ABSTRACT
Background
Human plasma and tissue lycopene concentrations are heterogeneous even when consuming controlled amounts of tomato or lycopene.
Objectives
Our objective is to determine whether single nucleotide polymorphisms (SNPs) in or near known or putative carotenoid metabolism genes [β-carotene 15,15’ monooxygenase 1 (BCO1), scavenger receptor class B type 1 (SCARB1), ATP-binding cassette transporter subfamily A member 1 (ABCA1), microsomal triglyceride transfer protein (MTTP), apolipoprotein B-48, elongation of very long chain fatty acids protein 2 (ELOVL2), and ATP-binding cassette subfamily B member 1 (ABCB1), and an intergenic superoxide dismutase 2, mitochondrial-associated SNP] are predictive of plasma lycopene responses to steady state tomato juice consumption.
Methods
Secondary linear regression analyses of data from a dose-escalation study of prostate cancer patients [n = 47; mean ± SEM age: 60 ± 1 y; BMI (in kg/m2): 32 ± 1] consuming 0, 1, or 2 cans of tomato-soy juice/d (163 mL/can; 20.6 mg lycopene 1.2 mg β-carotene/can) for 24 ± 0.7 d before prostatectomy were conducted to explore 11 SNP genotype effects on the change in plasma lycopene and plasma and prostate tissue concentrations of lycopene, β-carotene, phytoene, and phytofluene.
Results
Two BCO1 SNP genotypes were significant predictors of the change in plasma lycopene, with SNP effects differing in magnitude and direction, depending on the level of juice intake (rs12934922 × diet group P = 0.02; rs6564851 × diet group P = 0.046). Further analyses suggested that plasma β-carotene changes were predicted by BCO1 rs12934922 (P < 0.01), prostate lycopene by trending interaction and main effects of BCO1 SNPs (rs12934922 × diet group P = 0.09; rs12934922 P = 0.02; rs6564851 P = 0.053), and prostate β-carotene by BCO1 SNP interaction and main effects (rs12934922 × diet group P = 0.01; rs12934922 P < 0.01; rs7501331 P = 0.02).
Conclusions
In conclusion, SNPs in BCO1 and other genes may modulate human plasma and prostate tissue responses to dietary lycopene intake and warrant validation in larger, human controlled feeding intervention and cohort studies. Genetic variants related to carotenoid metabolism may partially explain heterogeneous human blood and tissue responses and may be critical covariates for population studies and clinical trials. This trial was registered at clinicaltrials.gov as NCT01009736.
Keywords: lycopene, carotenoids, genetics, prostate cancer, tomato
Introduction
Inverse associations between tomato product intake, the estimated intake of lycopene (the red carotenoid in tomatoes), or blood lycopene concentrations with prostate cancer risk have been noted in a series of evaluations of the Health Professionals Follow-up Study (1–5). Recent systematic reviews or meta-analyses support these relations (5–8), and studies employing experimental models of prostate carcinogenesis demonstrate inhibitory benefits of tomato (9, 10) or lycopene feeding (11–13). Yet, there is often great variation in the epidemiologic cohort data regarding tomatoes, lycopene, and prostate cancer (14). Many factors may contribute to this, including imprecision in estimating tomato product consumption, variations in bioactive content of tomato varieties (15), and the impacts of food processing (16, 17) or impacts of co-consumed foods on lycopene bioavailability (18–20). Relations between estimated lycopene intake and blood concentrations are modest (21). In our highly controlled studies of tomato product or lycopene intake, we have observed dramatic person-to-person heterogeneity in changes in plasma or tissue carotenoids (16, 22–28), strongly suggesting that individual genetic variation may contribute to this heterogeneity.
Physiologic responses to dietary carotenoid intake are impacted by a number of processes, including extrinsic variables such as molecular structure of the carotenoid, amount consumed, food matrix and co-consumed lipids, drug interactions, and intrinsic variables such as the person's anthropometrics, age, adiposity, and physiologic state [reviewed in (29)]. Genetic factors are emerging as important determinants of responses to carotenoids [reviewed in (29)]. Variation as a result of single nucleotide polymorphisms (SNPs) in genes encoding proteins involved in carotenoid and lipid absorption, distribution, and metabolism are associated with marked differences in postprandial responses to lycopene (27) as well as random blood lycopene concentrations measured in observational studies (30–32). To date, only 1 small study has investigated whether SNPs in the carotenoid metabolizing enzyme β-carotene oxygenase 1 (BCO1) are related to the steady state plasma carotenoid responses to dietary interventions (33), with results suggesting that particular combinations of BCO1 SNP genotypes are associated with plasma lycopene responses.
Quantifying the impact of genetic variation on lycopene plasma and tissue responses may enhance our ability to define the causes of interperson variability in both epidemiologic studies and clinical interventions. This may provide greater precision and insight into diet and cancer relations, and lead to personalized nutritional interventions. The objective of this study was to explore whether a set of candidate SNPs, along with other factors, may be significant determinants of plasma and prostatic carotenoid concentrations in prostate cancer patients consuming a tomato-based juice.
Materials and methods
Participant samples and study design
To explore the impact of candidate SNPs on plasma responses to dietary lycopene, SNP genotypes and associations with carotenoid kinetic outcomes were determined for participants in a study in which men consumed 0, 1, or 2 cans of tomato-soy juice for 24 ± 0.7 d before prostatectomy. The primary outcomes of this study were to evaluate compliance, safety, and biomarkers of exposure in the participants consuming the juice (28). All analyses herein are secondary outcomes. Details of the juice production process, its phytochemical constituents, and this study are found in other publications (28, 34). Procedures were in accordance with the ethical standards set forth by the Ohio State University Institutional Review Board. In brief, men choosing to undergo radical prostatectomy for clinically localized prostate cancer consented and enrolled using a dose-escalation study design, where each arm of the study (none, 1, or 2 cans/d) was sequentially filled. At the baseline visit, subjects provided a nonfasting blood sample and completed a simple questionnaire about their usual lycopene intake from commonly consumed lycopene-containing foods (28). Each 5.5 oz. (163 mL) can of tomato-soy juice provided lycopene (20.6 mg total lycopene/can, 18 mg as all-trans lycopene), β-carotene (1.15 mg/can), phytoene (2.4 mg/can), and phytofluene (1.1 mg/can). During the study, subjects consumed a restricted dietary lycopene diet, which limited lycopene intakes from nonintervention foods to <5 mg/d using a dietary lycopene tracking worksheet (28). The end of the study was defined by the scheduling of the prostatectomy, and on the day of the surgery, after an overnight fast, subjects provided a blood sample. Fresh prostate tissue was collected for carotenoid analysis, and noncancerous areas were used for genetic analysis. Genotype data were not obtainable for 12 of the initial 60 subjects because there was insufficient tissue available, and 1 subject did not complete baseline dietary questionnaires, thus the group sizes were n = 12, n = 10, and n = 25 for 0, 1, and 2 cans/d groups, respectively. Whether SNP genotypes were associated with changes in other plasma tomato carotenoids (phytoene, phytofluene, and β-carotene), with baseline plasma carotenoids, or with end-of-study prostate carotenoid concentrations were also analyzed as additional exploratory outcomes.
Plasma and prostate tissue carotenoid analysis
Plasma and prostate tissue concentrations of lycopene, β-carotene, phytoene, and phytofluene were analyzed by high pressure liquid chromatography with photodiode array detection, as described elsewhere (28). Mean plasma and prostate carotenoid concentrations and statistical analyses of these outcomes for the complete population (N = 56) have been described previously (28).
Selection of candidate gene SNPs
Two separate experiments of the associations of different groupings of candidate SNPs with carotenoid outcomes were conducted. Details about the SNPs examined in each study are described in Supplemental Table 1 and below.
Experiment 1.
The first group of candidate gene SNPs was selected on the basis of publications reporting that variation in these particular SNPs is associated with differences in steady state blood carotenoid concentrations in humans or because of specific hypotheses relating the gene function with carotenoid assimilation. Because the sample size was small, we chose a list of candidate gene SNPs reported to frequently occur in the reference Caucasian population. The candidate list was composed of 10 genes: 2 carotenoid oxygenases known to cleave lycopene and β-carotene: BCO1 (35) and β-carotene oxygenase 2 (36); proteins previously shown to be involved in the absorption of carotenoids: cluster of differentiation 36 (37), scavenger receptor class B member 1 (SCARB1) (38), intestine-specific homeobox (39); and proteins involved in lipid metabolism, which may be involved in carotenoid metabolism but for which there are not yet mechanistic data to support this: ATP-binding cassette, subfamily A member 1 (ABCA1), microsomal triglyceride transfer protein (MTTP), apolipoprotein B, apolipoprotein A-IV, and secretion associated, ras related GTPase 1B (26). Using the National Center for Biotechnology Information SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP), we evaluated the SNPs in these genes and selected those meeting the following criteria: minor allele frequency >10% in the reference population, and either results in a missense mutation or, if intronic, literature supports a relation with carotenoid status. Based on these criteria, we found 5 SNPs in 4 genes which met our criteria, and included 2 additional SNPs based on previous literature. The intronic SCARB1 rs11057841 SNP met our prevalence criteria, and was included as it was previously found to be associated with serum lutein in 2 cohorts, whereby each T-allele was associated with a 24% increase in serum lutein (40). The BCO1 rs6564851 SNP was not found by our dbSNP search because it is upstream of the gene, but was included in our analysis because the G-allele was previously associated with blood carotenoid concentrations (30). β-carotene oxygenase 2, cluster of differentiation 36, intestine-specific homeobox, apolipoprotein A-IV, and secretion associated, ras related GTPase 1B did not have known SNPs that met our inclusion criteria for the reference populations.
The objective of this current analysis was to determine whether any of these candidate SNPs are significant predictors of the change in plasma lycopene concentration in response to 0, 1, or 2 cans of tomato-soy juice/d. Before the genetic analysis, a set of additional questions leveraging the available data were defined to determine whether the SNPs were predictors of baseline plasma carotenoid concentrations, prostatic carotenoid concentrations, or the change in plasma phytoene, phytofluene, and β-carotene in response to the dietary treatments.
Experiment 2.
A second set of candidate gene SNPs were identified from a previously published list of 28 SNPs in 16 genes found to explain 72% of the variance in postprandial plasma chylomicron lycopene responses to lycopene consumption (27). The same allele frequency criteria described above were used to identify candidate SNPs; however, because many of the significant SNPs related to lycopene bioavailability (27) were not in the coding region of the genes, we did not require that the SNP led to a missense mutation. Thus, the objective was to assess the relation of 6 SNPs [elongation of very long chain fatty acids protein 2 (ELOVL2) rs911196, rs3798709, and rs9468304; intergenic SNP rs9365046 (previously associated with superoxide dismutase 2, mitochondrial) (27); MTTP rs17029173; and ATP-binding cassette, subfamily B, ), member 1 (ABCB1) rs10248420] with the change in plasma lycopene concentration in response to 0, 1, or 2 cans of tomato-soy juice/d. As in Experiment 1, additional exploratory hypotheses were defined to assess SNP relations with baseline plasma carotenoids, prostate carotenoids, and plasma phytoene, phytofluene, and β-carotene responses.
Linkage disequilibrium of the 3 BCO1 SNPs and the 3 ELOVL2 SNPs was evaluated using LDmatrix with LDlink 1.1 (analysistools.nci.nih.gov/LDlink/, NIH, National Cancer Institute) (41) using the CEU (Utah Residents from North and Western Europe) reference population, which most closely reflected our 98% Caucasian, North American study population.
DNA extraction and SNP genotyping
Normal prostate DNA was isolated from formalin-fixed paraffin-embedded tissue (5 µm in thickness), which was noncancerous. Slides were stripped of paraffin using xylene and rehydrated in subsequent ethanol/water washes. Tissue was scraped from slides and DNA was isolated as previously described (42).
SNP genotypes were determined using Taqman genotyping assays (Applied Biosystems) according to the manufacturer's recommended conditions. Analyses were run on the Applied Biosystems 7900HT Fast Real-Time PCR System for 40 cycles. An endpoint plate read was performed for allelic discrimination. The following Taqman genotyping assays were used to identify genotypes of specific gene SNPs: BCO1 rs12934922 (C__25,745,282_10), BCO1 rs7501331 (C__25,772,261_20), rs6564851 (C__28,949,771_10) (upstream of BCO1), SCARB1 rs11057841 (C__11,274,631_10), ABCA1 rs2230808 (C__2,741,104_1_), MTTP rs2306985 (C__16,195,242_10), MTTP rs170291173 (custom-order; sequences available upon request), and apolipoprotein B rs679899 (C_1,026,583_10), ELOVL2 rs3798709 (C__27,518,037_10), ELOVL2 rs9468304 (C__31,002,050_10), and ELOVL2 rs911196 (custom-order; sequences available upon request), and ABCB1 rs10248420 (C__30,375,194_10), and rs9365046 (intergenic (27)) (C__29,781,433_10). Water and duplicate control DNA samples were used for assay controls.
Blood chemistry measurements
Serum lipid analysis was conducted by the Ohio State Clinical Research Center Analytical Laboratory using the Dimension Xpand Clinical Chemistry System (Siemens Medical Diagnostics). The analytical sensitivity was 3.0 mg/dL for HDL cholesterol, 5 mg/dL for LDL cholesterol, 50 mg/dL for cholesterol, and 15 mg/dL for triglyceride.
Statistics
All statistical analyses were conducted using SigmaPlot 13.0 (Systat Software, Inc.) with 1 exception described below. Plasma and prostate carotenoid concentration differences between diet groups of men for whom SNPs were analyzed were determined by Kruskal-Wallis 1-factor ANOVA with Dunn's post hoc pairwise test. The effects of diet group (0, 1, or 2 cans of juice/d), SNP genotype (0, 1, or 2 effect-alleles), diet group × SNP genotype interaction, along with covariates for intervention duration (d), baseline plasma lycopene concentration (nmol/mg LDL), body mass (kg), and extraneous dietary lycopene were evaluated using a 2-step multiple linear regression process: first SNP model screening, then combined SNP model building. SNP genotypes were independent of the covariates as determined by Spearman's Rank Order correlation method (data not shown). Plasma carotenoids are carried in the lipoprotein-containing fractions of plasma (43), with carotenoids being preferentially incorporated into different lipoprotein cholesterol fractions (43), therefore plasma lipoprotein cholesterol concentrations are often covariates for plasma carotenoid concentrations. Plasma carotenoid concentrations were expressed in relation to plasma lipoprotein cholesterol fraction (total, LDL cholesterol, and HDL cholesterol) concentrations with which they were most strongly correlated at baseline as determined by Pearson Product Moment Correlation. This was done to reduce the number of covariates in the statistical models and because the baseline blood samples collected were from nonfasting subjects, while the end-of-study plasma concentrations were from fasting subjects, which can impact the plasma lipoprotein cholesterol concentrations in the blood. Plasma lycopene concentrations were adjusted to LDL cholesterol concentrations because it was the most strongly correlated cholesterol fraction (of total cholesterol, LDL cholesterol, and HDL cholesterol) at baseline. The SNP screening models evaluated the effect of SNP genotype, diet group, and genotype × diet group interactions in the context of covariates: body mass (kg), intervention duration (d), baseline plasma lycopene concentration adjusted for LDL cholesterol (nmol/mg LDL cholesterol), and nonintervention lycopene intake from the diet as assessed by the daily lycopene intake worksheet (mean mg/d). In this exploratory study, significance of the SNP genotype × diet group interaction and SNP genotype main effects were considered significant for an unadjusted P ≤ 0.05, and suggestive for 0.05 < P ≤ 0.10. In the combined SNP model-building step, significant and suggestive SNPs and SNP × diet group variables along with retained covariates (unadjusted P ≤ 0.10 and a positive contribution to the total adjusted R2) were combined into 1 model, which was evaluated using backwards stepwise elimination to determine what amount of the variance (adjusted R2) in the plasma lycopene response could be attributable to each variable.
Additional questions were pursued to leverage the existing data for hypothesis-generation. In brief, we explored the predictors of change in plasma phytoene (nmol/mg total cholesterol), phytofluene (µmol/L), and β-carotene (µmol/L), of baseline plasma lycopene, phytoene, and phytofluene, and of end-of-study prostate carotene concentrations (all in nmol/g). The same 2-step approach as above was used to determine the effect of SNP genotype, diet group, and SNP × diet group on the change in plasma carotenes and on final prostate carotene. To improve normality, the changes in plasma phytoene, phytofluene, and β-carotene were log-transformed. Prostate carotenoid model covariates were carotenoid intake from nonintervention foods (mean mg/d), baseline plasma carotenoid concentration (as a marker of baseline carotenoid status), and intervention duration (d). The relations of baseline plasma lycopene, phytoene, phytofluene, and β-carotene concentrations with SNP genotype, usual intake of the carotenoid (by baseline questionnaire), and SNP × usual carotenoid intake interactions were assessed in the context of a covariate for body mass (kg). Significance cutoffs were as described above. To improve normality, baseline plasma phytoene, phytofluene, and β-carotene were log-transformed. A list of all of the first-step, screening models are found in Supplemental Table 2, with corresponding model P values and false discovery rates (44) calculated using R (www.r-project.org). Significant SNPs, interactions, and covariates were combined into a predictive model for each respective experiment to evaluate the contribution of each variable to the outcome variance (adjusted R2). To visualize the magnitude and direction of the effects of SNP genotypes, model outcomes in response to SNP and dietary variables main effects were calculated with covariates held constant and presented as bar graphs.
Results
Baseline plasma carotenoid concentrations and estimated baseline carotenoid intakes
Subject characteristics, and baseline plasma lipid profile, plasma carotenoid concentrations, and calculated usual carotene intakes from lycopene-rich foods are found in Table 1.
TABLE 1.
Baseline characteristics of genotyped men with prostate cancer1
| N | 47 |
| Age, y | 60 ± 1 |
| Race, n (%) | |
| Caucasian | 46 (98%) |
| African American | 1 (2%) |
| Body mass, kg | 101 ± 3 |
| Body mass index, kg/m2 | 32 ± 1 |
| Plasma analytes | |
| Total cholesterol2, mg/dL | 168 ± 5 |
| LDL cholesterol, mg/dL | 107 ± 4 |
| HDL cholesterol, mg/dL | 42 ± 1 |
| Triglycerides, mg/dL | 179 ± 17 |
| Lycopene, µmol/L | 1.17 ± 0.07 |
| β-carotene, µmol/L | 0.22 ± 0.01 |
| Phytoene, µmol/L | 0.14 ± 0.01 |
| Phytofluene, µmol/L | 0.21 ± 0.027 |
| Usual intake3, mg/d | |
| Lycopene | 8.1 ± 0.97 |
| β-carotene | 0.43 ± 0.05 |
| Phytoene | 1.4 ± 0.19 |
| Phytofluene | 0.97 ± 0.09 |
1Values are means ± SEMs.
240% (19/47) of subjects reported taking cholesterol or triglyceride-lowering drugs.
3Usual carotenoid intake is based on subject responses to a questionnaire administered at baseline on the usual number of servings consumed weekly of common high lycopene foods.
Daily carotenoid intake, compliance, and responses to dietary intervention
The participants in this study were compliant with the intervention [0 cans/d group was 100% compliant, 1 can/d group consumed 97.5 ± 1.5% (mean ± SEM) of the assigned cans, 2 cans/d group consumed 94.6 ± 1.5% of the assigned cans]. Subjects tracked intake of nonintervention lycopene-rich foods, which contributed 0.66 ± 0.11 mg lycopene/d, 0.06 ± 0.01 mg β-carotene/d, 0.20 ± 0.03 mg phytoene/d, and 0.09 ± 0.01 mg phytofluene/d. The mean total lycopene (nonintervention lycopene foods + intervention lycopene) consumed was 1.1 ± 0.29 mg/d, 21 ± 0.20 mg/d, and 42 ± 0.10 mg/d for the 0, 1, and 2 can/d groups, respectively. Plasma and prostate carotenoid concentrations of men with prostate cancer consuming tomato-soy juice included in SNP analyses are presented in Supplemental Tables 3and4.
Correlation between plasma carotenoids and plasma lipids
Baseline plasma lycopene concentrations were correlated by Pearson's correlation with baseline LDL cholesterol (r = 0.556; P = 8.46 × 10−6) and total cholesterol (r = 0.509; P = 6.11 × 10−5) concentrations, but not HDL cholesterol concentrations. Baseline plasma phytoene concentrations were correlated with LDL cholesterol (r = 0.282, P= 0.04), total cholesterol (r = 0.313, P= 0.02), but not with HDL cholesterol concentrations. Baseline plasma phytofluene concentrations were not correlated with HDL cholesterol, total cholesterol, or LDL cholesterol concentrations. Baseline β-carotene plasma concentrations were not significantly correlated with plasma HDL cholesterol, LDL cholesterol, or total cholesterol concentrations.
SNP genotype frequencies, linkage disequilibrium, and associations with covariates
The frequencies of investigated SNP genotypes are found in Tables 2 and 3. All SNPs were in Hardy-Weinberg equilibrium with the HapMap CEU population. Of the 3 BCO1 SNPs, rs12934922 and rs6564851 were not in linkage disequilibrium (D’ = 0.041 and R2 = 0.001). However, rs7501331 and rs6564851 were in weak linkage disequilibrium (D’ = 0.28, R2 = 0.037), as were rs12934922 and rs7501331 (D’ = 0.547, R2 = 0.077). The candidate ELOVL2 SNP genotypes were all in strong linkage disequilibrium with one another, rs911196 and rs3498709 with a D’ = 1.0, R2 = 1.0, rs911196 and rs9468304 with a D’ = 0.967, R2 = 0.936, and rs3798709 and rs9468304 with a D’ = 0.967, R2 = 0.936. Intergenic rs9365046 linkage disequilibrium with the ELOVL2 SNPs was evaluated because they reside on chromosome 6, and rs9365046 was not in linkage disequilibrium with the 3 ELOVL2 SNPs (D’ = 0.256, R2 = 0.001 for all 3). SNP genotypes were not correlated with baseline usual intake of lycopene, phytoene, phytofluene, or β-carotene from high lycopene foods, body mass, intervention duration, or baseline plasma LDL cholesterol or total cholesterol concentrations (P > 0.05).
TABLE 2.
Genotype frequencies in men with prostate cancer by tomato-soy juice diet group for Experiment 1 and genotype distributions in the reference population1
| BCO1 rs12934922 | BCO1 rs7501331 | BCO1 rs6564851 | SCARB1 rs11057841 | ABCA1 rs2230808 | APOB rs679899 | MTTP rs2306985 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | |
| Diet group (cans/d) | ||||||||||||||
| 0 | AA | 3 (6%) | CC | 9 (19%) | GG | 3 (6%) | CC | 7 (15%) | GG | 6 (13%) | GG | 2 (4%) | CC | 3 (6%) |
| — | AT | 5 (11%) | CT | 2 (4%) | GT | 4 (9%) | CT | 5 (11%) | AG | 4 (9%) | AG | 8 (17%) | CG | 7 (15%) |
| — | TT | 4 (9%) | TT | 1 (2%) | TT | 5 (11%) | TT | 0 (0%) | AA | 1 (2%) | AA | 1 (2%) | GG | 2 (4%) |
| 1 | AA | 4 (9%) | CC | 4 (9%) | GG | 3 (6%) | CC | 6 (13%) | GG | 5 (11%) | GG | 5 (11%) | CC | 4 (9%) |
| — | AT | 4 (9%) | CT | 5 (11%) | GT | 6 (13%) | CT | 4 (9%) | AG | 4 (9%) | AG | 4 (9%) | CG | 4 (9%) |
| — | TT | 2 (4%) | TT | 1 (2%) | TT | 1 (2%) | TT | 0 (0%) | AA | 1 (2%) | AA | 1 (2%) | GG | 2 (4%) |
| 2 | AA | 4 (9%) | CC | 12 (26%) | GG | 8 (17%) | CC | 18 (38%) | GG | 15 (33%) | GG | 8 (17%) | CC | 11 (23%) |
| — | AT | 17 (36%) | CT | 11 (23%) | GT | 8 (17%) | CT | 6 (13%) | AG | 9 (20%) | AG | 12 (26%) | CG | 11 (23%) |
| — | TT | 4 (9%) | TT | 2 (4%) | TT | 9 (19%) | TT | 1 (2%) | AA | 1 (2%) | AA | 5 (11%) | GG | 3 (6%) |
| All | AA | 11 (23%) | CC | 25 (53%) | GG | 14 (30%) | CC | 31 (66%) | GG | 26 (57%) | GG | 15 (33%) | CC | 18 (38%) |
| — | AT | 26 (55%) | CT | 18 (38%) | GT | 18 (38%) | CT | 15 (32%) | AG | 17 (36%) | AG | 24 (52%) | CG | 22 (47%) |
| — | TT | 10 (21%) | TT | 4 (9%) | TT | 15 (32%) | TT | 1 (2%) | AA | 3 (7%) | AA | 7 (15%) | GG | 7 (15%) |
| N | — | 47 | — | 47 | — | 47 | — | 47 | — | 46 | — | 46 | — | 47 |
| CEU2 reference population | AA3 | 31% | CC4 | 45% | GG5 | 32% | CC6 | 75% | GG7 | 3% | GG8 | 31% | CC9 | 45% |
| — | AT | 50% | CT | 52% | GT | 49% | CT | 22% | AG | 25% | AG | 49% | CG | 42% |
| — | TT | 19% | TT | 3% | TT | 19% | TT | 3% | AA | 71% | AA | 21% | GG | 13% |
1 ABCA1, ATP-binding cassette transporter subfamily A, member 1; APOB, apolipoprotein B; BCO1, β-carotene 15,15’ monooxygenase 1; MTTP, microsomal triglyceride transfer protein; SCARB1, scavenger receptor class B type 1.
2CEU, reference population based on 180 samples from Utah residents with Northern and Western European ancestry from phase 3 of the International HapMap project.
3CEU reference data are from ss48414159.
4CEU reference data are from ss69351277.
5CEU reference data are from ss11320807.
6CEU reference data are from ss17455704.
7CEU reference data are from ss66130841.
8CEU reference data are from ss71648483.
9CEU reference data are from ss44554337.
TABLE 3.
Genotype frequencies in men with prostate cancer by tomato-soy juice diet group for Experiment 2 and distributions in the reference population1
| ELOVL2 rs911196 | ABCB1 rs10248420 | “SOD2” rs9365046 | MTTP rs17029173 | |||||
|---|---|---|---|---|---|---|---|---|
| Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | Genotype | n (%) | |
| Diet group (cans/d) | ||||||||
| 0 | AA | 6 (13%) | AA | 10 (21%) | GG | 8 (17%) | TT | 7 (15%) |
| — | AC | 6 (13%) | AG | 2 (4%) | GA | 3 (7%) | TG | 3 (7%) |
| — | CC | 0 (0%) | GG | 0 (0%) | AA | 0 (0%) | GG | 2 (4%) |
| 1 | AA | 8 (17%) | AA | 6 (13%) | GG | 9 (20%) | TT | 10 (22%) |
| — | AC | 2 (4%) | AG | 3 (6%) | GA | 1 (2%) | TG | 0 (0%) |
| — | CC | 0 (0%) | GG | 1 (2%) | AA | 0 (0%) | GG | 0 (0%) |
| 2 | AA | 19 (40%) | AA | 16 (34%) | GG | 23 (50%) | TT | 17 (37%) |
| — | AC | 6 (13%) | AG | 9 (19%) | GA | 2 (4%) | TG | 7 (15%) |
| — | CC | 0 (0%) | GG | 0 (0%) | AA | 0 (0%) | GG | 0 (0%) |
| All groups | AA | 33 (70%) | AA | 32 (70%) | GG | 41 (87%) | TT | 34 (74%) |
| — | AC | 14 (30%) | AG | 14 (30%) | GA | 6 (13%) | TG | 10 (22%) |
| — | CC | 0 (0%) | GG | 1 (2%) | AA | 0 (0%) | GG | 2 (4%) |
| N | — | 47 | — | 47 | — | 47 | — | 462 |
| Reference population, CEU3 | AA4 | 63% | AA5 | 67% | GG6 | 92% | TT7 | 78% |
| — | CA | 35% | AG | 32% | GA | 8% | TG | 20% |
| — | CC | 2% | GG | 1% | AA | 0% | GG | 2% |
1 ABCB1, ATP-binding cassette subfamily B, member 1; ELOVL2, elongation of very long chain fatty acids protein 2; MTTP, microsomal triglyceride transfer protein; SOD2, superoxide dismutase 2, mitochondrial.
2One of the subjects did not have a discernable genotype for MTTP rs17029173 and was therefore not included in the analysis.
3CEU, reference population based on 180 samples from Utah residents with Northern and Western European ancestry from phase 3 of the International HapMap project.
4CEU reference data are from ss71646511.
5CEU reference data are from ss14118214.
6CEU reference data are from ss12830088.
7CEU reference data are from ss23331365.
Variables predictive of the plasma lycopene response to tomato-soy juice consumption
The plasma carotenoid responses to the dietary interventions are shown in Figure 1 and the baseline plasma carotenoid, change in plasma carotenoid, and end-of-study prostate carotenoid concentrations are found in Supplemental Tables 3 and 4.
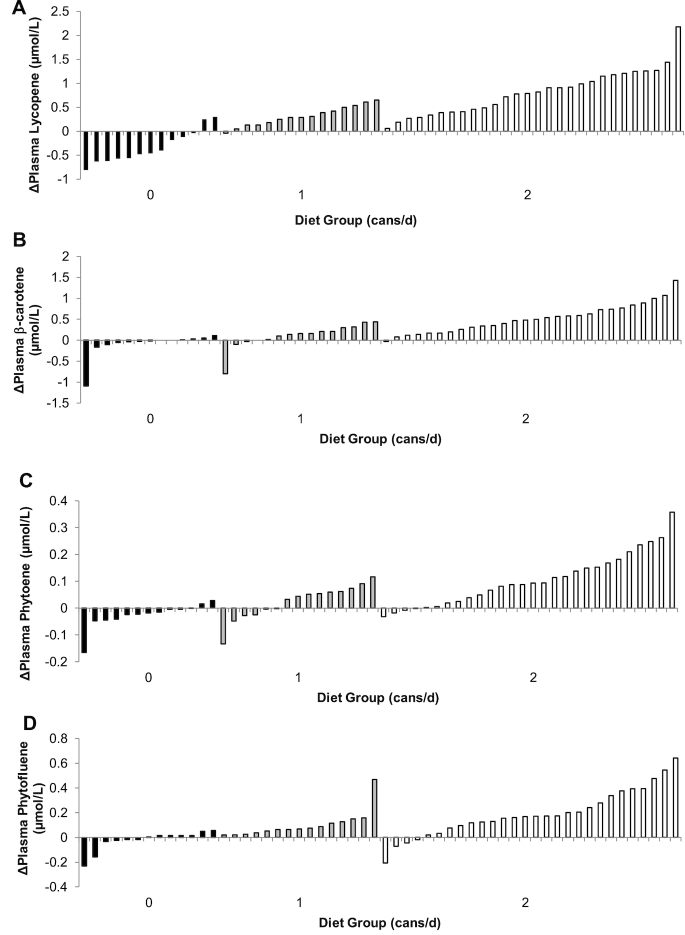
FIGURE 1.
Individual changes in plasma lycopene (A), β-carotene (B), phytoene (C), and phytofluene (D) concentrations in response to tomato-soy juice consumption in men with prostate cancer.
Experiment 1.
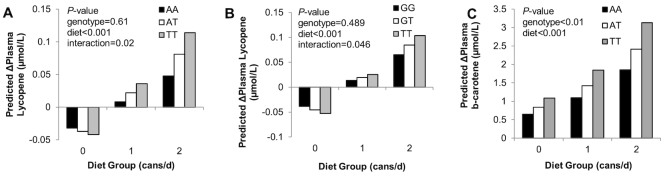
Several variables were predictors of an individual's change in plasma lycopene concentration over the course of the study, with intervention tomato juice consumption accounting for the greatest proportion of variance in plasma lycopene response (Table 4), followed by covariates of baseline plasma lycopene concentration and body mass, which were negative predictors. The proportions of variance in plasma lycopene change explained by the variables are graphically depicted in Supplemental Figure 1. The effects of BCO1 rs12934922 and rs6564851 genotypes on plasma lycopene response both differed by diet group (Table 4). Model-estimated diet group × genotype interactions impacting plasma lycopene responses adjusted for covariates are shown in Figure 2. Specifically, the BCO1 rs12934922 genotype × diet group interaction was such that, when covariates and rs6564851 genotype were held constant, the T-allele was associated with greater increases in plasma lycopene in the men consuming 1 can/d (0.01 µmol/L per T-allele) and 2 cans/d (0.03 µmol/L per T-allele), but greater reductions in plasma lycopene in the 0 cans/d group (0.005 µmol/L per T-allele). BCO1 rs6564851 genotype was associated with the change in plasma lycopene concentration, with the T-allele being associated with greater increases in plasma lycopene in the 1 and 2 cans/d groups, but with greater reductions in plasma lycopene in the 0 cans/d group. Other tested SNPs were not associated with a change in plasma lycopene in this experiment.
TABLE 4.
Experiment 1: variables predictive of the change in plasma carotenoid concentrations in men with prostate cancer consuming tomato-soy juice1
| Carotenoid | Units | Transformation | Model goodness of fit (adjusted R2) | Model P value | Variable | β ± SE | P value for variable | Estimated proportion of response variability attributable to variable2 |
|---|---|---|---|---|---|---|---|---|
| Lycopene | nmol/mg LDL cholesterol | None | 0.757 | <0.001 | Constant3 | −0.030 ± 0.013 | 0.02 | — |
| — | — | — | — | — | Diet group (cans/d) | 0.073 ± 0.010 | <0.001 | 54.7% |
| — | — | — | — | — | BCO1 rs12934922 genotype × diet group | −0.018 ± 0.007 | 0.02 | 3.6% |
| — | — | — | — | — | BCO1 rs6564851 genotype × diet group | −0.013 ± 0.006 | 0.046 | 2.0% |
| — | — | — | — | — | BCO1 rs12934922 genotype | 0.005 ± 0.010 | 0.61 | 0% |
| — | — | — | — | — | BCO1 rs6564851 genotype | 0.007 ± 0.010 | 0.49 | 0% |
| — | — | — | — | — | Baseline plasma lycopene (nmol/mg LDL cholesterol) (difference from mean) | −0.549 ± 0.101 | <0.001 | 15.0% |
| — | — | — | — | — | Body mass (kg) (difference from mean) | −0.0006 ± 00002 | 0.02 | 2.0% |
| β-carotene | µmol/L | Log10 | 0.619 | <0.001 | Constant | 0.015 ± 0.062 | 0.81 | — |
| — | — | — | — | — | Diet group (cans/d) | 0.230 ± 0.032 | <0.001 | 37.3% |
| — | — | — | — | — | BCO1 rs12934922 genotype | −0.114 ± 0.040 | <0.01 | 19.4% |
| — | — | — | — | — | Nonintervention β-carotene from high lycopene foods (mg/d) (difference from mean) | −1.231 ± 0.463 | 0.01 | 5.2% |
| Phytoene | nmol/mg total cholesterol | Log10 | 0.469 | <0.001 | Constant | −0.121 ± 0.045 | 0.01 | — |
| — | — | — | — | — | Diet group (cans/d) | 0.189 ± 0.030 | <0.001 | 39.7% |
| — | — | — | — | — | Baseline Log10 plasma phytoene (nmol/mg total cholesterol) (difference from mean) | −0.410 ± 0.103 | <0.001 | 14.9% |
| Phytofluene | µmol/L | Log10 | 0.667 | <0.001 | Constant | 0.014 ± 0.050 | 0.79 | — |
| — | — | — | — | — | Diet group (cans/d) | 0.164 ± 0.026 | <0.001 | 30.0% |
| — | — | — | — | — | MTTP rs2306985 genotype | −0.055 ± 0.032 | 0.09 | 7.4% |
| — | — | — | — | — | Baseline Log10 plasma phytofluene (µmol/L) (difference from mean) | −0.531 ± 0.085 | <0.001 | 23.7% |
| — | — | — | — | — | Body mass (kg) (difference from mean) | −0.004 ± 0.001 | <0.01 | 5.6% |
1 BCO1, β-carotene 15,15’ monooxygenase 1; MTTP, microsomal triglyceride transfer protein.
2As determined by stepwise removal of variables and quantifying the contribution of the variable to the model's adjusted R2. See Supplemental Figure 1 for graphical depiction of the proportion of variance in the outcome explained by each variable.
3“Constant” represents the y-intercept for the model equation.
FIGURE 2.
Model-predicted changes of plasma lycopene and β-carotene concentrations by BCO1 rs12934922 (A), rs6564851 (B), rs12934922 (C) genotypes and tomato-soy juice treatments (∼23 d) in prostate cancer patients. Bars represent predicted outcomes from the multiple linear regression model while holding covariate effects constant. Model variables are presented in Table 4. P values are for the effects in the multivariable models. Significant effects are P ≤ 0.05, and nonsignificantly trending effects are 0.05 < P ≤ 0.10.
Experiment 2.
We observed no association between SNPs previously reported to impact lycopene bioavailability (27) and the change in plasma lycopene concentration in response to the tomato juice intake.
Variables predictive of the changes in plasma β-carotene, phytoene, and phytofluene in response to tomato-soy juice consumption
Using a model-building approach, we were able to identify relations between genetic variables and plasma carotenoid responses to dietary intervention (Tables 5 and 6, and Supplemental Figures 1 and 2). For lycopene, juice intake was consistently the most important, positive predictor contributing to the change in plasma carotenoid response for β-carotene, phytoene, and phytofluene. Baseline plasma concentrations were the second greatest determinants of phytoene and phytofluene plasma responses, and were negative predictors. Body mass was a negative predictor of plasma lycopene and phytofluene responses, but not β-carotene or phytoene responses. β-carotene consumed from nonintervention tomato products was also a negative predictor of the change in plasma β-carotene. Tomato phytoene, phytofluene, or lycopene from nonintervention tomato products were not predictors of respective plasma responses for these carotenes.
TABLE 5.
Experiment 2: variables predictive of the change in plasma carotenoid concentrations in men with prostate cancer consuming tomato-soy juice1
| Carotenoid | Transformation | Model Adjusted R2 | Model P value | Variable | β ± SE | P value for variable | Estimated proportion of response variability attributable to variable2 |
|---|---|---|---|---|---|---|---|
| Lycopene, nmol/mg LDL cholesterol | None | 0.752 | <0.001 | Constant3 | −0.013 ± 0.004 | <0.01 | — |
| — | — | — | — | Diet group | 0.029 ± 0.003 | <0.001 | 60% |
| — | — | — | — | Baseline plasma lycopene (nmol/mg LDL cholesterol) (difference from mean) | −0.301 ± 0.056 | <0.001 | 12% |
| — | — | — | — | Body mass (difference from mean) | −0.0003 ± 0.0001 | 0.01 | 3% |
| β-carotene, µmol/L | Log10 | 0.605 | <0.001 | Constant | −0.115 ± 0.055 | 0.04 | — |
| — | — | — | — | Diet group | 0.270 ± 0.038 | <0.001 | 37% |
| — | — | — | — | ABCB1 rs10248420 genotype × diet group | −0.148 ± 0.074 | 0.053 | 3% |
| — | — | — | — | ABCB1 rs10248420 genotype | 0.099 ± 0.112 | 0.38 | 7% |
| — | — | — | — | Extraneous dietary β-carotene (difference from mean) | −1.358 ± 0.474 | <0.07 | 14% |
| Phytoene, nmol/mg total cholesterol | Log10 | 0.522 | <0.001 | Constant | −1.057 ± 0.298 | <0.001 | — |
| — | — | — | — | Diet group | 0.192 ± 0.034 | <0.001 | 12% |
| — | — | — | — | “SOD2” rs9365046 genotype | −0.156 ± 0.0844 | 0.07 | 32% |
| — | — | — | — | Baseline Log10 [plasma phytoene (nmol/mg total cholesterol)] (difference from mean) | −0.435 ± 0.125 | 0.001 | 9% |
| Phytofluene, µmol/L | Log10 | 0.705 | <0.001 | Constant | −0.085 ± 0.041 | 0.02 | — |
| — | — | — | — | Diet group | 0.182 ± 0.025 | <0.001 | 30% |
| — | — | — | — | ELOVL2 rs911196 genotype | 0.124 ± 0.048 | 0.01 | 10% |
| — | — | — | — | Baseline Log10 [plasma phytofluene (µmol/L)] (difference from mean) | −0.584 ± 0.083 | <0.001 | 24% |
| — | — | — | — | Body mass (kg) (difference from mean) | −0.004 ± 0.001 | <0.001 | 5% |
1 ABCB1, ATP-binding cassette subfamily B member 1; ELOVL2, elongation of very long chain fatty acids protein 2; SOD2, superoxide dismutase 2, mitochondrial.
2As determined by stepwise removal of variables and quantifying the contribution of the variable to the model's adjusted R2. See Supplemental Figure 2 for graphical depiction of the proportion of variance in the outcome explained by each variable.
3“Constant” represents the y-intercept for the model.
TABLE 6.
Experiment 1: variables predictive of end-of-study prostate and baseline plasma carotenoid concentrations in men with prostate cancer consuming tomato-soy juice1
| Tissue carotenoid concentration outcome | Transformation | Model adjusted R2 | Model P value | Variable | β ± SE | P value for variable | Estimated proportion of response variability attributable to variable2 |
|---|---|---|---|---|---|---|---|
| Prostate | |||||||
| Lycopene, nmol/g | None | 0.276 | 0.004 | Constant3 | 0.421 ± 0.108 | <0.001 | — |
| — | — | — | — | Diet group (cans/d) | 0.187 ± 0.086 | 0.04 | 1.2% |
| — | — | — | — | BCO1 rs12934922 genotype | 0.211 ± 0.086 | 0.02 | 4.2% |
| — | — | — | — | BCO1 rs12934922 genotype × diet group | −0.129 ± 0.073 | 0.09 | 4% |
| — | — | — | — | BCO1 rs6564851 genotype | −0.097 ± 0.049 | 0.053 | 1.9% |
| — | — | — | — | Baseline plasma lycopene (nmol/mg LDL cholesterol) | 3.27 ± 0.979 | <0.01 | 16.3% |
| β-carotene, nmol/g | None | 0.343 | 0.001 | Constant | −0.024 ± 0.070 | 0.737 | — |
| — | — | — | — | Diet group | 0.143 ± 0.050 | <0.01 | 0% |
| — | — | — | — | Body mass | −0.003 ± 0.001 | 0.03 | 7% |
| — | — | — | — | Baseline plasma β-carotene (umol/L)* | 0.173 ± 0.047 | <0.001 | 28% |
| — | — | — | — | BCO1 rs12934922 genotype | 0.147 ± 0.052 | <0.01 | 0% |
| — | — | — | — | BCO1 rs12934922 genotype × diet group | −0.108 ± 0.042 | 0.01 | 10% |
| — | — | — | — | BCO1 rs7501331 genotype | −0.093 ± 0.039 | 0.02 | 5% |
| Phytoene, nmol/g | None | — | — | No significant predictors | — | — | |
| Phytofluene, nmol/g | Log10 | 0.343 | <0.001 | Constant | −0.063 ± 0.149 | 0.68 | — |
| — | — | — | — | Diet group | −0.058 ± 0.054 | 0.30 | 0% |
| — | — | — | — | ABCA1 rs2230808 | −0.111 ± 0.100 | 0.28 | 0% |
| — | — | — | — | ABCA1 rs2230808 × diet group | 0.153 ± 0.07 | 0.04 | 6% |
| — | — | — | — | Log10 [Baseline plasma phytofluene (µmol/L)] (difference from mean) | 0.671 ± 0.149 | <0.001 | 33% |
| Baseline plasma | |||||||
| Lycopene, nmol/mg LDL cholesterol | None | 0.257 | 0.005 | Constant | 0.094 ± 0.022 | <0.001 | — |
| — | — | — | — | Usual daily lycopene intake (mg/d) | 0.003 ± 0.002 | 0.13 | 11% |
| — | — | — | — | BCO1 rs12934922 genotype | 0.013 ± 0.016 | 0.42 | 6.6% |
| — | — | — | — | BCO1 rs12934922 genotype × usual daily lycopene intake | −0.003 ± 0.002 | 0.07 | 7.3% |
| — | — | — | — | ABCA1 rs2230808 genotype | −0.024 ± 0.014 | 0.10 | 0% |
| — | — | — | — | ABCA1 rs2230808 genotype × usual daily lycopene intake | 0.002 ± 0.001 | 0.08 | 4.3% |
| β-carotene, µmol/L | Log10 | — | — | No significant predictors | — | — | |
| Phytoene, nmol/mg total cholesterol | Log10 | 0.138 | 0.026 | Constant | −2.186 ± 0.060 | <0.001 | — |
| — | — | — | — | Usual daily phytoene intake (mg/d) | 0.037 ± 0.026 | 0.17 | 0% |
| — | — | — | — | BCO1 rs7501331 genotype | 0.026 ± 0.079 | 0.74 | 7.2% |
| — | — | — | — | BCO1 rs7501331 genotype × usual daily phytoene intake | −0.091 ± 0.041 | 0.03 | 10.5% |
| Phytofluene, µmol/L | Log10 | 0.131 | 0.018 | Constant | −0.663 ± 0.126 | <0.001 | — |
| — | — | — | — | Usual daily phytofluene intake | 0.108 ± 0.049 | 0.04 | 7.6% |
| — | — | — | — | SCARB1 rs11057841 genotype | −0.128 ± 0.066 | 0.06 | 5.5% |
1 ABCB1, ATP-binding cassette subfamily B member 1; BCO1, β-carotene 15,15’ monooxygenase 1; SCARB1, scavenger receptor class B type 1.
2As determined by stepwise removal of variables and quantifying the contribution of the variable to the model's adjusted R2. See Supplemental Figure 3 for graphical depiction of the proportion of variance in the outcome explained by each variable.
3“Constant” represents the y-intercept for the model.
Experiment 1.
The change in plasma β-carotene was impacted by a main effect of the BCO1 rs12934922 genotype (Table 4), with the T-allele being predictive of greater increases in plasma β-carotene (Figure 2), whereas none of the tested SNPs was associated with the change in plasma phytoene. The MTTP rs2306985 (Figure 3) genotype was suggestively predictive of the change in plasma phytofluene, with each additional G-allele reducing the increase in plasma phytofluene.
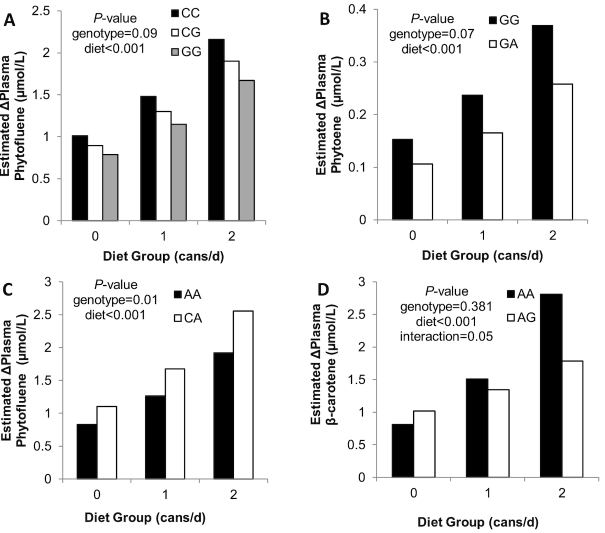
FIGURE 3.
Model-predicted changes of plasma carotenoids by non-BCO1 candidate SNP genotypes MTTP rs2306985 (A), “SOD” rs9365046 (B), ELOVL2 rs911196 (C), ABCB1 rs10248420 (D), and tomato-soy juice treatment. Bars represent the predicted mean response adjusted for other significant covariates as found in Table 5. Panels (C) and (D) show only 2 genotypes because the third genotype was either not present in our population or only 1 subject in the study carried that genotype. P values correspond with the variable effects from the multiple linear regression. Significant effects are P ≤ 0.05, and nonsignificantly trending effects are 0.05 < P ≤ 0.10. ABCB1, ATP-binding cassette subfamily B member 1; BCO1, β-carotene 15,15' monooxygenase; ELOVL2, elongation of very long chain fatty acids protein 2; MTTP, microsomal triglyceride transfer protein; SNP, single nucleotide polymorphism; SOD, superoxide dismutase, mitochondrial.
Experiment 2.
Several genetic variables previously associated with lycopene bioavailability by Borel et al. (27) were predictive of the change in plasma phytoene, β-carotene, and phytofluene, and are shown in Table 5 and Figure 3. The change in plasma β-carotene concentration could be predicted by diet group, β-carotene consumed from nonintervention foods, and a suggestive interaction between ABCB1 rs10248420 genotype × diet group (P = 0.053). The change in plasma phytoene was predicted by diet group, baseline plasma phytoene concentration (adjusted for total cholesterol), and suggestively by the rs9365046 genotype. The change in plasma phytofluene was predicted by diet group, baseline plasma phytofluene concentration, body mass, and ELOVL2 rs911196 genotype.
Factors predicting end-of-study prostate carotenoid concentrations
An effect of diet group alone on prostate carotenoid concentrations was not detectable in this study, likely because of the short intervention period as previously discussed (28). Because enrollment prostate carotenoid concentrations could not be measured, we could not determine changes from dietary intervention.
Experiment 1.
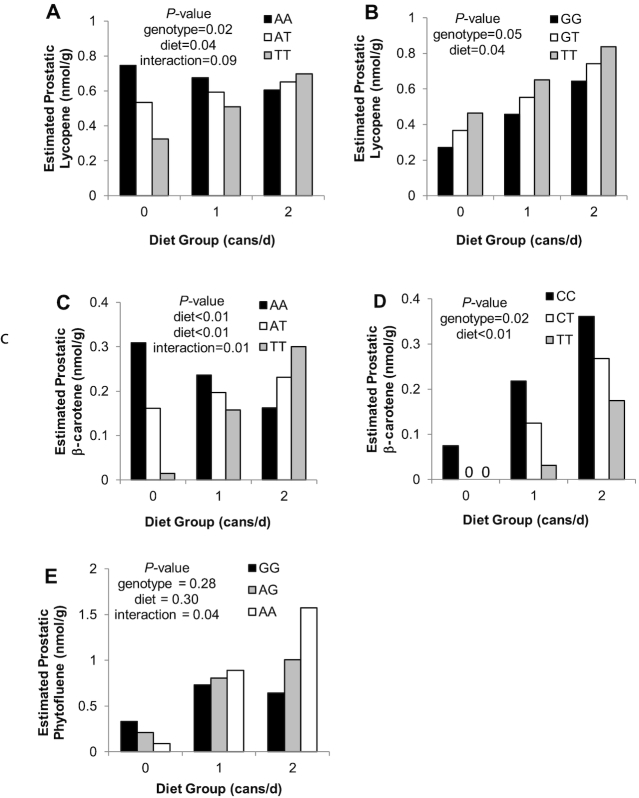
The predictive model variables for end-of-study prostate carotenoid concentrations are shown in Table 6 (Supplemental Figure 3). Prostatic lycopene concentrations could be predicted by a combination of diet group, baseline plasma lycopene concentration, and a suggestive interaction between the BCO1 rs12934922 genotype with diet group, with the T-allele being associated with lower prostate lycopene concentrations in the 0 cans/d group (0.21 nmol/g per T-allele) and 1 can/d group (0.8 nmol/g per T-allele), but greater concentrations in the 2 cans/d group (0.05 nmol/g per T-allele) (Figure 4). The BCO1 rs12934922 genotype also had a significant main effect and BCO1 rs6564851 genotype was also associated with prostate lycopene concentrations, with the T-allele being associated with a greater prostate lycopene concentration (0.1 nmol/g per T-allele), regardless of diet group (Figure 4). Similarly, the BCO1 rs12934922 genotype interacted with diet group to impact prostate β-carotene concentrations, in the same pattern as that for prostate lycopene (Figure 4), with each T-allele being associated with lower prostate β-carotene concentrations in the 0 and 1 can/d groups and greater concentrations in the 2 cans/d group. The BCO1 rs7501331 T-allele was associated with lower concentrations of prostate β-carotene (Figure 4). In addition to these 2 BCO1 SNPs impacting prostate β-carotene, baseline plasma β-carotene, a marker of longer-term β-carotene status, and body mass were also predictors. The ABCA1 rs2230808 genotype significantly interacted with diet group to be predictive of prostate phytofluene concentrations, with each A-allele conferring a decrease in concentration in the 0 cans/d group, an increase in the 1 can/d group, and greater increases in the 2 cans/d group (Figure 4). None of the SNP genotypes were predictive of prostatic phytoene.
FIGURE 4.
Model-predicted end-of-study prostate carotenoid concentrations by diet group and BCO1 SNP genotypes rs12934922 (A), rs6564851 (B), rs12934922 (C), rs7501331 (D), and by ABCA1 rs2230808 genotype (E). Bars represent predicted mean outcomes adjusted for significant covariates and variables found in Table 6. P values correspond with the variable effects from the multiple linear regression models. Significant effects are P ≤ 0.05, and nonsignificantly trending effects are 0.05 < P ≤ 0.10. ABCA1, ATP-binding cassette subfamily B member 1; BCO1, β-carotene 15,15’ monooxygenase; SNP, single nucleotide polymorphism.
Experiment 2.
Genotypes of the 4 candidate SNPs previously found to be related to lycopene bioavailability were not significantly predictive of prostate carotenoid concentrations (Table 7; Supplemental Figure 4).
TABLE 7.
Experiment 2: variables predictive of end-of-study prostate and baseline plasma carotenoid concentrations in men with prostate cancer consuming tomato-soy juice
| Tissue carotenoid concentration outcome | Transformation | Model adjusted R2 | Model P value | Variable | β ± SE | P value for variable | Estimated proportion of response variability attributable to variable1 |
|---|---|---|---|---|---|---|---|
| Prostate | |||||||
| Lycopene, nmol/g | None | 0.204 | 0.001 | Constant2 | 0.636 ± 0.037 | <0.001 | — |
| — | — | — | Intervention duration (d) (difference from mean) | 0.756 ± 0.408 | 0.07 | 4.1% | |
| — | — | — | Baseline plasma lycopene (nmol/mg LDL cholesterol) (difference from mean) | 2.89 ± 0.898 | <0.0 | 16.3% | |
| β-carotene, nmol/g | None | 0.333 | <0.001 | Constant | 0.098 ± 0.033 | <0.01 | — |
| — | — | — | Log10 [Baseline plasma β-carotene (µmol/L)] (difference from mean) | 0.192 ± 0.041 | <0.001 | 28.3% | |
| — | — | — | Body mass (kg) (difference from mean) | −0.003 ± 0.001 | 0.03 | 5.0% | |
| Phytoene, nmol/g | None | — | — | No significant predictors | — | — | |
| Phytofluene, nmol/g | Log10 | 0.372 | <0.001 | Constant | −0.077 ± 0.097 | 0.431 | — |
| — | — | — | Log10 [Baseline plasma phytofluene (µmol/L)] (difference from mean) | 0.696 ± 0.124 | <0.001 | 35.3% | |
| Baseline plasma | |||||||
| Lycopene, nmol/mg LDL cholesterol | None | 0.116 | 0.006 | Constant | 0.093 ± 0.008 | <0.001 | — |
| — | — | — | Usual daily lycopene intake (mg/d) | 0.002 ± 0.001 | <0.01 | 11.6% | |
| β-carotene, µmol/L | Log10 | 0 | n/a | No significant predictors | — | — | |
| Phytoene, nmol/mg total cholesterol | Log10 | 0 | n/a | No significant predictors | — | — | |
| Phytofluene, µmol/L | Log10 | 0.0757 | 0.024 | Usual daily phytofluene intake (mg/d) | 0.111 ± 0.048 | 0.02 | 7.6% |
1As determined by stepwise removal of variables and quantifying the contribution of the variable to the model's adjusted R2. See Supplemental Figure 4 for graphical depiction of the proportion of variance in the outcome explained by each variable.
2“Constant” represents the y-intercept for the model.
Factors predicting baseline plasma carotenoid concentrations
Experiment 1.
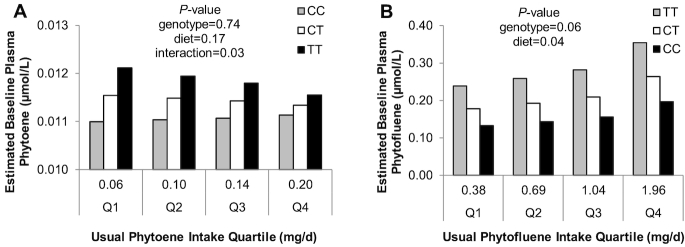
The variables predicting baseline plasma carotenoid concentrations are shown in Table 6, and Supplemental Figure 3. Baseline plasma lycopene is suggestively (but nonsignificantly) predicted by estimated average daily lycopene intake, BCO1 rs12934922 genotype interaction with estimated average daily lycopene intake, ABCA1 rs2230808 genotype interaction with estimated average daily lycopene intake, and a main effect of ABCA1 rs2230808 genotype. Baseline plasma phytoene could be predicted by an interaction between BCO1 rs7501331 genotype and usual daily phytoene intake (Figure 5). Baseline plasma phytofluene could be predicted by usual daily phytofluene intake and a nonsignificant trend (0.05 < P < 0.10) SCARB1 rs11057841 genotype effect (Figure 5). Baseline plasma β-carotene concentrations were not associated with body mass index or the evaluated SNPs.
FIGURE 5.
Model-predicted effects of SNP genotypes BCO1 rs7501331 (A) and SCARB1 rs11057841 (B) on baseline carotenoid concentrations. Bars represent predicted mean outcomes adjusted for significant covariates and variables found in Table 6. Mean usual carotenoid intake values are presented for each quartile. Significant effects are P ≤ 0.05, and nonsignificantly trending effects are 0.05 < P ≤ 0.10. BCO1, β-carotene 15,15’ monooxygenase; SCARB1, scavenger receptor class B type 1; SNP, single nucleotide polymorphism.
Experiment 2.
None of the candidate SNPs previously found to be predictive of lycopene bioavailability was a predictor of baseline plasma lycopene, phytoene, β-carotene, or phytofluene concentrations in our study (Table 7 and Supplemental Figure 4).
Discussion
In previous intervention studies with tomato products we observed significant changes in plasma lycopene concentrations over a 2–4-wk period (24, 25, 28, 34, 45). However, we have also seen a very wide range of achieved plasma carotenoid concentrations as in the current study (Figure 1), which is not explained by a lack in intervention compliance or controlled lycopene background diet compliance. The goal was to determine whether common candidate SNPs, hypothesized to impact carotenoid assimilation, could explain variability in plasma lycopene responses to a controlled tomato juice intervention. Secondarily, we explored whether these SNPs were predictors of plasma and tissue responses to other carotenoids. In the first set of SNPs, BCO1 rs6564851 and rs12934922 interactions with the dose of tomato juice explained 5.6% of the variation in plasma lycopene responses, with a total of 75.7% of the variance being explained by a combination of baseline characteristics, diet group, and genetics. SNPs previously associated with lycopene bioavailability (27) were not predictive of the plasma lycopene response in this steady state intervention. Perhaps a critical conclusion from this study is that carotenoid absorption and achieved plasma and tissue concentrations are the results of complex, dynamically regulated, but possibly differing metabolic processes.
While lesser proportions of total variance were explained for the additional outcomes, BCO1 rs12934922 explained 19% of variance in the change in plasma β-carotene. BCO1 SNPs repeatedly emerged as predictors of additional outcomes. BCO1 is an oxidative cleavage enzyme best known for converting provitamin A carotenoids, such as β-carotene, to retinal (vitamin A), but also cleaves lycopene in vitro, despite being a nonprovitamin A carotenoid (35, 46). However, a murine study did not indicate that BCO1 cleaves lycopene in vivo (47), thus further study is needed to confirm BCO1 cleavage activity of lycopene. The rs12934922 genotype appeared to have the strongest impact on carotenoid measures in this study. A previous study reported the rs12934922 T-allele to be associated with reduced in vitro and in vivo catalytic activity of BCO1 for β-carotene, resulting in greater plasma β-carotene concentrations in female volunteers after consuming a single, 120 mg β-carotene dose (48). This SNP was predictive of the change in plasma β-carotene and prostate β-carotene. Suggestive trends emerged between rs12934922 genotype and prostate lycopene as well as baseline plasma lycopene. These results suggest that rs12934922 might, as for β-carotene (48), impact BCO1’s potential lycopene cleavage efficiency, with the T-allele leading to greater lycopene accumulation. Alternatively, if rs12934922 reduces conversion of β-carotene to vitamin A, this may have an indirect effect on lycopene absorption by upregulating uptake via SCARB1 as a result of reduced retinoic acid status (39, 49, 50).
It is interesting to note that the different dose-levels provided in the current study revealed, for the first time, BCO1 SNP × diet group interactions; however, the potential mechanisms underlying the dose-dependent effects of the BCO1 SNPs will require future investigation. These BCO1 SNP × diet interactions may illustrate the complexities of genetic impacts on carotenoid pharmacokinetics. It is currently known that expression of BCO1 and SCARB1, a membrane-associated protein involved in carotenoid absorption, are under negative-feedback regulation by retinoic acid, a downstream product of BCO1-catalyzed β-carotene cleavage (39, 49). Thus, it is possible that the current observed interactions are the result of a dynamic system in which BCO1-generated metabolites regulate uptake and subsequent cleavage of dietary carotenoids in a concentration-dependent manner.
Other BCO1 SNPs also emerged as predictors of carotene status. The BCO1 rs7501331 genotype was a determinant of end-of-study prostate β-carotene, with the T-allele being associated with lower prostate β-carotene and baseline plasma phytoene. In contrast, previously the rs7501331 T-allele was associated with reduced catalytic activity of BCO1 for β-carotene and greater plasma β-carotene in women after a single 120 mg dose (48), but the impact on tissue carotene concentrations was not previously reported. Herein, BCO1 rs6564851 × diet group was a predictor of the change in plasma lycopene and trended toward an association with prostate lycopene, with the T-allele being associated with greater increases in plasma lycopene in the 1 and 2 cans/d groups, with greater decreases in plasma lycopene in the 0 cans/d group, and greater prostate lycopene across diet groups. The T-allele of rs6564851, located 5’ upstream of BCO1, was previously associated with lower plasma β- and α-carotene, but greater lycopene in several large cohorts (30). Another study found the T-allele to be associated with greater postprandial retinyl palmitate: β-carotene, indicative of increased β-carotene cleavage (51). However, this is the first clinical support for association of the rs6564851 T-allele with lycopene. BCO1 SNPs were generally less impactful on phytoene and phytofluene status, and at this time, it is unclear these carotenes are BCO1 substrates.
Of the additional hypotheses, SNPs in MTTP, ABCB1, ABCA1, and SCARB1 were associated with changes in plasma carotenes in response to the intervention. MTTP is involved in intestinal chylomicron and hepatic VLDL and LDL assembly (52), thus gene defects could impair packaging of lipid-soluble cargoes. ABCB1 is a “drug” efflux pump with broad substrate specificity (53), which could include carotenoids. ABCA1 is involved in HDL lipidation, variants have been associated with blood lutein, and it may be involved in carotenoid transport (54, 55). Lastly, SCARB1 rs101057841 was predictive of baseline plasma phytofluene in the current study, and its T-allele was previously associated with greater serum lutein (40).
This is the first analysis of genetic determinants of both plasma and tissue lycopene responses to a controlled tomato product intervention. It is important to acknowledge that the tomato juice contained an isoflavone-rich soybean extract, which may have components that could impact the magnitude of carotenoid responses and variable effect sizes. Importantly, the study design incorporated a dose-effect element superimposed over a self-selected controlled and documented carotenoid diet, revealing several diet group × genotype interactions. Weaknesses include the modest power and that not all genotype × diet combinations were equally sampled. As an exploratory study, we examined both significant and nonsignificantly trending SNPs and SNP × diet interactions from the single-SNP screening models in the combined SNP models, and presented the significant and nonsignificant trend relations. A table of all screening models tested, model P values, and multicomparison adjusted model false discovery rates are presented in Supplemental Table 2. Readers are advised to interpret both negative and positive results with caution because of the small size and post hoc nature of this study.
On the whole, the results from this study suggest that several common BCO1 SNPs may be determinants of the change in plasma lycopene. The findings support the hypothesis that genetic variation is an important cause of person-to-person variance in dietary lycopene responses. Furthermore, if these variants impact the plasma and tissue concentrations of lycopene, it is also plausible that they modulate the efficacy of lycopene for disease risk modification. To better understand humans’ gene-carotenoid interactions, future larger studies of groups consuming controlled doses of dietary carotenoids should be investigated.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—NEM, JMT-A, JLF, JPM, EMG, AET, and SKC: designed the research; NEM, JMT-A, JLF, RM, EMG, and SKC: conducted the research; NEM and JPM: conducted the statistical analysis; NEM, JMT-A, JLF, JPM, RM, EMG, KMR, AET, SJS, and SKC: analyzed the data; NEM: principal author; and all authors: read and approved the final paper.
Notes
Supported by the National Cancer Institute of the National Institutes of Health by Award Numbers R01CA112632, P30CA016058 and the National Center for Advancing Translational Sciences by Award Number UL1TR001070. NEM was funded by the National Center for Complementary and Integrative Health and the Office of Dietary Supplements under award number K99/R00 AT008576 and by The USDA Agricultural Research Service under CRIS 3092-51000-056-03S. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the USDA, or NIH NCI, NCCIH, or ODS. Additional support was provided by The Ohio State University's Food Innovation Center and The Center for Advancement of Functional Foods Research and Entrepreneurship.
Author disclosures: NEM, JMT-A, JLF, JPM, RM, EMG, KMR, AET, SJS, and SKC, no conflicts of interest.
Supplemental Tables 1−4 and Supplemental Figures 1−4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ABCA1, ATP-binding cassette transporter subfamily A member 1; ABCB1, ATP-binding cassette subfamily B member 1; BCO1, β-carotene 15,15’ monooxygenase 1; ELOVL2, elongation of very long chain fatty acids protein 2; MTTP, microsomal triglyceride transfer protein; SCARB1, scavenger receptor class B type 1; SNP, single nucleotide polymorphism.
References
- 1. Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87(23):1767–76. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Jacobs EJ, Newton CC, McCullough ML. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer Prevention Study-II Nutrition Cohort. Int J Cancer. 2016;138(12):2846–55. [DOI] [PubMed] [Google Scholar]
- 3. Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, Wilson KM, Rider JR, Fiorentino M, Finn S, Kenfield SA et al.. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103(3):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, Giovannucci E. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106(2):djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, Black A, Boeing H, Bueno-de-Mesquita HB, Chan JM et al.. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr. 2015;102(5):1142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowles JL 3rd, Ranard KM, Smith JW, An R, Erdman JW Jr.. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20(4):361–77. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Cui R, Xiao Y, Fang J, Xu Q. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. PLoS One. 2015;10(9):e0137427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen P, Zhang W, Wang X, Zhao K, Negi DS, Zhuo L, Qi M, Wang X, Zhang X. Lycopene and risk of prostate cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(33):e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95(21):1578–86. [DOI] [PubMed] [Google Scholar]
- 10. Zuniga KE, Clinton SK, Erdman JW Jr.. The interactions of dietary tomato powder and soy germ on prostate carcinogenesis in the TRAMP model. Cancer Prev Res (Phila). 2013;6(6):548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konijeti R, Henning S, Moro A, Sheikh A, Elashoff D, Shapiro A, Ku M, Said JW, Heber D, Cohen P et al.. Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate. 2010;70(14):1547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan H-L, Thomas-Ahner JM, Moran NE, Erdman JW Jr, Young GS, Clinton SK. β-carotene 9’,10’ oxygenase modulates the anticancer activity of dietary tomato or lycopene on prostate carcinogenesis in the TRAMP model. Cancer Prev Res (Phila). 2017;10(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan L, Tan HL, Thomas-Ahner JM, Pearl DK, Erdman JW Jr., Moran NE, Clinton SK. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev Res (Phila). 2014;7(12):1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, Ambrosone CB, Thompson IM. Serum lycopene concentration and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2011;20(4):638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cichon MJ, Riedl KM, Schwartz SJ. A metabolomic evaluation of the phytochemical composition of tomato juices being used in human clinical trials. Food Chem. 2017;228:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grainger EM, Hadley CW, Moran NE, Riedl KM, Gong MC, Pohar K, Schwartz SJ, Clinton SK. A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br J Nutr. 2015;114(4):596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohn W, Thurmann P, Tenter U, Aebischer C, Schierle J, Schalch W. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur J Nutr. 2004;43(5):304–12. [DOI] [PubMed] [Google Scholar]
- 18. Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135(3):431–6. [DOI] [PubMed] [Google Scholar]
- 19. Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80(2):396–403. [DOI] [PubMed] [Google Scholar]
- 20. Conlon LE, King RD, Moran NE, Erdman JW Jr.. Coconut oil enhances tomato carotenoid tissue accumulation compared to safflower oil in the Mongolian gerbil (Meriones unguiculatus). J Agric Food Chem. 2012;60(34):8386–94. [DOI] [PubMed] [Google Scholar]
- 21. Scott KJ, Thurnham DI, Hart DJ, Bingham SA, Day K. The correlation between the intake of lutein, lycopene and β-carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50–65 years in the UK. Br J Nutr. 1996;75(3):409–18. [DOI] [PubMed] [Google Scholar]
- 22. Moran NE, Cichon MJ, Riedl KM, Grainger EM, Schwartz SJ, Novotny JA, Erdman JW Jr, Clinton SK. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am J Clin Nutr. 2015;102(6):1436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moran NE, Novotny JA, Cichon MJ, Riedl KM, Rogers RB, Grainger EM, Schwartz SJ, Erdman JW Jr, Clinton SK. Absorption and distribution kinetics of the 13C-labeled tomato carotenoid phytoene in healthy adults. J Nutr. 2016;146(2):368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alien CM, Smith AM, Clinton SK, Schwartz SJ. Tomato consumption increases lycopene isomer concentrations in breast milk and plasma of lactating women. J Am Diet Assoc. 2002;102(9):1257–62. [DOI] [PubMed] [Google Scholar]
- 25. Allen CM, Schwartz SJ, Craft NE, Giovannucci EL, De Groff VL, Clinton SK. Changes in plasma and oral mucosal lycopene isomer concentrations in healthy adults consuming standard servings of processed tomato products. Nutr Cancer. 2003;47(1):48–56. [DOI] [PubMed] [Google Scholar]
- 26. Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res. 2012;56(2):228–40. [DOI] [PubMed] [Google Scholar]
- 27. Borel P, Desmarchelier C, Nowicki M, Bott R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic Biol Med. 2015;83:238–44. [DOI] [PubMed] [Google Scholar]
- 28. Grainger EM, Moran NE, Francis D, Wan L, Kopec RE, Riedl KM, Abaza R, Schwartz SJ, Clinton SK. A novel tomato-soy juice induces a dose-response increase in urinary and plasma phytochemical biomarkers in men with prostate cancer. J Nutr. 2018; doi: 10.1093/jn/nxy232. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moran NE, Mohn ES, Hason N, Erdman JW Jr, Johnson EJ. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv Nutr. 2018;9(4):465–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C et al.. Common variation in the β-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84(2):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zubair N, Kooperberg C, Liu J, Di C, Peters U, Neuhouser ML. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J Nutr. 2015;145(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Adamo CR, D'Urso A, Ryan KA, Yerges-Armstrong LM, Semba RD, Steinle NI, Mitchell BD, Shuldiner AR, McArdle PF. A common variant in the SETD7 gene predicts serum lycopene concentrations. Nutrients. 2016;8(2):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang TT, Edwards AJ, Clevidence BA. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to β-carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms. J Nutr Biochem. 2013;24(8):1538–46. [DOI] [PubMed] [Google Scholar]
- 34. Bohn T, Blackwood M, Francis D, Tian Q, Schwartz SJ, Clinton SK. Bioavailability of phytochemical constituents from a novel soy fortified lycopene rich tomato juice developed for targeted cancer prevention trials. Nutr Cancer. 2013;65(6):919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. dela Sena C, Narayanasamy S, Riedl KM, Curley RW Jr, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant human β-carotene 15,15'-oxygenase (BCO1). J Biol Chem. 2013;288(52):37094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281(28):19327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moussa M, Gouranton E, Gleize B, Yazidi CE, Niot I, Besnard P, Borel P, Landrier JF. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol Nutr Food Res. 2011;55(4):578–84. [DOI] [PubMed] [Google Scholar]
- 38. Reboul E, Abou L, Mikail C, Ghiringhelli O, Andre M, Portugal H, Jourdheuil-Rahmani D, Amiot MJ, Lairon D, Borel P. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem J. 2005;387(Pt 2):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production. FASEB J. 2010;24(6):1656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McKay GJ, Loane E, Nolan JM, Patterson CC, Meyers KJ, Mares JA, Yonova-Doing E, Hammond CJ, Beatty S, Silvestri G. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology. 2013;120(8):1632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31(21):3555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dworkin AM, Ridd K, Bautista D, Allain DC, Iwenofu OH, Roy R, Bastian BC, Toland AE. Germline variation controls the architecture of somatic alterations in tumors. PLoS Genet. 2010;6(9):e1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romanchik JE, Morel DW, Harrison EH. Distributions of carotenoids and α-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J Nutr. 1995;125(10):2610–7. [DOI] [PubMed] [Google Scholar]
- 44. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Method. 1995;57(1):289–300. [Google Scholar]
- 45. Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, Ferketich AK, Monk JP, Gong MC, Bahnson RR, DeGroff VL et al.. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145–54. [DOI] [PubMed] [Google Scholar]
- 46. Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX Jr.. Identification, expression, and substrate specificity of a mammalian β-carotene 15,15'-dioxygenase. J Biol Chem. 2001;276(9):6560–5. [DOI] [PubMed] [Google Scholar]
- 47. Ford NA, Elsen AC, Erdman JW Jr.. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res. 2013;33(9):733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15'-monoxygenase alter β-carotene metabolism in female volunteers. FASEB J. 2009;23(4):1041–53. [DOI] [PubMed] [Google Scholar]
- 49. Widjaja-Adhi MA, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet. 2015;24(11):3206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem. 2013;288(13):9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the β-carotene 15,15'-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142(1):161S–5S. [DOI] [PubMed] [Google Scholar]
- 52. Communications LHNCfB. MTTP. Genetics Home Reference. 2/22/2016 ed Bethesda, MD: U.S. National Library of Medicine; 2016. [Google Scholar]
- 53. Communications LHNCfB. ABCB1. Genetics Home Reference. 2/22/2016 ed Bethesda, MD: U.S. National Library of Medicine; 2016. [Google Scholar]
- 54. Niesor EJ, Chaput E, Mary JL, Staempfli A, Topp A, Stauffer A, Wang H, Durrwell A. Effect of compounds affecting ABCA1 expression and CETP activity on the HDL pathway involved in intestinal absorption of lutein and zeaxanthin. Lipids. 2014;49(12):1233–43. [DOI] [PubMed] [Google Scholar]
- 55. Meyers KJ, Mares JA, Igo RP Jr, Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK et al.. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Invest Ophthalmol Vis Sci. 2014;55(1):587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.