ABSTRACT
Background
When mother's milk is insufficient, pasteurized human donor milk (DM) is the recommended supplement for hospitalized very-low-birth-weight infants. The current method of pasteurization (Holder, 62.5°C, 30 min) negatively affects heat-sensitive nutrients and bioactive proteins.
Objectives
Objectives of this study were to compare changes in DM composition after thermal pasteurization (Holder and flash-heating) and nonthermal methods [UV-C irradiation and high hydrostatic pressure (HHP)]. We hypothesized that nonthermal techniques would result in fewer changes to composition.
Methods
Holder, flash-heating (brought to boil), UV-C irradiation (250 nm, 25 min), and HHP (500 MPa, 8 min) were studied. Pools of milk from 17 women known to contain bacteria at >5 × 107 colony forming units (CFU)/L were collected from the Rogers Hixon Ontario Human Milk Bank and underwent each pasteurization technique. Macronutrients, heat-sensitive micronutrients (vitamin C, folate), and bioactive components [bile-salt-stimulated lipase (BSSL), lysozyme, lactoferrin] were measured in raw and pools of pasteurized milk. Milk was cultured to determine how well each technique produced a culture negative result (detection limit <1 × 103 CFU/L).
Results
Folate was reduced by 24–27% after Holder, flash-heating, and UV-C (P < 0.05); no reduction was observed after HHP. All pasteurization methods reduced vitamin C (60–75%, P < 0.001). BSSL was abolished after Holder and flash-heating (P < 0.001), reduced after UV-C (48%, P < 0.001), but unaffected by HHP. Lysozyme activity was reduced after flash-heating (44%) and UV-C (74%, P < 0.004) but unaffected by Holder or HHP. Lactoferrin was reduced by all methods (P < 0.02) but most severely by flash-heating (74%) and least severely by HHP (25%). Holder and UV-C reduced lactoferrin by ∼48%. All pasteurization methods reduced the number of culture positive DM samples (P < 0.001).
Conclusions
HHP better preserves human milk composition than Holder pasteurization. Future research on the feasibility of HHP for pasteurizing human milk is warranted because its implementation may improve the nutritional status and health of DM-fed infants.
Keywords: donor human milk, Holder pasteurization, flash-heating, UV-C irradiation, high hydrostatic pressure processing
Introduction
For many reasons including its nutrient content and bioactive compounds, mother's milk is the optimal source of nutrition for all infants, including vulnerable infants (1). This includes both very-low-birth-weight infants in the neonatal intensive care unit and infants born to HIV+ mothers in low-income countries where antiretroviral therapy is not readily available to reduce the risk of mother-to-infant HIV transmission (2). Other occasions exist when mother's own milk may be unavailable.
In high-income countries, both not-for-profit human milk banks and for-profit companies provide a safe supply of pasteurized donor human milk (DM) when mother's milk is unavailable. In North America, not-for-profit milk banks are members of the Human Milk Banking Association of North America (HMBANA) and DM is triaged first to very-low-birth-weight infants at risk of necrotizing enterocolitis (3, 4). HMBANA guidelines stipulate that potential donors be serologically screened for blood-borne diseases, after which donated milk is pasteurized using the Holder method (62.5°C for 30 min) (5). This is to ensure it is safe for infant consumption and specifically free from pathogenic bacteria and viruses which could harm a vulnerable infant. However, thermal processing involved with the Holder method is known to negatively affect important heat-sensitive vitamins such as folate and vitamin C—nutrients that play important roles in DNA and RNA biosynthesis and repair, and collagen synthesis (6–8). Holder pasteurization also denatures key enzymes, such as bile-salt-stimulated lipase (BSSL) involved in fat absorption, and important bioactive components such as the antimicrobial proteins lactoferrin and lysozyme (9–11).
In low-resource countries, the WHO and UNICEF jointly recommend the flash-heating procedure as an interim feeding strategy for mothers who are HIV+ when antiretroviral drugs are unavailable or if a mother is temporarily unable to breastfeed and donated milk is required (12). Coincidentally, this is the method recommended on Internet-based milk-sharing sites (13, 14). Although flash-heating appears to be effective in inactivating HIV and other viruses, its impact on heat-sensitive nutrients and bioactive components is not clearly understood (15, 16).
Nonthermal alternatives to pasteurize human milk, such as UV-C irradiation and high hydrostatic pressure (HHP), show promise, but much more research is required to evaluate their impact on reducing bacterial load and milk composition (17–20). UV-C irradiation of human milk has been shown by one group to inactivate cytomegalovirus and improve retention of secretory immunoglobulin A, lactoferrin, and lysozyme compared with Holder pasteurization while achieving a 5-log reduction in bacterial load (21, 22). HHP is approved for use in Canada for a number of food products including meat, and fruit and vegetable–based juices/smoothies, making it an attractive alternative to pasteurization; however, few studies have examined its impact on milk composition or reducing the bacterial load of human milk.
The aim of this study, then, was to compare changes in human milk composition before and after conventional thermal pasteurization (Holder and flash-heating) alongside 2 promising nonthermal methods (UV-C irradiation and HHP). Specifically, the macronutrient, vitamin C, folate, BSSL, lysozyme, and lactoferrin concentrations of human milk pre- and postpasteurization were examined. It was hypothesized that nonthermal techniques would result in fewer changes to milk composition.
Methods
Before collection of human donor milk, research ethics board approval was obtained from Mount Sinai Hospital, The Hospital for Sick Children, and the HMBANA. Consent was obtained from each mother at the time of milk donation to use the milk for research.
Human milk collection
Expressed human milk from 17 different women (∼2 L each) donated to the Rogers Hixon Ontario Human Milk Bank and subsequently determined to contain a total bacterial load >5 × 107 CFU/L was used for the present study (Figure 1). Donated milk with bacterial loads >5 × 107 CFU/L is not processed or dispensed for human consumption according to the policy and procedures of this milk bank. All donated milk from each woman was pooled, warmed (until a temperature of 37°C was maintained for 5 min), and agitated to ensure homogeneity. Aliquots from each of the 17 pools were collected and frozen (−80°C) for subsequent microbiology and nutrient analysis. The remaining milk was equally divided into 400-mL aliquots and frozen at −20°C until pasteurization. To each aliquot of raw and pasteurized milk for folate analysis, 1% wt:vol sodium ascorbate was added before freezing to prevent possible degradation during prolonged storage at −80°C (23). Similarly, to inhibit possible degradation of ascorbic acid during storage at −80°C, a solution of 10% metaphosphoric acid containing 1% oxalic acid was added to both raw and pasteurized samples in a 1:1 ratio (24).
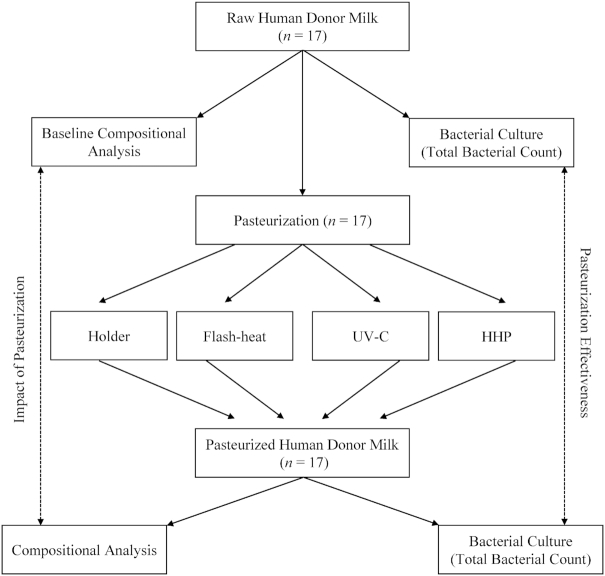
FIGURE 1.
Experimental design and overview of the study. Human donor milk was collected from 17 women (∼2 L each) and frozen by the Rogers Hixon Ontario Human Milk Bank. After undergoing baseline culture and composition analysis (macronutrients, micronutrients, bioactive components), each of the 17 pools underwent each of the 4 pasteurization techniques. Postpasteurization culture and composition analyses were conducted for each pool for each technique. HHP, high hydrostatic pressure processing.
Pasteurization
Milk was thawed overnight in a refrigerator (4°C), warmed in a water bath at 37°C, and gently inverted to ensure homogeneity. Four pasteurization techniques were assessed: Holder (62.5°C, 30 min), flash-heating (brought to boil), UV-C irradiation (250 nm, 25 min), and HHP (500 MPa, 8 min). After each pasteurization, aliquots for bacteriology and milk composition as described for raw milk were collected, frozen on dry ice, and then stored at −80°C until analysis.
For Holder pasteurization, 125 mL of human milk from each woman (n = 17) was poured into specialized bottles (Sterifeed, Medicare Colgate Ltd.), capped, and heat sealed. Heat treatment was carried out using a tabletop pasteurizer (Sterifeed T30, Medicare Colgate Ltd.), equipped with a temperature probe and data logger. Milk was heated in submerged bottles for 30 min until a temperature of 62.5°C was reached. After holding for 30 min, the bottles of milk were immediately placed in water at 4°C for 1 h.
Flash-heating was carried out according to a previously published protocol with minor modification (16). Milk (125 mL) samples (n = 17) were poured into a 250-mL glass media jar that could withstand heat, and covered with a lid. Jars were then placed in a beaker filled with water (1 L, room temperature). The beaker and media bottle were then simultaneously heated rapidly using a hot plate until the water began to boil, which took ∼16 min. The media bottles were then placed in an ice bath for 1 h to equilibrate to 4°C.
UV-C irradiation of human milk was adapted from the protocol published by Christen et al. (20) with modification. A UV-C germicidal lamp (Philips TUV PL-L, 2.3 Watt) was used as the source of UV-C irradiation that was inserted in a polypropylene graduated cylinder containing 125 mL of milk. In the present study the approximate UV-C dose (250 nm, ∼15 min) utilized by Christen et al. (20) to yield a 5-log reduction in bacterial load was initially used. However, pilot microbiology data suggested an additional 10 min of UV-C exposure was required to ensure a reduction in bacterial load to <1 × 103 CFU/L.
HHP pasteurization (Model 135, Hiperbaric) used water as the pressure transmission medium at a temperature of 4°C. Milk (125 mL) from each woman was poured into plastic bottles and placed into the HHP vessel. Based on literature values and pilot experiments, a pressure of 500 MPa for 8 min was selected for pasteurization (18, 25).
Analysis of nutrients, bioactive components, and bacteriology
The macronutrient content of raw and pasteurized milk samples was determined using a mid-infrared human milk analyzer (Miris Human Milk Analyzer), calibrated against wet-chemistry techniques. Precision of the instrument was assessed daily using an aliquot of pooled DM (n = 5). The CV for each macronutrient was <5%.
The folate concentration of milk samples was assessed after the tri-enzyme treatment by microbial assay using Lactobacillus rhamnosus (ATCC#7469) as the test organism, and 5-methyltetrahydrofolate to generate the calibrator curve (26, 27). A pool of human milk and certified reference material (BCR-487, European Commission, Joint Research Centre) were used to test reproducibility and accuracy. The interassay (n = 23) CV after the analysis of the pool of human milk and the BCR-487 was 5.5% and 10%, respectively, with a mean ± SD value for BCR-487 of 11.7 ± 1.7 mg/kg (certified value: 13.3 ± 1.3 mg/kg folate).
Vitamin C analyses of milk samples were carried out by HPLC using the previously published method of Romeu-Nadal et al. (28) with modification. An Agilent 1260 Infinity II (Agilent Technologies) HPLC was used, fitted with Poroshell 120-EC-C18 guard and analytical columns. Briefly, samples (30 µL) were injected into the column at 25°C and a flow rate of 1.3 mL/min. Isocratic separation was conducted using a mobile phase of 0.1% vol:vol acetic acid in deionized water and methanol, in a relative proportion of 97.5:2.5 vol:vol. Mean ± SD recovery of vitamin C from spiked DM samples was 106.1% ± 6% (n = 5). The interassay CV for vitamin C in pooled DM was 14% (n = 5).
Lipase activity (primarily BSSL) was determined on defatted milk samples using a commercially available kit (QuantiChrom Lipase Assay Kit, BioAssay Systems) validated for use in human milk (20). The interday CV of repeated measurements of BSSL activity in aliquots of pooled DM was 7.4% (n = 9).
Lactoferrin concentration was determined by HPLC using the aforementioned Agilent 1260 Infinity II system and columns according to the method of Yao et al. (29). Purchased human milk lactoferrin (Sigma-Aldrich) was used to generate the standard curve. HPLC separation was conducted using a mobile phase consisting of <0.1% trifluoroacetic acid in acetonitrile (Buffer A: 95:5 vol:vol and Buffer B: 5:95 vol:vol). The injection volume was 2 µL, the flow rate was 0.85 mL/min, and the column temperature was set to 37°C. Isocratic separation initially began at 30% Buffer B for 1 min, followed by a gradient of 30–60% over 7 min. The interday assay CV (n = 6) assessed using pooled DM was 7% and the mean ± SD percentage recovery of spiked DM samples was 90% ± 13% (n = 5).
Lysozyme activity in defatted milk samples was determined using the commercially available Micrococcus lysodeikticus turbidimetric assay kit modified to enable use of 96-well microplates (Sigma-Aldrich) (30, 31). Lyophilized lysozyme isolated from chicken egg white (99% purity) (Sigma-Aldrich L6876) was used as a reference material to validate the assay, with a mean ± SD recovery of 99% ± 11% (n = 13) and an interday assay CV of <10%.
Total bacterial counts on raw and pasteurized milk samples were determined using MacConkey agar and blood agar (incubated for 48 h at 35°C with 5% CO2) at The Hospital for Sick Children core microbiology laboratory. A negative culture was considered to be representative of a total bacterial count <1 × 103 CFU/L.
Statistical analysis
The anticipated change in folate concentration between raw and Holder pasteurized milk was used to estimate the sample size for the current study given the sensitivity of this nutrient to light and heat. Using the 0.75 SD reduction in folate concentration we previously reported as an effect size with an α = 0.05 and power = 80%, 14 milk samples in each of 2 groups were required for this pairwise comparison (32).
Statistical analyses were conducted using SAS version 9.4 (SAS Institute). All outcome variables (nutrient and bioactive compound composition) were checked to ensure they followed a normal distribution (PROC UNIVARIATE). Mean nutrient and bioactive compound concentrations were compared across groups (i.e., raw, Holder, flash-heat, UV-C, and HHP) using mixed models (PROC MIXED). When a statistically significant result was found, post hoc pairwise comparisons were conducted using LS-MEANS. The proportion of samples with negative cultures (pass, <1 × 103 CFU/L) for each pasteurization technique was assessed using logistic regression (PROC GENMOD). For all statistical analyses, P values <0.05 were considered significant.
Results
The changes in macronutrients (carbohydrate, fat, protein), energy, and micronutrients (folate, vitamin C) after the 4 pasteurization techniques are summarized in Table 1.
TABLE 1.
Concentrations of macronutrients and micronutrients per liter of raw and pasteurized human donor milk1
| Analyte | Raw | Post-Holder | Post–flash heat | Post–UV-C | Post-HHP |
|---|---|---|---|---|---|
| Carbohydrate, g/L | 69 ± 3a | 69 ± 4a | 69 ± 3a | 69 ± 3a | 67 ± 4b |
| Fat, g/L | 31 ± 8 | 31 ± 8 | 31 ± 8 | 31 ± 8 | 29 ± 8 |
| Crude protein, g/L | 10 ± 2 | 11 ± 2 | 10 ± 2 | 11 ± 2 | 11 ± 2 |
| Energy, kcal/L | 616 ± 72 | 613 ± 74 | 611 ± 75 | 610 ± 75 | 591 ± 74 |
| Folate, nmol/L | 191 ± 83a | 139 ± 39b | 145 ± 55b | 143 ± 56b | 177 ± 62a,b |
| Total vitamin C, mg/L | 15 ± 12a | 5.4 ± 6.7b | 5.9 ± 8.5b | 4.2 ± 4.1b | 3.7 ± 2.8b |
| Ascorbic acid, mg/L | 14 ± 11.2a | 5.4 ± 6.5b | 5.5 ± 6.8b | 2.9 ± 3.3b | 3.3 ± 3.4b |
1Values are means ± SDs, n = 17 for each group. Data were analyzed using linear regression models (PROC MIXED) followed by pairwise comparisons as appropriate (LS-MEANS). Labelled means in a row without a common letter differ, P < 0.05. HHP, high hydrostatic pressure processing.
Macronutrients
Overall, there were no statistically significant changes in the mean macronutrient content of DM after pasteurization by any method except for a statistically significant reduction in mean total carbohydrate after HHP (β-coefficient = −2.4 g/L; 95% CI: −2.6, −2.2 g/L, P = 0.04).
Micronutrients
In terms of micronutrients, there were statistically significant reductions in the mean folate concentration in DM after Holder (β = −52 nmol/L; 95% CI: −94, −10 nmol/L, P = 0.02), flash-heat (−46 nmol/L; 95% CI: −88, −4 nmol/L, P = 0.03), and UV-C (−48 nmol/L; 95% CI: −90, −6 nmol/L, P = 0.02) pasteurization; however, there was no statistically significant loss of folate after HHP (Table 1). There were no statistically significant differences related to loss of folate when individual methods were compared with one another.
Similarly, Holder, flash-heating, UV-C, and HHP all reduced concentrations of total vitamin C (β = −9.4 mg/L; 95% CI: −14.5, −4.3 mg/L; β = −8.9 mg/L; 95% CI: −14.0, −3.8 mg/L; β = −11.0 mg/L; 95% CI: −16.0, −5.9 mg/L; and β = −11.0 mg/L; 95% CI: −16.0, −5.9 mg/L for each pasteurization method, respectively). There were no statistically significant differences when comparisons were made between pasteurization methods.
Bioactive compounds
The activity of BSSL was abolished (<1% of original activity) after thermal pasteurization, whether using Holder or flash-heating (P < 0.0001, Figure 2A). There was also a significant reduction in BSSL activity after UV-C irradiation (β = −26 U/mL; 95% CI: −33, −19 U/mL, P < 0.0001); however, this was less than observed for the thermal methods. There was no loss of BSSL activity after HHP compared with raw milk. Accordingly, levels of BSSL activity were significantly lower (P < 0.0001) with Holder (β = −48 U/mL; 95% CI: −55, −41 U/mL), flash-heating (β = −48 U/mL; 95% CI: −55, −41 U/mL), and UV-C (β = −20 U/mL; 95% CI: −27, −13U/mL) pasteurization methods than with HHP.
FIGURE 2.
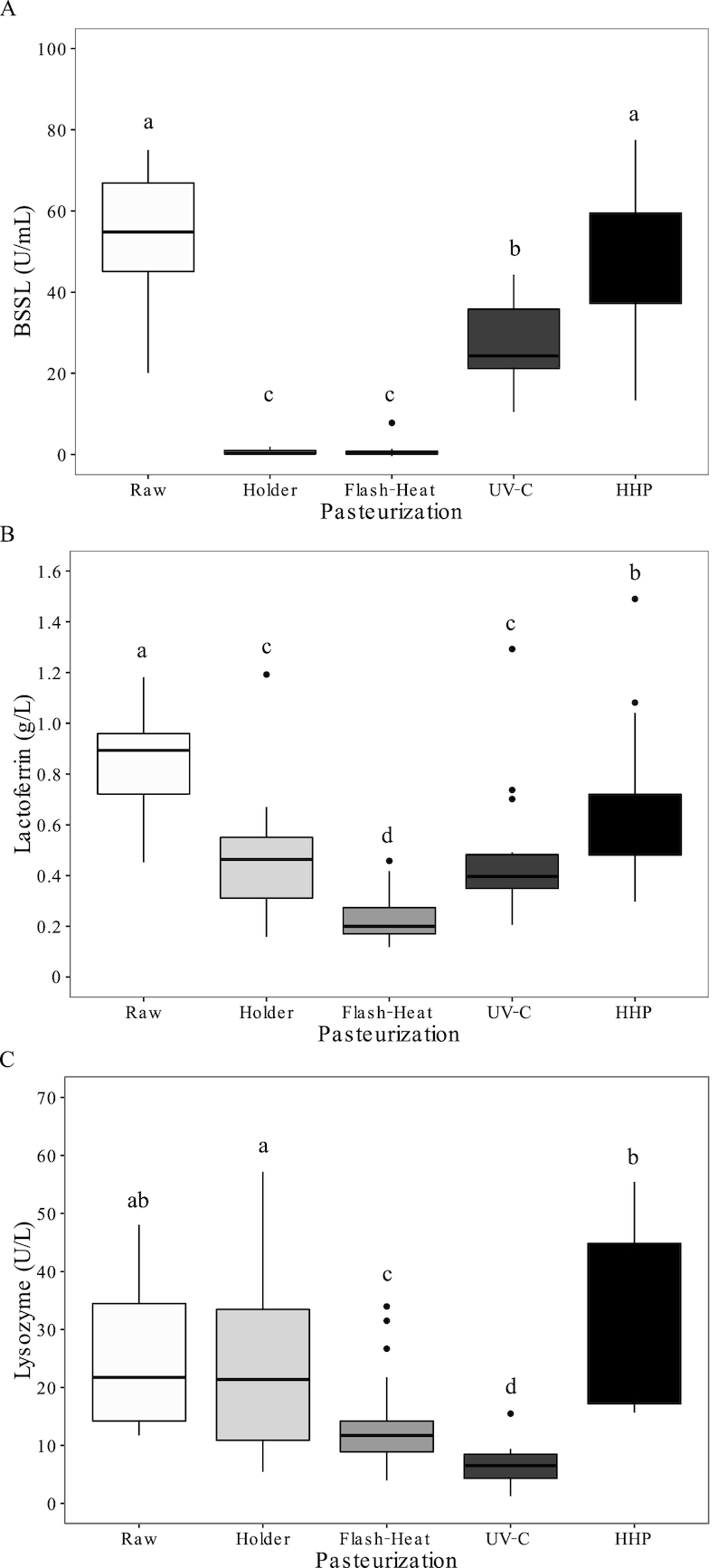
Concentration of human milk bioactive compounds before and after pasteurization. Data are presented as medians (first and third quartiles), n = 17 for each group. Whiskers were calculated as far as the data extended to a maximum of 1.5 times the IQR. Mean differences were assessed using linear regression models (PROC MIXED) followed by pairwise comparisons (LS-MEANS) if P value was <0.05. Different superscript letters indicate statistical significance for pairwise comparisons (P < 0.05). (A) BSSL activity; (B) lactoferrin concentration; (C) lysozyme activity. BSSL, bile-salt-stimulated lipase; HHP, high hydrostatic pressure processing.
The mean lactoferrin concentration of milk was reduced after pasteurization, regardless of the technique utilized; however, the magnitude of the reduction differed (Figure 2B). Flash-heating resulted in the greatest reduction in lactoferrin as compared with Holder (β = −0.23 g/L; 95% CI: −0.41, −0.05 g/L, P = 0.01), UV-C (β = −0.23 g/L; 95% CI: −0.41, −0.05 g/L, P = 0.01), and HHP (β = −0.44 g/L; 95% CI: −0.62, −0.26 g/L, P < 0.0001). Of the pasteurization methods, HHP had the smallest impact on the concentration of lactoferrin.
Lysozyme activity was also significantly affected by some of the pasteurization techniques (Figure 2C). There was a significant loss of activity after flash-heating (β = −11.3 U/L; 95% CI: −18.9, −3.7 U/L, P = 0.004) and UV-C (β = −19.0 U/L; 95% CI: −26.6, −11.4 U/L, P < 0.0001); however, there was no statistically significant loss of activity after Holder or HHP. Lysozyme activity after Holder and HHP was significantly higher than after flash-heating (β = 8.5 U/L; 95% CI: 0.92, 16.1 U/L, P < 0.0001 and β = 16.4 U/L; 95% CI: 8.8, 23.9 U/L, P = 0.03, respectively). Further, the lysozyme activity after HHP was higher (P = 0.04) when compared with the Holder method (β = 7.8 U/L; 95% CI: 0.22, 15.4 U/L).
Bacteriology
All methods of pasteurization increased the number of culture negative milk samples (<1 × 103 CFU/L) compared with prepasteurization (Table 2). There was a significantly higher proportion of samples with negative cultures post-HHP compared with post-UV-C (P = 0.02), which was trending towards significance when compared with after Holder (P = 0.06) and flash-heat (P = 0.08). There were no other statistically significant differences in the number of culture negative samples between methods postpasteurization (all P > 0.3); however, our sample size was limited when it came to detecting such a difference.
TABLE 2.
Summary of microbiology results of raw and pasteurized human donor milk1
| Colony count, CFU/L | Raw | Post-Holder | Post–flash heat | Post–UV-C | Post-HHP |
|---|---|---|---|---|---|
| <1000 (Pass) | 0 (0)a | 9 (53)b,c | 7 (41)b,c | 6 (35)b | 12 (71)c |
| ≥1000 (Fail) | 17 (100)2 | 8 (47) | 10 (59) | 11 (65) | 5 (29) |
1Values are presented as count (%), n = 17 for each group. The proportion of samples with negative cultures (<1000 CFU/L) for each pasteurization was assessed using logistic regression (PROC GENMOD). Labelled counts (percentages) in a row without a common letter differ, P < 0.05. HHP, high hydrostatic pressure processing.
2Raw milk of 2 mothers cultured positive for Bacillus spp.
Discussion
Of the pasteurization techniques studied (Holder, flash-heating, UV-C, and HHP), HHP appeared to be the best at retaining BSSL, lactoferrin, and lysozyme activity. Significant retention of these bioactive compounds may improve the nutritional status and health of vulnerable infants. For example, BSSL found in human milk is activated by bile salts in the duodenum and facilitates removal of fatty acids from the TG backbone (33). During infancy, endogenous digestive lipases are low and thus human milk BSSL is thought to be critical for adequate provision of fatty acids (providing 50% of total energy) and specifically long-chain PUFAs important for neurodevelopment (34). Results presented herein demonstrate almost complete destruction of BSSL with Holder pasteurization which is consistent with other reports in the literature (35, 36). Andersson et al. (37) demonstrated in a randomized crossover trial a 17% reduction in fat absorption in preterm infants fed mother's milk that had been pasteurized using the Holder method compared with raw mother's milk. As far as we are aware, this is the first study to report preservation of BSSL with HHP. We propose that preservation of BSSL by utilizing HHP technology may help increase fat absorption and, ultimately, the growth of infants.
Lactoferrin is a glycoprotein found in high concentrations in raw mother's milk compared with infant formula (38). It has broad spectrum antimicrobial activity thought to be due to its ability to sequester iron or to its lytic effect on microbial cells. Pammi and Suresh (38), in a recent Cochrane review of randomized control trials, concluded there was evidence, though of low quality, to suggest that lactoferrin supplementation of enteral feeds may decrease the rate of late-onset sepsis and necrotizing enterocolitis without adverse effects in preterm infants. Although all pasteurization methods examined in our study resulted in significant reductions in lactoferrin, HHP was the least impactful. The 28% reduction in lactoferrin observed in the present study using HHP conditions of 500 MPa for 8 min is similar to that reported by Mayayo et al. (39) who reported a 34% reduction with HHP using 500 MPa for 15 min. The magnitude of lactoferrin reduction observed after Holder pasteurization (−48%) and flash-heating (−74%) is comparable with current literature estimates (15, 25, 40–42). UV-C irradiation in the present study resulted in a 48% reduction in mean lactoferrin concentration, which is significantly higher than the 13% reduction reported by Christen et al. (21) using a lower UV-C dose (250 nm for 15 min). A higher UV-C dose in the present study was administered to achieve culture negative results postpasteurization (<1 × 103 CFU/L).
Lysozyme is also an antimicrobial enzyme found in higher concentration in human milk than in cow milk and is generally considered thermolabile. No significant reductions in lysozyme activity were observed in the present study after HHP or Holder pasteurization. In fact, to a small but statistically significant degree, HHP resulted in higher mean lysozyme activity than Holder. We speculate that this may be attributable to partial unfolding of the lysozyme protein during HHP, increasing the surface area and lytic activity of the enzyme against the M. lysodeikticus cell wall—a central component of the turbidimetric assay (43). An increase in lysozyme activity after HHP was similarly reported in the literature for bovine milk (44, 45). Reductions in lysozyme activity after Holder pasteurization vary widely in the literature (from no difference to 60% reduction) (19, 40–42, 46–48). Conversely, flash-heating severely affected lysozyme activity (49), in line with previous reports of a 45% reduction in activity. Likely the higher peak temperature attained with flash heating (100°C) than with Holder pasteurization (62.5°C) accounted for the varying effects of the 2 procedures on lysozyme activity. A greater reduction in lysozyme activity was observed after UV-C irradiation in the present study (74%) compared with a previously reported study in the literature (21). The use of the higher dose of UV-C to reduce the bacterial load to <103 CFU/L may account for the differences observed.
There was no clear advantage for any pasteurization method over another regarding retention of macronutrients or energy. The concentration of macronutrients and energy appears to be unaffected by pasteurization per se as is reported by others (50, 51), although very few studies have systematically investigated the impact of flash heating, UV-C irradiation, and HHP on macronutrient and energy composition. There is some variability in the literature regarding the impact of Holder pasteurization on fat concentration which likely reflects sampling of milk before analyses (e.g., warming and homogenization of milk before sampling ensuring quantitative transfer), and whether other steps in pasteurized DM production processes were considered such as multiple freeze and thaw cycles and the container changes required for pooling of milk. The small but statistically significant reduction in carbohydrate concentration after HHP treatment could reflect sampling technique because a similar numerical difference was noted for fat. It is worthwhile noting the mean differences pre- and post-HHP for carbohydrate and fat are within manufacturing specifications for the precision of the equipment for these nutrients (e.g., <2 g/L).
HHP appeared to better retain folate after pasteurization but not vitamin C. There did not appear to be an advantage of one pasteurization method over another in terms of retention of vitamin C. As far as we are aware this is the first study to examine the effect of HHP on the folate concentration of human milk. Our results showing a reduction in vitamin C concentration post-HHP differ from those of other studies (52–54). The reasons for this are unclear; however, it may be due to a variety of reasons including the oxygen concentration in the samples, initial vitamin C concentration, sample matrix, pH, and the HHP parameters (e.g., time, pressure, and temperature), which differ among studies.
It has been clearly established in the literature that Holder pasteurization affects the folate and vitamin C concentrations in human milk, consistent with the known sensitivity of these nutrients to heat (25, 55). Results of this study are consistent with previous reports of between a 20% and 36% reduction in vitamin C and a 10–35% reduction in folate after Holder pasteurization (6, 7, 53, 56, 57). To the best of our understanding this is the first study to report losses of vitamin C after UV-C pasteurization, a reasonable finding given the sensitivity of vitamin C to light in bovine milk (58). A previous study of flash-heat pasteurization reported that folate increases after processing, perhaps due to evaporation of liquid and concentration of folate as jars were uncovered during pasteurization (16). Moreover, a significant fraction of milk folate is bound to folate-binding protein and heat treatment may have released folates, making them more readily available for immunoassay (59).
To our knowledge, this is the first study to systematically investigate both conventional thermal and novel nonthermal approaches of pasteurizing human milk together in one experiment, taking microbiological safety and the nutritional and bioactive compound composition into account. We acknowledge a few limitations of our study. First, although we demonstrated a statistically significant improvement in the number of milk pools meeting criteria for being dispensed (e.g., <1 × 103 CFU/L) for all pasteurization methods compared with raw milk, the study was underpowered to evaluate between-group postpasteurization differences (e.g., Holder compared with HHP). Second, we do not have data on why some postpasteurization milk samples failed to meet dispensing criteria. For this study, we used donor human milk that did not meet the Rogers Hixon Ontario Human Milk Bank prepasteurization bacterial load criteria (>5 × 107 CFU/L) and thus would not normally be pasteurized or used for infant feeding. This allowed us to test the effectiveness of pasteurization but likely none of the methods would have eliminated the spore-forming bacteria Bacillus cereus. Out of the 17 pools of raw milk that underwent pasteurization, 2 cultured positive for Bacillus spp. Finally, we acknowledge that there may be other approaches to pasteurization of human milk that were not examined in the current study that warrant further investigation, including high-temperature short-time pasteurization (e.g., 72°C for a minimum of 10 s) and thermo-ultrasonication (25, 60). As far as we are aware, there is no available equipment to perform these methods under the conditions of a milk bank.
In conclusion, of the pasteurization methods studied, HHP appears to be the most viable alternative to the current Holder method owing to its superior ability to retain important bioactive compounds (BSSL, lysozyme, and lactoferrin) and the vitamin folate. Additional research is necessary before full-scale implementation of HHP technology in milk banks, especially to establish whether viral pathogens in milk, including cytomegalovirus and HIV, are eliminated. Preliminary reports on cells lines, culture media, and nonmilk biological matrices are promising (61, 62). In low-resource settings where nonthermal technologies may not be feasible, flash-heating maternal milk may be a suitable way to prevent HIV transmission from mother to child; however, health care professionals and policy makers need to be aware of the impact of heat on important bioactive compounds and heat-sensitive nutrients, including folate and vitamin C. It is unclear whether DM pasteurized using any technique could be used as the sole source of nutrition to meet the vitamin C requirements of an infant.
Acknowledgments
We gratefully acknowledge Sheena Ragoo and Carleigh Jenkins at the Rogers Hixon Ontario Human Milk Bank who facilitated donor screening and sample collection. The authors’ contributions were as follows— MAP, SU, AD, YP, and DLOC: designed the research; MAP, SA, AD, DS and YP: conducted the research; MAP and AK: performed the statistical analyses; MAP: Wrote the initial draft of the paper; DLOC: had primary responsibility for final content; and all authors: provided important critical revision and approved the final manuscript.
Notes
Supported by Canadian Institutes of Health Research grant FDN #143233 (to DLOC, SU, and YP).
Author disclosures: MAP, SU, AD, YP, SA, DS, AK, and DLOC, no conflicts of interest.
Abbreviations used: BSSL, bile-salt-stimulated lipase; DM, human donor milk; HHP, high hydrostatic pressure processing; HMBANA, Human Milk Banking Association of North America.
References
- 1. Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. [DOI] [PubMed] [Google Scholar]
- 2. Breast is always best, even for HIV-positive mothers. [Internet]. Geneva, Switzerland: World Health Organization; 2010; [cited 29 Sept, 2017]. Available from: http://www.who.int/bulletin/volumes/88/1/10-030110/en/. [Google Scholar]
- 3. O'Connor DL, Gibbins S, Kiss A, Bando N, Brennan-Donnan J, Ng E, Campbell DM, Vaz S, Fusch C, Asztalos E et al.. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA. 2016;316(18):1897–905. [DOI] [PubMed] [Google Scholar]
- 4. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6:CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guidelines for the Establishment and Operation of a Donor Human Milk Bank. 10th ed Fort Worth, TX: Human Milk Banking Association of North America; 2018. [Google Scholar]
- 6. Van Zoeren-Grobben D, Schrijver J, Van den Berg H, Berger HM. Human milk vitamin content after pasteurization, storage, or tube feeding. Arch Dis Child. 1987;62:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romeu-Nadal M, Castellote AI, Gayà A, López-Sabater MC. Effect of pasteurisation on ascorbic acid, dehydroascorbic acid, tocopherols and fatty acids in pooled mature human milk. Food Chem. 2008;107:434–8. [Google Scholar]
- 8. Chin KY, Ima-Nirwana S. Vitamin C and bone health: evidence from cell, animal and human studies. Curr Drug Targets. 2018;19(5):439–50. [DOI] [PubMed] [Google Scholar]
- 9. Sharma D, Shastri S. Lactoferrin and neonatology – role in neonatal sepsis and necrotizing enterocolitis: present, past and future. J Matern Fetal Neonatal Med. 2016;29(5):763–70. [DOI] [PubMed] [Google Scholar]
- 10. Underwood MA. Human milk for the premature infant. Pediatr Clin North Am. 2013;60:189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Connor DL, Ewaschuk JB, Unger S. Human milk pasteurization. Curr Opin Clin Nutr Metab Care. 2015;18:269–75. [DOI] [PubMed] [Google Scholar]
- 12. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. [Internet]. Geneva, Switzerland: World Health Organization; 2016; [cited 11 Nov, 2017]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK379874/. [PubMed] [Google Scholar]
- 13. Frequently asked questions, [Internet]. Human Milk 4 Human Babies: Informed Milk Sharing Network; [cited 26 June, 2018]. Available from: http://hm4hb.net/faq/. [Google Scholar]
- 14. Resource for informed breastmilk sharing. [Internet]. Eats On Feets; [cited 26 June, 2018]. Available from: http://www.eatsonfeets.org/. [Google Scholar]
- 15. Israel-Ballard K, Chantry C, Dewey K, Lönnerdal B, Sheppard H, Donovan R, Carlson J, Sage A, Abrams B. Viral, nutritional, and bacterial safety of flash-heated and Pretoria-pasteurized breast milk to prevent mother-to-child transmission of HIV in resource-poor countries: a pilot study. J Acquir Immune Defic Syndr. 2005;40:175–81. [DOI] [PubMed] [Google Scholar]
- 16. Israel-Ballard KA, Abrams BF, Coutsoudis A, Sibeko LN, Cheryk LA, Chantry CJ. Vitamin content of breast milk from HIV-1-infected mothers before and after flash-heat treatment. J Acquir Immune Defic Syndr. 2008;48:444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viazis S, Farkas BE, Jaykus LA. Inactivation of bacterial pathogens in human milk by high-pressure processing. J Food Prot. 2008;71:109–18. [DOI] [PubMed] [Google Scholar]
- 18. Permanyer M, Castellote C, Audí C, Castell M. Maintenance of breast milk immunoglobulin A after high-pressure processing. J Dairy Sci. 2010;93:877–83. [DOI] [PubMed] [Google Scholar]
- 19. Sousa SG, Delgadillo I, Saraiva JA. Effect of thermal pasteurisation and high-pressure processing on immunoglobulin content and lysozyme and lactoperoxidase activity in human colostrum. Food Chem. 2014;151:79–85. [DOI] [PubMed] [Google Scholar]
- 20. Christen L, Lai CT, Hartmann B, Hartmann PE, Geddes DT. Ultraviolet-C irradiation: a novel pasteurization method for donor human milk. PLoS One. 2013;8:e68120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christen L, Lai CT, Hartmann B, Hartmann PE, Geddes DT. The effect of UV-C pasteurization on bacteriostatic properties and immunological proteins of donor human milk. PLoS One. 2013;8:e85867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lloyd ML, Hod N, Jayaraman J, Marchant EA, Christen L, Chiang P, Hartmann P, Shellam GR, Simmer K. Inactivation of cytomegalovirus in breast milk using ultraviolet-C irradiation: opportunities for a new treatment option in breast milk banking. PLoS One. 2016;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 24. Elisia I, Kitts DD. Differences in vitamin E and C profile between infant formula and human milk and relative susceptibility to lipid oxidation. Int J Vitam Nutr Res. 2013;83:311–9. [DOI] [PubMed] [Google Scholar]
- 25. Peila C, Emmerik NE, Giribaldi M, Stahl B, Ruitenberg JE, van Elburg RM, Moro GE, Bertino E, Coscia A, Cavallarin L. Human milk processing: a systematic review of innovative techniques to ensure the safety and quality of donor milk. J Pediatr Gastroenterol Nutr. 2017;64(3):353–61. [DOI] [PubMed] [Google Scholar]
- 26. O'Connor DL, Tamura T, Picciano F. Pteroylpolyglutamates in human milk. Am J Clin Nutr. 1991;53:930–4. [DOI] [PubMed] [Google Scholar]
- 27. Hyun TH, Tamura T. Trienzyme extraction in combination with microbiologic assay in food folate analysis: an updated review. Exp Biol Med. 2005;230:444–54. [DOI] [PubMed] [Google Scholar]
- 28. Romeu-Nadal M, Morera-Pons S, Castellote AI, Lopez-Sabater MC. Rapid high-performance liquid chromatographic method for vitamin C determination in human milk versus an enzymatic method. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830(1):41–6. [DOI] [PubMed] [Google Scholar]
- 29. Yao X, Bunt C, Cornish J, Quek SY, Wen J. Improved RP-HPLC method for determination of bovine lactoferrin and its proteolytic degradation in simulated gastrointestinal fluids. Biomed Chromatogr. 2013;27(2):197–202. [DOI] [PubMed] [Google Scholar]
- 30. Shugar D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952;8:302–9. [DOI] [PubMed] [Google Scholar]
- 31. Lee YC, Yang D. Determination of lysozyme activities in a microplate format. Anal Biochem. 2002;310:223–4. [DOI] [PubMed] [Google Scholar]
- 32. Donnelly-Vanderloo M, O'Connor DL, Shoukri M. Impact of pasteurization and procedures commonly used to rethermalize stored human milk on folate content. Nutr Res. 1994;14(9):1305–16. [Google Scholar]
- 33. Hernell O, Blackberg L. Human milk bile salt-stimulated lipase: functional and molecular aspects. J Pediatr. 1994;125(5 Pt 2):S56–61. [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Cui Q, Yan C. The effect of supplementation of long-chain polyunsaturated fatty acids during lactation on neurodevelopmental outcomes of preterm infant from infancy to school age: a systematic review and meta-analysis. Pediatr Neurol. 2016;59:54–61..e1. [DOI] [PubMed] [Google Scholar]
- 35. Baro C, Giribaldi M, Arslanoglu S, Giuffrida MG, Dellavalle G, Conti A, Tonetto P, Biasini A, Coscia A, Fabris C et al.. Effect of two pasteurization methods on the protein content of human milk. Front Biosci. 2011;3:818–29. [DOI] [PubMed] [Google Scholar]
- 36. Henderson TR, Fay TN, Hamosh M. Effect of pasteurization on long chain polyunsaturated fatty acid levels and enzyme activities of human milk. J Pediatr. 1998;132(5):876–8. [DOI] [PubMed] [Google Scholar]
- 37. Andersson Y, Sävman K, Bläckberg L, Hernell O. Pasteurization of mother's own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 2007;96:1445–9. [DOI] [PubMed] [Google Scholar]
- 38. Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2017;6:CD007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mayayo C, Montserrat M, Ramos SJ, Martínez-Lorenzo MJ, Calvo M, Sánchez L, Pérez MD. Kinetic parameters for high-pressure-induced denaturation of lactoferrin in human milk. Int Dairy J. 2014;39:246–52. [Google Scholar]
- 40. Czank C, Prime DK, Hartmann B, Simmer K, Hartmann PE. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr Res. 2009;66:374–9. [DOI] [PubMed] [Google Scholar]
- 41. Akinbi H, Meinzen-Derr J, Auer C, Ma Y, Pullum D, Kusano R, Reszka KJ, Zimmerly K. Alterations in the host defense properties of human milk following prolonged storage or pasteurization. J Pediatr Gastroenterol Nutr. 2010;51:347–52. [DOI] [PubMed] [Google Scholar]
- 42. Daniels B, Schmidt S, King T, Israel-Ballard K, Amundson Mansen K, Coutsoudis A. The effect of simulated flash-heat pasteurization on immune components of human milk. Nutrients. 2017;9(2):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nash DP, Jonas J. Structure of pressure-assisted cold denatured lysozyme and comparison with lysozyme folding intermediates. Biochemistry. 1997;36(47):14375–83. [DOI] [PubMed] [Google Scholar]
- 44. Iucci L, Patrignani F, Vallicelli M, Guerzoni ME, Lanciotti R. Effects of high pressure homogenization on the activity of lysozyme and lactoferrin against Listeria monocytogenes. Food Control. 2007;18:558–65. [Google Scholar]
- 45. Vannini L, Lanciotti R, Baldi D, Guerzoni ME. Interactions between high pressure homogenization and antimicrobial activity of lysozyme and lactoperoxidase. Int J Food Microbiol. 2004;94:123–35. [DOI] [PubMed] [Google Scholar]
- 46. Viazis S, Farkas BE, Allen JC. Effects of high-pressure processing on immunoglobulin A and lysozyme activity in human milk. J Hum Lact. 2007;23(3):253–61. [Google Scholar]
- 47. Guerra AF, Mellinger-Silva C, Rosenthal A, Luchese RH. Hot topic: Holder pasteurization of human milk affects some bioactive proteins. J Dairy Sci. 2018;101(4):2814–8. [DOI] [PubMed] [Google Scholar]
- 48. Lima HK, Wagner-Gillespie M, Perrin MT, Fogleman AD. Bacteria and bioactivity in Holder pasteurized and shelf-stable human milk products. Curr Dev Nutr. 2017;1(8):e001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chantry CJ, Wiedeman J, Buehring G, Peerson JM, Hayfron K, K'Aluoch O, Lonnerdal B, Israel-Ballard K, Coutsoudis A, Abrams B. Effect of flash-heat treatment on antimicrobial activity of breastmilk. Breastfeed Med. 2011;6(3):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. García-Lara NR, Vieco DE, De la Cruz-Bértolo J, Lora-Pablos D, Velasco NU, Pallás-Alonso CR. Effect of Holder pasteurization and frozen storage on macronutrients and energy content of breast milk. J Pediatr Gastroenterol Nutr. 2013;57(3):377–82. [DOI] [PubMed] [Google Scholar]
- 51. Goes HC, Torres AG, Donangelo CM, Trugo NM. Nutrient composition of banked human milk in Brazil and influence of processing on zinc distribution in milk fractions. Nutrition. 2002;18(7–8):590–4. [DOI] [PubMed] [Google Scholar]
- 52. Martysiak-Zurowska D, Puta M, Barczak N, Dabrowska J, Malinowska-Panczyk E, Kielbratowska B, Kolodziejsa I. Effect of high pressure and sub-zero temperature on total antioxidant capacity and the content of vitamin C, fatty acids and secondary products of lipid oxidation in human milk. Pol J Food Nutr Sci. 2017;67(2):117–22. [Google Scholar]
- 53. Moltó-Puigmartí C, Permanyer M, Castellote AI, López-Sabater MC. Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk. Food Chem. 2011;124:697–702. [Google Scholar]
- 54. Jandhyala M, Barbosa-Canovas G, Swanson BG. Note: retention of ascorbic acid, thiamin and pyridoxal after high hydrostatic pressure or thermal treatments. Food Sci Tech Int. 2002;8(5):303–8. [Google Scholar]
- 55. Fortification basics: stability. [Internet]. DSM; [cited 11 Nov, 2017]. Available from: https://www.dsm.com/content/dam/dsm/nip/en_US/documents/stability.pdf. [Google Scholar]
- 56. Buttner BE, Witthoft CM, Domellof M, Hernell O, Ohlund I. Effect of type of heat treatment of breastmilk on folate content and pattern. Breastfeed Med. 2014;9(2):86–91. [DOI] [PubMed] [Google Scholar]
- 57. Goldsmith SJ, Eitenmiller RR, Toledo RT, Barnhart HM. Effect of processing and storage on the water-soluble vitamin content of human milk. J Food Sci. 1983;48:994–5. [Google Scholar]
- 58. Guneser O, Karagul Yuceer Y. Effect of ultraviolet light on water- and fat-soluble vitamins in cow and goat milk. J Dairy Sci. 2012;95:6230–41. [DOI] [PubMed] [Google Scholar]
- 59. Gregory JF., 3rd Denaturation of the folacin-binding protein in pasteurized milk products. J Nutr. 1982;112(7):1329–38. [DOI] [PubMed] [Google Scholar]
- 60. Escuder-Vieco D, Espinosa-Martos I, Rodríguez JM, Corzo N, Montilla A, Siegfried P, Pallás-Alonso CR, Fernández L. High-temperature short-time pasteurization system for donor milk in a human milk bank setting. Front Microbiol. 2018;9:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakagami T, Shigehisa T, Ohmori T, Taji S, Hase A, Kimura T, Yamanishi K. Inactivation of herpes viruses by high hydrostatic pressure. J Virol Methods. 1992;38(2):255–61. [DOI] [PubMed] [Google Scholar]
- 62. Otake T, Mori H, Kawahata T, Kojima Y, Nishimura H, Oishi I, Shigehisa T. Reduction of HIV-1 infectivity and reverse transcriptase activity by high hydrostatic pressure treatment. Biocontrol Sci. 2000;5(2):127–9. [Google Scholar]