products with reduced trans-fatty acid content. J Am Diet Assoc. 2006;106(6):867–80. [DOI] [PubMed] [Google Scholar]
- 11. DeBonte L, Iassonova D, Liu L, Loh W. Commercialization of high oleic canola oils. Lipid Tech. 2012;24(8):175–7. [Google Scholar]
- 12. Huth PJ, Fulgoni VL, Larson BT. A systematic review of high-oleic vegetable oil substitutions for other oats and oils on cardiovascular disease risk factors: implications for novel high-oleic soybean oils. Adv Nutr. 2015;6(6):674–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. 2012;4(12):2097–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Food and Drug Administration. Final determination regarding partially hydrogenated oils (removing trans fat); 2018.
- 15. Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr. 2011;105(3):417–27. [DOI] [PubMed] [Google Scholar]
- 16. Jones PJ, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, Couture P, Charest A, Baril-Gravel L, West SG et al.. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014;100(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowen KJ, Sullivan VK, Kris-Etherton PM, Petersen KS. Nutrition and cardiovascular disease—an update. Curr Atheroscler Rep. 2018;20(2):8. [DOI] [PubMed] [Google Scholar]
- 18. Senanayake VK, Pu S, Jenkins DA, Lamarche B, Kris-Etherton PM, West SG, Fleming JA, Liu X, McCrea CE, Jones PJ. Plasma fatty acid changes following consumption of dietary oils containing n–3, n–6, and n–9 fatty acids at different proportions: preliminary findings of the Canola Oil Multicenter Intervention Trial (COMIT). Trials. 2014;15:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mackay DS, Jew S, Jones PJ. Best practices for design and implementation of human clinical trials studying dietary oils. Prog Lipid Res. 2017;65:1–11. [DOI] [PubMed] [Google Scholar]
- 20. U.S. Department of Agriculture Agricultural Research Service. Energy intakes: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age, what we eat in America, NHANES 2015–2016; 2018.
- 21. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 22. Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, McCrea CE, Pu S, Couture P, Connelly PW et al.. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity (Silver Spring). 2016;24(11):2261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM et al.. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. [DOI] [PubMed] [Google Scholar]
- 24. Raatz SK, Conrad Z, Jahns L, Belury MA, Pickl MJ. Modeled replacement of traditional soybean and canola oil with high-oleic varieties increases monounsaturated fatty acid and reduces both saturated fatty acid and polyunsaturated fatty acid intake in the US adult population. Am J Clin Nutr. 2017;108:1–9. [DOI] [PubMed] [Google Scholar]
- 25. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr.. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 26. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–4. [DOI] [PubMed] [Google Scholar]
- 27. Statistics Canada. Canadian health measures survey: metabolic syndrome in adults, 2012 to 2013 [Internet]. http://www.statcan.gc.ca/pub/82-625-x/2014001/article/14123-eng.htm - n1.
- 28. Ford ES, Schulze MB, Pischon T, Bergmann MM, Joost HG, Boeing H. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Cardiovasc Diabetol. 2008;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–14. [DOI] [PubMed] [Google Scholar]
- 30. Palomaki A, Pohjantahti-Maaroos H, Wallenius M, Kankkunen P, Aro H, Husgafvel S, Pihlava JM, Oksanen K. Effects of dietary cold-pressed turnip rapeseed oil and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome. Lipids Health Dis. 2010;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baxheinrich A, Stratmann B, Lee-Barkey YH, Tschoepe D, Wahrburg U. Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of alpha-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br J Nutr. 2012;108(4):682–91. [DOI] [PubMed] [Google Scholar]


